Abstract
Chemical modification of proteins with polyethylene glycol (PEGylation) can increase plasma half-lives, stability, and therapeutic potency. To make a PEGylated recombinant immunotoxin with improved therapeutic properties, we prepared a mutant of anti-Tac(Fv)-PE38 (LMB-2), a recombinant immunotoxin composed of a single-chain Fv fragment of the anti-human Tac monoclonal antibody to the IL-2 receptor α subunit fused to a 38-kDa fragment of Pseudomonas exotoxin. For site-specific PEGylation of LMB-2, one cysteine residue was introduced into the peptide connector (ASGCGPE) between the Fv and the toxin. This mutant LMB-2 (cys1-LMB-2), which retained full cytotoxic activity, was then site-specifically conjugated with 5 or 20 kDa of polyethylene glycol-maleimide. When compared with unmodified LMB-2, both PEGylated immunotoxins showed similar cytotoxic activities in vitro but superior stability at 37°C in mouse serum, a 5- to 8-fold increase in plasma half-lives in mice, and a 3- to 4-fold increase in antitumor activity. This was accompanied by a substantial decrease in animal toxicity and immunogenicity. Site-specific PEGylation of recombinant immunotoxins may increase their therapeutic potency in humans.
Keywords: Pseudomonas exotoxin, cancer therapy, immunotherapy, pharmacokinetics, liver toxicity
Recombinant immunotoxins are chimeric proteins in which a truncated toxin that serves as the cytotoxic moiety is fused to an Fv portion of an antibody that serves as the targeting moiety. We have produced many different recombinant immunotoxins in which the Fv portion of an antibody to a tumor-related antigen is fused to a 38-kDa mutant form of Pseudomonas exotoxin A (PE) that has a deletion of its cell binding domain (1–6). Five of these recombinant immunotoxins [anti-Tac(Fv)-PE38, B3(Fv)-PE38, B3(dsFv)-PE38, RFB4(dsFv)-PE38, and e23(dsFv)-PE38] have recently been evaluated in Phase I trials in patients with cancer (7, 8). All of these immunotoxins have produced complete regressions of human cancer xenografts in nude mice and are relatively well tolerated by mice and monkeys.
Anti-Tac(Fv)-PE38 (LMB-2), which contains the Fv fragment of the anti-human Tac monoclonal antibody to the IL-2 receptor α subunit (also referred to as Tac, p55, or CD25) (Fig. 1) has produced major clinical responses in hematologic malignancies (7, 9). LMB-2 was administered to 35 patients with CD25+ hematologic malignancies who had failed standard and salvage therapies. One patient with hairy cell leukemia had a complete remission, ongoing at 18 months, and seven partial responses were observed in hairy cell leukemia (3) cutaneous T-cell lymphoma (1), chronic lymphocytic leukemia (1), Hodgkin's disease (1), and adult T-cell leukemia (1).
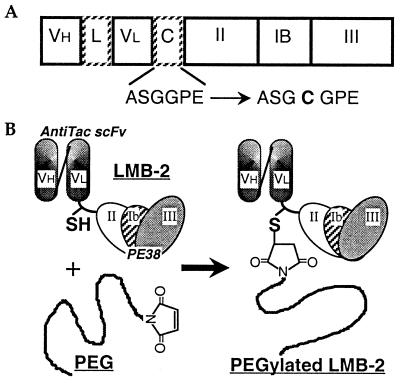
Figure 1.
(A) Scheme of mutant LMB-2 [anti-Tac(Fv)-PE38] with one cysteine in the connector. The positions of amino acids that span PE sequences are numbered. The amino acid sequence of the connector (C) of mutant LMB-2 (cys1-LMB-2) is changed from ASGGPE to ASGCGPE. VH, heavy chain fragment of anti-Tac antibody; L, peptide linker (GGGGS)3; VL, light chain fragment; C, connector; II, PE domain II; Ib, PE domain Ib; III, PE domain III. (B) Site-specific PEGylation to a cysteine residue in the connector of mutant LMB-2. cys1-LMB-2 is site-specifically conjugated with PEG via the formation of a thioether bond between a free thiol group in the connector of mutant LMB-2 and maleimide at one end of the PEG chain.
However, LMB-2 exhibited side-effects that limited the amount of immunotoxin that could be given to patients. These toxic side-effects are at least in part caused by the nonspecific binding of LMB-2 to normal tissues, because one dose-limiting toxicity was damaging to liver cells that do not express IL-2 receptors (10). Side-effects resulting from specific targeting of CD25+ normal cells may also have occurred. In addition, we found that human anti-PE antibodies and occasionally anti-mouse Fv antibodies were formed in some patients treated with LMB-2 (9). This immunogenicity of recombinant immunotoxins reduces their therapeutic usefulness. If side-effects and immunogenicity can be prevented, one should be able to give more immunotoxin and obtain improved responses in human malignancies. We recently described a new strategy designed to decrease the side effects of LMB-2 in which mutations are introduced into the framework region of the Fv to lower its isoelectric point (11). These mutant immunotoxins are less toxic to mice, allowing higher doses to be given with a substantial increase in antitumor activity. However, liver damage was still the dose-limiting toxicity, and this approach is not designed to decrease immunogenicity.
