Abstract
The Staphylococcus aureus cid and lrg operons have been shown to control cell death and lysis in a manner thought to be analogous to programmed cell death (apoptosis) in eukaryotic organisms. Although orthologous operons are present in a wide variety of bacterial species, members of the Bacillus cereus group are unique in that they have a total of four cid-/lrg-like operons. Two of these operons are similar to the S. aureus cid and lrg operons, while the other two (designated clhAB1 and clhAB2) are unique to this group. In the present study, the functions and regulation of these loci were examined. Interestingly, the Bacillus anthracis lrgAB mutant displayed decreased stationary-phase survival, whereas the clhAB2 mutant exhibited increased stationary-phase survival compared to the parental and complementation strains. However, neither mutation had a dramatic effect on murein hydrolase activity or autolysis. Furthermore, a quantitative analysis of the sporulation efficiency revealed that both mutants formed fewer spores than did the parental strain. Similar to S. aureus, B. anthracis lrgAB transcription was shown to be induced by gramicidin and CCCP, agents known to dissipate the proton motive force, in a lytSR-dependent manner. Northern blot analyses also demonstrated a positive role for lytSR in the clhAB2 transcription. Taken together, the results of the present study demonstrate that B. anthracis lrgAB and clhAB2 play important roles in the control of cell death and lysis and reveal a previously unrecognized role of this system in sporulation.
Studies have shown that the Staphylococcus aureus cidABC and lrgAB operons play an important role in the control of cell death and lysis (22). The cidA gene exerts its effect by increasing murein hydrolase activity to increase cell lysis (21, 24, 25), while the lrgAB operon inhibits murein hydrolase activity and lysis (11). Based on the predicted structural features of the CidA and LrgA proteins, along with the antagonistic effects of cid and lrg mutations on murein hydrolase activity, CidA and LrgA have been proposed to regulate cell death and lysis in a manner analogous to bacteriophage-encoded holins and antiholins, respectively (4). One biological function of the cidA gene is in the control of cell lysis during biofilm development (26). The resulting genomic DNA released (designated eDNA) was found to provide an important structural role in the biofilm matrix. These studies have led to the hypothesis that the cid and lrg gene products comprise the molecular machinery mediating bacterial programmed cell death (4, 22).
Analysis of S. aureus cidABC and lrgAB transcription have revealed two overlapping regulatory pathways, one mediated by the LysR-type transcriptional regulator, CidR, and responding to glucose metabolism (33), and the other induced by changes in membrane potential and involving the two-component regulatory system, LytSR (20). The cidABC operon lies downstream from the cidR gene (25, 33), and analysis of a cidR mutant indicated that CidR enhances cidABC and lrgAB expression in the presence of acetic acid generated by the metabolism of high levels of glucose (33). By comparison, expression of lrgAB is positively regulated by the lytSR operon (6) located immediately upstream of lrgAB. Recent studies in S. aureus have shown that this regulatory system is required for the induction of lrgAB expression in response to various agents affecting membrane potential (20). Typical of two-component regulatory systems, LytS is predicted to be a membrane protein that upon stimulation interacts with its cognate response regulator, LytR, which then increases the expression of the lrgAB operon. Initial studies of the S. aureus lytSR operon revealed that this novel regulatory system affects murein hydrolase activity and autolysis, presumably due to its regulation of lrgAB expression (6). Furthermore, recent studies demonstrate that lytSR is also involved in regulating biofilm development in S. aureus (unpublished data).
A sequence analysis of other microbial genomes has revealed the presence of cidAB and lrgAB homologues, as well as the cidR and lytSR regulatory genes, in a wide variety of gram-positive and gram-negative bacteria and several archaeal species (4). One such organism is Bacillus anthracis, which contains operons with open reading frames similar to the previously characterized cid and lrg operons of S. aureus. As in S. aureus, B. anthracis cid and lrg operons are located downstream of genes encoding a LysR-type transcriptional regulator and a two-component regulatory system, respectively (1). Unlike in S. aureus, the cidR gene is divergently transcribed in B. anthracis, and there is no cidC gene, encoding a pyruvate oxidase, in the B. anthracis genome (1). The B. anthracis CidR homologue was shown to play an important role in the regulation of cid and lrg expression, as well as cell death in the stationary phase (1). That study also showed that the genes encoding S-layer proteins are regulated by CidR and their gene products, Sap and EA1, possess murein hydrolase activity.
In the present study, we examined the B. anthracis LytSR two-component regulatory system and have begun the characterization of two additional cid/lrg homologues (designated clhAB1 and clhAB2 [for cid/lrg homologues AB-1 and AB-2]) within the B. anthracis genome. The results presented here confirm that the lytSR operon of B. anthracis, like that of the S. aureus lytSR, positively regulates lrgAB expression and that lrgAB transcription is induced by dissipation of the proton motive force in a lytSR-dependent fashion. In addition, the lrgAB and clhAB2 mutations were shown to affect stationary-phase survival and lysis. Finally, the results presented reveal for the first time that this system affects sporulation.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in the present study are listed in Table 1. B. anthracis Sterne was grown in brain heart infusion (BHI; Difco Laboratories, Detroit, MI) broth or filter-sterilized NZY broth (3% [wt/vol] N-Z Amine A and 1% [wt/vol] yeast extract - adjusted to pH 7.5). Escherichia coli strains were grown in Luria-Bertani (LB) medium. Agar was added (15 g per L) when needed. All liquid cultures were grown at 37°C with constant shaking (250 rpm) with a culture volume to flask ratio of no greater than 1:10. Chemicals were purchased from either Sigma Chemical Co. (St. Louis, MO) or Fisher Scientific (Fair Lawn, NJ). Sporulation assays were carried out in Schaeffer's medium (12). When necessary, antibiotics were used at the following concentrations: ampicillin (50 μg ml−1), erythromycin (5 μg ml−1), kanamycin (50 μg ml−1), and chloramphenicol (10 μg ml−1).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| B. anthracis | ||
| Sterne | B. anthracis(pXO1+ pXO2−) | 31 |
| KB5000 | Sterne lytSR::Km; Kmr | This study |
| KB5050 | KB5000 with pLC17 | This study |
| KB5400 | Sterne lrgAB::Km; Kmr | This study |
| KB5450 | KB5400 with pLC20 | This study |
| KB6000 | Sterne clhAB2::Km; Kmr | This study |
| KB6050 | KB6000 with pJA22 | This study |
| E. coli | ||
| DH5α | Host strain for construction of recombinant plasmids | Invitrogen |
| JM110 | Dam− Dcm− strain | 34 |
| Plasmid | ||
| pDG780 | Source of Kmr cassette; Kmr Ampr | 12 |
| pKS1 | Temperature-sensitive plasmid for B. anthracis; Kmr | 28 |
| pJA71 | Derivative of pKS1 containing the kanamycin cassette flanked by lytSR fragments; Kmr | This study |
| pCN51 | Shuttle vector; Emr Ampr | 4 |
| pLC17 | lytSR complementation plasmid | This study |
| pBKJ236 | Integration plasmid for B. anthracis | 14 |
| pJA22 | clhAB2 complementation plasmid | This study |
| pLC20 | lrgAB complementation plasmid | This study |
Emr, erythromycin resistance; Kmr, kanamycin resistance; Ampr, ampicillin resistance.
