Abstract
Formation of photosynthesis complexes in Rhodobacter sphaeroides is regulated in a redox- and light-dependent manner by the AppA/PpsR and PrrB/PrrA systems. While on the one hand, blue light is sensed by the flavin adenine dinucleotide-binding BLUF domain of AppA, on the other, light is absorbed by bacteriochlorophyll signals through PrrB/PrrA. We show that much smaller quantities initiate the AppA-mediated response to blue light than the bacteriochlorophyll-mediated response.
Rhodobacter sphaeroides is a facultatively photosynthetic alphaproteobacterium that synthesizes photosynthetic complexes in response to light and oxygen signals. Formation of these complexes is maximal under low-oxygen conditions (28). Increasing the oxygen concentration to semiaerobic levels (∼50% oxygen saturation) leads to a small decrease in the transcription rate in the dark, but under these conditions, blue light strongly represses photosynthetic complex formation (1, 25). This blue-light response is mediated by AppA, which recognizes and integrates redox and light signals (1, 18). AppA is an antagonist to PpsR which represses photosynthesis genes under high oxygen tension by binding consensus sequences upstream of these genes (5). Light signaling (Fig. 1) is controlled by the N-terminal flavin adenine dinucleotide (FAD)-binding BLUF domain (6), which functions as a blue-light photoreceptor in various prokaryotes and can also be found in eukaryotes, e.g., in the Pac proteins of Euglena species (11). The C-terminal part of AppA is sufficient for redox regulation, while the BLUF domain alone is unable to transmit the blue light signal (8). Interestingly, light and redox regulation is maintained even if there is no covalent linkage of the two domains, demonstrating the modular structure of AppA (8). A heme cofactor is needed for redox regulation and also plays a role in the interaction between the N- and C-terminal parts of AppA (9, 20). While the AppA/PpsR system is responsible for blue-light-dependent repression of photosynthesis genes in aerobic and semiaerobic cultures, the PrrB/PrrA system activates their transcription under low-oxygen conditions (21, 22). This two-component system is regulated by the flow of electrons through the respiratory chain and consequently by photosynthetic electron transport, which reduces electron flow through the cbb3 oxidase (10). Light absorption by bacteriochlorophylls thus leads to activation of the PrrB/PrrA system. Under low oxygen tension in the light, the PrrB/PrrA system releases the AppA/PpsR-mediated repression of photosynthesis genes (10). The release of PpsR repression alone is insufficient to allow photosynthesis gene expression, but activation by PrrB/PrrA is required (12).
FIG. 1.
Model of the coordinated influence of AppA/PpsR and PrrB/PrrA on photosynthesis gene expression in R. sphaeroides. Positive regulation is mediated through the PrrB/PrrA two-component-system. Light illumination initiates cyclic photosynthetic electron transport, which consequently reduces electron flow through the respiratory cbb3 oxidase. This further enhances PrrB/PrrA-mediated gene activation. PpsR represses photosynthesis genes under high oxygen tension. At intermediate or low oxygen levels, PpsR is bound to AppA and released from the DNA. Under semiaerobic conditions, blue light illumination leads to a dissociation of AppA and PpsR that allows PpsR to strongly inhibit photosynthesis gene expression.
We determined the light sensitivity of the AppA/PpsR-mediated response initiated by the FAD-binding BLUF domain and the PrrB/PrrA-mediated response initiated by bacteriochlorophyll absorption (Fig. 1). To this end, we monitored the levels of puc mRNA in cells grown under different oxygen tensions by Northern blot analysis and the levels of puc and bchL mRNAs by real-time reverse transcription (RT)-PCR. In R. sphaeroides, the puc operon encodes the protein components of light-harvesting complex II and bchL encodes protochlorophyllid reductase. Both operons are under the direct control of PrrA (3) and PpsR (19). A binding site for the redox regulator FnrL (28) is present upstream of the puc operon but not upstream of bchL. The puc and bchL mRNA levels in wild-type cultures grown at intermediate oxygen levels showed a strong decrease after illumination with very low quantities of blue light. In contrast, the same light quantities do not affect puc mRNA levels in anaerobically grown wild-type cultures. In a PrrB mutant, the light-dependent increase in puc mRNA under anaerobic conditions is lost due to the lack of the sensor kinase. In this strain, AppA/PpsR-mediated repression takes place even in the absence of oxygen (10). To prove the higher light sensitivity of the AppA/PpsR-mediated response under redox conditions identical to those that lead to blue light stimulation of PrrB/PrrA in the wild type, we also analyzed the puc and bchL mRNA levels in an anaerobically grown PrrB mutant. We also show the effects of different light quantities on the level of bacteriochlorophyll, which correlates with the amount of photosynthetic complexes.