These recombinant immunotoxins (molecular weight: 63,000) have a lower molecular weight than conventional antibody-toxin conjugates (molecular weight: ≈190,000), and this leads to a shorter survival in the circulation and increased distribution into tumors as well as various normal tissues such as kidney and liver. Preclinical studies have shown that antitumor activity is enhanced if recombinant immunotoxins survive longer in the circulation (12). Thus, an increase in their blood-residency time should lead to an increase in their antitumor activities and a decrease in their distribution into normal tissues such as liver that is a major source of the unfavorable side-effects. The net result would be an augmentation of their therapeutic potency.
The most useful ways to increase the blood-residency of proteins is to modify them with polyethylene glycol (PEG). Chemical modification of proteins with PEG (PEGylation) increases their molecular size and steric hindrance, both of which depend on the PEG attached to the protein. This results in an improvement of plasma half-lives and in proteolytic-stability, and a decrease in immunogenicity and hepatic uptake (13, 15). PEGylation of interleukin-2 has been reported to increase its antitumor potency in vivo (16), and PEGylation of an F(ab′)2 derived from the monoclonal antibody A7 has improved its tumor localization (17). We previously reported that a PEGylated chimeric toxin composed of transforming growth factor-α and PE showed an improvement in its blood-residency time and a decrease in its immunogenicity resulting in enhanced in vivo antitumor potency and reduced in vivo toxicity (18). However, PEGylation was accompanied by a significant loss of specific cytotoxic activity. Unlike PEGylation of enzymes that act on small substrates, PEGylation of recombinant immunotoxins may cause a decrease in their activity attributable to loss of antigen-binding, translocation to the cytosol, or ADP-ribosylation activity, because these steps are based on macromolecular interactions that are easily sterically hindered by the attached PEG. In most cases, PEGylation of proteins is nonspecific and targeted at all of the lysine residues in the protein, some of which may be in or near the active-site. To overcome this drawback, we previously attempted to do site-specific PEGylation of mutant PE molecules that were engineered to contain one or two cysteine residues on the surface of PE (19, 20). Free thiol chemistry was used for the attachment of PEG to these residues. This approach was not successful because there was a low yield of PEGylated immunotoxin and a significant loss in activity.
In this study, we chose a different approach to site-specific PEGylation. To keep the antigen-binding, translocation, and ADP-ribosylation activities intact, we prepared a mutant of LMB-2 (cys1-LMB-2) with one cysteine in the peptide connector that attaches the Fv to the toxin (Fig. 1A) and modified the cys1-LMB-2 with PEG-maleimide. The PEGylated LMB-2s had comparable in vitro specific cytotoxicity against CD25+ tumor cells, but other properties including stability, plasma half-life, antitumor activity, immunogenicity, and nonspecific toxicity were greatly improved.
Materials and Methods
Materials.
Methoxy-polyethylene glycol-maleimide (PEG-maleimide; molecular weight: 5,000 or 20,000) was obtained from Fluka or Shearwater Polymers (Huntsville, AL). Other reagents and solvents were obtained from standard sources.
Bacterial Strains and Plasmid.
Escherichia coli DH5α (MAX efficiency) from Bethesda Research Laboratories was used for propagation of plasmids. E. coli CJ236 from Bio-Rad was used for preparation of single-stranded uracil-containing phagemid DNA, to be used as template for oligodeoxynucleotide-directed mutagenesis. E. coli BL21 (λDE3), which carries the T7 RNA polymerase gene under the control of an inducible promoter on a λ prophage, was used as a host for expression of recombinant immunotoxins. Plasmid pRK79 encodes anti-Tac(Fv)-PE38 (LMB-2) (2).
Mutagenesis of LMB-2.
Mutagenesis of LMB-2 was done by the method of Kunkel et al. (21) with some modifications as described (11). Mutations in the plasmid were confirmed by DNA sequencing.
Expression and Purification of Recombinant Immunotoxins.
The components of LMB-2 (native LMB-2) and cys1-LMB-2 were produced in E. coli BL21 (λDE3) containing the corresponding expression plasmids (pRK79 or mutant pRK79) as described (11).
PEGylation of cys1-LMB-2 with One Cysteine.
