Abstract
The Nef protein of human immunodeficiency virus type 1 downregulates the CD4 coreceptor from the surface of host cells by accelerating the rate of CD4 endocytosis through a clathrin/AP-2 pathway. Herein, we report that Nef has the additional function of targeting CD4 to the multivesicular body (MVB) pathway for eventual delivery to lysosomes. This targeting involves the endosomal sorting complex required for transport (ESCRT) machinery. Perturbation of this machinery does not prevent removal of CD4 from the cell surface but precludes its lysosomal degradation, indicating that accelerated endocytosis and targeting to the MVB pathway are separate functions of Nef. We also show that both CD4 and Nef are ubiquitinated on lysine residues, but this modification is dispensable for Nef-induced targeting of CD4 to the MVB pathway.
Primate immunodeficiency viruses infect helper T lymphocytes and cells of the macrophage/monocyte lineage by binding of their viral envelope glycoprotein, Env, to a combination of two host cell-specific surface proteins, CD4 and either the CCR5 or CXCR4 chemokine receptors (reviewed in reference 62). Ensuing fusion of the viral envelope with the host cell plasma membrane delivers the viral genetic material into the cytoplasm. Remarkably, the most highly transcribed viral gene in the early phase of infection does not encode an enzyme or structural protein but an accessory protein named Nef. Early expression of Nef is thought to reprogram the host cell for optimal replication of the virus. Indeed, Nef has been shown to enhance virus production (19, 24, 59, 74) and to promote progression to AIDS (23, 47, 48), making it an attractive candidate for pharmacologic intervention.
Nef is an N-terminally myristoylated protein with a molecular mass of 27 kDa for human immunodeficiency virus type 1 (HIV-1) and 35 kDa for HIV-2 and simian immunodeficiency virus (27, 29, 50, 65). Nef has been ascribed many functions, the best characterized of which is the downregulation of the CD4 coreceptor from the surface of infected cells (28, 35, 57). CD4 downregulation is believed to prevent superinfection (8, 52) and to preclude the cellular retention of newly synthesized Env (8, 49), thus allowing the establishment of a robust infection (30, 71).
The molecular mechanism by which Nef downregulates CD4 has been extensively studied. A consensus has emerged that Nef accelerates the endocytosis of cell surface CD4 (2, 64) by linking the cytosolic tail of CD4 to the heterotetrameric (α-β2-μ2-σ2) adaptor protein-2 (AP-2) complex (17, 25, 34, 45, 67). Determinants in the CD4 tail bind to a hydrophobic pocket comprising tryptophan-57 and leucine-58 on the folded core domain of Nef (34). On the other hand, a dileucine motif (i.e., ENTSLL, residues 160 to 165) (14, 22, 32) and a diacidic motif (i.e., DD, residues 174 and 175) (3) (residues correspond to the NL4-3 clone of HIV-1) within a C-terminal, flexible loop of Nef bind to the α and σ2 subunits of AP-2 (17, 18, 25, 51). AP-2, in turn, binds to clathrin, leading to the concentration of CD4 within clathrin-coated pits (15, 33). These pits eventually bud from the plasma membrane as clathrin-coated vesicles that deliver internalized CD4 to endosomes. In essence, then, Nef acts as a connector that confers on CD4 the ability to be rapidly internalized in a manner similar to endocytic receptors (75).
Unlike typical endocytic recycling receptors like the transferrin receptor or the low-density lipoprotein receptor, however, CD4 that is forcibly internalized by Nef does not return to the cell surface but is delivered to lysosomes for degradation (4, 64, 68). Thus, expression of Nef decreases both the surface and total levels of CD4. What keeps internalized CD4 from returning to the plasma membrane? We hypothesized that Nef might additionally act on endosomes to direct CD4 to lysosomes. This is precisely the fate followed by signaling receptors, transporters, and other transmembrane proteins that undergo ubiquitination-mediated internalization and targeting to the multivesicular body (MVB) pathway (40, 46). This targeting involves the endosomal sorting complex required for transport (ESCRT), including the ESCRT-0, -I, -II, and -III complexes, which function to sort ubiquitinated cargoes into intraluminal vesicles of MVBs for eventual degradation in lysosomes (40, 46). Herein, we show that Nef indeed plays a novel role in targeting internalized CD4 from endosomes to the MVB pathway in an ESCRT-dependent manner. We also show that both Nef and CD4 undergo ubiquitination on lysine residues, but, strikingly, this modification is not required for CD4 targeting to the MVB pathway.
