Abstract
Quorum sensing (QS) plays important roles in the pathogenicity of Burkholderia glumae, the causative agent of bacterial rice grain rot. We determined how QS is involved in catalase expression in B. glumae. The QS-defective mutant of B. glumae exhibited less catalase activity than wild-type B. glumae. A β-glucuronidase assay of a katG::Tn3-gusA78 reporter fusion protein revealed that katG expression is under the control of QS. Furthermore, katG expression was upregulated by QsmR, a transcriptional activator for flagellar-gene expression that is regulated by QS. A gel mobility shift assay confirmed that QsmR directly activates katG expression. The katG mutant produced toxoflavin but exhibited less severe disease than BGR1 on rice panicles. Under visible light conditions and a photon flux density of 61.6 μmol−1 m−2, the survival rate of the katG mutant was 105-fold lower than that of BGR1. This suggests that KatG is a major catalase that protects bacterial cells from visible light, which probably results in less severe disease caused by the katG mutant.
The bacterium Burkholderia glumae produces toxoflavin, which is an essential virulence factor for bacterial rice grain rot (17). The expression of genes involved in toxoflavin biosynthesis and transport is regulated by quorum sensing (QS), which depends on N-octanoyl homoserine lactone (C8-HSL), which is biosynthesized by TofI and its cognate receptor, TofR (17). The TofR-C8-HSL complex activates the expression of an IclR-type transcriptional regulator gene, qsmR. QsmR then activates the expression of a final master regulator for flagellar-gene expression, flhDC (19).
Toxoflavin is a very effective electron carrier under aerobic conditions and generates hydrogen peroxide; thus, the phytotoxic effects of toxoflavin may be caused by peroxides (20). Toxoflavin is similar to other photosensitizers, such as porphyrins and flavins, which are activated by light and produce reactive oxygen species (ROS) with oxygen (26).
Protection against ROS is important for cell survival and the pathogenesis of pathogens because ROS has toxic effects on cells (21). Bacteria protect themselves against oxidative stress by possessing superoxide dismutase, catalase, or alkyl hydroperoxide reductase (6, 13). Catalases are antioxidant enzymes that catalyze the breakdown of hydrogen peroxide into water and oxygen. There are four main groups: monofunctional catalases, bifunctional catalase-peroxidases, nonheme catalases, and minor catalases (27).
Catalases play critical roles in the growth in hosts and in the virulence of pathogenic bacteria. For example, the major catalase KatA of Pseudomonas aeruginosa is important for full virulence (9). KatA and KatB of Legionella pneumophila detoxify the phagosomal milieu to promote intracellular growth of the bacterium (2). In Mycobacterium tuberculosis, KatG is important for survival in ROS-producing macrophages and for virulence in mice (29).
QS regulates genes involved in resistance to ROS in gram-negative bacteria (8). In P. aeruginosa, QS activates the expression of sets of genes to relieve oxidative stress (9). QS enhances the ability of Vibrio cholerae to overcome oxidative stress and contributes to its survival in the environment (16). In Burkholderia pseudomallei, the QS system regulates the response to oxidative stress via the BpsR/C8-HSL-dependent regulation of DpsA (24). On the other hand, the expression of many of the hydrogen peroxide-inducible genes in Escherichia coli is regulated by OxyR (4, 13).
Given that toxoflavin is a major virulence factor for B. glumae in rice, we reasoned that the bacterial cells should have internal and external protection mechanisms against ROS produced by toxoflavin or other compounds. We also hypothesized that genes involved in detoxifying ROS could be regulated by QS because toxoflavin biosynthesis is tightly regulated by QS. Here, we report that katG expression is under the control of QS and that KatG plays important roles in the pathogenicity of B. glumae and in its protection from visible light.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. All of the B. glumae strains were derivatives of the wild-type strain BGR1. The strains were cultured in Luria broth (LB) (10 g liter−1 Difco Bacto tryptone, 10 g liter−1 Difco yeast extract, and 5 g liter−1 NaCl, pH 7.2) with or without 1.5% (wt vol−1) agar. The B. glumae cells and E. coli DH5α cells were cultured at 37°C. Antibiotics were used at the following concentrations: ampicillin, 100 μg ml−1; rifampicin (rifampin), 50 μg ml−1; and tetracycline, 10 μg ml−1.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Characteristicsa | Source or reference |
|---|---|---|
| E. coli | ||
| DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF) U169 recA1 endA1 hsdR17(rK+ mK+) supE44 thi-1 gyrA relA1 | Gibco BRL |
| B. glumae | ||
| BGR1 | Wild-type; Rifr | 15 |
| BGS2 | BGR1 tofI::Ω | 17 |
| BGS6 | BGR1 toxR′ toxA′::Ω | 18 |
| BGS9 | BGR1 qsmR::Ω | 17 |
| BGC2 | BGR1 katG::Tn3-gusA78 | This study |
| S2C2 | BGS2 katG::Tn3-gusA78 | This study |
| S6C2 | BGS6 katG::Tn3-gusA78 | This study |
| S9C2 | BGS9 katG::Tn3-gusA78 | This study |
| Plasmids | ||
| pBluescript II | Cloning vehicle; phagemid; pUC derivative; Ampr | Stratagene |
| pLAFR3 | Tra− Mob+ RK2 replicon; Tetr | 32 |
| pBGT63 | 2.2-kb DNA fragment harboring qsmR cloned into pLAFR3 | 19 |
| pJ9 | Cosmid carrying katG in pLAFR3 | This study |
| pHJ4 | 5.5-kb PstI fragment including katG from pJ9 cloned into pLAFR3 | This study |
Ampr, ampicillin resistance; Rifr, rifampin resistance; Tetr, tetracycline resistance.