A typical procedure for preparation of PEGylated LMB-2 is as follows. LMB-2 with a free thiol group in PBS was allowed to react with 30-fold molar excess of 5- or 20-kDa PEG-maleimide (PEG5k or PEG20k) at 25°C for 12 h. The reaction mixture was diluted with 20 volumes of Buffer A (20 mM Tris⋅HCl/1 mM EDTA, pH 7.5), then was directly loaded on a Q-Sepharose column that was previously equilibrated with Buffer A. The column was washed with 50 volumes of Buffer A for removing uncoupled and uncoupled PEG5k or PEG20k, then was eluted with Buffer B (1 M NaCl in Buffer A). The appropriate Q-Sepharose (Amersham Pharmacia) fractions containing PEGylated LMB-2s were collected and diluted with 5 volumes of Buffer A, then were loaded on a Mono-Q (Amersham Pharmacia) column that was previously equilibrated with Buffer A, to roughly separate PEGylated LMB-2s from unmodified cys1-LMB-2. The column was washed with 10 volumes of Buffer A, then eluted with a NaCl gradient (0–1 M) in Buffer A. After concentrating the appropriate Mono-Q fractions by Centricon YM-10 (Amicon), the crude PEGylated LMB-2s were separated on TSK G3000SW that was equilibrated and eluted with PBS. The obtained PEGylated LMB-2s were extremely pure, according to SDS/PAGE analysis.
Free Sulfhydryl Content.
The free sulfhydryl group content was determined by the 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) method (22).
PEG Content.
PEG content was assayed by a modification of the method of Childs (23). PEG5k or PEG20k was used as a standard.
Cytotoxicity Assay.
The specific cytotoxicity of native, cys1-LMB-2s, and the PEGylated LMB-2s was assessed by protein synthesis inhibition assay on ATac-4 cells (2).
Stability Assay.
The stability of LMB-2, cys1-LMB-2, and PEGylated LMB-2s was determined by incubating them at a final concentration of 10 μg/ml at 37°C in mouse serum. The amount of active immunotoxin remaining after different times of incubation was determined by ATac4 cytotoxicity assay.
Nonspecific Toxicity Assay.
Groups of four, five, or seven female BALB/c mice (6–7 weeks old; about 20 g) were injected i.v. with 200 μl of increasing doses of LMB-2, cys1-LMB-2, and PEGylated LMB-2s. PBS containing 0.2% BSA was used as a diluent. The LD50 is the calculated dose of recombinant immunotoxin that kills 50% of the animals.
Antitumor Study.
Antitumor activities of the immunotoxins were determined in mice bearing ATac-4 tumors. ATac-4 cells (2 × 106) were inoculated s.c. on day 0 into nude mice (Athymic Ncr nu/nu; 7 weeks old; about 20 g). Starting on day 4, mice were treated with i.v. injections of immunotoxins. PBS containing 0.2% BSA was used as a diluent, and 10 μg/mouse of PEG5k or PEG20k were used as controls. Therapy was given on days 4, 6, and 8. Each treatment group consisted of five mice. Tumors were measured with a caliper every 2 days, and the volume of the tumor was calculated by using the formula: tumor volume (in mm3) = length × (width)2 × 0.4.
Pharmacokinetic Assays.
Normal female BALB/c mice (6–7 weeks old; about 20 g) were injected intravenously with 2 μg of various immunotoxins. Blood samples were drawn at different times. The level of recombinant immunotoxin was measured in a bioassay in which diluted serum samples were incubated with ATac-4 cells, and the ability of serum samples to inhibit protein synthesis was measured. The data were analyzed by an exponential curve fitting program rstrip 5 (MicroMath Scientific Software, Salt Lake City).
Immunogenicity Assays.
Female BALB/c mice (6–7 weeks old; about 20 g) in groups of five were immunized i.p. with LMB-2, cys1-LMB-2, and PEGylated LMB-2s at doses of 4 μg/mouse or 40 μg/mouse in PBS containing 0.2% mouse serum albumin. Two weeks after the first immunization, mice were once again immunized with the appropriate antigen. Blood samples were collected every 7 days after the first immunization. The specific IgG levels in serum were determined by ELISA, using goat anti-mouse IgG antibody conjugated to alkaline phosphatase and p-nitrophenyl phosphate as substrate. The plates were coated with LMB-2.
Results
Preparation and Characterization of PEGylated LMB-2s.
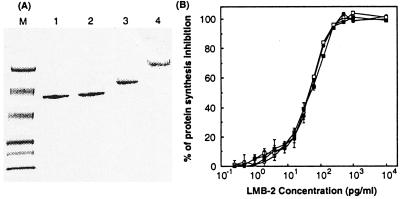
In LMB-2, the Fv portion of the anti-Tac antibody is linked to PE38 by a peptide connector (ASGGPE). To prevent loss of the antigen-binding, translocation, and ADP-ribosylation functions of LMB-2 that are necessary for its specific cytotoxic activity against CD25+ tumor cells, we prepared a mutant form of LMB-2 with one cysteine in the peptide (ASGCGPE) that connects the Fv to PE38 (Fig. 1A). The cysteine was used for site-specific PEGylation using free thiol chemistry. As shown in Fig. 2, the PEGylated LMB-2 molecules ran as a single band on SDS/PAGE when eluted from a TSK size exclusion column as a single peak (data not shown). Fig. 2 and Table 1 show that the specific in vitro cytotoxicity of cys1-LMB-2 against the ATac-4 cell (CD25+ cell line) was similar to that of native LMB-2. As expected, each cys1-LMB-2 molecule contained one free thiol group whereas native LMB-2 did not. The yield of highly purified cys1-LMB-2 prepared from inclusion bodies was ≈7.0%, which is the same as that of native LMB-2 (7.3%).
