Abstract
The gram-positive bacterium Bacillus subtilis contains two minimal Tat translocases, TatAdCd and TatAyCy, which are each involved in the secretion of one or more specific protein substrates. We have investigated the subcellular localization of the TatA components by employing C-terminal green fluorescent protein (GFP) fusions and fluorescence microscopy. When expressed from a xylose-inducible promoter, the TatA-GFP fusion proteins displayed a dual localization pattern, being localized peripherally and showing bright foci which are predominantly located at the division sites and/or poles of the cells. Importantly, the localization of TatAd-GFP was similar when the protein was expressed from its own promoter under phosphate starvation conditions, indicating that these foci are not the result of artificial overexpression. Moreover, the TatAd-GFP fusion protein was shown to be functional in the translocation of its substrate PhoD, provided that TatCd is also present. Furthermore, we demonstrate that the localization of TatAd-GFP in foci depends on the presence of the TatCd component. Remarkably, however, the TatAd-GFP foci can also be observed in the presence of TatCy, indicating that TatAd can interact not only with TatCd but also with TatCy. These results suggest that the formation of TatAd complexes in B. subtilis is controlled by TatC.
The bacterial twin-arginine translocation (Tat) machinery is able to transport folded proteins across the cytoplasmic membrane (26). Preproteins translocated by the Tat pathway are characterized by a twin-arginine (RR) motif in their signal sequences.
In Escherichia coli, the Tat system consists of three components, the TatA, TatB, and TatC proteins. In the currently favored model for its mode of action, a TatB-TatC complex is involved in initial RR signal peptide recognition and binding of precursor proteins. Multiple TatA subunits then associate with this complex to form a protein-conducting channel (1). TatA, which is homologous to TatB, can be found complexed to TatBC but also forms a wide range of large, homooligomeric complexes (7, 23). In a few cases, the TatB protein can be functionally replaced by the TatA protein, indicating that TatA and TatC are able to form an active, minimal translocase (6, 10).
Most gram-positive bacteria contain only two types of Tat subunit, a TatC protein and a TatA protein which has characteristics and the ability to perform the function of both TatA and TatB of E. coli (2, 13). Bacillus subtilis contains two substrate-specific Tat systems: a TatAyCy translocase that is required for translocation of the iron-dependent DyP peroxidase YwbN and a TatAdCd translocase which translocates the phosphodiesterase PhoD (12). In addition, B. subtilis contains a third TatA component, designated TatAc. This protein is dispensable for Tat-dependent translocation of YwbN or PhoD, and its function is currently unknown.
TatAd is the most-studied TatA component of B. subtilis, and like TatA of E. coli, it is able to form both homooligomeric complexes and complexes with TatCd (2, 31). Despite the fact that it contains an N-terminal transmembrane segment (17), TatAd was also found in the cytosol, where it appears to interact with its substrate, pre-PhoD, via the signal sequence (24). TatCd was proposed to act as a receptor for the anchoring at and subsequent incorporation into the membrane of this TatAd-PhoD complex (28).
The subcellular localization of Tat components in E. coli has been extensively investigated by fluorescence microscopy. Green fluorescent protein (GFP) fusions of TatA were localized at the periphery of the cells, but punctate regions of fluorescence were also reported (4, 25). In these studies, TatB was localized all over the membrane, with some accumulation at the cell poles. TatC was mainly distributed evenly throughout the periphery of the cells, with some small punctate regions. Recently, the oligomeric state of TatA-yellow fluorescent protein (YFP) in living E. coli cells was determined by single-molecule imaging (18). TatA complexes with a broad range of stoichiometries were observed as fluorescent foci, and TatA was also present in a dispersed state in the membrane.
For B. subtilis, the subcellular localization of only one Tat component has been reported so far. Both N- and C-terminal fusions of GFP to TatCy were shown to be localized throughout the membrane, with frequent foci at the cell poles and division septa, and this localization pattern was classified as “polar” (20).
In this study, we have investigated the subcellular localization of the three TatA proteins of B. subtilis by using GFP fusions, functionality assessments, and fluorescence microscopy. TatAc and TatAd showed a dual localization pattern, with fluorescence in the membrane as well as in foci which were enriched at the cell poles. Notably, the localization of TatAd-GFP in foci was shown to depend on the presence of a TatC component, suggesting that TatC drives complex formation by TatAd.
MATERIALS AND METHODS
Plasmids, bacterial strains, and media.
Table 1 lists the strains and plasmids used in this study. Strains were grown at 37°C. TY (tryptone-yeast extract) medium contained Bacto tryptone (1%), Bacto yeast extract (0.5%), and NaCl (1%). High-phosphate (HPDM) and low-phosphate (LPDM) defined media were prepared as described previously (21), and phosphate was added as KH2PO4 to a final concentration of 3.5 mM (HPDM) or 0.42 mM (LPDM). When required, the medium for E. coli or B. subtilis culture was supplemented with spectinomycin (Sp; 100 μg/ml), ampicillin (Ap; 100 μg/ml), chloramphenicol (Cm; 5 μg/ml), kanamycin (Km; 10 μg/ml), and/or erythromycin (Em; 5 μg/ml).
TABLE 1.