DNA manipulation and mutagenesis.
Small-scale preparation of plasmid DNA from E. coli and B. glumae was performed using the alkaline lysis method (30). The construction of the genomic library of BGR1 was reported previously (17). To identify a cosmid clone carrying a katG gene, two primers, katG1 (5′-GATCGCGGGCGGCCACAC-3′) and katG2 (5′-AGCTTGAACCAGGCGCGC-3′), which were deduced from sequence information of B. pseudomallei (11), were used to amplify the katG gene of B. glumae. The amplified 454-bp fragment was used as a probe DNA in colony hybridization to identify a cosmid clone carrying a katG homolog from the previously constructed genomic library of BGR1 (30). The resulting plasmid, pJ9 (Table 1), was digested with PstI, and the 5.5-kb PstI fragment showing a positive hybridization signal with the probe DNA was subcloned into pLAFR3 (Table 1), resulting in pHJ4 (Table 1). The plasmid pHJ4 was mutagenized with Tn3-gusA as described previously (17). The insertion site and orientation of Tn3-gusA in each mutant were mapped using restriction enzyme digestion analysis and direct sequencing of the plasmid with the primer Tn3gus (5′-CCGGTCATCTGAGACCATTAAAAGA-3′), which allows sequencing out of Tn3-gusA. The mutagenized plasmids that carried Tn3-gusA insertions were introduced individually and marker exchanged into the strains BGR1, BGS2 (BGR1 tofI::Ω), and BGS9 (BGR1 qsmR::Ω), as described previously (17). All marker exchanges were confirmed by Southern hybridization analysis using pHJ4 as the probe DNA.
Overexpression and purification of QsmR.
QsmR was overexpressed and purified as described previously (19).
Gel mobility shift assay.
The 364-bp upstream region of katG was amplified by PCR using the KA1 (5′-ATCTAGGCCTGCCGCTG-3′) and KA2 (5′-GTATTCTCCTTTGATCGC-3′) primers. The DNA fragments were eluted from an agarose gel and labeled with biotin for chemiluminescence, using a light shift chemiluminescent electrophoretic mobility shift assay kit (Pierce Biotechnology, Rockford, IL). For specific competitor DNA, we used the 242-bp upstream region of a catalase gene E (katE) of B. glumae, which was amplified using the KEN1 and KEN2 primers as described previously (17). Purified QsmR-His (100 nM) was incubated with 2 nM labeled DNA in binding buffer [10 mM Tris-HCl (pH 7.6), 10 mM KCl, 0.5 mM EDTA, 1 mM dithiothreitol, 5% (vol vol−1) glycerol, and 1 μg μl−1 poly(dI-dC)] for 15 min at 28°C. For the competitor DNA, a 20-fold molar excess of unlabeled target DNA was added to the reaction mixture, along with the extract, before the labeled DNA target was added. The mixtures were size fractionated in a nondenaturing 4% polyacrylamide gel, followed by transfer to nitrocellulose membranes and detection of biotin-labeled probes by streptavidin-horseradish peroxidase chemiluminescence.
Catalase activity staining.
Bacterial cells were washed twice with ice-cold 20 mM Tris-HCl (pH 7.7) buffer and sonicated with a VCX-400 sonicator (Sonics & Materials, Inc., Newtown, CT). The sample was clarified by centrifugation at 12,000 × g for 20 min at 4°C, and the proteins in the cleared lysate were separated in a 9% nondenaturing polyacrylamide gel. The gel was soaked in 0.003% hydrogen peroxide for 10 min and then stained with 2% potassium ferricyanide as described previously (34). Protein concentrations were determined as described by the manufacturer (Bio-Rad, Hercules, CA).
Western blot analysis.