Figure 2.
Properties of LMB-2 molecules. (A) SDS/PAGE analysis of PEGylated LMB-2s. SDS/PAGE was performed under non-reducing conditions. Lanes: M, molecular weight standards from top to bottom: 111,000, 73,000, 47,500, 33,900, 28,800, and 20,500; 1, LMB-2; 2, cys1-LMB-2; 3, PEG5k-LMB-2; 4, PEG20k-LMB-2. (B) In vitro specific cytotoxicity of various forms of LMB-2 on ATac4 cells. Various LMB-2s were diluted with 0.2% BSA in DPBS. ATac-4 cells were seeded at 2.0 × 104 cells/well in 96-well plates 24 h before the addition of LMB-2 (○), cys1-LMB-2 (●), PEG5k-LMB-2 (□), PEG20k-LMB-2 (■) and were incubated with them at 37°C for 24 h, and then were assayed by measuring inhibition of incorporation of 3H-leucine.
Table 1.
Characterization of PEGylated LMB-2s
| Connector | Molar ratio of free SH/LMB-2 | Molar ratio of PEG/LMB-2 | IC50, pg/ml | LD50, mg/kg | |
|---|---|---|---|---|---|
| LMB-2 | ASGGPE | 0.1 | 0.0 | 60 | 0.51 |
| Mutant LMB-2 | ASG C GPE | 1.1 | 0.0 | 60 | 0.54 |
| PEG5k-mLMB-2 | 0.0 | 1.1 | 60 | 2.92 | |
| PEG20k-mLMB-2 | 0.0 | 1.0 | 70 | 3.09 |
Purified cys1-LMB-2 with a free thiol group in the connector was site-specifically modified with PEG5k- or PEG20k-maleimide by the formation of a thioether bond (Fig. 1B). After purification, both types of PEGylated LMB-2s had similar cytotoxic activities, and these were the same as the unmodified native and mutant LMB-2 (Fig. 2 and Table 1). After site-specific PEGylation of cys1-LMB-2, the PEGylated LMB-2 did not have a free thiol group and instead contained about 1 mole of PEG/mole of protein.
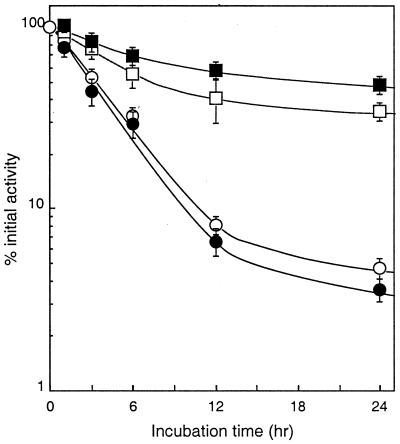
Stability of PEGylated LMB-2 in Mouse Serum.
The stabilities of various LMB-2 molecules were assessed by incubating them in mouse serum at 37°C for various periods of time (Fig. 3). Both native and mutant LMB-2 were relatively stable for 1 h, and then their cytotoxic activities diminished in a time-dependent manner. In contrast, both PEGylated LMB-2s were quite stable. After 24 h, PEG5k- and PEG20k-LMB-2s retained about 40 and 50% of their initial activities.
Figure 3.
Stability of PEGylated LMB-2s in mouse serum. The stability of LMB-2 (○), cys1-LMB-2 (●), PEG5k-LMB-2 (□), and PEG20k-LMB-2 (■) was determined by incubation at 10 μg/ml at 37°C in mouse serum from female BALB/c mice (50 μl of 40 μg/ml immunotoxin + 150 μl of mouse serum). The amount of active immunotoxin remaining after different times of incubation was determined by a cytotoxicity assay.
Toxicity of PEGylated LMB-2s.
For toxicity studies, several different doses of each type of immunotoxin were injected intravenously into BALB/c mice. Almost all of the deaths occurred within 4 days after treatment. Table 2 indicates the number of dead mice and the total number of mice injected recorded 14 days after treatment. The LD50 of native and mutant LMB-2 was found to be about 0.5 mg/kg, as shown in Table 1. By contrast, the PEGylated LMB-2s were well tolerated; the LD50s of PEG5k-LMB-2 and PEG20k-LMB-2 were about 3.0 mg/kg.
Table 2.
Acute in vivo toxicity of a single i.v. injection of PEGylated LMB-2s in mice
| Groups | Injected dose, mg protein/kg
|
||||||
|---|---|---|---|---|---|---|---|
| 0.23 | 0.45 | 0.68 | 0.90 | 1.8 | 3.6 | 4.8 | |
| LMB-2 | 1/7* | 3/7 | 5/7 | 7/7 | |||
| Mutant LMB-2 | 0/7 | 3/7 | 5/7 | 7/7 | |||
| PEG5k-mLMB-2 | 0/5 | 0/7 | 2/7 | 5/7 | 4/4 | ||
| PEG20k-mLMB-2 | 0/5 | 0/7 | 1/7 | 5/7 | 4/4 | ||
Values reported are number of mice dying divided by number of mice treated.