MATERIALS AND METHODS
Expression plasmids.
pCMV-CD4 encoding human CD4 was provided by Klaus Strebel (National Institute of Allergy and Infectious Diseases, NIH) and used for site-directed mutagenesis using a QuikChange II kit (Stratagene, Cedar Creek, TX). pNL4-3 Nef.IRES.GFP (hereinafter referred to as pNef.IRES.GFP, where IRES is internal ribosome entry site and GFP is green fluorescent protein), pCIneo-Nef, and the respective dileucine mutants were described previously (17, 51). The DNA sequence encoding NL4-3 Nef with all 10 lysine codons mutated to arginine codons (Nef-10K/R) was chemically synthesized by DNA2.0 (Menlo Park, CA). Flanking EcoRI and SalI sites in this construct were used to insert the fragment into the pCIneo mammalian expression vector (Promega, Madison, WI), resulting in the pCIneo-Nef-10K/R plasmid. To produce pNef-GFP, the NL4-3 Nef cDNA was amplified from pCI-Nef and cloned as a HindIII/EcoRI fragment into pEGFP N1 plasmid (Clontech, Mountain View, CA). To obtain the pCIneo-Nef-wt-HA (where wt is wild type and HA is hemagglutinin) and pCIneo-Nef-10K/R-HA plasmids, the Nef open reading frame in pCI-Nef and pCI-Nef-10K/R was amplified by PCR to include an in-frame HA epitope tag at the C terminus of Nef. Subsequently the fragments were cloned in pCI-neo as an EcoRI/SalI insert. The pGFP-VPS4-wt and pGFP-VPS4-E/Q (where VPS4-E/Q is VPS4 with the mutation E223Q) plasmids were gifts from Philip Woodman (University of Manchester, United Kingdom). All mutagenesis and subcloning products were verified by DNA sequencing.
Cell culture, transfections, and RNA interference (RNAi).
HeLa cells (American Type Culture Collection, Manassas, VA) were grown in Dulbecco's modified Eagle medium (Invitrogen, Carlsbad, CA) supplemented with 100 U/ml penicillin, 0.1 μg/ml streptomycin, 2 mM l-glutamine, and 10% (vol/vol) fetal bovine serum. Cells were transiently transfected with the indicated plasmids by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). The CD4+ T-cell lines JM and A3.01 (a derivative of the CEM T-cell line) were obtained from the NIH AIDS Research and Reference Reagent Program (Germantown, MD) (deposited originally by G. Farrar and T. Folks, respectively). T-cell lines were grown in RPMI 1640 medium (Invitrogen, Carlsbad, CA) with the same additives indicated above and transfected using Amaxa Biosystems Nucleofector II and the Nucleofector V kit (Amaxa, Gaithersburg, MD). The small interfering RNA (siRNA) designed to target human TSG101 (5′-CCU CCA GUC UUC UCU CGU C-3′) was purchased from Dharmacon (Lafayette, CO). siRNA transfections were carried out using the Oligofectamine reagent from Invitrogen (Carlsbad, CA).
Antibodies.
