Abstract
Recent studies have established that several nonenveloped viruses utilize virus-encoded lytic peptides for host membrane disruption. We investigated this mechanism with the “gamma” peptide of the insect virus Flock House virus (FHV). We demonstrate that the C terminus of gamma is essential for membrane disruption in vitro and the rescue of immature virus infectivity in vivo, and the amphipathic N terminus of gamma alone is not sufficient. We also show that deletion of the C-terminal domain disrupts icosahedral ordering of the amphipathic helices of gamma in the virus. Our results have broad implications for understanding membrane lysis during nonenveloped virus entry.
The presence of membrane lytic peptides in many nonenveloped viruses is well established (3, 16), but how these peptides are deployed from the virus capsid during host cell entry and disrupt membranes remains unclear. These peptides are typically generated by a postassembly proteolytic processing event (1, 11) and are exposed from a previously buried position during conformational alterations in the capsid triggered by host cell conditions (2, 18). Flock House virus (FHV), an insect nodavirus, contains a 4-kDa peptide called “gamma” (γ), which shares many of the characteristics of other nonenveloped virus lytic peptides (3). The FHV capsid is made from 180 copies of a single-coat protein (α) enclosing a single-stranded bipartite RNA genome (9). Gamma is generated by the autocatalytic cleavage of α during virus maturation (α → β + γ) (15), remains localized in the capsid interior (9) with occasional externalization or “breathing” (6), and is exposed under low-pH conditions in the endosomes during entry (Odegard et al., submitted for publication). Covalently independent gamma is necessary for virus infection, since maturation-defective FHV (D75N/N363T FHV), which does not undergo the autocleavage of α, is not infectious (15, 17). The N-terminal ∼21 residues of gamma (corresponding to residues 364 to 384 of α) constitute an amphipathic helix which can disrupt membranes in vitro when synthetically produced (4, 5) and is recognized as the host membrane-interacting region of FHV during entry. The hydrophobic, ∼23-residue-long C terminus of gamma, especially certain phenylalanine residues (at positions 402, 405, and 407), is responsible for specifically packaging viral RNA into capsids during assembly (14).
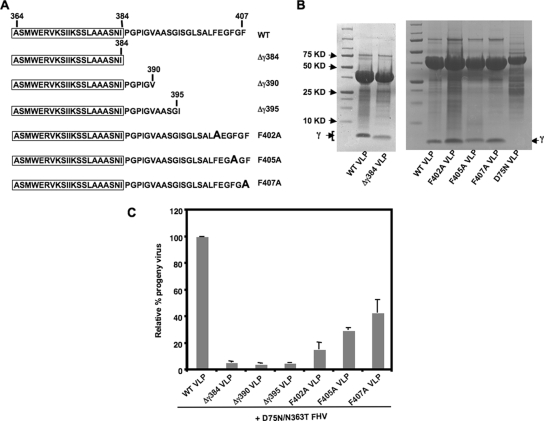
It was recently demonstrated that a supply of full-length gamma from noninfectious virus-like particles (VLPs) of FHV (13) during entry can restore infectivity to maturation-defective FHV (17), suggesting that gamma can function in trans to mediate access into host cells. This trans-complementation assay (17) was utilized to provide a quantitative readout of the effect of gamma mutations specifically on virus entry and to thus assess the region(s) of gamma required during virus entry. To determine the minimal sequence of gamma required for trans-complementation, FHV VLPs were produced that included the first 384, 390, or 395 amino acids of capsid protein α (designated Δγ384, Δγ390, and Δγ395, respectively). These VLPs contained only the amphipathic region of gamma, or that and additional parts of the C-terminal region (Fig. 1A), and underwent normal assembly and maturation cleavage (Fig. 1B). The amount of progeny virus produced by trans-complementing maturation-defective D75N/N363T FHV with these mutated VLPs was negligible (∼5%) compared to that produced by the wild-type (WT) VLPs (considered 100%) (Fig. 1C). Thus, the amphipathic region of gamma was not sufficient by itself to restore infectivity to maturation-defective FHV, and the C terminus of gamma, beyond residue 395, was essential for rescue. Three phenylalanines located beyond residue 395 in gamma were separately mutated to alanines, and mature VLPs were generated (Fig. 1A and B) and tested in the trans-complementation assay. The relative efficiencies of rescue demonstrated by the F402A, F405A, and F407A VLPs were 15%, 29%, and 43%, respectively (Fig. 1C), compared to that by the WT VLPs (100%). This rescue efficiency was similar to the relative infectivity previously determined for FHV containing the same point mutations (14). This suggests that the phenylalanines at the gamma C terminus are not only involved in the specific packaging of genomic RNA during FHV assembly (14) but are also required during virus entry.