Bacterial cells (5 ml) were collected by centrifugation (12,000 × g for 1 min) and suspended in 0.5 ml of 20 mM Tris-HCl (pH 7.7) buffer. The cells were disrupted via sonication with a VCX-400 sonicator (Sonics & Materials), and the lysate was cleared by centrifugation (12,000 × g for 20 min). The supernatant proteins were separated by electrophoresis in a 12% sodium dodecyl sulfate-polyacrylamide gel and then transferred to nitrocellulose membranes for Western blot analysis. A rabbit polyclonal anti-KatG antibody was raised against a synthetic peptide (LMSPARRKNKDFPDPVSN) by Peptron (Daejeon, Korea) and used as the primary antibody. The membranes were incubated with KatG immunoglobulin antibody, and rabbit anti-mouse immunoglobulin G (H+L) (Pierce) was used as a secondary antibody. Detection was performed using nitroblue tetrazolium/5-bromo-4-chloro-3-indolylphosphate as the chromogen, as described by the manufacturer (Pierce).
Disk diffusion assay with H2O2.
Bacterial cells were grown to stationary phase in LB medium at 37°C and then overlaid onto LB agar plates. A 0.8-mm-diameter disk soaked in 30% H2O2 was placed on each plate and incubated at 37°C overnight.
β-Glucuronidase assay.
The β-glucuronidase enzyme assay was performed as described previously, with some modifications (14). All of the BGR1 derivatives were grown in LB medium at 37°C for 12 h with shaking. C8-HSL was added at 1 μM when the cells were subcultured. The bacteria were collected by centrifugation, resuspended in β-glucuronidase extraction buffer, and lysed by sonication with a VCX-400 sonicator (Sonics & Materials). The extract was used in a β-glucuronidase enzyme assay with 4-methylumbelliferyl glucuronide as the substrate. The fluorescence was measured at 365-nm excitation and 460-nm emission in a Hoefer DQ 300 fluorometer (Hoefer Scientific Instruments, San Francisco, CA). One unit of β-glucuronidase activity was defined as the amount of enzyme required to release 1 nM of 4-methylumbelliferon per bacterium per minute.
Effect of visible light on bacterial viability.
Overnight cultures of the BGR1 derivatives were subcultured and grown for an additional 12 h. To determine viable-cell counts under dark and light conditions, 100-μl samples were removed, serially diluted 10-fold, and spotted on two sets of LB agar plates. To avoid light, the petri plates were entirely wrapped with aluminum foil. To evaluate bacterial viability under visible light, the upper part of the petri dish was illuminated 22 cm from seven 10-W fluorescent lamps (FL10D; Namyung Lighting Co., Seoul, Korea). The light intensity was 61.6 μmol/s/m2 and was measured with a light meter (LI-250; Li-Cor, Lincoln, NE). Colonies were counted 24 h after incubation under visible light at 37°C. The survival (percent) of the bacteria in visible light was calculated using the following formula: survival rate = number of cells surviving under light/number of cells grown in the dark × 100.
Plant inoculation.
Rice plants (Oryza sativa cv. Milyang 23) were grown in a greenhouse, inoculated at the flowering stage with a bacterial suspension (1 × 108 CFU/ml) using an atomizer (Binks Wren, Glendale Heights, IL), and kept in a greenhouse. Disease severity was evaluated daily for 10 days as described by Iiyama et al. (12) using the following scale: 0, healthy panicle; 1, panicle 0 to 20% discolored; 2, panicle 20 to 40% discolored; 3, panicle 40 to 60% discolored; 4, panicle 60 to 80% discolored; 5, panicle 80 to 100% discolored. Disease severity was determined using the following formula: disease degree = Σ(number of samples per score × score)/total number of panicles. Pathogenicity assays were repeated three times with three replications.
Nucleotide sequence accession number.
The complete DNA sequence of katG from B. glumae BGR1 was deposited in the GenBank database under accession number FJ716792.
RESULTS
QS mutants have less catalase activity.
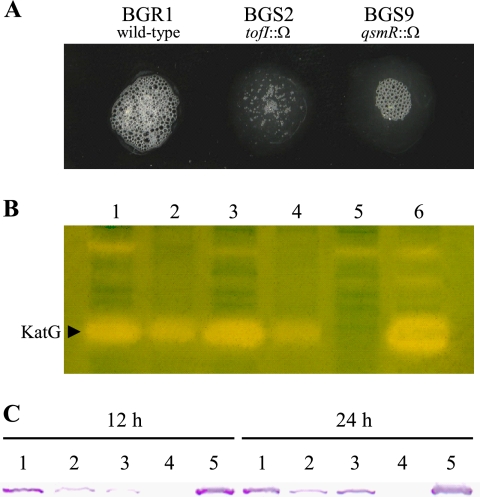
The BGR1 cells exposed to 100% hydrogen peroxide produced many more air bubbles than the tofI mutant BGS2 and the qsmR mutant BGS9 (Fig. 1A). Therefore, we investigated whether QS controls catalase activity in B. glumae. Catalase activity staining of BGR1 revealed at least three distinct bands (Fig. 1B). The BGS2 and BGS9 strains had produced fewer catalases at 24 h after inoculation (Fig. 1B), which was consistent with a preliminary observation. However, when 1 μM C8-HSL was added to the BGS2 cells, the activity staining pattern recovered to that of BGR1 (Fig. 1B), indicating that catalase activity is regulated by QS.