Plasmids and strains
| Plasmid or strain | Genotypea/relevant features | Reference |
|---|---|---|
| Plasmids | ||
| pSG1151 | gfpmut1 Apr Cmr | 19 |
| pSG1151-TatAd | Derivative of pSG1151; tatAd-gfpmut1 Apr Cmr | This work |
| pSG1154 | amyE3′ spc Pxyl-gfpmut1 amyE5′ Spr Apr | 19 |
| pSG1154-TatAc | Derivative of pSG1154; amyE3′ spc Pxyl-tatAc-gfpmut1 amyE5′ Spr Apr | This work |
| pSG1154-TatAd | Derivative of pSG1154; amyE3′ spc Pxyl-tatAd-gfpmut1 amyE5′ Spr Apr | This work |
| pSG1154-TatAy | Derivative of pSG1154; amyE3′ spc Pxyl-tatAy-gfpmut1 amyE5′ Spr Apr | This work |
| pGDL48 | Contains multiple cloning site to place genes under the control of the erythromycin promoter; Apr Kmr | 30 |
| pCAy | pGDL48 derivative containing the tatAy gene; Apr Kmr | 12 |
| pCACy | pGDL48 derivative containing the tatAyCy operon; Apr Kmr | 12 |
| pCCy | pGDL48 derivative containing the tatCy gene; Apr Kmr | 12 |
| pCCd | pGDL48 derivative containing the tatCd gene; Apr Kmr | This work |
| pECy20 | pUC21 derivative for the disruption of tatCy; Apr Emr | This work |
| B. subtilis strains | ||
| 168 | trpC2 | 15 |
| 168 X-Ac-gfp | trpC2 amyE::pSG1154-TatAc (Pxyl-tatAc-gfpmut1) Spr | This work |
| 168 X-Ad-gfp | trpC2 amyE::pSG1154-TatAd (Pxyl-tatAd-gfpmut1) Spr | This work |
| 168 X-Ay-gfp | trpC2 amyE::pSG1154-TatAy (Pxyl-tatAy-gfpmut1) Spr | This work |
| 168 tatAc1 | trpC2 tatAc::Km Kmr | 12 |
| 168 tatAc1 X-Ad-gfp | trpC2 tatAc::Km amyE::pSG1154-TatAd (Pxyl-tatAd-gfpmut1) Kmr Spr | This work |
| 168 ΔtatAdCd | trpC2 tatAd-tatCd::Km Kmr | 12 |
| 168 ΔtatAdCd X-Ad-gfp | trpC2 tatAd-tatCd::Km amyE::pSG1154-TatAd (Pxyl-tatAd-gfpmut1) Kmr Spr | This work |
| 168 ΔtatAy(Cy) | trpC2 tatAy::Cm Cmr | This work |
| 168 ΔtatAy(Cy) X-Ad-gfp | trpC2 tatAy::Cm amyE::pSG1154-TatAd (Pxyl-tatAd-gfpmut1) Cmr Spr | This work |
| 168 Ad-gfp | trpC2 chr::pSG1151-TatAd (tatAd-gfpmut1 ′tatAd) Cmr | This work |
| 168 ΔtatCy | trpC2 tatCy::Em Emr | This work |
| 168 ΔtatCy Ad-gfp | trpC2 tatCy::Em chr::pSG1151-TatAd (tatAd-gfpmut1 ′tatAd) Cmr Emr | This work |
gfpmut1 encodes a variant of GFP, described in reference 19.
DNA techniques.
Procedures for DNA purification, restriction, ligation, agarose gel electrophoresis, and transformation of E. coli cells were carried out essentially as described previously (27). Enzymes were from Roche Molecular Biochemicals or MBI Fermentas. PCR was performed using Expand polymerase (Roche Molecular Biochemicals) or Phusion polymerase (Finnzymes). DNA sequences of all plasmids used were confirmed by double-stranded DNA sequencing. B. subtilis was transformed as described previously (30).
Plasmids expressing TatAc-GFP, TatAd-GFP, and TatAy-GFP fusions were constructed as follows. The tatAc gene was amplified by PCR from chromosomal DNA of B. subtilis strain 168 by using primers 5′-TAA GGT ACC ATG GAA TTA AGC TTC AC-3′ (forward, KpnI site) and 5′-ACT AGA ATT CCA TTT GTT TGT CTT CTT TG-3′ (reverse, EcoRI site). The tatAd gene was amplified by using primers 5′-TAA GGT ACC ATG TTT TCA AAC ATT GG-3′ (forward, KpnI site) and 5′-ACT AGA ATT CGC CCG CGT TTT TGT CCT GC-3′ (reverse, EcoRI site). The tatAy gene was amplified by using primers 5′-TAA GGT ACC ATG CCG ATC GGT CCT GG-3′ (forward, KpnI site) and 5′-AAG CTT ATC GAT CTG ATC TTC TTT CTT TTT TTC C-3′ (reverse, ClaI site). The resulting PCR fragments were digested with the indicated restriction enzymes and ligated into the corresponding sites of pSG1154 (19), resulting in plasmids pSG1154-TatAc, pSG1154-TatAd, and pSG1154-TatAy. In addition, the TatAd-encoding PCR fragment was ligated into the KpnI and EcoRI sites of pSG1151 (19), resulting in plasmid pSG1151-TatAd.
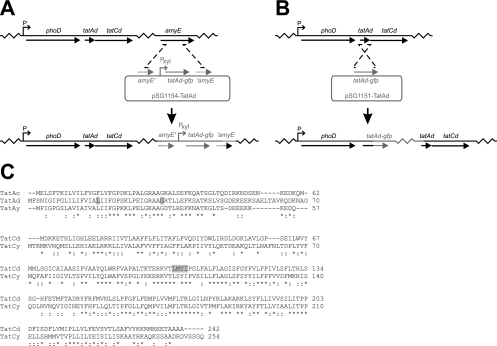
B. subtilis 168 X-Ac-gfp, 168 X-Ad-gfp, and 168 X-Ay-gfp (the X denotes the Pxyl promoter) were obtained by transformation of B. subtilis 168 with plasmids pSG1154-TatAc, pSG1154-TatAd, and pSG1154-TatAy, respectively (Fig. 1A). Colonies were screened for successful double crossover integration into the amyE gene by the loss of the ability to degrade starch. Strain 168 Ad-gfp was obtained by transforming B. subtilis 168 with plasmid pSG1151-TatAd, resulting in chromosomal integration via a single crossover event at the tatAd locus (Fig. 1B).
FIG. 1.