Antitumor Activity of PEGylated LMB-2s.
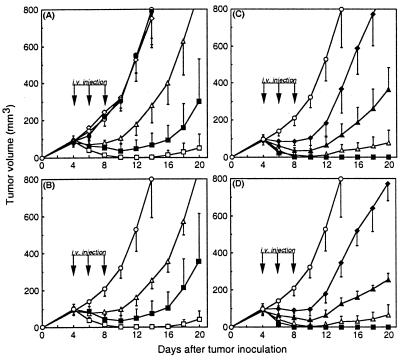
To assess antitumor activities, ATac-4 cells were inoculated s.c. in nude mice on day 0. Treatment was started on day 4 when the tumors measured about 100 mm3. Animals were treated i.v. with three doses given on days 4, 6, and 8. The control groups received vehicle (PBS containing 0.2% BSA) or 10 μg of PEG5k or PEG20k. As shown in Fig. 4, native and mutant LMB-2s inhibited tumor growth in a dose-dependent manner. The antitumor activities of both unPEGlyated LMB-2s were similar. The dose required to maintain the average tumor volume for 6 days at the level of the first injection was 0.025 mg/kg × 3 for LMB-2 and cys1-LMB-2 and 0.006 mg/kg × 3 for PEG5k-LMB-2 and PEG20k-LMB-2. Complete regressions, which were defined as disappearance of tumor without regrowth after more than 50 days, were observed in 2 of 5 mice or 1 of 5 mice at the dose of 0.1 mg/kg × 3 of native or mutant LMB-2 respectively. At the 0.2 mg/kg × 3 dose level, one of five mice administered either native or mutant LMB-2 died from toxicity during the therapeutic period, but complete regressions were observed in all four remaining mice (data not shown). The antitumor activities of both types of PEGylated LMB-2s were markedly improved. Complete regression was observed in 1 of 5 mice or 2 of 5 mice at the dose of 0.025 mg/kg × 3 of PEG5k- or PEG20k-LMB-2, respectively. At the dose of 0.05 mg/kg × 3, PEGylated LMB-2s caused complete regressions lasting over 50 days. When given by themselves, PEG5k and PEG20k (10 μg/mouse) had no antitumor activity. Overall, approximately 4 times the dose was required to produce comparable changes in tumor volume when either of the PEGylated LMB-2s is used.
Figure 4.
Antitumor effects of PEGylated LMB-2s on ATac-4 solid tumor. ATac-4 cells (2 × 106) were inoculated s.c. on day 0 into nude mice. Starting on day 4, mice were treated with i.v. injections of immunotoxins on days 4, 6, and 8. Groups of five mice were treated for each dose QOD × 3 at each dose level. (A) LMB-2. (B) cys1-LMB-2. (C) PEG5k-LMB-2. (D) PEG20k-LMB-2. The following symbols were used: ○, diluent; □, 0.100 mg/kg; ■, 0.050 mg/kg; ▵, 0.025; ▴, 0.013 mg/kg; ♦, 0.006 mg/kg; ●, PEG5k 0.5 mg/kg; ⋄, PEG20k 0.5 mg/kg.
Pharmacokinetics of PEGylated LMB-2s.
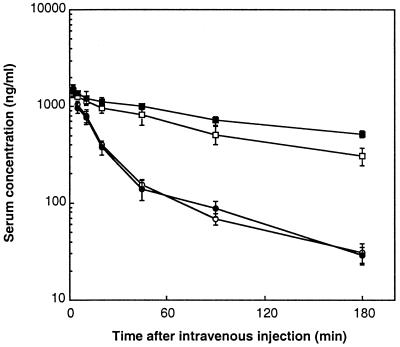
BALB/c mice were injected i.v. with a single dose of 2 μg of each form of LMB-2. Blood was drawn at different times after the injection and was assayed for immunotoxin levels on ATac-4 cells. As shown in Fig. 5, the serum concentration profiles of native and mutant LMB-2s showed a biexponential elimination curve. The plasma half-lives of the unmodified LMB-2s were about 13 min (Table 3). In contrast, the serum concentration profiles of both PEGylated LMB-2s showed monoexponential elimination curves. The plasma half-life of PEG5k-LMB-2 was increased about 5-fold and that of PEG20k-LMB-2 about 8-fold. The area under the curve and mean residence time of both PEGylated LMB-2s was also substantially increased.
Figure 5.
Pharmacokinetics of PEGylated LMB-2s in mice. Normal female BALB/c mice were injected intravenously with 2 μg of LMB-2 (○), cys1-LMB-2 (●), PEG5k-LMB-2 (□), PEG20k-LMB-2 (■). Blood samples were drawn at different times. The level of immunotoxin was measured by bioassay in which diluted serum samples were incubated with Atac-4 cells, and the ability of serum samples to inhibit protein synthesis was measured. A standard curve was made for each immunotoxin. Groups of four mice were used. Values are mean ± SD.