FIG. 1.
C-terminal region of gamma required for trans-complementation during the entry of maturation-defective FHV. (A) Schematic of truncations or single mutations in the gamma region of FHV capsid protein α in VLPs. The sequence of gamma in each of the mutated VLPs is shown, with the N-terminal amphipathic region boxed. The single F→A mutations in F402A, F405A, and F407A are indicated in boldface. (B) A total of 5 μl of WT, Δγ384, phenylalanine mutant, or D75N VLPs, at a concentration of 5 mg/ml, was subjected to SDS-PAGE on a 4 to 20% Tris-glycine gel (Invitrogen) and stained with Coomassie brilliant blue. The position of gamma is indicated. The cleavage-defective D75N VLPs do not have gamma. (C) Drosophila DL-1 cells (1 × 108) were coinfected with 1.5 × 103 particles/cell of D75N/N363T FHV and 9 × 103 particles/cell of WT, Δγ384, Δγ390, Δγ395, F402A, F405A, or F407A VLPs. [35S]methionine-cysteine-labeled progeny virus was quantified, with the amount of progeny produced during coinfection with D75N/N363T FHV and WT VLPs normalized at 100%. The standard deviation was calculated from three replicates.
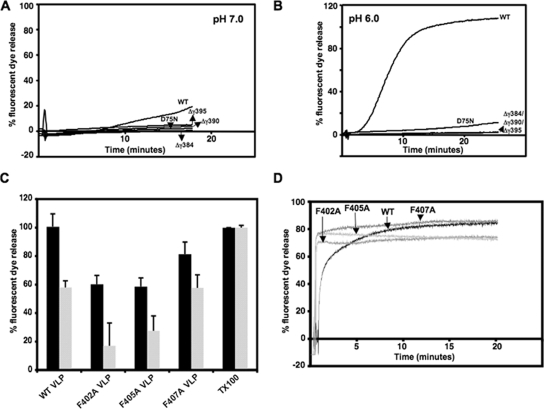
Since the primary function of gamma during FHV entry is expected to be host membrane disruption, the ability of the mutated VLPs to disrupt DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine)-treated liposomes and release enclosed fluorescent dye was determined in comparison to the WT VLPs. We found that the in vivo rescue behavior of VLPs containing truncated gamma correlated with their in vitro membrane disruption activities. WT VLPs, at a concentration of 0.1 mg/ml (6.37 × 1011 particles), released dye from liposomes at pH 7.0 (Fig. 2A) and at a much higher rate at pH 6.0 (Fig. 2B), which mimics the acidic endosomal environment. In contrast, the Δγ384, Δγ390, and Δγ395 VLPs were severely impaired in disrupting liposomes at both pH conditions (Fig. 2A and B), indicating that diminished endosomal membrane lysis by truncated gamma peptides could be responsible for the inability of mutated VLPs to rescue the infectivity of maturation-defective particles. The maturation-defective D75N VLP, which is unable to rescue infection (17), was also inefficient in liposome disruption at a neutral or low pH (Fig. 2A and B), indicating that in vitro membrane disruption by VLPs is a reliable indicator of their in vivo entry behavior.
FIG. 2.