Strain construction and protein sequences. (A) Construction of strain 168 X-Ad-gfp by double crossover integration of plasmid pSG1154-TatAd into the amyE locus of B. subtilis 168. The resulting strain contains the native phoD-tatAd-tatCd locus, as well as one copy of tatAd-gfp under the control of the xylose-inducible Pxyl promoter. (B) Construction of strain 168 Ad-gfp by single crossover integration of plasmid pSG1151-TatAd into the tatAd locus of B. subtilis 168. This results in a strain in which phoD and tatAd-gfp are under the control of their native promoters, while the downstream tatAd and tatCd genes become promoterless. (C) Sequence comparison of TatAc, TatAd, and TatAy and of TatCd and TatCy of Bacillus subtilis. Sequences were aligned with the ClustalW multiple sequence alignment program (29). Identical amino acids (*) or conservative substitutions (:) are marked. Amino acids that were found to be different from the sequences listed in SubtiList are indicated by shading.
To construct plasmid pCCd, the tatCd gene was amplified from chromosomal DNA of B. subtilis 168 by PCR using primers 5′-ACG CGT CGA CGA AAG GGA GGG CTT TTT TG-3′ (forward, SalI site) and 5′-GGA ATT CGA AGT CAC CGG GTG GTA CG-3′ (reverse, EcoRI site). The resulting PCR fragment was purified from a 1% agarose gel, cleaved with SalI and EcoRI, and ligated into the corresponding sites of pGDL48 (30).
The construction of B. subtilis 168 ΔtatAy(Cy) was performed as described for the Em-resistant tatAy mutant strain (12), except for the use of a Cm resistance marker. Since it was shown previously that the replacement of the tatAy gene by an antibiotic resistance marker has polar effects on the transcription of tatCy, which is located downstream of the tatAy gene (12) (see Fig. 3C), this Cm-resistant mutant was named 168 ΔtatAy(Cy).
FIG. 3.
Localization of TatAd-GFP depends on TatCy. (A) Localization of TatAd-GFP in Tat deletion strains. Strains 168 tatAc1 X-Ad-gfp (ΔtatAc), 168 ΔtatAdCd X-Ad-gfp (ΔtatAdCd), and 168 ΔtatAy(Cy) X-Ad-gfp (ΔtatAyCy) were grown in TY medium containing 1% xylose for 5 h and examined by fluorescence microscopy. Phase-contrast (left) and fluorescence (right) pictures of the same cells are shown, and the scale bars indicate 5 μm. (B) Expression levels of TatAd-GFP. At the same time as the samples whose images are shown in Fig. 3A, samples containing equal amounts of cells were taken and analyzed by SDS-PAGE and Western blotting using anti-GFP antibodies. Mobilities of molecular mass markers (in kDa) are shown on the right. Lane 1, 168 tatAc1 X-Ad-gfp; lane 2, 168 ΔtatAdCd X-Ad-gfp; lane 3, 168 ΔtatAy(Cy) X-Ad-gfp; lane 4, 168 ΔtatAy(Cy) X-Ad-gfp/pCAy; lane 5, 168 ΔtatAy(Cy) X-Ad-gfp/pCACy; lane 6, 168 ΔtatAy(Cy) X-Ad-gfp/pCCy. (C) Localization of TatAd-GFP is influenced by TatCy. Strain 168 ΔtatAy(Cy) X-Ad-gfp containing complementation plasmid pCAy (+ TatAy), pCACy (+ TatAyCy), or pCCy (+ TatCy) was grown, and fluorescence was investigated as described for panel A.
B. subtilis 168 ΔtatCy was constructed as follows: the Sp resistance marker in plasmid pJCy2 (11) was removed by digestion with PstI and replaced by an Em resistance marker derived from plasmid pLR300 (14) digested with BglII and ClaI. The cleaved pJCy2 and Em resistance marker-encoding fragments were blunt ended with T4 DNA polymerase, and ligation was performed. The resulting plasmid, pECy20, contains the Em resistance marker integrated into the tatCy gene in such a way that the Emr gene is oriented in the same direction as the tatCy gene. Next, B. subtilis 168 ΔtatCy was obtained by a double crossover recombination between the disrupted tatCy gene of pECy20 and the chromosomal tatCy gene. Correct integration was checked by PCR analysis, and successful disruption of the tatCy gene was confirmed by checking the inability of the resulting strain to secrete the TatCy substrate, YwbN.
Finally, tat mutant strains expressing TatA-GFP fusions were obtained by chromosomal integration of the pSG1154 or pSG1151 derivatives into the respective tat mutant strains. During DNA sequencing of the pSG1154-TatAd and pSG1151-TatAd constructs, we identified two differences from the tatAd sequence that was previously published (11, 15) and is available on the SubtiList webpage (http://genolist.pasteur.fr/SubtiList/). We discovered that the isoleucine residue at position 18 is in fact a leucine residue and that the lysine residue at position 34 is in fact a glycine residue. At both positions, these are the same amino acids that are present at those positions in the TatAy protein, indicating that TatAd and TatAy are even more homologous than previously assumed (Fig. 1C). Similarly, the sequence of TatCd differs from the one available on the SubtiList webpage in such a way that an apparent internal nucleotide repeat is actually not present. As a result, the isoleucine residue at position 101 is in fact a leucine residue and the three amino acids downstream of this residue, methionine, tyrosine, and isoleucine, are not present. In short, the amino acid sequence IMYIMYI is in fact the sequence LMYI. Again, the sequence of TatCd is thus more similar to that of TatCy than previously reported.
Fluorescence microscopy.
For fluorescence microscopy, overnight cultures grown in media containing antibiotics were diluted in fresh medium without antibiotics to an optical density at 600 nm of 0.05 (in TY containing 1% xylose) or 0.1 (in HPDM or LPDM) and grown at 37°C. At appropriate time intervals, 6 μl of culture was applied directly to a microscope slide and examined using a 100× oil immersion objective on a Zeiss microscope (Carl Zeiss) and an Axion Vision camera (Axion Technologies).