FIG. 1.
(A) Catalase assay based on the production of air bubbles from BGR1, BGS2 (tofI::Ω), and BGS9 (qsmR::Ω) cells with hydrogen peroxide. (B) Catalase activity staining. The arrowhead indicates a band that disappeared from the katG mutant. Lanes: 1, BGR1; 2, BGS2; 3, BGS2 with 1 μM C8-HSL; 4, BGS9; 5, BGC2 (katG::Tn3-gusA); 6, BGC2(pHJ4). (C) Western blot analysis. Lanes: 1, BGR1; 2, BGS2; 3, BGS9; 4, BGC2; 5, BGC2(pHJ4).
Expression of katG is regulated by QS.
To identify the protein responsible for catalase production based on the results of catalase activity staining, we first constructed a katG mutant by Tn3-gusA mutagenesis of pHJ4 carrying the katG gene in the 5.5-kb PstI fragment (Table 1). The size of the katG gene of B. glumae was 2,181 bp, encoding a 79.6-kDa protein, and exhibited 63% deduced amino acid sequence identity to KatG of B. pseudomallei (data not shown). The KatG belonged to a bifunctional catalase-peroxidase group. We found one Tn3-gusA78 mutant inserted between bp 152 and 153 downstream of the putative start codon of katG (data not shown). The katG::Tn3-gusA78 mutation was marker exchanged into the BGR1, BGS2 (tofI::Ω), and BGS9 (qsmR::Ω) strains, resulting in BGC2 (katG::Tn3-gusA), S2C2 (tofI::Ω and katG::Tn3-gusA), and S9C2 (qsmR::Ω and katG::Tn3-gusA), respectively (Table 1). Among the three distinct bands revealed via catalase activity staining, the KatG band disappeared from the katG mutant strains (Fig. 1B). The production of KatG in the BGC2 strain was recovered when the plasmid pHJ4 carrying the katG gene was mobilized into the mutant in trans (Fig. 1B). In addition, at least two other positive bands appeared to be regulated by QS; however, we could not identify the catalase types. To further confirm that KatG production is regulated by QS, we detected KatG by Western blot analysis using an anti-KatG antibody raised in a mouse. As shown in Fig. 1C, BGR1 contained much more KatG than BGS2 and BGS9 at 12 h after incubation, although low basal levels of KatG were detected in the BGS2 and BGS9 mutants (Fig. 1C). The katG mutant strain BGC2 did not produce KatG but recovered KatG production when pHJ4 was provided (Fig. 1C). These data prove that the bottom band on the gel was KatG. The BGC2 strain produced toxoflavin (data not shown), which indicates that toxoflavin production is not affected by KatG.
Expression of katG is directly regulated by QsmR.
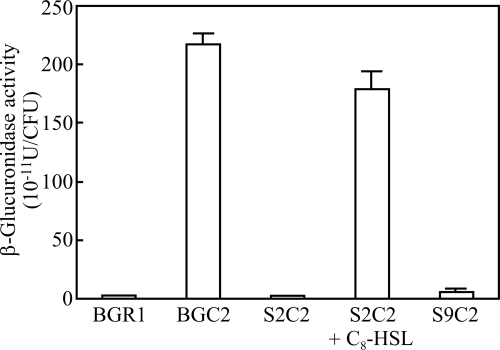
To determine if katG expression is regulated by QsmR at the transcriptional level, we measured β-glucuronidase activities in the BGC2, S2C2, and S9C2 strains. katG was expressed at a high level in the BGC2 strain but at very low levels in S2C2 and S9C2 (Fig. 2). When 1 μM C8-HSL was added to the S2C2 strain, β-glucuronidase activity recovered to the level in the BGC2 strain (Fig. 2). β-Glucuronidase activity in the S9C2 strain carrying qsmR in pBGT63 also recovered to the level in the BGC2 strain (Fig. 2). This indicates that katG expression is controlled by QsmR.
FIG. 2.
QsmR activates katG expression. Expression of katG was reduced in the tofI mutant strain BGS2 and the qsmR mutant strain BGS9, but it recovered to wild-type levels after the addition of 1 μM C8-HSL to the BGS2 culture. Values are means and standard deviations from triplicate experiments.