Disruption of liposomes and fluorescent dye release by VLPs in vitro. In each case, total fluorescence is normalized to dye release achieved by the addition of 0.1% Triton X-100 to liposomes under the same conditions. In the case of panels A, B, and D, closely similar results were obtained in three different experiments, whereas the standard deviations in panel C were calculated from three replicates. Fluorescence measurements were carried out at excitation/emission maxima of 492/514 nm for 6-carboxyfluorescein and 535/585 nm for SulfoB. (A) Kinetic study of 6-carboxyfluorescein release from DOPC-treated liposomes upon the addition of 6.37 × 1011 particles (lipid/particle molar ratio, 481:1) of WT, gamma-truncated, or maturation-defective D75N VLPs to liposomes in 50 mM HEPES (pH 7.0). (B) SulfoB release from DOPC-treated liposomes in 50 mM Bis-Tris (pH 6.0) upon the addition of 6.37 × 1011 particles of VLPs. (C) SulfoB release from DOPC-treated liposomes by 6.37 × 1011 particles of WT, F402A, F405A, or F407A VLPs after 1 h at pH 7.0 (black) or by 2 × 1011 particles (lipid/particle molar ratio, 1,387:1) of each of the VLPs after 15 min at pH 6.0 (gray). (D) SulfoB fluorescence upon the addition of heat-released gamma peptide with the WT sequence or with phenylalanine mutations at F402, F405, and F407 to dye-filled liposomes.
VLPs containing single phenylalanine mutations in gamma were able to disrupt liposomes but were overall less effective than the WT. At a concentration of 0.1 mg/ml at pH 7.0, F402A and F405A VLPs displayed ∼60% liposome disruption (1 h postincubation) compared to that of the WT (Fig. 2C). At pH 6.0, these VLPs caused significantly less liposome disruption than the WT at an early time point (15 min postincubation) at a concentration of 0.035 mg/ml (2 × 1011 particles) (Fig. 2C), although after 45 min of incubation, the extent of the liposome disruption approached the WT levels (data not shown). The F407A mutant VLPs, approximately half as competent as the WT VLPs in rescuing infection (Fig. 1C), were nonetheless as efficient as the WT VLPs in membrane disruption at low pH, although they were ∼80% as competent at a neutral pH (Fig. 2C). When gamma was isolated from the WT and phenylalanine mutant VLPs, by heating equal amounts (80 μg) at 65°C in Bis-Tris (pH 6.0) and centrifuging the heated material on a 30% sucrose cushion, the top fraction, which contained WT or mutated gamma peptides, caused the immediate release of fluorescent dye from liposomes (Fig. 2D). Given the dissimilar behavior of particle-associated phenylalanine mutant gamma and free mutant gamma, we hypothesized that the mutations in the C terminus could affect the organization of gamma within the particle.
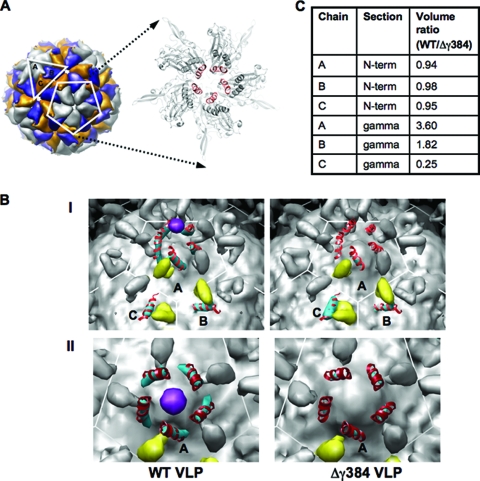
To test this hypothesis, cryoelectron microscopy (CryoEM) and image reconstruction were carried out to locate and compare the gamma peptides of WT FHV VLPs and Δγ384 VLPs, which had the most drastic truncation in gamma and could provide robust structural evidence. Data were collected on frozen hydrated samples of the VLPs (concentrated to 11 mg/ml in 50 mM HEPES [pH 7.0]) at the National Resource for Automated Molecular Microscopy on an FEI Tecnai F20 electron microscope operating at 120 kV. Images were processed using the EMAN suite (10), with a map calculated from the protein atomic coordinates of FHV (PDB entry no. 2Z2Q) as the starting model for image reconstructions. Real space and rigid body refinement was carried out using Chimera (12) and CNS (7), respectively. The estimated resolution for each image reconstruction was 8.8 Å, and the final R/Rfree were 28.4%/28.3% for WT and 29.5%/29.3% for Δγ384 VLPs. While the WT and Δγ384 reconstructions were nearly indistinguishable at the surface, striking changes were detected in the gamma