The AppA photoreceptor responds to blue light quantities as low as 0.2 μmol m−2 s−1 in vivo.
The ability of the AppA BLUF domain to react to blue light signals has been investigated intensively in vitro (4, 7, 15, 17). To further analyze the in vivo light sensitivity of the full-length AppA photoreceptor under semiaerobic conditions, an overnight culture of wild-type R. sphaeroides 2.4.1 was split into light and dark cultures and diluted to an optical density at 660 nm of 0.2. The diluted cultures were adjusted to semiaerobic conditions (∼100 ± 20 μM O2) and monitored with a Pt/Ag oxygen electrode (Micro Oxygen Sensor 501; UMS). Illumination with blue light was done through a 450-nm filter starting at 20 μmol m−2 s−1. Light quantity was measured at the position of the flask's center (Li-200SA Pyranometer Sensor). The light quantity was decreased stepwise by using different dim settings on a slide projector (250 W, 24 V; General Electric). RNA isolation, Northern blot analysis, and real-time RT-PCR were performed as described before (1, 23).
AppA-dependent repression of puc mRNA levels showed a high sensitivity to blue light down to 0.5 μmol m−2 s−1 (Fig. 2 and 3). Even at a light quantity of 0.2 μmol m−2 s−1, some puc repression was observed on Northern blots (Fig. 2). Lower light quantities showed no influence on semiaerobically grown cultures (Fig. 2 and 3). It has to be considered that blue light quantities were measured at the center of the flask; therefore, the light quantity per cell might be somewhat different from that value. Levels of bchL mRNA showed the same response as puc mRNA levels when monitored by real-time RT-PCR (Fig. 3). A similar sensitivity of puc and bchl expression is also visible in a FnrL mutant strain (data not shown), excluding a direct regulatory effect of the protein on light-dependent regulation. To test how the different light quantities affect the amount of photosynthetic complexes, we also determined the bacteriochlorophyll levels in the cultures. The results confirm that light quantities of 20 and 1 μmol m−2 s−1 strongly inhibit the formation of photosynthetic complexes under semiaerobic conditions. Blue light at 0.1 μmol m−2 s−1 no longer lead to significant inhibition, and blue light at 0.2 μmol m−2 s−1 is sufficient for slight repression (Fig. 4A). These results suggest a sensitivity of AppA to very low fluence rates of light similar to that previously shown for plant phytochromes (26) and cryptochromes (16).
FIG. 2.
(A) Northern blot assay, with puc and 14S rRNA-specific probes, of total RNA isolated from R. sphaeroides 2.4.1 grown in the dark or after illumination with blue light. Cultures were illuminated with decreasing amounts of blue light. Samples were taken at the time points shown, total RNA was isolated, and a Northern blot assay was performed as described before (1). (B) Response of puc expression to illumination of R. sphaeroides 2.4.1 with blue light. The ratios of mRNA levels of cultures grown in the light to those of the dark control were calculated after normalizing the puc phosphorimaging signals to the rRNA signals. Percentages of inhibition were calculated according to the formula 100 − (puclight/rRNAlight)/(pucdark/rRNAdark). Mean values and maximal deviations of two different experiments are shown.
FIG. 3.
Relative levels of puc and bchL mRNAs as determined by real-time RT-PCR of total RNA isolates from semiaerobic (A) cultures of R. sphaeroides 2.4.1 and anaerobic (B) cultures of R. sphaeroides PrrB1 after illumination with different quantities of blue light. Responses to illumination with 20, 1, 0.5, and 0.1 μmol m−2 s−1 are shown. Relative (rel.) expression levels of puc and bchL were quantified and normalized against mRNA levels of the rpoZ gene as described before (23).The n-fold change in target genes was calculated relative to that of rpoZ and control cultures grown in the dark (24). Mean values and standard deviations of three different experiments are shown.
FIG. 4.