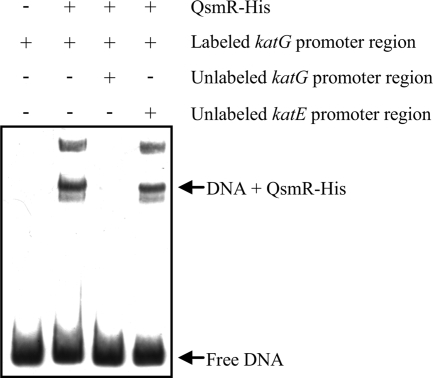
To further confirm that katG expression is directly regulated by QsmR, we performed an electrophoretic mobility shift assay with QsmR-His and the promoter region of katG. As shown in Fig. 3, QsmR-His bound to the promoter region of katG, but not to the katE promoter region, which proves that katG expression is directly activated by QsmR.
FIG. 3.
Gel mobility shift assays using purified QsmR-His and a DNA fragment containing the katG promoter region.
The KatG mutant is highly sensitive to exogenous H2O2.
To evaluate the role of KatG against oxidative stress, the susceptibility of the katG mutant BGC2 to H2O2 was compared with the susceptibilities of the wild-type BGR1, BGS2, and BGS9. The zones of inhibition with BGR1, BGC2, BGS2, and BGS9 were 30, 56, 35, and 37 mm, respectively (data not shown), indicating that the katG mutant is highly sensitive to exogenous H2O2.
KatG is responsible for protecting against visible light.
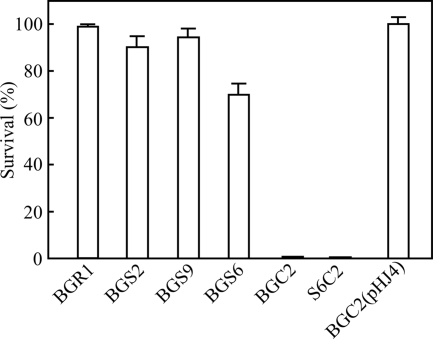
To determine if KatG helped protect cells from visible light, we exposed BGR1 and the mutant strains to visible light. The survival rate of BGR1 under visible light was more than 95% (average of three independent experiments) compared with that under dark conditions (Fig. 4). Survival in the BGS2 and BGS9 strains was more than 90% (Fig. 4). In contrast, the survival rate of the BGC2 strain was less than 1% under visible light but recovered to the survival rate of BGR1 when pHJ4 was provided in trans (Fig. 4). Both strains S2C2 and S9C2 were sensitive to visible light, as expected (data not shown). When the toxoflavin-deficient mutant BGS6 (toxR′ toxA′::Ω) was tested for visible light sensitivity, the survival rate was approximately 70% (Fig. 4). However, no colonies appeared when the double mutant (S6C2) lacking toxoflavin biosynthesis and KatG was exposed to visible light (Fig. 4). These results indicate that KatG plays an important role in protecting cells exposed to visible light.
FIG. 4.
Survival rates of BGR1, BGS2 (tofI::Ω), BGS9 (qsmR::Ω), BGS6 (toxR′ toxA′::Ω), BGC2 (katG::Tn3-gusA78), S6C2 (toxR′ toxA′::Ω and katG::Tn3-gusA78), and BGC2(pHJ4) to visible light. Values are means and standard deviations from triplicate experiments.
The KatG mutant exhibited less severe disease.
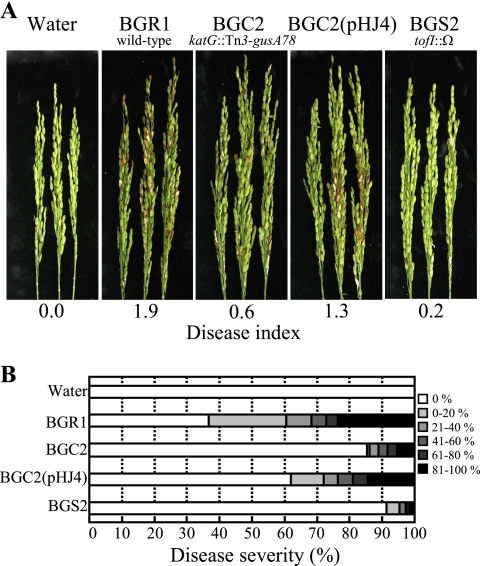
To assess the virulence of the BGC2 strain in rice panicles, BGR1, BGS2, BGC2, and BGC2 carrying pHJ4 were inoculated into rice panicles at the flowering stage. BGR1 caused severe grain rot, whereas the BGS2 and BGC2 strains exhibited much less severe disease (Fig. 5). When pHJ4 was introduced into the BGC2 strain, the disease severity increased to that of BGR1 (Fig. 5).
FIG. 5.
(A) Pathogenicity assay of BGR1, BGC2 (katG::Tn3-gusA78), BGC2(pHJ4), and BGS2 (tofI::Ω). The photographs were taken 7 days after inoculation. (B) Distribution patterns of disease severity for each treatment.