Unconjugated or Alexa-488- or allophycocyanin (APC)-conjugated monoclonal antibodies to human CD4, used for immunofluorescence and fluorescence-activated cell sorting (FACS) analysis, were purchased from Caltag (Burlingame, CA). Monoclonal antibodies to human CD4, clones NCL-CD4-368 (Leica Microsystems, Bannockburn, IL), and OKT4 (eBiosciences, San Diego, CA) were used for immunoblotting and immunoprecipitation experiments, respectively. Monoclonal antibodies to TSG101, actin, and CD63 (H5C6) were purchased from BD Biosciences (San Jose, CA). Rabbit polyclonal antiserum to HIV-1 Nef was obtained from the NIH AIDS Research and Reference Reagent Program (deposited originally by Ronald Swanstrom) (70). Rabbit antiserum to HRS and SNX2 were gifts from S. Urbé (University of Liverpool, United Kingdom) and C. Haft (National Institute of Diabetes and Digestive and Kidney Diseases, NIH), respectively. Rabbit antibody to the myc epitope was from Cell Signaling (Danvers, MA). Horseradish peroxidase-conjugated donkey anti-mouse immunoglobulin G (IgG) and donkey anti-rabbit IgG were from Amersham Biosciences (Piscataway, NJ). Secondary antibodies conjugated to fluorophores indicated in the figure legends were from Molecular Probes (Eugene, OR).
FACS analysis.
Cells transfected with the plasmids indicated in the figure legends were stained with APC-conjugated anti-CD4 and prepared for FACS analysis as described previously (17, 51). Untransfected cells were used to control for nonspecific antibody labeling. In all cases, GFP fluorescence was used to identify and select transfected cells. The levels of APC fluorescence in cells expressing GFP were measured with a FACSCalibur flow cytometer and analyzed using the CellQuest software (Becton Dickinson, Franklin Lakes, NJ).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis.
Cells were lysed for 30 min at 4°C using lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 10% [vol/vol] glycerol, 5 mM EDTA, 1% [vol/vol] Triton X-100) supplemented with complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). Cell lysates were spun for 15 min at 20,000 × g, and supernatants were recovered and mixed with NuPAGE SDS sample buffer (Invitrogen, Carlsbad, CA). Samples were boiled, and proteins were resolved on NuPAGE Bis-Tris gels (Invitrogen). Immunoblot analysis was performed as previously described (55).
Metabolic labeling and immunoprecipitation.
Metabolic labeling and immunoprecipitation were performed as previously described (12) with minor changes. In brief, cells grown on 12-well plates were pulse-labeled for 15 min at 37°C using 0.1 mCi/ml [35S]methionine-cysteine (Express Protein Label; Perkin Elmer-Cetus, Boston, MA). Cells were rinsed twice with ice-cold phosphate-buffered saline (PBS) and subsequently chased for different times at 37°C in regular culture medium supplemented with excess methionine (0.06 mg/ml) and cysteine (0.1 mg/ml). At each time point, cells were rinsed twice with ice-cold PBS and frozen for later analysis. Cell extracts were immunoprecipitated with antibodies to CD4 and analyzed by SDS-PAGE and fluorography. Quantification of signal intensity was performed using a Typhoon 9200 PhosphorImager and ImageQuant analysis software (Amersham Biosciences).
In vivo ubiquitination assays.
Cell extracts were prepared by incubating cells for 30 min at 4°C in lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 10% [vol/vol] glycerol, 5 mM EDTA, 1% [vol/vol] Triton X-100), supplemented with 20 mM N-ethylmaleimide (Calbiochem, Gibbstown, NJ) to minimize deubiquitination during extract preparation and with 1× complete protease inhibitor cocktail. Cell extracts were subjected to immunoprecipitation using a monoclonal antibody to CD4 or an immobilized rat antibody against the HA epitope (Roche Applied Science, Indianapolis, IN), to immunoprecipitate Nef-HA proteins. Immunoprecipitates were analyzed by SDS-PAGE and immunoblotting.
Immunofluorescence microscopy.
Cells grown on coverslips and transiently expressing the proteins of interest were fixed with either 4% (wt/vol) paraformaldehyde (PFA) in PBS for 15 min at room temperature or methanol for 3 min at room temperature. PFA-fixed cells were permeabilized with 0.2% (vol/vol) Triton X-100 in PBS. Cells were incubated with primary and secondary antibodies in blocking solution (0.2% [wt/vol] pork skin gelatin in PBS). Coverslips were mounted on slides, and cells were analyzed with an Olympus FluoView FV1000 scanning unit on an inverted Olympus IX81 microscope, as previously described (55).
Immunoelectron microscopy.