DNA manipulations.
B. anthracis genomic DNA was isolated by using a Qiagen (Valencia, CA) genomic DNA purification kit according to the manufacturer's instructions. Plasmid DNA was purified by using the Wizard Plus SV DNA purification kit (Promega, Inc., Madison, WI). Restriction endonucleases and T4 DNA ligase were purchased from either New England Biolabs (Beverly, MA) or Invitrogen Life Technologies (Carlsbad, CA). E. coli competent cells were prepared as performed by Dagert and Ehrlich (9) and electroporation of B. anthracis cells were carried out as described by Koehler et al. (15).
Allele replacement of lytSR.
A lytSR mutation was generated in B. anthracis Sterne using an allele replacement strategy as follows. A 1,025-bp DNA fragment spanning a region 5′ to the lytSR genes was PCR-amplified using the forward primer, LytSRF1, and the reverse primer, LytSRR1 (Table 2), incorporating XhoI and HindIII-BglII restriction sites, respectively. This DNA fragment was then ligated into the XhoI and BglII sites of pKS1 (28). Next, a 1,000-bp 3′ lytSR fragment was amplified by using the forward primer, LytSRF2, and the reverse primer, LytSRR2 (Table 2), incorporating PstI and SpeI restriction sites, respectively. This fragment was subsequently ligated into the PstI and SpeI sites of pKS1 containing the 5′ lytSR fragment. This plasmid (designated pJA52) was transformed into E. coli JM110, reisolated, and then used for electroporation into B. anthracis (17). Single colonies of the transformed B. anthracis cells were grown overnight at 37°C in BHI broth containing erythromycin and then subcultured with 1:1000 dilutions in antibiotic-free BHI broth at 30°C each day for 3 days. After the third day, dilutions of the culture were spread on BHI agar plates containing erythromycin and isolated colonies were subsequently screened for an erythromycin-sensitive, kanamycin-resistant phenotype. Replacement of the lytSR operon with the kanamycin resistance gene was confirmed by PCR analysis, and the mutant strain was designated KB5000 (Table 1). An lrgAB mutation was made by using a similar strategy using the primer sets LrgABKOF3a and LrgABKOR3 and LrgABKOF4 and LrgABKOR4 (Table 2). This strain was designated KB5400 (Table 1). A similar allele replacement strategy was also used to make a clhAB2 mutation using the primer sets Clh2F1/Clh2R1 and Clh2F2/Clh2R2 (Table 2). This strain was designated KB6000 (Table 1).
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide | Sequence (5′-3′) |
|---|---|
| LytSRF1 | GGCGCTCGAGGTTGCTTTTGTTCCCATATTTTCGG |
| LytSRR1 | CCGAAGCTTAGATCTATAAGTCCGACGCGTTCAATC |
| LytSRF2 | CAACTGCAGTCAGCCATGGTTCAACTCTA |
| LytSRR2 | CCGCACTAGTCGATAGCTGATAAAATTTGCCACC |
| Clh2F1 | ATAGGGCCCCCAAGTGACAATCCAACGACA |
| Clh2R1 | TGTCTCGAGATGCCAGTTGCGATGAGTGTT |
| Clh2F2 | GATCTGCAGTGTACCCACGTTCCAACCATA |
| Clh2R2 | GCGTCTAGAATAGAGTTATCAAGTAAAGCG |
| LrgABKOF3a | GGACTCGAGGTGTAGAGGACGCATTAGAAG |
| LrgABKOR3 | CGGAGATCTAATGACTAACCCGATTACCGATG |
| LrgABKOF4 | TAACTGCAGAACAGCGGGTCACGCATTAGGAG |
| LrgABKOR4 | GCGACTAGTTTATTTTGTTCTAACGTCCTTCTCCAT |
| 5LytSRCOMP | CCCGGATCCGTAAAAGCTCAATACCTCACCTCG |
| 3LytSRCOMP | CCCGAATTCGGAAACGCTCTCTAAATTTCAC |
| Clh2F3 | ACTCTGCAGTCCGTTAGTCACATTCCC |
| Clh2R3 | TGCTCTAGACTTGGCGTACCTCCTACA |
| 5FwdlrgABCOMP | CGCGGTACCAAAGAGGTGGCCAAAATGAG |
| 3RevlrgABCOMP | CCCGGATCCGTTTTCCTATCCAATAAACGGCATA |
| lrgAF | ATTTACCAATTCCAATGCCCTCA |
| lrgAR | CGTGCTTGCGTCCTTTATTTACT |
| lrgBF | AATCGCATACGGAATCGGAACA |
| lrgBR | TCCAATAAACGGCATAAACATCG |
| Clh2AB-1F | CTGGATTGCAAAGCTGCTC |
| Clh2AB-1R | TTCATCGCTCTCATCACACC |
| Clh2AB-2F | TCATCGCGACTCTCTTTCCT |
| Clh2AB-2R | TCTTGTCCAAATTGCTGCTC |
Complementation plasmid construction.