Protein techniques.
To detect PhoD, cells were separated from the medium by centrifugation. Proteins in the medium were concentrated by precipitation with 10% trichloroacetic acid, and samples for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were prepared as described previously (16). After separation by SDS-PAGE, proteins were transferred to a polyvinylidene difluoride membrane (Molecular Probes) and PhoD was visualized with PhoD-specific antibodies (22) and horseradish peroxidase-conjugated anti-rabbit antibodies (Amersham Biosciences). GFP fusion proteins were visualized with GFP-specific antibodies (Molecular Probes) and horseradish peroxidase-conjugated anti-rabbit antibodies.
RESULTS
Localization of TatA proteins.
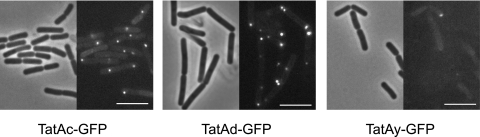
In order to investigate the subcellular localization of the TatA proteins of Bacillus subtilis, we constructed C-terminal GFP fusions of TatAc, TatAd, and TatAy under the control of a xylose-inducible promoter in plasmid pSG1154 (19). The resulting constructs were subsequently integrated into the chromosome of B. subtilis strain 168 via a double crossover event at the amyE locus, leaving the native tat genes intact (Fig. 1A). To visualize the TatA-GFP fusion proteins in living cells, the Bacillus strains producing these fusions were grown in TY medium containing 1% xylose and examined by fluorescence microscopy (Fig. 2). The localization patterns of TatAc-GFP and TatAd-GFP were similar, with most cells showing one or two bright foci that were primarily located at the poles or division septa of the cells. Since division sites will become the new cell poles, and in analogy to the reported localization pattern of TatCy-GFP (20), we will refer to these foci as being “polar.” In addition, these two TatA-GFP fusions exhibit a peripheral green staining suggestive of homogeneous membrane localization. The TatAy-GFP fusion shows much weaker fluorescence signals, making its localization more difficult to detect. It is visible in foci at the cell poles, at least, and it might be localized homogeneously in the membrane as well. These results suggest that the TatA proteins of B. subtilis are similarly localized at dual locations: an even distribution throughout the membrane and in polar foci. We have also constructed similar GFP fusions with TatCd and TatCy, but unfortunately, the resulting fluorescence was generally too weak to draw any conclusions (data not shown).
FIG. 2.
Localization of TatA proteins of B. subtilis. Strains 168 X-Ac-gfp, 168 X-Ad-gfp, and 168 X-Ay-gfp were grown in TY medium containing 1% xylose for 7 h until early stationary phase and examined by fluorescence microscopy. Phase-contrast (left) and fluorescence (right) pictures of the same cells are shown, and the scale bars indicate 5 μm.
Focal localization of TatAd-GFP depends on the presence of TatCy.
Since the TatAd-GFP-producing strain showed the brightest fluorescence signals and, therefore, the dual localization pattern of this fusion protein was most evident, we investigated the localization of this fusion protein in more detail (the most important results are summarized in Table 2). To investigate whether the localization of TatAd-GFP depends on the presence of other Tat proteins, we introduced the pSG1154-TatAd construct into strains in which one or more tat genes were deleted. The resulting strains were subsequently grown in TY medium containing 1% xylose and examined by fluorescence microscopy (Fig. 3A). In strain 168 tatAc1, in which the tatAc gene is disrupted, the localization of TatAd-GFP was similar to that in B. subtilis 168, displaying membrane localization and polar foci. The deletion of both the tatAd and tatCd genes also did not have an effect on the localization of the xylose-induced TatAd-GFP. However, since this experiment was carried out in TY medium, in which the phosphate starvation-inducible tatAd and tatCd genes are not expressed (11), this is not surprising. In contrast, in strain 168 ΔtatAy(Cy), in which TatAy and TatCy are both depleted, the bright foci of TatAd-GFP were no longer present and only the peripheral staining was observed (Fig. 3A). Similar experiments were performed with TatAc-GFP, and for this fusion protein also, the localization in polar foci did not depend on the presence of either TatAc or TatAdCd but was abolished in strain 168 ΔtatAy(Cy) (data not shown). To investigate whether the loss of foci of TatAd-GFP in strain 168 ΔtatAy(Cy) might be the result of lower levels of expression of the fusion protein, the level of production of TatAd-GFP in the three tat deletion strains was investigated by SDS-PAGE and Western blot analyses using anti-GFP antibodies. As shown in Fig. 3B, in all strains, a band of around 34 kDa, corresponding to TatAd-GFP, was detected. Importantly, the levels of TatAd-GFP were similar in all three tat deletion strains, indicating that the alteration of the localization pattern of TatAd-GFP in strain 168 ΔtatAy(Cy) compared to its localization in the other two tat deletion strains is not due to a difference in the amount of fusion protein produced. In some cases, a minor degradation product was detected, but this did not correlate with the localization patterns. Next, we investigated whether the altered localization of TatAd-GFP in strain 168 ΔtatAy(Cy) was due to the absence of TatAy, TatCy, or both by expressing these proteins from a plasmid in which the corresponding genes are placed under the control of a constitutive promoter and examining these strains by fluorescence microscopy (Fig. 3C). When TatAy was introduced, the TatAd-GFP localization pattern did not change. However, when TatAyCy or TatCy alone was introduced, foci of TatAd-GFP were visible again. These results clearly indicate that the localization of TatAd-GFP in foci depends on the presence of TatCy (Table 2).
TABLE 2.