Cells were fixed with 4% (wt/vol) PFA in PBS for 1 h, rinsed with 50 mM glycine in PBS for 15 min, and blocked with 1% (wt/vol) bovine serum albumin in PBS. Cells were then permeabilized with 0.05% (wt/vol) saponin and 1% (wt/vol) bovine serum albumin in PBS, incubated with antibody to CD4 (Caltag, Burlingame, CA) for 1 h at room temperature or overnight at 4°C, rinsed, and subsequently incubated with nanogold-conjugated secondary antibodies (Nanoprobes, Yaphank, NY) for 1 h at room temperature. Cells were then fixed with glutaraldehyde (2.5% [vol/vol] in 0.1 M cacodylate buffer) for 1 h, rinsed, treated with a gold enhancement mixture (Nanoprobes, Yaphank, NY) for 6 min, and postfixed in reduced osmium prior to embedding in Epon. Sections of 70 to 100 nm were stained with lead citrate prior to imaging with a Tecnai 20 transmission electron microscope (FEI Company, Netherlands) operating at 120 kV. Images were captured on a 2,000- by 2,000-pixel charge-coupled-device camera (Gatan, Pleasanton, CA).
RESULTS
Nef mediates postendocytic targeting of CD4 for lysosomal degradation.
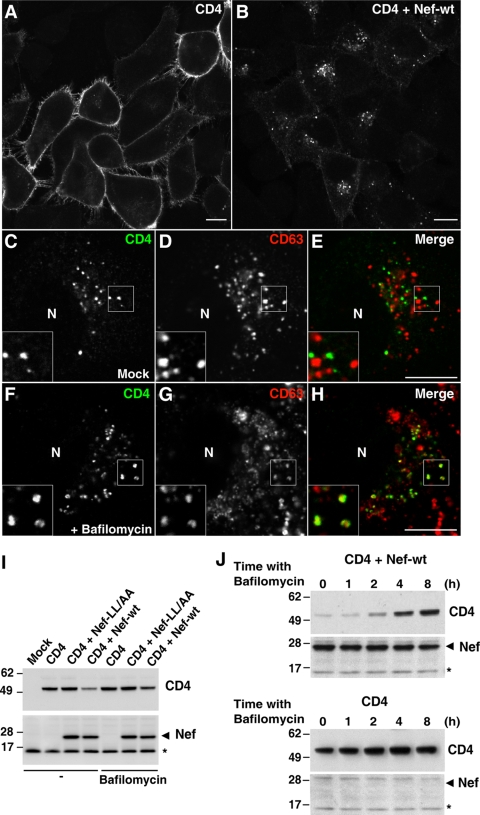
The ability of Nef to downregulate CD4 is not restricted to the natural host cells, primate T lymphocytes and macrophages/monocytes, but can be fully recapitulated in other cell types. We chose to perform most of our experiments with HeLa cells because of their lack of endogenous CD4, which allows analysis of CD4 mutants. In addition, they are highly amenable to transfection, RNAi, and high-resolution morphological analyses. Key findings, however, were replicated in other cell types, including T cells. Immunofluorescence microscopy analysis showed that Nef caused a dramatic redistribution of CD4 from the plasma membrane (Fig. 1A) to cytoplasmic vesicles (Fig. 1B) in HeLa cells. These vesicles were distinct from lysosomes stained for the lysosomal membrane proteins, CD63 (Fig. 1C to E) and Lamp-1 (data not shown). However, inhibition of lysosomal acidification and degradation with either monensin (see Fig. S1 in the supplemental material) or bafilomycin A1 (Fig. 1F to H) resulted in detection of CD4 within a population of lysosomes that contained CD63 (Fig. 1F to H; see also Fig. S1 in the supplemental material) as well as Lamp-1 (data not shown). These observations indicated that downregulated CD4 localized to a population of vesicular intermediates en route to lysosomes, and that, once in lysosomes, CD4 was rapidly degraded. Immunoblot analysis confirmed that expression of Nef decreased the total levels of CD4 (Fig. 1I) and showed that this decrease could be partially prevented by incubation with bafilomycin A1 (Fig. 1I) in a time-dependent manner (Fig. 1J). These effects were specific, as a Nef construct with mutations of leucine residues to alanines in the endocytic dileucine motif (Nef-LL/AA) failed to decrease the total levels of CD4 (Fig. 1I). Nef-LL/AA was subsequently used in most experiments as a negative control. We concluded that expression of Nef caused not only rapid internalization of cell surface CD4 but also its targeting to lysosomes in HeLa cells.