Complementation of the lytSR mutation in KB5000 was achieved by PCR amplification of the lytSR open reading frames, along with 540 bp upstream region using Platinum Pfx High Fidelity DNA polymerase (Invitrogen) and the primers 5LytSRCOMP and 3LytSRCOMP (Table 2), incorporating SphI and BamHI sites, respectively. The resulting DNA fragment was then ligated into the SphI and BamHI sites of pBK123, a derivative of pCN51 (7), in which the erythromycin cassette has been replaced with chloramphenicol cassette from pC194, generating the plasmid pLC17. This plasmid was introduced into KB5000 by electroporation, generating the lytSR complementation strain designated KB5050 (Table 1). Complementation of clhAB2 in KB6000 was achieved by PCR amplification of the clhAB2 open reading frames, along with a 575-bp upstream region, using the primers Clh2F3 and Clh2R3 (Table 2) incorporating PstI and XbaI sites, respectively. The resulting DNA fragment was then ligated into the PstI and XbaI sites of pHT304 (3), generating pJA22. This plasmid was introduced into KB6000 by electroporation, generating the clhAB2 complementation strain KB6050 (Table 1). Complementation of the lrgAB mutation in KB5400 was attempted by PCR amplification of the lrgAB open reading frames, along with a 500-bp upstream region, using the primers 5FwdlrgABCOMP and 3RevlrgABCOMP (Table 2), incorporating SphI and BamHI sites, respectively. The resulting DNA fragment was then ligated into the SphI and BamHI sites of pCN51 (7), generating the plasmid pLC20. This plasmid was introduced into KB5000 by electroporation, generating strain KB5450 (Table 1).
Isolation of RNA.
Overnight cultures of B. anthracis strains were inoculated into 100 ml of prewarmed NZY broth (with or without the addition of 35 mM glucose) in a 1-liter flask to an optical density at 600 nm (OD600) of 0.1 and incubated with shaking at 250 rpm for 12 h at 37°C. Cells were harvested at 2, 6, and 12 h postinoculation. Harvested cells were transferred to lysing matrix B tubes (Qbiogene, La Jolla, CA) containing 0.1-mm beads and lysed by shaking them in a Fastprep FP120 instrument (Qbiogene) for 23 s at a setting of 6 (19). The lysates were centrifuged at 13,000 × g for 10 min at 4°C, and the supernatants were collected for RNA purification by using an RNeasy kit (Qiagen) according to the protocols provided by the manufacturer.
Northern blot analysis.
RNA samples (5.0 μg) were separated by electrophoresis in a 1% agarose gel containing 0.66 M formaldehyde and morpholinepropanesulfonic acid running buffer (20 mM morpholinepropanesulfonic acid, 10 mM sodium acetate, 2.0 mM EDTA [pH 7.0]). The RNA samples were subsequently transferred to a nylon membrane (Micron Separations, Inc., Westboro, MA) by capillary transfer in 20× SSC buffer (0.3 M sodium citrate, 3.0 M NaCl [pH 7.0]) and fixed to the membrane by UV cross-linking using a UV Stratalinker 1800 (Stratagene, Cedar Creek, TX). Hybridization with gene-specific probes was performed using the digoxigenin (DIG) system (Roche Applied Science, Indianapolis, ID) according to the manufacturer's recommendations. DIG-labeled DNA probes were PCR amplified using the gene-specific primers listed in Table 2. Northern hybridization experiments were performed on RNA extracted from the lytSR, lrgAB, and clhAB2 mutants and the expected transcripts were absent in each case, confirming the specificity of the probes used (unpublished results). The sizes of each transcript were determined by comparison to an RNA ladder (Invitrogen) run in the same gel.
Murein hydrolase assays.
Cell wall-associated proteins were collected essentially as described previously for B. subtilis (10). Fresh overnight cultures of B. anthracis strains were used to inoculate 50 ml of NZY broth to an OD600 of 0.1, followed by incubation for 6 h at 37°C and 250 rpm. The cells were harvested by centrifugation at 12,000 × g for 10 min and resuspended in 1/100 volumes of sample buffer as described previously (10). The cell pellet was boiled for 10 min and centrifuged at 13,000 × g, and the supernatants were collected. The extracted proteins were analyzed by zymography as described previously (16).
Determination of stationary-phase survival.
Overnight cultures of B. anthracis were diluted to an OD600 of 0.1 in 50 ml of NZY broth (with or without the addition of 35 mM glucose) in 500-ml flasks, followed by incubation for several days at 37°C with shaking at 250 rpm. At intervals, an aliquot of the cultures were collected to assess viable cell counts and the OD600.
Sporulation assay.
Cultures were grown in Schaeffer's medium (13) and samples were taken 48 h after inoculation, diluted in phosphate-buffered saline, and plated on BHI agar before and after heat treatment at 65°C for 30 min. The sporulation efficiency was calculated as the total number of cells surviving heat treatment divided by the total number of cells present prior to heat treatment and then multiplying that value by 100 (32).
Fluorescence microscopy.
A Live/Dead BacLight bacterial viability kit (Molecular Probes, Inc.) was used to assess differences in cell morphology and to distinguish between live and dead cells. The method utilizes a mixture of Syto-9 and propidium iodide, which results in the staining of live cells green and dead cells red. Cells were stained according to the manufacturer's instructions and then visualized under ×1,000 magnification with a Nikon Eclipse TS 100 microscope (Nikon Instruments, Inc., Melville, NY). Representative images of each strain were captured by using a Nikon Digital Sight DS-L1 camera.