Summary of the most important fluorescence microscopy results presented in this papera
| Strain genotype | Medium | Presence of:
|
||
|---|---|---|---|---|
| TatCy | TatCd | TatAd-GFP foci | ||
| X-tatAd-gfp | TY | + | − | + |
| tatAy(Cy) X-tatAd-gfp | TY | − | − | − |
| tatAy(Cy) X-tatAd-gfp/pCCy | TY | + | − | + |
| tatAd-gfp | LPDM | + | − | + |
| tatCy2 tatAd-gfp | LPDM | − | − | − |
| tatCy2 tatAd-gfp/pCCy | LPDM | + | − | + |
| tatCy2 tatAd-gfp/pCCd | LPDM | − | + | + |
Data show whether (+) or not (−) TatCy and/or TatCd are produced under the conditions we used to visualize TatAd-GFP.
TatAd-GFP is functional in PhoD translocation.
In the experiments described above, the TatAd-GFP fusion protein was overexpressed from a xylose-inducible promoter, and strains were grown in TY medium in which the tatAd gene is normally not expressed. Both factors might influence the localization of TatAd-GFP, so to observe its localization under more physiological conditions, a strain was constructed in which the TatAd-GFP-encoding DNA was integrated at the native tatAd locus. This was achieved by cloning the tatAd gene in the pSG1151 vector (19) and integrating this construct via a single crossover event into the chromosome of B. subtilis strain 168. This resulted in strain 168 Ad-gfp, with the tatAd-gfp gene under the control of the native promoter of the phoD-tatAd-tatCd operon, which is located in front of the phoD gene and induced by phosphate starvation (Fig. 1B). Next, the strain was grown in a synthetic medium containing either a large or a small amount of phosphate, and the level of production of the fusion protein was investigated by SDS-PAGE and Western blotting using anti-GFP antibodies. After 7 h of growth in HPDM, no production of TatAd-GFP could be observed (Fig. 4A). However, cells grown in LPDM showed clear production of TatAd-GFP, indicating that the expression of the gene encoding the fusion protein is correctly induced by phosphate starvation. A potential drawback of the single crossover integration of plasmid pSG1151-TatAd at the tatAd locus might be that it causes polar effects on the expression of the tatCd gene, which lies downstream of the tatAd gene (Fig. 1B). Indeed, when this strain was grown under conditions of phosphate limitation, pre-PhoD (∼63 kDa) was produced, but no PhoD could be detected in the extracellular medium (Fig. 4B, lanes 1 and 3). However, when TatCd was introduced into this strain on a plasmid in which the corresponding gene is placed under the control of a constitutive promoter, the amount of pre-PhoD in the cells was shown to be decreased (Fig. 4B, lane 2), while a band corresponding to mature PhoD (∼57 kDa) could be detected in the extracellular medium, indicating that PhoD is secreted (Fig. 4B, lane 4). The difference between these two strains in the ability to secrete PhoD is not due to differences in the levels of TatAd-GFP protein produced (Fig. 4A, lanes 2 and 3) but depends on the presence of TatCd. Moreover, the anti-GFP antibodies only detected the intact TatAd-GFP fusion protein, indicating that GFP is not cleaved off to produce free TatAd. Therefore, we conclude that the TatAd-GFP fusion protein is functional in PhoD secretion.
FIG. 4.
Expression and localization of TatAd-GFP under phosphate starvation conditions. (A) Strain 168 Ad-gfp was grown in HPDM (lane 1) or LPDM (lane 2), and strain 168 Ad-gfp/pCCd was grown in LPDM (lane 3). After 7 h, samples were taken and analyzed by SDS-PAGE and Western blotting using anti-GFP antibodies. Mobilities of molecular mass markers (in kDa) are shown on the right. (B) Strains 168 Ad-gfp and 168 Ad-gfp/pCCd were grown in LPDM for 8 h. The supernatant was concentrated by trichloroacetic acid precipitation, and cell samples and supernatants were analyzed by SDS-PAGE and Western blotting using anti-PhoD antibodies. Mobilities of molecular mass markers (in kDa) are shown on the right. Lane 1, cells of strain 168 Ad-gfp; lane 2, cells of strain 168 Ad-gfp/pCCd; lane 3, supernatant from strain 168 Ad-gfp culture; lane 4, supernatant from strain 168 Ad-gfp/pCCd culture. The panels are from nonadjacent lanes of the same Western blot membrane. (C) Strain 168 Ad-gfp was grown in HPDM (high) or LPDM (low), and strain 168 Ad-gfp/pCCd (+ TatCd) was grown in LPDM. These strains were examined by fluorescence microscopy after 7 h of growth. Phase-contrast (left) and fluorescence (right) pictures of the same cells are shown, and the scale bars indicate 5 μm.
Next, the subcellular localization of TatAd-GFP expressed from its native promoter was investigated by fluorescence microscopy (Fig. 4C). As expected, when grown in HPDM, no fluorescence signals were detected. When strain 168 Ad-gfp was grown in LPDM, the subcellular localization of TatAd-GFP was similar to that observed in cells with the xylose-inducible fusion grown in TY medium, showing localization at the cell periphery and in bright polar foci (compare Fig. 4C and Fig. 2). This demonstrates that the TatAd foci are not the result of artificial overproduction of the fusion protein and, since TatAd-GFP is shown to be functional, are probably not due to aggregation. However, since strain 168 Ad-gfp apparently lacks TatCd, the observed foci of TatAd-GFP might be an artifact caused by the absence of a binding partner, its natural TatC counterpart. Therefore, we also investigated the localization of TatAd-GFP in strain 168 Ad-gfp in which TatCd was produced from a plasmid. Under these conditions, where PhoD secretion takes place, the polar foci are still present (Fig. 4C), suggesting that the foci of TatAd-GFP might correspond to localization of the biologically active protein.
Foci of TatAd-GFP depend on TatCd or TatCy.