Relative bacteriochlorophyll contents of semiaerobic (A) and anaerobic (B) cultures of R. sphaeroides 2.4.1 after illumination with different quantities of blue light. Responses to illumination with 20 (□), 1 (▪), 0.2 (▤), and 0.1 (░⃞) μmol m−2 s−1 and dark conditions (▨) are shown. The relative bacteriochlorophyll content of illuminated cultures is given as absorbance at 770 nm after acetone-methanol (7:2) extraction of 1.5 ml cells normalized against the optical density at 660 nm and against values from control cultures grown in the dark. Values for dark conditions are blotted but without normalization against a control culture. Results from one of at least two independent experiments with similar results are shown.
The PrrB/PrrA-dependent signal chain requires higher blue light quantities for a light-dependent response than does AppA/PpsR.
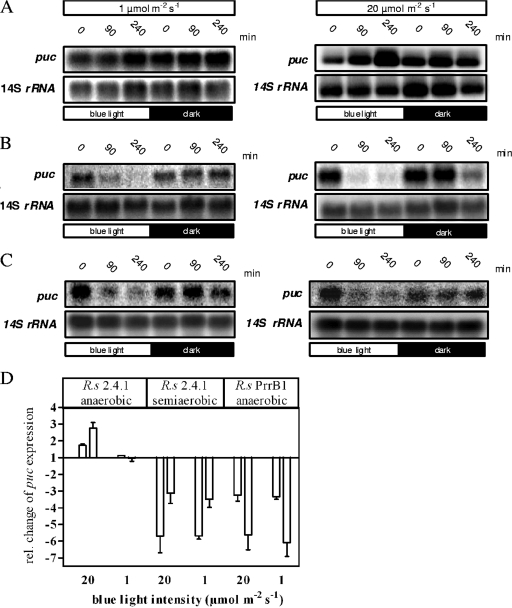
As shown before (10), light-dependent transcriptional control under anaerobic conditions is coordinated by an interplay of the AppA/PpsR and PrrB/PrrA regulatory systems. While AppA directly senses blue light through the FAD bound to the N-terminal BLUF domain (1, 7, 27), PrrB phosphorylation is regulated in an indirect manner via electron flow through the respiratory chain (13, 14, 22). To test the light sensitivity of the PrrB/PrrA-mediated response, puc expression was monitored in anaerobic cultures that were grown in a malate minimal salt medium in completely filled screw-capped flasks with 60 mM dimethyl sulfoxide added as a terminal electron acceptor. When required, spectinomycin was added to the culture to a concentration of 10 μg ml−1. Quantification of puc mRNA levels in wild-type strain 2.4.1 by Northern blotting showed an increase in gene expression after illumination with 20 μmol m−2 s−1, as described before (10), while illumination with 1 μmol m−2 s−1 showed no effect (Fig. 5). An even stronger puc mRNA increase was observed after 240 min of illumination with blue light at 20 μmol m−2 s−1 (10), while 1 μmol m−2 s−1 again had no influence (Fig. 5A). The light-dependent puc repression found under semiaerobic conditions at the same time points is shown in Fig. 5B. This lower sensitivity of the PrrB/PrrA-dependent signal chain to light was also observed when bacteriochlorophyll levels were monitored. Light at 1 μmol m−2 s−1 had no effect on the level of bacteriochlorophyll, while 20 μmol m−2 s−1 led to a twofold increase (Fig. 4B). This suggests that absorption by bacteriochlorophyll and the photosynthetic electron flow need a higher blue light threshold to trigger the PrrB/PrrA-dependent response, in contrast to the very low threshold needed by the AppA photoreceptor.
FIG. 5.
Northern blot assays, with puc and 14S rRNA-specific probes, of total RNA isolated from anaerobic (A) and semiaerobic (B) cultures of R. sphaeroides (R.s) 2.4.1 and anaerobic (C) cultures of R. sphaeroides PrrB1 grown in the dark or after illumination with blue light at 20 and 1 μmol m−2 s−1. Samples were taken at the time points shown, total RNA was isolated, and Northern blot assays were performed as described before (1). Note that the exposure times for the blot with the RNA from anaerobically grown cultures were shorter due to higher puc expression levels. (D) Relative (rel.) change in puc expression after illumination with blue light at 20 and 1 μmol m−2 s−1 after 90 (left bars) and 240 (right bars) min. Ratios of mRNA levels of cultures grown in the light to those of the dark control were calculated after normalizing the puc phosphorimaging signals to the rRNA signals according to the formula (puclight/rRNAlight)/(pucdark/rRNAdark). Mean values and the maximal deviation of two different experiments are shown.