RESULTS
Identification of cidAB/lrgAB orthologues in B. anthracis.
A recent study established the existence of the Cid/Lrg regulatory network in B. anthracis and has demonstrated its role in the control of cell death and lysis in this pathogen (1). That study also established the importance of the CidR regulatory protein in the control of cid and lrg expression and demonstrated its impact on cell death in the stationary phase. The goal of the present study was to focus on LytSR-mediated control of this system and to begin to define the transcriptional organization and the functions of the cid and lrg homologues. Interestingly, a BLAST search of the B. anthracis Sterne genome (http://www.ncbi.nlm.nih.gov) revealed the presence of two additional cidAB/lrgAB orthologues (designated clhAB1 and clhAB2) besides those associated with the B. anthracis cidR and lytSR operons (Fig. 1). Sequence analysis of the available genomes revealed that clhAB1 and clhAB2 are unique to the B. cereus group of bacteria, including B. anthracis and Bacillus thuringiensis. The B. anthracis clhA1 and clhB1 genes (accession numbers BAS3599 and BAS3598) are predicted to encode 13.2- and 23.3-kDa proteins, respectively, sharing 35 and 30% amino acid sequence identity with the cidA- and cidB-encoded proteins of B. anthracis. The B. anthracis clhA2 and clhB2 genes (accession numbers BAS4960 and BAS4959) encode proteins with 32 and 37% amino acid sequence identity compared to B. anthracis cidA and cidB. Based on the sequence similarities of the clhAB1 and clhAB2 genes to cidAB and lrgAB, these genes are proposed to participate in the control of cell death and lysis in B. anthracis.
FIG. 1.
Schematic diagram of cid and lrg orthologues of B. anthracis. The cid and lrg operons are located adjacent to cidR and lytSR loci, respectively, as in S. aureus. Two additional loci with similarities to the S. aureus cid/lrg operons are also present in the B. anthracis genome. These genes have been designated clhA1 (BAS3599), clhB1 (BAS3598), clhA2 (BAS4960), and clhB2 (BAS4959). The gntR (BAS3600) and secG (BAS4958) genes encode a potential transcriptional regulator and a subunit of the SecG preprotein translocase, respectively. Open reading frames whose putative products do not match known proteins are labeled “orf.” Arrows above the genes represent the direction and sizes of transcripts identified by Northern blot analyses presented here and in previous studies (1).
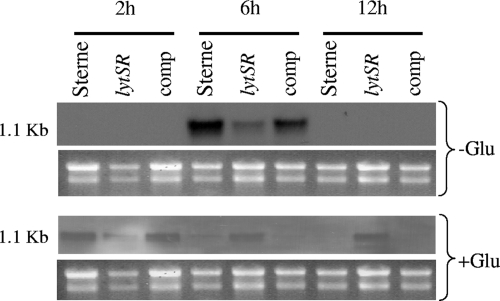
Considering the similarities of the clh1 and clh2 loci to the cidAB and lrgAB operons, we performed transcription analyses to determine whether these genes also form dicistronic operons. Although transcription of the clhA1 and clhB1 genes could not be detected under a variety of growth conditions tested using either Northern blot or reverse transcriptase PCR analyses, transcription of the clhA2 and clhB2 genes was readily detected by Northern blotting (Fig. 2). Furthermore, probes specific for clhA2 and clhB2 genes both hybridized to 1.1-kb transcripts, suggesting that these genes form a dicistronic operon similar to the cidAB and lrgAB operons (1). We also examined the regulation of clhAB2 in response to glucose, a factor known to affect expression of the cid and lrg operons. Previously, it has been demonstrated that the B. anthracis cidAB transcription is most abundant in the early exponential phase in the presence or absence of glucose (1). Furthermore, lrgAB transcription was found to be most abundant in lag to early exponential phase in the presence or absence of glucose. In the absence of glucose, the clhAB2 operon was found to be expressed in the parental strain during late-exponential (6-h) phase, while growth in the presence of glucose resulted in clhAB2 transcription primarily during early exponential growth (Fig. 2).
FIG. 2.
Northern blot analysis of clhAB2 transcription. B. anthracis Sterne, its lytSR mutant derivative (lytSR), and the lytSR complementation strain (comp) were grown in the presence (+Glu) or absence (−Glu) of 35 mM glucose, and RNA was collected at 2, 6, and 12 h. Portions (5 μg) of samples were separated in a 1.0% formaldehyde-agarose gel, transferred to a nylon membrane, and hybridized to DIG-labeled probes derived from the clhB2 gene. An ethidium bromide-stained gel of the RNA used in these experiments is also shown as a loading control.
LytSR-mediated transcriptional control.
It was shown previously that the transcription of the B. anthracis cidAB and lrgAB operons is dependent on the cidR gene encoding a putative LysR-type transcription regulator (1). To investigate the impact of lytSR on the transcription of B. anthracis cidAB and lrgAB, we generated a lytSR mutant and performed Northern blot analyses on RNA isolated from these cells grown in the presence or absence of glucose. As shown in Fig. 3, dramatically reduced levels of the 1.2-kb lrgAB transcripts, both in the presence and in the absence of glucose, were observed in the lytSR mutant compared to the parental and complemented strains. In contrast, cidAB expression was found to be unaffected by the lytSR mutation under the conditions tested (data not shown), which is similar to what was observed in S. aureus.
FIG. 3.
Northern blot analysis of lrgAB transcription. B. anthracis Sterne, its lytSR mutant derivative (lytSR), and the lytSR complementation strain (comp) were grown in the presence (+Glu) or absence (−Glu) of 35 mM glucose, and RNA was collected at 2, 6 and 12 h. Portions (5 μg) of samples were separated in a 1.0% formaldehyde-agarose gel, transferred to a nylon membrane, and hybridized to DIG-labeled probes derived from the lrgB gene. An ethidium bromide-stained gel of the RNA used in these experiments is also shown.