Since we observed that the foci of xylose-induced TatAd-GFP depend on the presence of TatCy, we also investigated the localization pattern of TatAd-GFP expressed from its own promoter after integration of plasmid pSG1151-TatAd in strain 168 ΔtatCy, in which the tatCy gene is deleted. In this strain, foci of the fusion protein are completely absent and only the peripheral fluorescence signal is visible. However, TatAd-GFP foci could be observed when TatCy was reintroduced into strain 168 ΔtatCy via production from plasmid pCCy (Fig. 5A). These different localization patterns are not the result of different intracellular amounts of the TatAd-GFP fusion protein, since these are similar in the presence or absence of TatCy (Fig. 5B). This demonstrates that the foci of TatAd-GFP expressed from its native promoter depend on the presence of TatCy, as was also observed for the xylose-induced TatAd-GFP. In addition, these results indicate that the foci of TatAd-GFP observed in strain 168 Ad-gfp cells grown in LPDM (Fig. 4C) are caused by TatCy (Table 2), which is known to be produced under these conditions (11).
FIG. 5.
Foci of TatAd-GFP depend on a TatC component. (A) Strains 168 ΔtatCy Ad-gfp (ΔtatCy), 168 ΔtatCy Ad-gfp/pCCy (+ TatCy), and 168 ΔtatCy Ad-gfp/pCCd (+ TatCd) were grown in LPDM for 7 h and examined by fluorescence microscopy. Phase-contrast (left) and fluorescence (right) pictures of the same cells are shown, and the scale bars indicate 5 μm. (B) At the same time as the samples whose images are shown in panel A, samples containing equal amounts of cells were taken and analyzed by SDS-PAGE and Western blotting using anti-GFP antibodies. Lane 1, 168 ΔtatCy Ad-gfp; lane 2, 168 ΔtatCy Ad-gfp/pCCy; lane 3, 168 ΔtatCy Ad-gfp/pCCd. Mobilities of molecular mass markers (in kDa) are shown on the right.
As demonstrated by the results described above, strain 168 Ad-gfp does not produce TatCd. Therefore, we investigated whether TatAd-GFP would be localized in foci when TatCd, encoded by plasmid pCCd, was introduced into strain 168 ΔtatCy Ad-gfp. As can be observed from the results in Fig. 5A, like that of TatCy, the presence of TatCd also results in foci of TatAd-GFP in strain 168 ΔtatCy Ad-gfp. This demonstrates that the localization of TatAd in foci depends on the presence of a TatC component (Table 2) and suggests that TatAd can functionally interact with both TatCd and TatCy.
DISCUSSION
The subcellular localization of Tat components in E. coli has been extensively investigated by using fluorescence microscopy (4, 18, 25). The Tat machineries of gram-positive bacteria have a different composition than those of gram-negative organisms, lacking a TatB protein. Much research has been done to elucidate the working mechanism of Tat translocation in the model gram-positive organism B. subtilis, but only the subcellular localization of TatCy has been reported so far (20).
In this study, we have investigated the subcellular localization of the three TatA proteins of B. subtilis by using GFP fusions, functionality assessments, and fluorescence microscopy. In order to investigate the subcellular localization of the TatA proteins of B. subtilis, we have constructed fusions of these proteins with GFP. Recently, it has been suggested that TatA of E. coli has a dual topology, with its N terminus residing on the cytoplasmic side of the membrane. The location of its C terminus depends on the membrane potential and is cytoplasmic in the active, presumably channel-forming state (8). It is currently unknown whether the Bacillus TatA proteins assume the same topology or the Nout-Cin topology that has previously been assumed. In both cases, however, the C terminus will be cytoplasmic and can probably be fused to GFP without adverse effects (see below).
From the fluorescence microscopy results presented in this paper, we conclude that TatAd-GFP can be localized at two places in the cell, namely, peripherally and in bright foci which are enriched at the cell poles and/or division sites. These foci of TatAd-GFP might consist of inclusion bodies, but for several reasons, we consider this possibility unlikely. First, the TatAd-GFP fusion protein is shown to be functional in PhoD secretion (Fig. 4). Second, the formation of foci is not caused by the intracellular level of TatAd-GFP, since in the absence of a TatC component, no foci are seen, although the total amount of TatAd-GFP is similar (Fig. 3B and 5B). In addition, the results obtained upon the expression of TatAd-GFP from a xylose-induced promoter were in agreement with those obtained upon expression from its native promoter. Third, the presence of a TatC component is necessary for the functionality of its TatA counterpart and is therefore unlikely to promote the formation of inclusion bodies or other types of inactive aggregates of TatA. The dual localization of the TatA proteins of B. subtilis is similar to that of TatCy, which was investigated using the same xylose-inducible GFP fusion system and also displayed membrane fluorescence and polar foci (20). In E. coli, a similar dual localization of TatA-GFP has been observed (4, 25), although there has been some controversy about whether these foci represent active or inactive assemblies and about whether Tat translocation takes place at the poles or not. In a recent study, TatA-YFP was expressed at native levels in E. coli and was localized in a dispersed state in the membrane and in foci. These foci were shown to be individual TatA complexes with, on average, 25 TatA molecules (18).
The foci of TatAd-GFP are only observed when a TatC component is present (for a summary, see Table 2). When TatAd-GFP is expressed from a xylose-inducible promoter in TY medium, its natural binding partner, TatCd, is not expressed (11), and therefore, TatCy must be responsible for the formation of TatAd-GFP foci (Fig. 2). In the strain that expresses TatAd-GFP from its native locus, polar effects lead to the absence of TatCd. However, TatCy is also present under phosphate starvation conditions (11), and therefore, TatAd-GFP foci can still be formed (Fig. 4). When TatCy is deleted, however, no foci are visible and only peripheral fluorescence is observed under both conditions (Fig. 3A and 5A). By subsequently expressing TatC components from a plasmid, it was demonstrated that both TatCy and TatCd are able to promote the formation of foci of TatAd-GFP (Fig. 5). Thus, the foci of TatAd-GFP depend on the presence of a TatC component, similar to E. coli, where TatA complexes were not formed in cells lacking TatBC and it was concluded that TatBC controls the oligomerization of TatA (18). In another study, overexpressed E. coli TatA formed ordered assemblies of tubes which depended on the presence of TatC (5).