FIG. 1.
Nef targets CD4 to lysosomes. (A and B) HeLa cells grown on coverslips were transfected with pCMV-CD4 (A) or pCMV-CD4 plus pCIneo-Nef-wt (B). After 16 h cells were fixed, permeabilized, and stained with mouse monoclonal antibody to CD4 followed by Alexa-488-conjugated donkey polyclonal antibody to mouse IgG. (C to H) HeLa cells were cotransfected with pCMV-CD4 and pCIneo-Nef-wt and, 16 h later, were incubated for an additional 5 h with no additions (C to E) or with 1 μM bafilomycin A1 (F to H). Cells were then fixed, permeabilized, and stained with Alexa-488-conjugated mouse monoclonal antibody to CD4 (green channel) and mouse IgG1 monoclonal antibody to CD63. This was followed by incubation with Alexa-594-conjugated goat polyclonal antibody specific to mouse IgG1 isotype (red channel). Cells were imaged by confocal laser scanning microscopy. Yellow in the merged images indicates colocalization. Bar, 10 μm. Insets are images of the boxed areas at a magnification of ×2. N, nucleus. (I) HeLa cells were transfected with pCMV-CD4 together with either pCIneo, pCIneo-Nef-wt, or pCIneo-Nef-LL/AA. After transfection, cells were incubated for 16 h and then for an additional 3 h either in the absence (−) or presence of 1 μM bafilomycin A1. Total cell extracts were analyzed by SDS-PAGE and immunoblotting with antibodies to CD4 (upper panel) and Nef (lower panel). The antibody to Nef detects a nonspecific band (asterisk) that is also present in untransfected cell extracts and serves as an internal loading control. (J) HeLa cells were transfected with pCMV-CD4 (lower panel) or pCMV-CD4 plus pCIneo-Nef-wt (upper panel) and, after 16 h, were incubated in the absence or presence of 1 μM bafilomycin A1 for the different periods indicated in the figure. Total cell extracts were analyzed by SDS-PAGE and immunoblotting as in panel I. Molecular mass (in kDa) markers are indicated on the left.
Nef directs CD4 to MVBs.
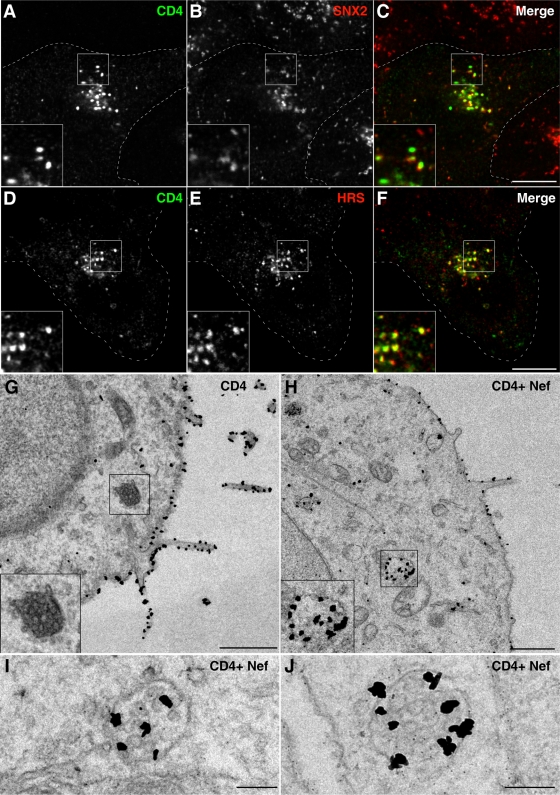
To investigate the pathway by which Nef directs CD4 to lysosomes, we further characterized the vesicular intermediates that contained downregulated CD4 at steady state. Immunofluorescence microscopy in the absence of lysosomal inhibitors showed colocalization of CD4 with endosomal markers (Fig. 2A to F). In the presence of Nef, ∼40% of the CD4-containing vesicles labeled for the endosomal marker SNX2 (Fig. 2A to C), a component of the retromer complex that mediates retrograde transport from endosomes to the trans-Golgi network (13). A greater proportion (∼60%) of CD4-containing vesicles labeled for HRS (Fig. 2D to F), a subunit of the ESCRT-0 complex responsible for targeting cargo proteins to the MVB pathway (40, 46). These findings indicated that vesicles containing downregulated CD4 corresponded to endosomes that were enriched in a component of the MVB machinery (i.e., HRS).