region (Fig. 3B). To quantify these changes, the electron densities for the pseudoatomic models of FHV, lacking the interior RNA, the gamma helices, and the capsid protein N termini, and calculated at the resolution of the CryoEM image reconstructions, were subtracted from the WT and Δγ384 VLP image reconstructions. The volume of the density associated with the gamma helices and the capsid protein N termini was calculated above a threshold of 0.4σ. While the densities of the capsid protein N termini (residues 59 to 72), calculated as controls, appeared similar for the A, B, and C subunits in the icosahedral asymmetric unit (iASU) between the two reconstructions, the densities of the gamma helices (residues 364 to 381) in the A and B subunits were increased approximately fourfold and twofold, respectively, in the WT reconstruction, whereas the density of C-subunit gamma was fourfold stronger in the Δγ384 reconstruction (Fig. 3C). The magnitude of the difference in density represents a clear structural dissimilarity between the WT and Δγ384 VLPs, with the gamma amphipathic helices becoming less icosahedrally ordered overall in the Δγ384 reconstruction. Interestingly, the pentameric helical bundles formed by gamma N termini at the fivefold axis of symmetry of the FHV capsid (Fig. 3A), and thought to be primarily involved in membrane interaction (8), were visible in the WT VLP reconstruction, but the corresponding density weakened significantly in the Δγ384 VLP (Fig. 3B), indicating significant disorder.
FIG. 3.
CryoEM image reconstructions of WT and Δγ384 VLPs. (A) Model of WT FHV showing the iASU consisting of the A, B, and C subunits, as well as the A subunits at the fivefold axis of symmetry. An expanded view of the fivefold axis shows the amphipathic region of the gamma peptides (red) forming a pentameric helical bundle. (B) Panel I, inner surface of WT VLP and Δγ384 VLP image reconstructions contoured at 0.4σ. The coloring is as follows: density for the capsid protein N termini (residues 59 to 72) in the iASU is in yellow, density for the gamma amphipathic helices (residues 364 to 381) is in cyan, and a potential pocket factor is in purple. Modeled into the density for the gamma amphipathic helices are the corresponding residues from the FHV crystal structure (PDB entry 2Z2Q) in red. Panel II, inner view, looking down on the fivefold axis of symmetry of WT and Δγ384 VLP image reconstructions. The density for the gamma amphipathic helices from the A subunits is in cyan, with the corresponding residues from the FHV crystal structure modeled in red. A pocket factor is in purple. (C) Volume ratios corresponding to the gamma amphipathic helices and the capsid protein N termini for subunits A, B, and C in the iASU. The reported ratio for each volume pair is the WT VLP density to the corresponding Δγ384 VLP density.
Our data show that the N-terminal amphipathic helix of gamma, previously thought to be the key to infection, is not sufficient for membrane permeabilization during FHV entry and is coincidentally less icosahedrally ordered in the capsid interior in the absence of the C terminus. Although the role of the gamma C terminus in entry is not clear, one interesting possibility is that the phenylalanine residues in this region interact with other capsid elements or packaged RNA to maintain the N-terminal helices in a structural conformation essential for biological activity. We demonstrate that other regions of nonenveloped virus lytic peptides, in addition to the membrane-interacting domains, might be essential in order to gain maximum leverage during entry.
Acknowledgments
We thank Glen R. Nemerow and Jeffrey A. Speir for critically reading the manuscript.
This work was supported by NIH grant GM034220 to J.E.J.
Footnotes
Published ahead of print on 15 April 2009.