DISCUSSION
In addition to controlling many phenotypes in bacteria, QS contributes to the survival of bacteria in vivo under stressful conditions (10). For example, QS plays a role in survival in vivo by enhancing proliferation and adjustment to environmental change in Vibrio vulnificus (31). QS also enhances the viability of V. cholerae under certain stress conditions by upregulating the expression of rpoS via HapR (16). The mechanism by which QS enhances the viability of bacteria is related to biofilm formation (16).
When cells of B. glumae produce toxoflavin under the control of QS, they are exposed to oxidative stress in the form of hydrogen peroxide generated by toxoflavin autorecycling oxidation (26). Therefore, it is essential for B. glumae to overcome this oxidative stress in order for it to survive infection and changing environmental conditions. The expression of a number of antioxidant enzymes helps protect bacteria against oxidative-stress damage (13). In P. aeruginosa, QS controls a major catalase (katA) and sod genes, and mediates biofilm susceptibility to hydrogen peroxide (10).
In general, the expression of genes for antioxidant enzymes, such as catalase and alkyl hydroperoxide reductase, is regulated by OxyR (25, 28). In B. glumae, QsmR, a TofR regulon, controls katG expression. This is not unusual; QS activates the expression of numerous genes involved in the oxidative-stress response in P. aeruginosa (9). Considering our previous report that QsmR regulates flhDC, which is a key regulator of flagellar-gene expression, QsmR probably is involved in controlling more biological processes than previously thought. Given that OxyR is a major regulator of the oxidative-stress responses of other gram-negative bacteria, such as Xanthomonas campestris and B. pseudomallei, we cannot rule out the possibility that OxyR, in addition to QsmR, directly or indirectly regulates katG expression under oxidative stress in B. glumae.
Based on the catalase activity staining and immunoblot analysis, the basal expression levels of katG were substantial in the BGS2 and BGS9 strains, consistent with the fact that these strains exhibit sensitivity to hydrogen peroxide similar to that of BGR1. These results may explain why approximately 90% of BGS2 and BGS9 cells survived visible light, whereas the survival rate of BGC2 was extremely low. High baseline expression of katG was also observed in B. pseudomallei (23). OxyR represses katG expression in B. pseudomallei in the absence of oxidative stress; however, a certain level of katG promoter activity was detected in BGR1 (23). Under oxidative-stress conditions, KatG of B. pseudomallei plays important roles in cell survival (22).
The phototoxic effect of visible light on bacteria is oxygen dependent (7). For example, exposing Porphyromonas gingivalis and Fusobacterium nucleatum to blue light under anaerobic conditions eliminates the phototoxic effect seen under aerobic conditions (7). The katG mutation in B. glumae did not affect survival at the stationary growth phase but was lethal when the strain was exposed to visible light. The results suggest that katG is a major catalase that protects cells from phototoxic effects induced by visible light. We postulated that toxoflavin may be a major source of phototoxic hydrogen peroxide production in B. glumae. However, it is very unlikely that it is the only major source because no significant differences were observed in the survival of the BGS6 and S6C2 mutant strains. Therefore, other compounds may also generate phototoxins in B. glumae under visible light.
Toxoflavin probably does not act alone, and it remains unknown how phototoxicity occurs when B. glumae cells are exposed to visible light. Sanguinarine is a phototoxic hydrogen peroxide-producing alkaloid that is phototoxic to catalase-deficient strains of E. coli (33). Therefore, it is clear that catalases are necessary to protect bacteria against phototoxic effects generated by hydrogen peroxide-producing compounds. As B. glumae cells cannot grow under anaerobic conditions, we could not evaluate whether the phototoxic effect on B. glumae was directly dependent on oxygen.
Pathogenic microorganisms encounter ROS when interacting with host cells during infection (1, 3), and catalases are associated with virulence in various bacterial pathogens, including L. pneumophila and Agrobacterium tumefaciens (2, 35). KatA of Campylobacter jejuni is essential for its survival in macrophages (5). We found a very similar phenomenon with the katG mutant BGC2, which failed to prompt severe bacterial grain rot even though the mutant still produced toxoflavin. The reduced disease severity caused by the katG mutant might be due to low survival on rice panicles under light. Therefore, it is very likely that KatG is vital for the survival of B. glumae on the surfaces of rice panicles under light conditions.
Acknowledgments
This work was supported by a Korea Science and Engineering Foundation (KOSEF) grant funded by the Korean government (MEST [Ministry of Education, Science, and Technology] no. 500-20080104); grant no. CG2130 from the Crop Functional Genomics Center of the 21st Century Frontier Research Programs, which is funded by MEST; and a Korea Research Foundation Grant funded by the Korean government (MOEHRD, Basic Research Promotion Fund; KRF-2006-005-J04701).
Footnotes
Published ahead of print on 24 April 2009.