Light-dependent AppA/PpsR regulation can be restored under low-oxygen conditions in the PrrB1 mutant strain (2), which lacks PrrB and therefore no longer shows the electron flow-dependent response. Here mRNA levels of puc and bchL were strongly repressed shortly after illumination with high quantities of blue light (10), as also described for semiaerobically grown wild-type cultures (1) (Fig. 3B). This effect was also visible when cultures of both strains were submitted to a lower blue light quantity of 1 μmol m−2 s−1 (Fig. 3 and 5C and D) and reflected by decreasing amounts of bacteriochlorophyll (data not shown). We conclude from these data that blue-light-dependent regulation by AppA/PpsR is more sensitive than blue-light-dependent PrrB/PrrA regulation. Our data further prove the important role of AppA/PpsR in the avoidance of photosynthesis gene expression when oxygen and light are present, down to very low light quantities. Additionally, we show that the higher sensitivity of AppA/PpsR can shut down gene expression at low blue light quantities before the PrrB/PrrA-dependent influence sets in at higher light quantities.
Footnotes
Published ahead of print on 24 April 2009.
REFERENCES
- 1.Braatsch, S., M. Gomelsky, S. Kuphal, and G. Klug. 2002. A single flavoprotein, AppA, integrates both redox and light signals in Rhodobacter sphaeroides. Mol. Microbiol. 45827-836. [DOI] [PubMed] [Google Scholar]
- 2.Eraso, J. M., and S. Kaplan. 1995. Oxygen-insensitive synthesis of the photosynthetic membranes of Rhodobacter sphaeroides: a mutant histidine kinase. J. Bacteriol. 1772695-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eraso, J. M., J. H. Roh, X. Zeng, S. J. Callister, M. S. Lipton, and S. Kaplan. 2008. Role of the global transcriptional regulator PrrA in Rhodobacter sphaeroides 2.4.1: combined transcriptome and proteome analysis. J. Bacteriol. 1904831-4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gauden, M., J. S. Grinstead, W. Laan, I. H. van Stokkum, M. Avila-Perez, K. C. Toh, R. Boelens, R. Kaptein, R. van Grondelle, K. J. Hellingwerf, and J. T. Kennis. 2007. On the role of aromatic side chains in the photoactivation of BLUF domains. Biochemistry 467405-7415. [DOI] [PubMed] [Google Scholar]
- 5.Gomelsky, M., and S. Kaplan. 1995. appA, a novel gene encoding a trans-acting factor involved in the regulation of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 1774609-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomelsky, M., and G. Klug. 2002. BLUF: a novel FAD-binding domain involved in sensory transduction in microorganisms. Trends Biochem. Sci. 27497-500. [DOI] [PubMed] [Google Scholar]
- 7.Grinstead, J. S., S. T. Hsu, W. Laan, A. M. Bonvin, K. J. Hellingwerf, R. Boelens, and R. Kaptein. 2006. The solution structure of the AppA BLUF domain: insight into the mechanism of light-induced signaling. Chembiochem 7187-193. [DOI] [PubMed] [Google Scholar]
- 8.Han, Y., S. Braatsch, L. Osterloh, and G. Klug. 2004. A eukaryotic BLUF domain mediates light-dependent gene expression in the purple bacterium Rhodobacter sphaeroides 2.4.1. Proc. Natl. Acad. Sci. USA 10112306-12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han, Y., M. H. Meyer, M. Keusgen, and G. Klug. 2007. A haem cofactor is required for redox and light signalling by the AppA protein of Rhodobacter sphaeroides. Mol. Microbiol. 641090-1104. [DOI] [PubMed] [Google Scholar]
- 10.Happ, H. N., S. Braatsch, V. Broschek, L. Osterloh, and G. Klug. 2005. Light-dependent regulation of photosynthesis genes in Rhodobacter sphaeroides 2.4.1 is coordinately controlled by photosynthetic electron transport via the PrrBA two-component system and the photoreceptor AppA. Mol. Microbiol. 58903-914. [DOI] [PubMed] [Google Scholar]
- 11.Iseki, M., S. Matsunaga, A. Murakami, K. Ohno, K. Shiga, K. Yoshida, M. Sugai, T. Takahashi, T. Hori, and M. Watanabe. 2002. A blue-light-activated adenylyl cyclase mediates photoavoidance in Euglena gracilis. Nature 4151047-1051. [DOI] [PubMed] [Google Scholar]