REFERENCES
- 1.Arias, C. F., P. Romero, V. Alvarez, and S. Lopez. 1996. Trypsin activation pathway of rotavirus infectivity. J. Virol. 705832-5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arita, M., S. Koike, J. Aoki, H. Horie, and A. Nomoto. 1998. Interaction of poliovirus with its purified receptor and conformational alteration in the virion. J. Virol. 723578-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee, M., and J. E. Johnson. 2008. Activation, exposure and penetration of virally encoded, membrane-active polypeptides during non-enveloped virus entry. Curr. Protein Pept Sci. 916-27. [DOI] [PubMed] [Google Scholar]
- 4.Bong, D. T., A. Janshoff, C. Steinem, and M. R. Ghadiri. 2000. Membrane partitioning of the cleavage peptide in Flock House virus. Biophys. J. 78839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bong, D. T., C. Steinem, A. Janshoff, J. E. Johnson, and M. Reza Ghadiri. 1999. A highly membrane-active peptide in Flock House virus: implications for the mechanism of nodavirus infection. Chem. Biol. 6473-481. [DOI] [PubMed] [Google Scholar]
- 6.Bothner, B., X. F. Dong, L. Bibbs, J. E. Johnson, and G. Siuzdak. 1998. Evidence of viral capsid dynamics using limited proteolysis and mass spectrometry. J. Biol. Chem. 273673-676. [DOI] [PubMed] [Google Scholar]
- 7.Brunger, A. T., P. D. Adams, G. M. Clore, W. L. DeLano, P. Gros, R. W. Grosse-Kunstleve, J. S. Jiang, J. Kuszewski, M. Nilges, N. S. Pannu, R. J. Read, L. M. Rice, T. Simonson, and G. L. Warren. 1998. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54905-921. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, R. H., V. S. Reddy, N. H. Olson, A. J. Fisher, T. S. Baker, and J. E. Johnson. 1994. Functional implications of quasi-equivalence in a T = 3 icosahedral animal virus established by cryo-electron microscopy and X-ray crystallography. Structure 2271-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher, A. J., and J. E. Johnson. 1993. Ordered duplex RNA controls capsid architecture in an icosahedral animal virus. Nature 361176-179. [DOI] [PubMed] [Google Scholar]
- 10.Ludtke, S. J., P. R. Baldwin, and W. Chiu. 1999. EMAN: semiautomated software for high-resolution single-particle reconstructions. J. Struct. Biol. 12882-97. [DOI] [PubMed] [Google Scholar]
- 11.Odegard, A. L., K. Chandran, X. Zhang, J. S. Parker, T. S. Baker, and M. L. Nibert. 2004. Putative autocleavage of outer capsid protein μ1, allowing release of myristoylated peptide μ1N during particle uncoating, is critical for cell entry by reovirus. J. Virol. 788732-8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pettersen, E. F., T. D. Goddard, C. C. Huang, G. S. Couch, D. M. Greenblatt, E. C. Meng, and T. E. Ferrin. 2004. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 251605-1612. [DOI] [PubMed] [Google Scholar]
- 13.Schneemann, A., R. Dasgupta, J. E. Johnson, and R. R. Rueckert. 1993. Use of recombinant baculoviruses in synthesis of morphologically distinct viruslike particles of Flock House virus, a nodavirus. J. Virol. 672756-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneemann, A., and D. Marshall. 1998. Specific encapsidation of nodavirus RNAs is mediated through the C terminus of capsid precursor protein alpha. J. Virol. 728738-8746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneemann, A., W. Zhong, T. M. Gallagher, and R. R. Rueckert. 1992. Maturation cleavage required for infectivity of a nodavirus. J. Virol. 666728-6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai, B. 2007. Penetration of nonenveloped viruses into the cytoplasm. Annu. Rev. Cell Dev. Biol. 2323-43. [DOI] [PubMed] [Google Scholar]
- 17.Walukiewicz, H. E., M. Banerjee, A. Schneemann, and J. E. Johnson. 2008. Rescue of maturation-defective Flock House virus infectivity with noninfectious, mature, viruslike particles. J. Virol. 822025-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiethoff, C. M., H. Wodrich, L. Gerace, and G. R. Nemerow. 2005. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J. Virol. 791992-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]