To determine whether transcription of clhAB1 and clhAB2 is affected by B. anthracis lytSR, we performed Northern blot analysis as described above using probes specific for these operons. As shown in Fig. 2, transcription of clhAB2 in the lytSR mutant was lower than in the parental and complemented strains during the late exponential phase in the absence of glucose. Interestingly, clhAB2 transcription was higher in the lytSR mutant in the presence of glucose during late-exponential and stationary phases of growth compared to the parental and complementation strains (Fig. 2), indicating that LytSR can have both positive and negative effects on clhAB2 expression depending on the growth conditions. Finally, to determine the role of cidR in the regulation of clhAB2 transcription, RNA was isolated from the previously characterized B. anthracis cidR mutant (1) and analyzed by Northern blot analysis. These experiments, however, demonstrated no effect of the cidR mutant on clhAB2 expression, indicating that CidR is not involved in the control of this operon under these conditions (data not shown). Again, transcription of the clhA1 and clhB1 genes was not detected in either of the lytSR or cidR mutants.
Studies in S. aureus have also demonstrated that lrgAB transcription is induced in a lytSR-dependent fashion in response to agents that dissipate membrane potential (Δψ) (20). To examine whether B. anthracis lrgAB and clhAB2 transcription is similarly regulated, the parental, lytSR mutant, and complementation strains were grown to exponential phase and treated with 25 μg of gramicidin/ml or 10 μM carbonyl cyanide m-chlorophenylhydrazone (CCCP) to dissipate the Δψ, and RNA was collected for Northern blot analyses. As shown in Fig. 4a, lrgAB transcription was induced by both of these agents in the parental strain but was not induced in the lytSR mutant. Furthermore, Δψ-mediated induction of lrgAB expression was restored to wild-type levels in the complementation strain. Again, in contrast, clhAB2 expression was found to be repressed by treatment with agents that dissipate the Δψ and was also absent in the lytSR mutant and complemented strains (Fig. 4b). Combined, these studies demonstrate that the B. anthracis LytSR two-component regulatory system is functionally similar to that of S. aureus and that it mediates both Δψ-dependent (lrgAB) and -independent (clhAB2) transcriptional control in B. anthracis.
FIG. 4.
Δψ-mediated control of lrgAB and chlAB2 transcription. B. anthracis Sterne, its lytSR mutant derivative (lytSR), and the lytSR complementation strain (comp) were grown without glucose for 6 h and treated with gramicidin or CCCP for 15 min prior to RNA isolation. Portions (5 μg) of samples were separated in a 1.0% formaldehyde-agarose gel, transferred to a nylon membrane, and hybridized to DIG-labeled probes derived from the lrgB gene (a) or the clhB2 gene (b). An ethidium bromide-stained gel of the RNA used in these experiments is also shown.
Assessing the role of lytSR in cell death and lysis.
Preliminary studies revealed that the lytSR mutant grew at the same rate as the Sterne and complemented strains as determined by measurements of optical density and viability in a time course assay (unpublished results). To assess the impact of the lytSR mutation on cell death and lysis during the stationary phase, these strains were grown for 9 days, and measurements of the cell viability and the optical density were obtained. Unlike the cidR mutant, which exhibited a rapid cell death (RCD) phenotype in the stationary phase (1), the B. anthracis lytSR mutation did not have a discernible effect on stationary-phase survival and lysis (data not shown). However, the lytSR mutant had a significant effect on expression of the 85-kDa Sap S-layer protein (see Fig. S1a in the supplemental material) previously found to contain murein hydrolase activity (1). Similar to results described previously (1), the absence of Sap in the lytSR mutant strain corresponded with the loss of an 85-kDa murein hydrolase (see Fig. S1b in the supplemental material). These results are also similar to the finding that the cidR mutation in B. anthracis caused reduced Sap production (1) and that, as in S. aureus, the B. anthracis LytSR two-component regulatory system plays a role in regulating murein hydrolase activity. However, this effect on murein hydrolase activity had no discernible impact on cell lysis under the conditions tested.
Analysis of lrgAB and clhAB2 mutants.
Since the lytSR mutation in B. anthracis only had observable effects on transcription of the lrgAB and clhAB2 operons, we elected to focus on the functions of lrgAB and clhAB2. Thus, we generated mutations in the lrgAB and clhAB2 operons by allele replacement and, as described above, subjected these mutants to assays of cell death and lysis in the stationary phase. Interestingly, similar to the B. anthracis cidR mutant (1), the lrgAB mutant exhibited a RCD phenotype in the stationary phase, displaying a complete loss of viability after 12 and 48 h of incubation in the presence or absence of glucose, respectively (Fig. 5b). Unlike in S. aureus, measurements of the optical density revealed that the B. anthracis lrgAB mutant displayed a lysis pattern similar to that of the wild-type strain (Fig. 5a), indicating that while the lrgAB mutation had a dramatic effect on cell viability in sthe tationary phase it had little impact on cell lysis. Consistent with this is the observation that the lrgAB mutation had little effect on murein hydrolase activity in a zymographic assay (unpublished results). It should also be noted that expression of the lrgAB operon from a plasmid failed to restore the wild-type phenotype in the lrgAB mutant (Fig. 5a and b), despite the fact that lrgAB transcription was restored in this strain (data not shown). Although the reason for the inability to complement this mutation is unknown, these results are similar to studies of the S. aureus cidA mutation, which is also not complementable by expression of cidA in trans (24, 26). In contrast to the lrgAB mutant, the clhAB2 mutant exhibited decreased cell death in the stationary phase in the absence of glucose, maintaining a viability that was ∼10-fold higher than the parental and complemented strains (Fig. 5d and see Fig. S2 in the supplemental material). In the presence of glucose, the viabilities of the parental, clhAB2 mutant, and complementation strains were indistinguishable and remained lower at approximately 8 × 103 CFU per ml. In addition, the optical density measurements of the clhAB2 mutant were similar to the parental and complementation strains, both in the presence and in the absence of glucose (Fig. 5c).