Translocation via the Tat pathway can only occur if both TatA and TatC components are present to form a translocase (12), and under conditions where translocation of PhoD via TatAd-GFP and TatCd occurs, the fluorescent foci are indeed present (Fig. 4). Therefore, these foci most likely represent a form of TatAd that is functional during Tat translocation. However, we cannot conclude from our results whether the foci of TatAd-GFP are caused by complexes of TatA alone or in combination with TatC and/or substrate. Therefore, elucidation of the subcellular location of the actual translocation process itself will require further investigation.
In the complete absence of a TatC component, only peripheral fluorescence of TatAd-GFP can be observed, which thus represents free TatAd. This could be a membrane-integral form of TatAd-GFP but might also represent cytosolic TatAd complexes (24), since TatAd was shown to have a basal affinity for membranes (28).
Interestingly, TatCy is also able to promote the formation of foci of TatAd-GFP, although these are not functional in the translocation of PhoD (Fig. 4), probably due to lack of substrate recognition. Recent results show that TatAd is able to form a complex with TatCy that can translocate YwbN (9) when TatAd is overproduced, at least, but not when it is present at natural levels. This indicates that functional interaction of TatAd with TatCy is indeed possible, although with low efficiency. Since the substrate specificity of Tat translocases is determined by the TatC component (11), it is logical that TatAd and TatCy can translocate YwbN but not PhoD.
Although we have only investigated TatAd in detail, TatAc-GFP and TatAy-GFP seem to be similarly localized in foci at the cell poles. This is in agreement with the facts that TatAyCy and TatAdCd are organized in similar complexes and that their mode of action is probably the same (3). Remarkably, the foci of TatAc are also absent in the TatAyCy deletion strain. TatAc is dispensable for the translocation of PhoD or YwbN, but it is tempting to speculate that it may be involved in secretion of the recently discovered B. subtilis Tat substrate QcrA or YkuE (32), possibly in conjunction with TatCy.
Acknowledgments
We thank J. P. Müller for the anti-PhoD antibodies.
This work was supported by a grant of the Nederlandse stichting voor Wetenschappelijk Onderzoek (NWO) within the Molecule to Cell (MtC) program.
Footnotes
Published ahead of print on 24 April 2009.
REFERENCES
- 1.Alami, M., I. Lüke, S. Deitermann, G. Eisner, H. G. Koch, J. Brunner, and M. Müller. 2003. Differential interactions between a twin-arginine signal peptide and its translocase in Escherichia coli. Mol. Cell 12937-946. [DOI] [PubMed] [Google Scholar]
- 2.Barnett, J. P., R. T. Eijlander, O. P. Kuipers, and C. Robinson. 2008. A minimal Tat system from a Gram-positive organism: a bifunctional TatA subunit participates in discrete TatAC and TatA complexes. J. Biol. Chem. 2832534-2542. [DOI] [PubMed] [Google Scholar]
- 3.Barnett, J. P., R. van der Ploeg, R. T. Eijlander, A. Nenninger, S. Mendel, R. Rozeboom, O. P. Kuipers, J. M. van Dijl, and C. Robinson. 2009. The twin-arginine translocation (Tat) systems from Bacillus subtilis display a conserved mode of complex organization and similar substrate recognition requirements. FEBS J. 276232-243. [DOI] [PubMed] [Google Scholar]
- 4.Berthelmann, F., and T. Brüser. 2004. Localization of the Tat translocon components in Escherichia coli. FEBS Lett. 56982-88. [DOI] [PubMed] [Google Scholar]
- 5.Berthelmann, F., D. Mehner, S. Richter, U. Lindenstrauss, H. Lünsdorf, G. Hause, and T. Brüser. 2008. Recombinant expression of tatABC and tatAC results in the formation of interacting cytoplasmic TatA tubes in Escherichia coli. J. Biol. Chem. 28325281-25289. [DOI] [PubMed] [Google Scholar]
- 6.Blaudeck, N., P. Kreutzenbeck, M. Müller, G. A. Sprenger, and R. Freudl. 2005. Isolation and characterization of bifunctional Escherichia coli TatA mutant proteins that allow efficient tat-dependent protein translocation in the absence of TatB. J. Biol. Chem. 2803426-3432. [DOI] [PubMed] [Google Scholar]
- 7.Bolhuis, A., J. E. Mathers, J. D. Thomas, C. M. L. Barrett, and C. Robinson. 2001. TatB and TatC form a functional and structural unit of the twin-arginine translocase from Escherichia coli. J. Biol. Chem. 27620213-20219. [DOI] [PubMed] [Google Scholar]
- 8.Chan, C. S., M. R. Zlomislic, D. P. Tieleman, and R. J. Turner. 2007. The TatA subunit of Escherichia coli twin-arginine translocase has an N-in topology. Biochemistry 467396-7404. [DOI] [PubMed] [Google Scholar]
- 9.Eijlander, R. T., J. D. Jongbloed, and O. P. Kuipers. 2009. Relaxed specificity of the Bacillus subtilis TatAdCd translocase in Tat-dependent protein secretion. J. Bacteriol. 191196-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ize, B., F. Gérard, M. Zhang, A. Chanal, R. Voulhoux, T. Palmer, A. Filloux, and L. F. Wu. 2002. In vivo dissection of the Tat translocation pathway in Escherichia coli. J. Mol. Biol. 317327-335. [DOI] [PubMed] [Google Scholar]