Table 3.
Pharmacokinetic parameters of immunotoxins
| t1/2, min | MRT, min | AUC, ng⋅min/ml | |
|---|---|---|---|
| LMB-2 | 13 | 34.1 | 31,604 |
| Mutant LMB-2 | 13 | 35.3 | 31,385 |
| PEG5k-LMB-2 | 66 | 67.9 | 111,589 |
| PEG20k-LMB-2 | 106 | 75.3 | 145,835 |
Values are calculated from data in Fig. 5.
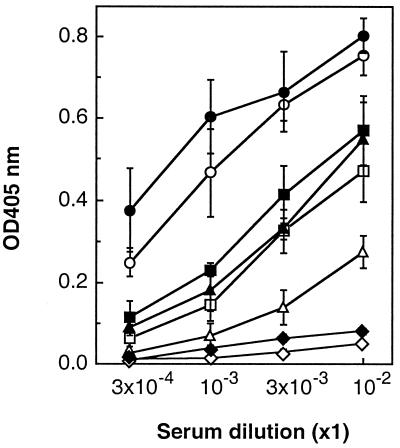
Immunogenicity of PEGylated in LMB-2s.
To assess immunogenicity, BALB/c mice were immunized i.p. with each form of LMB-2 at a dose of 4 μg/mouse or 40 μg/mouse on days 0 and 14. Serum samples were collect on day 21 after the first immunization, and mouse anti-LMB-2 IgG antibody levels in serum were determined by ELISA using native LMB-2 as the coating antigen. As shown in Fig. 6, native LMB-2 and mutant LMB-2 at a dose of 4 μg/mouse × 2 markedly induced anti-LMB-2 IgG antibodies in mice. Higher doses of native LMB-2 and mutant LMB-2 could not be administered because of toxicity to the mice. In contrast, the immunogenicity of both PEGylated LMB-2s was found to be much lower. Native LMB-2 was used to detect the antibody response in this study, but very similar results were obtained when mutant LMB-2 or PEGylated LMB-2s were used to coat the plates (data not shown).
Figure 6.
IgG responses in mice to PEGylated LMB-2s. Mice in groups of five were injected i.p. with LMB-2 (○), cys1-LMB-2 (●), PEG5k-LMB-2 (□, ▪), and PEG20k-LMB-2 (▵, ▴) at a dose of 4 μg/mouse (○, ●, □, ▵) or 40 μg/mouse (▪, ▴) in PBS containing 0.2% mouse serum albumin. Two weeks after the first immunization, mice were once again immunized with the appropriate antigen. The specific IgG levels were determined by ELISA on day 21. The following symbols were used: ⋄, no treatment; ♦, 0.2% MSA/PBS.
Discussion
We show here that site-specific PEGylation of recombinant immunotoxin LMB-2 increases its stability, blood residence time, and antitumor activity while decreasing its nonspecific toxicity and immunogenicity. Overall, the therapeutic window is increased by over 20-fold. These results have important clinical implications for the use of immunotoxins in patients.
In a recently completed clinical trial, LMB-2 was given to 35 patients and showed antitumor activity in a variety of malignancies. One complete response was observed in hairy cell leukemia, which has lasted over 22 months. Several different toxicities were observed in this trial. These included transaminase elevations caused by liver damage, weight gain, hypotension, and fever. Toxic side-effects of recombinant immunotoxins are of two types. One type results from specific targeting of normal cells that express the same antigen as the tumor cells. To overcome this toxicity, it may be necessary to discover a target antigen that is not expressed on normal cells. We are currently searching for new tumor-related antigens by analysis of the expressed sequence tag database analysis (24). The other type of toxicity arises from undefined nonspecific adsorption and uptake by CD25-negative normal cells. In the Phase I trial with LMB-2 and in toxicity studies of LMB-2 using mice whose CD25 does not react with LMB-2 or monkeys whose CD25 does react, liver damage as evidenced by transaminase elevations in the blood was frequently encountered (7, 9, 10). If this type of toxicity can be reduced, it should be possible to raise the dose of immunotoxin used to treat patients and obtain more frequent and substantial clinical responses. One approach to reducing nonspecific toxicity is to restrict the distribution of immunotoxin from the blood to normal tissues by increasing its blood-residency time. Additionally, prolongation of plasma half-life may lead to enhanced antitumor efficacy (11). In this study, we attempted to improve the blood-residency of LMB-2 and to enhance its therapeutic potency by PEGylation.