FIG. 5.
Stationary-phase survival assays. B. anthracis Sterne (squares), its lrgAB mutant derivative (a and b, triangles), the lrgAB complementation strain (a and b, circles), its clhAB2 mutant derivative (c and d, diamonds), and the clhAB2 complementation strain (c and d, circles) were grown in the presence (open symbols) or absence (solid symbols) of 35 mM glucose, and samples were removed and assayed to determine the optical density (OD600 [a and c]) and cell viability (log CFU/ml [b and d]). The data presented are representative of three independent experiments. Additional replicates are presented in Fig. S2 in the supplemental material.
To more closely examine the effects of the lrgAB and clhAB2 mutations on B. anthracis, we examined cell viability using a fluorescent Live/Dead BacLight bacterial viability kit (Molecular Probes). In cultures grown overnight in the absence of glucose, both the parental and the lrgAB mutant strains showed normal rod-shaped cells (unpublished results). At the 48-h time point, cultures of the parental strain contained a mixture of live and dead cells exhibiting green and red fluorescence, respectively (Fig. 6). However, at 48 h, the time in which the lrgAB mutant viability drops to undetectable levels, the majority of the lrgAB mutant cells stained red as expected but appeared unevenly stained within the cells. Furthermore, the cells failed to separate normally and thus formed long chains. Strikingly, the staining pattern and the appearance of the clhAB2 mutant were drastically different compared to the parental and lrgAB mutant strains. As shown in Fig. 6, the clhAB2 mutant appeared shorter and formed more rounded cells. Similar to the lrgAB mutant, the clhAB2 mutant also failed to separate normally. Furthermore, the clhAB2 mutant cells were only weakly stained with Syto-9, suggesting that the cells may be less robust and/or contained a compromised cell membrane. Importantly, the wild-type appearance of cells was restored when clhAB2 was expressed in trans.
FIG. 6.
Microscopic examination of cell viability. Fluorescence microscopy using Live/Dead BacLight staining of the B. anthracis Sterne, its lrgAB mutant, its clhAB2 mutant derivative, and the clhAB2 complementation strain after incubation in NZY broth for 48 h. Red staining is indicative of dead or damaged cells, while green staining indicates live cells.
Effect of lrgAB and clhAB2 mutations on sporulation.
Given the dramatic effect of the lrgAB and clhAB2 mutations on the cells in the stationary phase, we predicted that these mutations would also affect the ability of the cells to undergo sporulation. Thus, the abilities of the lrgAB and clhAB2 mutants to sporulate were assessed. As shown in Fig. 7, the parental strain had a sporulation efficiency of ∼88% after 48 h of growth in the Schaeffer's medium. In contrast, the lrgAB and clhAB2 mutants showed dramatically reduced sporulation efficiencies (ca. 5 and 11%, respectively) after 48 h. As a negative control, the spo0A mutant was also tested and found not to form spores in this assay as expected. As described above, the sporulation defect of the clhAB2 mutant was restored to nearly wild-type levels in the complementation strain. These results demonstrate that the lrgAB and clhAB2 gene products have a dramatic impact on sporulation in B. anthracis.
FIG. 7.
Impact of lrgAB and clhAB2 mutations on sporulation. B. anthracis Sterne, lrgAB mutant, clhAB2 mutant, clhAB2 complementation strain KB6050 (comp), and spo0A mutant were grown in Schaeffer's medium. After 48 h of incubation at 37°C, dilutions were spread on BHI agar before and after heat treatment at 65°C for 30 min. The sporulation efficiency was calculated as the ratio of the number of spores to the number of viable cells for each strain.
DISCUSSION
Previously, our laboratory has demonstrated that expression of the B. anthracis cidAB and lrgAB operons is affected by the cidR gene encoding an LysR-type transcriptional regulator (1). Disruption of the cidR gene resulted in altered murein hydrolase activity and RCD in the stationary phase. In the present study we focused on the B. anthracis LytSR two-component regulatory system and show that, as in S. aureus, this system plays a positive role in lrgAB expression but had no apparent effect on cidAB expression under the growth conditions tested. The data presented here also demonstrate that, just as in S. aureus, Δψ-mediated induction of lrgAB expression in B. anthracis is dependent on the LytSR two-component regulatory system, indicating that this regulator is involved in sensing changes associated with Δψ. Thus, these data indicate that that the LytSR-mediated control of lrgAB expression is well conserved between S. aureus and B. anthracis.
We also examined two additional cidAB/lrgAB homologues in B. anthracis, designated clhAB1 and clhAB2, in addition to those associated with lytSR and cidR. These homologues are unique to the B. cereus group and, based on their sequence similarity to cid and lrg, were hypothesized to be involved in cell death and lysis. Similar to the B. anthracis cidAB and lrgAB operons, clhA2 and clhB2 genes form a dicistronic operon and were induced by growth in the presence of glucose. However, in contrast to the B. anthracis lrgAB expression, the glucose-dependent induction of clhAB2 was independent of cidR. Furthermore, our studies demonstrated that clhAB2 expression is affected by lytSR, but whether expression was increased or decreased by this mutation was dependent on the presence of glucose in the culture medium. Our results also demonstrate that clhAB2 expression was repressed by agents that dissipate Δψ, unlike lrgAB expression in both B. anthracis and S. aureus, which was induced by these compounds. These data clearly indicate that the regulatory strategies utilized to control lrgAB and clhAB2 expression in response to carbohydrate metabolism and Δψ are similar but that subtle species-specific variations exist. As in S. aureus, the B. anthracis LytSR two-component system may be involved in sensing key metabolic signals including but not limited to dissipation of Δψ to affect lrgAB and clhAB2 expression. Although the precise signal and molecular mechanism by which this signal transduction pathway functions is unknown, we speculate that this system provides a means by which the bacteria can assess its overall metabolic state. However, it is likely that the differences in regulation observed reflects the unique metabolic requirements of B. anthracis and S. aureus. Unfortunately, the expression of clhA1 and clhB1 could not be detected under any of the conditions tested, so no information regarding its regulatory control could be assessed.