REFERENCES
- 1.Abramovitch, R. B., and G. B. Martin. 2004. Strategies used by bacterial pathogens to suppress plant defenses. Curr. Opin. Plant Biol. 7356-364. [DOI] [PubMed] [Google Scholar]
- 2.Bandyopadhyay, P., B. Byrne, Y. Chan, M. S. Swanson, and H. M. Steinman. 2003. Legionella pneumophila catalase-peroxidases are required for proper trafficking and growth in primary macrophages. Infect. Immun. 714526-4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, S. M., M. L. Howell, M. L. Vasil, A. J. Anderson, and D. J. Hassett. 1995. Cloning and characterization of the katB gene of Pseudomonas aeruginosa encoding a hydrogen peroxide-inducible catalase: purification of KatB, cellular localization, and demonstration that it is essential for optimal resistance to hydrogen peroxide. J. Bacteriol. 1776536-6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christman, M. F., G. Storz, and B. N. Ames. 1989. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium, is homologous to a family of bacterial regulatory proteins. Proc. Natl. Acad. Sci. USA 863484-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day, W. A., Jr., J. L. Sajecki, T. M. Pitts, and L. A. Joens. 2000. Role of catalase in Campylobacter jejuni intracellular survival. Infect. Immun. 686337-6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farr, S. B., and T. Kogoma. 1991. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 55561-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feuerstein, O., I. Ginsburg, E. Dayan, D. Veler, and E. I. Weiss. 2005. Mechanism of visible light phototoxicity on Porphyromonas gingivalis and Fusobacterium nucleatum. Photochem. Photobiol. 811186-1189. [DOI] [PubMed] [Google Scholar]
- 8.Fuqua, C., S. C. Winans, and E. P. Greenberg. 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 176269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassett, D. J., E. Alsabbagh, K. Parvatiyar, M. L. Howell, R. W. Wilmott, and U. A. Ochsner. 2000. A protease-resistant catalase, KatA, released upon cell lysis during stationary phase is essential for aerobic survival of a Pseudomonas aeruginosa oxyR mutant at low cell densities. J. Bacteriol. 1824557-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassett, D. J., J.-F. Ma, J. G. Elkins, T. R. McDermott, U. A. Ochsner, S. E. H. West, C.-T. Huang, J. Fredericks, S. Burnett, P. S. Stewart, G. McFeters, L. Passador, and B. H. Iglewski. 1999. Quorum sensing in Pseudomonas aeruginosa controls expression of catalase and superoxide dismutase genes and mediates biofilm susceptibility to hydrogen peroxide. Mol. Microbiol. 341082-1093. [DOI] [PubMed] [Google Scholar]
- 11.Holden, M. T. G., R. W. Titball, S. J. Peacock, A. M. Cerdeño-Tárraga, T. Atkins, L. C. Crossman, T. Pitt, C. Churcher, K. Mungall, S. D. Bentley, M. Sebaihia, N. R. Thomson, N. Bason, I. R. Beacham, K. Brooks, K. A. Brown, N. F. Brown, G. L. Challis, I. Cherevach, T. Chillingworth, A. Cronin, B. Crossett, P. Davis, D. DeShazer, T. Feltwell, A. Fraser, Z. Hance, H. Hauser, S. Holroyd, K. Jagels, K. E. Keith, M. Maddison, S. Moule, C. Price, M. A. Quail, E. Rabbinowitsch, K. Rutherford, M. Sanders, M. Simmonds, S. Songsivilai, K. Stevens, S. Tumapa, M. Vesaratchavest, S. Whitehead, C. Yeats, B. G. Barrell, P. C. F. Oyston, and J. Parkhill. 2004. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc. Natl. Acad. Sci. USA 10114240-14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iiyama, K., N. Furuya, Y. Takanimi, and N. Matsuyama. 1995. A role of phytotoxin in virulence of Pseudomonas glumae. Ann. Phytopathol. Soc. Jpn. 61470-476. [Google Scholar]
- 13.Imlay, J. A. 2003. Pathways of oxidative damage. Annu. Rev. Microbiol. 57395-418. [DOI] [PubMed] [Google Scholar]
- 14.Jefferson, R. A., T. A. Kavanagh, and M. W. Bevan. 1987. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 63901-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeong, Y., J. Kim, S. Kim, Y. Kang, T. Nagamatsu, and I. Hwang. 2003. Toxoflavin produced by Burkholderia glumae causing rice grain rot is responsible for inducing bacterial wilt in many field crops. Plant Dis. 87890-895. [DOI] [PubMed] [Google Scholar]
- 16.Joelsson, A., B. Kan, and J. Zhu. 2007. Quorum sensing enhances the stress response in Vibrio cholerae. Appl. Environ. Microbiol. 733742-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, J., J.-G. Kim, Y. Kang, J. Y. Jang, G. J. Jog, J. Y. Lim, and I. Hwang. 2004. Quorum sensing and the LysR-type transcriptional activator ToxR regulate toxoflavin biosynthesis and transport in Burkholderia glumae. Mol. Microbiol. 54921-934. [DOI] [PubMed] [Google Scholar]
- 18.Kim, J. 2006. Ph.D. thesis. Seoul National University, Seoul, Republic of Korea.