PEGylation of proteins increases their plasma half-lives by reducing proteolysis and restricting tissue-distribution such as glomerular filtration by the kidney and hepatic uptake (12–20). This effect is attributed to the increased molecular size and steric hindrance that result from attaching PEG to proteins. In most cases, the application of PEGylation has been limited to enzymes whose substrates are very small molecules, such as adenosine deaminase and superoxide disumutase, because the attached PEG chain can sterically inhibit ligand-receptor and antigen-antibody binding, which are based on macromolecular interactions (19, 20). Additionally, PEGylation usually occurs at lysine residues, some of which may be in or near the active site of the protein, and this lysine modification by PEG is random and difficult to control (25). Recombinant immunotoxins have three important functions (1–6). These are antigen-binding, translocation, and ADP-ribosylation of EF2. All three involve macromolecular interactions. Both the antigen-binding domain and the ADP-ribosylation domain contain lysine residues. To circumvent interference with macromolecular interactions, we made a mutant LMB-2 (Fig. 1) with one cysteine residue in the nonfunctional connector between the Fv and PE moieties of LMB-2 and used the thiol of the cysteine for site-specific PEGylation (Fig. 1).
The specific in vitro cytotoxicity of cys1-LMB-2 containing the extra cysteine residue against CD25+ tumor cells (ATac-4) is about the same as that of parental LMB-2 (Fig. 2 and Table 1). cys1-LMB-2 was specifically PEGylated by formation of a thioether bond between the free thiol group in the linker region and a terminal maleimide group of PEG. The PEG5k- and PEG20k-LMB-2 produced retained full cytotoxic activity when compared with unmodified native and mutant LMB-2s (Fig. 2 and Table 1). This is in part because LMB-2 is processed within the cell into two fragments by a proteolytic cleavage between amino acids 279 and 280 PE. The PEG remains with the Fv whereas most of the toxin (amino acids 280–613) is transported to the endoplasmic reticulum, where it translocates to the cytosol and kills the cell. Usually, the activity of PEGylated proteins decreases with increasing molecular weight of the attached PEG because the steric hindrance caused by the attached PEG increases with the increasing length of the PEG chain (26). However, in this case PEG20k-LMB-2 had almost the same activity as PEG5k-LMB-2. The reason for this is not clear but may be related to the relatively long distance between the PEG attachment site in the connector and the active sites of LMB-2. The stability of the PEGylated LMB-2s at 37°C in mouse serum that contains proteases and other inactivating activities was enhanced compared with the unmodified native and mutant LMB-2 (Fig. 3). It is possible that the PEG sterically hinders LMB-2 from proteolytic attack or dissociation of the VH and VL chains, which leads to aggregation, and that PEG20k is more effective than PEG5k.
Both types of PEGylated LMB-2 showed a 3- to 4-fold higher antitumor activity than unmodified native and mutant LMB-2. In addition, their toxicity to mice was reduced about 6-fold (Fig. 4 and Tables 1 and 2). Consequently, PEGylation led to a 20-fold increase in therapeutic efficacy. PEGylation of LMB-2 effectively increased its blood-residency and area under the curve, which are caused by an increase in molecular size and enhanced stability (Fig. 5). The plasma half-lives were 5-fold longer with PEG5k-LMB-2 and 8-fold longer with PEG20k-LMB-2 than with unmodified LMB-2s. The increase in the antitumor activities is probably caused by the increase in plasma half-life, which allows more immunotoxin to enter the tumor over time through the leaky capillaries that are often found in tumors. It is well known that the transport of PEGylated proteins from blood to normal tissues is limited and that the nonspecific cellular absorption and uptake of PEGylated molecules is reduced (13, 27, 28). Thus, the decrease in the toxicity of LMB-2 may in part be caused by its reduced distribution from blood into normal tissues such as liver due to its increased molecular size (13). Moreover, the nonspecific binding and uptake of LMB-2 by normal cells may be suppressed by PEGylation, which shields ionic interactions. PEGylated LMB-2s were also found to have decreased immunogenicity, which could be attributable to reduced degradation by antigen-presenting cells such as macrophages, shielding of some epitopes of peptides after degradation (18), or the prevention of binding to receptors on B cells. Additionally, as mentioned above, the transport of PEGylated LMB-2s from blood to immune tissues such as spleen, bone marrow, and lymphoid tissue may be limited, and the nonspecific binding and uptake of PEGylated proteins by antigen-presenting cells may be reduced. Detailed studies on tissue distribution and cell-binding are necessary to clarify these issues.
In summary, site-specific PEGylation of LMB-2 greatly improves its therapeutic potency as an antitumor agent in mice by increasing its plasma half-life and decreasing its nonspecific toxicity. The approach used with LMB-2 should be applicable to other recombinant immunotoxins and could increase their activity in patients.
Acknowledgments
We thank Verity Fogg for cell culture assistance and Robb Mann for editorial assistance.