Based on previous studies in S. aureus, we proposed that cidA and lrgA gene products regulate murein hydrolase activity in a manner similar to holins and antiholins, respectively (5, 11, 22, 23). In contrast to previous findings in S. aureus, disruption of B. anthracis lrgAB and clhAB2 had a minimal effect on murein hydrolase activity, possibly as a result of the redundancy of these genes. However, the lrgAB and clhAB2 mutants displayed altered stationary-phase viability compared to the parental strain (Fig. 5). Similar to the B. anthracis cidR mutant, the lrgAB mutant exhibited an RCD phenotype in stationary phase that was enhanced by growth in the presence of excess glucose. Consistent with this, Live/Dead staining of stationary-phase cells revealed that the lrgAB mutant population was comprised of mostly dead cells in comparison to the parental strain. Although the lrgAB mutant displayed a dramatic affect on cell viability, it did not have any effect on stationary-phase lysis. In contrast to the lrgAB mutant, the clhAB2 mutant enhanced stationary phase survival when grown in the absence of glucose (Fig. 5c and d). Interestingly, Live/Dead staining revealed that although the clhAB2 mutant strain exhibited enhanced stationary-phase survival after 48 h of incubation, the cells of the clhAB2 mutant were visibly shorter and stained weakly with Syto-9, indicating that the cells may be less robust and/or contained a compromised cell membrane. Collectively, these results suggest that, in B. anthracis, lrgAB and clhAB2 operons regulate cell death in the stationary phase in an opposing manner without having a pronounced effect on stationary-phase lysis.
The control of bacterial cell death and lysis is proposed to be important in biofilm adherence and maturation (4). In S. aureus, CidA-dependent lysis during biofilm development results in the release of DNA, which becomes a structural component of the biofilm matrix (2, 26). Studies in different organisms have shown that the regulation of cell death and lysis is important for other bacterial developmental processes such as natural competence, fruiting body formation, and sporulation (18, 27). In the present study we demonstrate that mutations in the B. anthracis lrgAB and clhAB2 operons dramatically reduced sporulation efficiencies compared to the parental strain. Studies in sporulating bacteria such as B. subtilis have shown that the differentiation processes of sporulation require a number of rearrangements and modifications of cell wall peptidoglycan that include at least four events in which autolysins are likely to be involved (29, 30). These four events include (i) asymmetric cell division, (ii) engulfment of the prespore by the mother cell, (iii) lying down of peptidoglycan around the forespore, and (iv) lysis of the mother cell to release the mature endospore (29). It is possible that lrgAB and clhAB2 mutations may be involved in any of these four events of differentiation, thereby affecting the sporulation potential of the strain. Alternatively, the control of cell death and lysis of a subpopulation of cells may affect sporulation in a manner related to cannibalism as previously described (8, 22). Establishing the molecular mechanism by which the Cid/Lrg system affects sporulation is a current focus of our laboratory.
In conclusion, the results of the present study reveal that the B. anthracis lrgAB and clhAB2 play important roles in the control of cell death in the stationary phase and reveal a previously unrecognized role of this system in sporulation. Comparison of these systems in B. anthracis and S. aureus will be essential for elucidating the regulatory strategies underlying the control of cell death and lysis. Furthermore, continued investigation of this system in B. anthracis and other bacterial species will likely reveal additional insight into the biological roles of programmed cell death in bacteria.
Supplementary Material
Acknowledgments
We thank Theresa M. Koehler at the University of Texas Health Science Center at Houston for providing us with B. anthracis spo0A mutant strain.
This study was funded by NIH grant no. R01AI038901 and DOD grant no. DAAD 19-03-1-0191.