- 12.Jäger, A., S. Braatsch, K. Haberzettl, S. Metz, L. Osterloh, Y. Han, and G. Klug. 2007. The AppA and PpsR proteins from Rhodobacter sphaeroides can establish a redox-dependent signal chain but fail to transmit blue-light signals in other bacteria. J. Bacteriol. 1892274-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan, S., J. Eraso, and J. H. Roh. 2005. Interacting regulatory networks in the facultative photosynthetic bacterium, Rhodobacter sphaeroides 2.4.1. Biochem. Soc. Trans. 3351-55. [DOI] [PubMed] [Google Scholar]
- 14.Kim, Y. J., I. J. Ko, J. M. Lee, H. Y. Kang, Y. M. Kim, S. Kaplan, and J. I. Oh. 2007. Dominant role of the cbb3 oxidase in regulation of photosynthesis gene expression through the PrrBA system in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 1895617-5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laan, W., M. Gauden, S. Yeremenko, R. van Grondelle, J. T. Kennis, and K. J. Hellingwerf. 2006. On the mechanism of activation of the BLUF domain of AppA. Biochemistry 4551-60. [DOI] [PubMed] [Google Scholar]
- 16.Lin, C., H. Yang, H. Guo, T. Mockler, J. Chen, and A. R. Cashmore. 1998. Enhancement of blue-light sensitivity of Arabidopsis seedlings by a blue light receptor cryptochrome 2. Proc. Natl. Acad. Sci. USA 952686-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majerus, T., T. Kottke, W. Laan, K. Hellingwerf, and J. Heberle. 2007. Time-resolved FT-IR spectroscopy traces signal relay within the blue-light receptor AppA. Chemphyschem 81787-1789. [DOI] [PubMed] [Google Scholar]
- 18.Masuda, S., and C. E. Bauer. 2002. AppA is a blue light photoreceptor that antirepresses photosynthesis gene expression in Rhodobacter sphaeroides. Cell 110613-623. [DOI] [PubMed] [Google Scholar]
- 19.Moskvin, O. V., L. Gomelsky, and M. Gomelsky. 2005. Transcriptome analysis of the Rhodobacter sphaeroides PpsR regulon: PpsR as a master regulator of photosystem development. J. Bacteriol. 1872148-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moskvin, O. V., S. Kaplan, M. A. Gilles-Gonzalez, and M. Gomelsky. 2007. Novel heme-based oxygen sensor with a revealing evolutionary history. J. Biol. Chem. 28228740-28748. [DOI] [PubMed] [Google Scholar]
- 21.Oh, J. I., I. J. Ko, and S. Kaplan. 2001. The default state of the membrane-localized histidine kinase PrrB of Rhodobacter sphaeroides 2.4.1 is in the kinase-positive mode. J. Bacteriol. 1836807-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh, J. I., I. J. Ko, and S. Kaplan. 2004. Reconstitution of the Rhodobacter sphaeroides cbb3-PrrBA signal transduction pathway in vitro. Biochemistry 437915-7923. [DOI] [PubMed] [Google Scholar]
- 23.Pappas, C. T., J. Sram, O. V. Moskvin, P. S. Ivanov, R. C. Mackenzie, M. Choudhary, M. L. Land, F. W. Larimer, S. Kaplan, and M. Gomelsky. 2004. Construction and validation of the Rhodobacter sphaeroides 2.4.1 DNA microarray: transcriptome flexibility at diverse growth modes. J. Bacteriol. 1864748-4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimada, H., K. Iba, and K. I. Takamiya. 1992. Blue-light irradiation reduces the expression of puf and puc operons of Rhodobacter sphaeroides under semi-aerobic conditions. Plant Cell Physiol. 33471-475. [Google Scholar]
- 26.Smith, H. 1982. Light quality, photoperception, and plant strategy. Annu. Rev. Plant Physiol. 33481-518. [Google Scholar]
- 27.van der Horst, M. A., W. Laan, S. Yeremenko, A. Wende, P. Palm, D. Oesterhelt, and K. J. Hellingwerf. 2005. From primary photochemistry to biological function in the blue-light photoreceptors PYP and AppA. Photochem. Photobiol. Sci. 4688-693. [DOI] [PubMed] [Google Scholar]
- 28.Zeilstra-Ryalls, J., M. Gomelsky, J. M. Eraso, A. Yeliseev, J. O'Gara, and S. Kaplan. 1998. Control of photosystem formation in Rhodobacter sphaeroides. J. Bacteriol. 1802801-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]