Abbreviation
- PE
Pseudomonas exotoxin
- PEG
polyethylene glycol
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.140210597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.140210597
References
- 1.Pastan I. Biochim Biophys Acta. 1997;1333:1–6. [Google Scholar]
- 2.Kreitman R J, Bailon P, Chaudhary V K, FitzGerald D J, Pastan I. Blood. 1994;83:426–434. [PubMed] [Google Scholar]
- 3.Kreitman R J, Wang Q C, FitzGerald D J, Pastan I. Int J Cancer. 1999;81:148–155. doi: 10.1002/(sici)1097-0215(19990331)81:1<148::aid-ijc24>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Brinkmann U, Pai L H, FitzGerald D J, Willingham M, Pastan I. Proc Natl Acad Sci USA. 1991;88:8616–8620. doi: 10.1073/pnas.88.19.8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiter Y, Pai L H, Brinkmann U, Wang Q C, Pastan I. Cancer Res. 1994;54:2714–2718. [PubMed] [Google Scholar]
- 6.Reiter Y, Brinkmann U, Jung S H, Lee B, Kasprzyk P G, King C R, Pastan I. J Biol Chem. 1994;269:18327–18331. [PubMed] [Google Scholar]
- 7.Kreitman R J, Wilson W H, Robbins D, Margulies I, Stetler-Stevenson M, Waldmann T A, Pastan I. Blood. 1999;94:3340–3348. [PubMed] [Google Scholar]
- 8.Pai-Scherf L H, Villa J, Pearson D, Watson T, Liu E, Willingham M C, Pastan I. Clin Cancer Res. 1999;5:2311–2315. [PubMed] [Google Scholar]
- 9.Kreitman R J, Wilson W H, White J D, Stetler-Stevenson M, Jaffe E S, Giardina S, Waldmann T A, Pastan I. J Clin Oncol. 2000;18:1622–1636. doi: 10.1200/JCO.2000.18.8.1622. [DOI] [PubMed] [Google Scholar]
- 10.Kreitman R J, Pastan I. Semin Cancer Biol. 1995;5:297–306. doi: 10.1006/scbi.1995.0038. [DOI] [PubMed] [Google Scholar]
- 11.Onda M, Kreitman R J, Vasmatzis G, Lee B, Pastan I. J Immunol. 1999;163:6072–6077. [PubMed] [Google Scholar]
- 12.Pai L, H, Gallo M G, FitzGerald D J, Pastan I. Cancer Res. 1991;51:2808–2812. [PubMed] [Google Scholar]
- 13.Chaffee S, Mary A, Stiehm E R, Girault D, Fischer A, Hershfield M S. J Clin Invest. 1992;89:1643–1651. doi: 10.1172/JCI115761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yabe Y, Nishikawa M, Tamada A, Takakura Y, Hashida M. J Pharmacol Exp Ther. 1999;289:1176–1184. [PubMed] [Google Scholar]
- 15.Pyatak P S, Abuchowski A, Davis F F. Res Commun Chem Pathol Pharmacol. 1980;29:113–127. [PubMed] [Google Scholar]
- 16.Katre N V, Knauf M J, Laird W J. Proc Natl Acad Sci USA. 1987;84:1487–1491. doi: 10.1073/pnas.84.6.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitamura K, Takahashi T, Takashina K, Yamaguchi T, Noguchi A, Tsurumi H, Toyokuni T, Hakomori S. Biochem Biophys Res Commun. 1990;28:1387–1394. doi: 10.1016/0006-291x(90)90839-f. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q C, Pai L H, Debinski W, FitzGerald D J, Pastan I. Cancer Res. 1993;53:4588–4594. [PubMed] [Google Scholar]
- 19.Benhar I, Wang Q C, FitzGerald D, Pastan I. J Biol Chem. 1994;269:13398–133404. [PubMed] [Google Scholar]
- 20.Kuan C T, Wang Q C, Pastan I. J Biol Chem. 1994;269:7610–7616. [PubMed] [Google Scholar]
- 21.Kunkel T A, Bebenek K, McClary J. Methods Enzymol. 1991;204:125–139. doi: 10.1016/0076-6879(91)04008-c. [DOI] [PubMed] [Google Scholar]
- 22.Riddles P W, Blakeley R L, Zerner B. Anal Biochem. 1979;94:75–81. doi: 10.1016/0003-2697(79)90792-9. [DOI] [PubMed] [Google Scholar]
- 23.Childs C E. Microchem J. 1975;20:190–192. [Google Scholar]
- 24.Vasmatzis G, Essand M, Brinkmann U, Lee B, Pastan I. Proc Natl Acad Sci USA. 1998;6:300–304. doi: 10.1073/pnas.95.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsutsumi Y, Kihira T, Tsunoda S, Kanamori T, Nakagawa S, Mayumi T. Br J Cancer. 1995;71:963–968. doi: 10.1038/bjc.1995.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsutsumi Y, Tsunoda S, Kamada H, Kihira T, Nakagawa S, Kaneda Y, Kanamori T, Mayumi T. Br J Cancer. 1996;74:1090–1095. doi: 10.1038/bjc.1996.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Illum L, Jacobsen L O, Muller R H, Mak E, Davis S S. Biomaterials. 1987;8:113–117. doi: 10.1016/0142-9612(87)90099-8. [DOI] [PubMed] [Google Scholar]
- 28.Woodle M C, Lasic D D. Biochim Biophys Acta. 1992;14:171–199. doi: 10.1016/0304-4157(92)90038-c. [DOI] [PubMed] [Google Scholar]