Footnotes
Published ahead of print on 1 May 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Ahn, J. S., L. Chandramohan, L. E. Liou, and K. W. Bayles. 2006. Characterization of CidR-mediated regulation in Bacillus anthracis reveals a previously undetected role of S-layer proteins as murein hydrolases. Mol. Microbiol. 621158-1169. [DOI] [PubMed] [Google Scholar]
- 2.Allesen-Holm, M., K. B. Barken, L. Yang, M. Klausen, J. S. Webb, S. Kjelleberg, S. Molin, M. Givskov, and T. Tolker-Nielsen. 2006. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 591114-1128. [DOI] [PubMed] [Google Scholar]
- 3.Arantes, O., and D. Lereclus. 1991. Construction of cloning vectors for Bacillus thuringiensis. Gene 108115-119. [DOI] [PubMed] [Google Scholar]
- 4.Bayles, K. W. 2007. The biological role of death and lysis in biofilm development. Nat. Rev. Microbiol. 5721-726. [DOI] [PubMed] [Google Scholar]
- 5.Bayles, K. W. 2000. The bactericidal action of penicillin: new clues to an unsolved mystery. Trends Microbiol. 8274-278. [DOI] [PubMed] [Google Scholar]
- 6.Brunskill, E. W., and K. W. Bayles. 1996. Identification of LytSR-regulated genes from Staphylococcus aureus. J. Bacteriol. 1785810-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charpentier, E., A. I. Anton, P. Barry, B. Alfonso, Y. Fang, and R. P. Novick. 2004. Novel cassette-based shuttle vector system for gram-positive bacteria. Appl. Environ. Microbiol. 706076-6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claverys, J. P., and L. S. Havarstein. 2007. Cannibalism and fratricide: mechanisms and raisons d'etre. Nat. Rev. Microbiol. 5219-229. [DOI] [PubMed] [Google Scholar]
- 9.Dagert, M., and S. D. Ehrlich. 1979. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene 623-28. [DOI] [PubMed] [Google Scholar]
- 10.Foster, S. J. 1992. Analysis of the autolysins of Bacillus subtilis 168 during vegetative growth and differentiation by using renaturing polyacrylamide gel electrophoresis. J. Bacteriol. 174464-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groicher, K. H., B. A. Firek, D. F. Fujimoto, and K. W. Bayles. 2000. The Staphylococcus aureus lrgAB operon modulates murein hydrolase activity and penicillin tolerance. J. Bacteriol. 1821794-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerout-Fleury, A. M., K. Shazand, N. Frandsen, and P. Stragier. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167335-336. [DOI] [PubMed] [Google Scholar]
- 13.Inaoka, T., Y. Matsumura, and T. Tsuchido. 1999. SodA and manganese are essential for resistance to oxidative stress in growing and sporulating cells of Bacillus subtilis. J. Bacteriol. 1811939-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janes, B. K., and S. Stibitz. 2006. Routine markerless gene replacement in Bacillus anthracis. Infect. Immun. 741949-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koehler, T. M., Z. Dai, and M. Kaufman-Yarbray. 1994. Regulation of the Bacillus anthracis protective antigen gene: CO2 and a trans-acting element activate transcription from one of two promoters. J. Bacteriol. 176586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mani, N., P. Tobin, and R. K. Jayaswal. 1993. Isolation and characterization of autolysis-defective mutants of Staphylococcus aureus created by Tn917-lacZ mutagenesis. J. Bacteriol. 1751493-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrero, R., and S. L. Welkos. 1995. The transformation frequency of plasmids into Bacillus anthracis is affected by adenine methylation. Gene 15275-78. [DOI] [PubMed] [Google Scholar]
- 18.Nugroho, F. A., H. Yamamoto, Y. Kobayashi, and J. Sekiguchi. 1999. Characterization of a new sigma-K-dependent peptidoglycan hydrolase gene that plays a role in Bacillus subtilis mother cell lysis. J. Bacteriol. 1816230-6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papakyriacou, H., D. Vaz, A. Simor, M. Louie, and M. J. McGavin. 2000. Molecular analysis of the accessory gene regulator (agr) locus and balance of virulence factor expression in epidemic methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 181990-1000. [DOI] [PubMed] [Google Scholar]
- 20.Patton, T. G., S. J. Yang, and K. W. Bayles. 2006. The role of proton motive force in expression of the Staphylococcus aureus cid and lrg operons. Mol. Microbiol. 591395-1404. [DOI] [PubMed] [Google Scholar]
- 21.Patton, T. G., K. C. Rice, M. K. Foster, and K. W. Bayles. 2005. The Staphylococcus aureus cidC gene encodes a pyruvate oxidase that affects acetate metabolism and cell death in stationary phase. Mol. Microbiol. 561664-1674. [DOI] [PubMed] [Google Scholar]
- 22.Rice, K. C., and K. W. Bayles. 2008. Molecular control of bacterial death and lysis. Microbiol. Mol. Biol. Rev. 7285-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice, K. C., and K. W. Bayles. 2003. Death's toolbox: examining the molecular components of bacterial programmed cell death. Mol. Microbiol. 50729-738. [DOI] [PubMed] [Google Scholar]
- 24.Rice, K. C., J. B. Nelson, T. G. Patton, S. J. Yang, and K. W. Bayles. 2005. Acetic acid induces expression of the Staphylococcus aureus cidABC and lrgAB murein hydrolase regulator operons. J. Bacteriol. 187813-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rice, K. C., B. A. Firek, J. B. Nelson, S. J. Yang, T. G. Patton, and K. W. Bayles. 2003. The Staphylococcus aureus cidAB operon: evaluation of its role in regulation of murein hydrolase activity and penicillin tolerance. J. Bacteriol. 1852635-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rice, K. C., E. E. Mann, J. L. Endres, E. C. Weiss, J. E. Cassat, M. S. Smeltzer, and K. W. Bayles. 2007. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 1048113-8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenbluh, A., and E. Rosenberg. 1990. Role of autocide AMI in development of Myxococcus xanthus. J. Bacteriol. 1724307-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shatalin, K. Y., and A. A. Neyfakh. 2005. Efficient gene inactivation in Bacillus anthracis. FEMS Microbiol. Lett. 245315-319. [DOI] [PubMed] [Google Scholar]
- 29.Smith, T. J., S. A. Blackman, and S. J. Foster. 2000. Autolysins of Bacillus subtilis: multiple enzymes with multiple functions. Microbiology 146(Pt. 2)249-262. [DOI] [PubMed] [Google Scholar]
- 30.Smith, T. J., S. A. Blackman, and S. J. Foster. 1996. Peptidoglycan hydrolases of Bacillus subtilis 168. Microb. Drug Resist. 2113-118. [DOI] [PubMed] [Google Scholar]
- 31.Sterne, M. 1937. Variation in Bacillus anthracis. Onderstepoort J. Vet. Res. 8271-349. [Google Scholar]
- 32.Tam, N. K., N. Q. Uyen, H. A. Hong, H. Duc le, T. T. Hoa, C. R. Serra, A. O. Henriques, and S. M. Cutting. 2006. The intestinal life cycle of Bacillus subtilis and close relatives. J. Bacteriol. 1882692-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang, S. J., K. C. Rice, R. J. Brown, T. G. Patton, L. E. Liou, Y. H. Park, and K. W. Bayles. 2005. A LysR-type regulator, CidR, is required for induction of the Staphylococcus aureus cidABC operon. J. Bacteriol. 1875893-5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33103-119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.