- 19.Kim, J., Y. Kang, O. Choi, Y. Jeong, J.-E. Jeong, J. Y. Lim, M. Kim, J. S. Moon, H. Suga, and I. Hwang. 2007. Regulation of polar flagellum genes is mediated by quorum sensing and FlhDC in Burkholderia glumae. Mol. Microbiol. 64165-179. [DOI] [PubMed] [Google Scholar]
- 20.Latuasan, H. E., and W. Berends. 1961. On the origin of the toxicity of toxoflavin. Biochim. Biophys. Acta 52502-508. [DOI] [PubMed] [Google Scholar]
- 21.Levine, A., R. Tenhaken, R. Dixon, and C. Lamb. 1994. H2O2 from oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79583-593. [DOI] [PubMed] [Google Scholar]
- 22.Loprasert, S., W. Whangsuk, R. Sallabhan, and S. Mongkolsuk. 2003. Regulation of the katG-dpsA operon and the importance of katG in survival of Burkholderia pseudomallei exposed to oxidative stress. FEBS Lett. 54217-21. [DOI] [PubMed] [Google Scholar]
- 23.Loprasert, S., R. Sallabhan, W. Whangsuk, and S. Mongkolsuk. 2002. The Burkholderia pseudomallei oxyR gene: expression analysis and mutant characterization. Gene 296161-169. [DOI] [PubMed] [Google Scholar]
- 24.Lumjiaktase, P., S. P. Diggle, S. Loprasert, S. Tungpradabkul, M. Daykin, M. Cámara, P. Williams, and M. Kunakorn. 2006. Quorum sensing regulates dpsA and the oxidative stress response in Burkholderia pseudomallei. Microbiology 1523651-3659. [DOI] [PubMed] [Google Scholar]
- 25.Mongkolsuk, S., W. Whangsuk, P. Vattanaviboon, S. Loprasert, and M. Fuangthong. 2000. A Xanthomonas alkyl hydroperoxide reductase subunit C (ahpC) mutant showed an altered peroxide stress response and complex regulation of the compensatory response of peroxide detoxification enzymes. J. Bacteriol. 1826845-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagamatsu, T. 2001. Syntheses, transformation, and biological activities of 7-azapteridine antibiotics: toxoflavin, fervenulin, reumycin and their analogs. Recent Res. Develop. Org. Bioorg. Chem. 497-121. [Google Scholar]
- 27.Nicholls, P., I. Fita, and P. C. Loewen. 2001. Enzymology and structure of catalases. Adv. Inorg. Chem. 5151-106. [Google Scholar]
- 28.Ochsner, U. A., M. L. Vasil, E. Alsabbagh, K. Parvatiyar, and D. J. Hassett. 2000. Role of the Pseudomonas aeruginosa oxyR-recG operon in oxidative stress defense and DNA repair: OxyR-dependent regulation of katB-ankB, ahpB, and ahpC-ahpF. J. Bacteriol. 1824533-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piddington, D. L., F. C. Fang, T. Laessig, A. M. Cooper, I. M. Orme, and N. A. Buchmeier. 2001. Cu, Zn superoxide dismutase of Mycobacterium tuberculosis contributes to survival in activated macrophages that are generating an oxidative burst. Infect. Immun. 694980-4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., Cold Spring Harbor Press, Cold Spring Harbor, NY.
- 31.Shin, N.-R., D. Y. Lee, and H. S. Yoo. 2007. Identification of quorum sensing-related regulons in Vibrio vulnificus by two-dimensional gel electrophoresis and differentially displayed reverse transcriptase PCR. FEMS Immunol. Med. Microbiol. 5094-103. [DOI] [PubMed] [Google Scholar]
- 32.Staskawicz, B., D. Dahlbeck, N. Keen, and C. Napoli. 1987. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J. Bacteriol. 1695789-5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuveson, R. W., R. A. Larson, K. A. Marley, G.-R. Wang, and M. R. Berenbaum. 2008. Sanguinarine, a phototoxic H2O2-producing alkaloid. Photochem. Photobiol. 50733-738. [DOI] [PubMed] [Google Scholar]
- 34.Wayne, L. G., and G. A. Diaz. 1986. A double staining method for differentiating between two classes of mycobacterial catalase in polyacrylamide electrophoresis gels. Anal. Biochem. 15789-92. [DOI] [PubMed] [Google Scholar]
- 35.Xu, X. Q., and S. Q. Pan. 2000. An Agrobacterium catalase is a virulence factor involved in tumorigenesis. Mol. Microbiol. 35407-414. [DOI] [PubMed] [Google Scholar]