- 11.Jongbloed, J. D. H., U. Martin, H. Antelmann, M. Hecker, H. Tjalsma, G. Venema, S. Bron, J. M. van Dijl, and J. Müller. 2000. TatC is a specificity determinant for protein secretion via the twin-arginine translocation pathway. J. Biol. Chem. 27541350-41357. [DOI] [PubMed] [Google Scholar]
- 12.Jongbloed, J. D. H., U. Grieger, H. Antelmann, M. Hecker, R. Nijland, S. Bron, and J. M. van Dijl. 2004. Two minimal Tat translocases in Bacillus. Mol. Microbiol. 541319-1325. [DOI] [PubMed] [Google Scholar]
- 13.Jongbloed, J. D., R. van der Ploeg, and J. M. van Dijl. 2006. Bifunctional TatA subunits in minimal Tat protein translocases. Trends Microbiol. 142-4. [DOI] [PubMed] [Google Scholar]
- 14.Kiewiet, R., S. Bron, K. de Jonge, G. Venema, and J. F. Seegers. 1993. Theta replication of the lactococcal plasmid pWVO2. Mol. Microbiol. 10319-327. [PubMed] [Google Scholar]
- 15.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, M. G. Bertero, P. Bessières, A. Bolotin, S. Borchert, R. Borriss, L. Boursier, A. Brans, M. Braun, S. C. Brignell, S. Bron, S. Brouillet, C. V. Bruschi, B. Caldwell, V. Capuano, N. M. Carter, S.-K. Choi, J.-J. Codani, I. F. Connerton, A. Danchin, et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390249-256. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 17.Lange, C., S. D. Müller, T. H. Walther, J. Bürck, and A. S. Ulrich. 2007. Structure analysis of the protein translocating channel TatA in membranes using a multi-construct approach. Biochim. Biophys. Acta 17682627-2634. [DOI] [PubMed] [Google Scholar]
- 18.Leake, M. C., N. P. Greene, R. M. Godun, T. Granjon, G. Buchanan, S. Chen, R. M. Berry, T. Palmer, and B. C. Berks. 2008. Variable stoichiometry of the TatA component of the twin-arginine protein transport system observed by in vivo single-molecule imaging. Proc. Natl. Acad. Sci. USA 10515376-15381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis, P. J., and A. L. Marston. 1999. GFP vectors for controlled expression and dual labelling of protein fusions in Bacillus subtilis. Gene 227101-110. [DOI] [PubMed] [Google Scholar]
- 20.Meile, J.-C., L. J. Wu, S. D. Ehrlich, J. Errington, and P. Noirot. 2006. Systematic localisation of proteins fused to the green fluorescent protein in Bacillus subtilis: identification of new proteins at the DNA replication factory. Proteomics 62135-2146. [DOI] [PubMed] [Google Scholar]
- 21.Müller, J. P., Z. An, T. Merad, I. C. Hancock, and C. R. Harwood. 1997. Influence of Bacillus subtilis phoR on cell wall anionic polymers. Microbiology 143947-956. [DOI] [PubMed] [Google Scholar]
- 22.Müller, J. P., and M. Wagner. 1999. Localisation of the cell wall-associated phosphodiesterase PhoD of Bacillus subtilis. FEMS Microbiol. Lett. 180287-296. [DOI] [PubMed] [Google Scholar]
- 23.Oates, J., C. M. L. Barrett, J. P. Barnett, K. G. Byrne, A. Bolhuis, and C. Robinson. 2005. The Escherichia coli twin-arginine translocation apparatus incorporates a distinct form of TatABC complex, spectrum of modular TatA complexes and minor TatAB complex. J. Mol. Biol. 346295-305. [DOI] [PubMed] [Google Scholar]
- 24.Pop, O. I., M. Westermann, R. Volkmer-Engert, D. Schulz, C. Lemke, S. Schreiber, R. Gerlach, R. Wetzker, and J. P. Müller. 2003. Sequence-specific binding of prePhoD to soluble TatAd indicates protein-mediated targeting of the Tat export in Bacillus subtilis. J. Biol. Chem. 27838428-38436. [DOI] [PubMed] [Google Scholar]
- 25.Ray, N., A. Nenninger, C. W. Mullineaux, and C. Robinson. 2005. Location and mobility of twin arginine translocase subunits in the Escherichia coli plasma membrane. J. Biol. Chem. 28017961-17968. [DOI] [PubMed] [Google Scholar]
- 26.Robinson, C., and A. Bolhuis. 2001. Protein targeting by the twin-arginine translocation pathway. Nat. Rev. Mol. Cell Biol. 2350-356. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 28.Schreiber, S., R. Stengel, M. Westermann, R. Volkmer-Engert, O. I. Pop, and J. P. Müller. 2006. Affinity of TatCd for TatAd elucidates its receptor function in the Bacillus subtilis twin arginine translocation (Tat) translocase system. J. Biol. Chem. 28119977-19984. [DOI] [PubMed] [Google Scholar]
- 29.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 224673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tjalsma, H., A. Bolhuis, M. L. van Roosmalen, T. Wiegert, W. Schumann, C. P. Broekhuizen, W. J. Quax, G. Venema, S. Bron, and J. M. van Dijl. 1998. Functional analysis of the secretory precursor processing machinery of Bacillus subtilis: identification of a eubacterial homolog of archaeal and eukaryotic signal peptidases. Genes Dev. 122318-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westermann, M., O. I. Pop, R. Gerlach, T. R. Appel, W. Schlörmann, S. Schreiber, and J. P. Müller. 2006. The TatAd component of the Bacillus subtilis twin-arginine protein transport system forms homo-multimeric complexes in its cytosolic and membrane embedded localisation. Biochim. Biophys. Acta 1758443-451. [DOI] [PubMed] [Google Scholar]
- 32.Widdick, D. A., R. T. Eijlander, J. M. van Dijl, O. P. Kuipers, and T. Palmer. 2008. A facile reporter system for the experimental identification of twin-arginine translocation (Tat) signal peptides from all kingdoms of life. J. Mol. Biol. 375595-603. [DOI] [PubMed] [Google Scholar]