FIG. 2.
Nef induces redistribution of CD4 to multivesicular bodies. (A to F) HeLa cells transfected with pCMV-CD4 and pCIneo-Nef-wt were fixed 16 h later and costained with mouse monoclonal antibody to CD4 and rabbit polyclonal antibodies to either SNX2 (A to C) or HRS (D to F), followed by Alexa-488-conjugated donkey antibody to mouse IgG (green channel) and Alexa-594-conjugated donkey antibody to rabbit IgG (red channel). Cells were imaged by confocal laser scanning microscopy. Cell outlines are indicated by dashed lines. Yellow in the merged images indicates colocalization. Bar, 10 μm. The insets represent the boxed areas at a magnification of ×2. (G to J) HeLa cells were transfected with pCMV-CD4 (G) or pCMV-CD4 plus pCIneo-Nef-wt (H to J). After 16 h, cells were fixed and processed for immunoelectron microscopy as described in the Materials and Methods section. Examples of MVBs are shown in the insets of panels G and H and in panels I and J. The insets in panels G and H show the boxed areas at a magnification of×2. Bars, 1 μm (G and H) 0.2 μm (I and J).
Immunoelectron microscopy allowed analysis of CD4 downregulation at the ultrastructural level. In the absence of Nef, most CD4 was found on the plasma membrane (Fig. 2G) and not in MVBs (Fig. 2G, inset). Expression of Nef, however, caused reduction in plasma membrane staining and the appearance of CD4 in both the limiting membrane and the interior of MVBs (Fig. 2H to J), leading us to conclude that Nef directed CD4 to the MVB pathway.
Requirement of the ESCRT machinery for Nef-induced CD4 degradation.
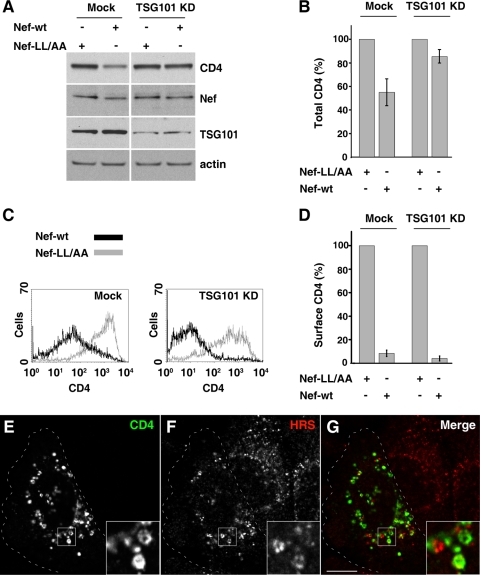
We next investigated whether Nef-induced CD4 targeting to the MVB pathway was dependent on the ESCRT machinery. To this end, we depleted HeLa cells of the TSG101 subunit of ESCRT-I by RNAi (Fig. 3A) (∼75% depletion was achieved) and analyzed total CD4 by immunoblotting (Fig. 3A and B) and surface CD4 by FACS analysis (Fig. 3C). We found that TSG101 depletion impaired the reduction of total CD4 levels (Fig. 3A and B) but not the reduction of surface CD4 upon expression of Nef (Fig. 3C and D). Immunofluorescence microscopy showed that, in TSG101-depleted, Nef-expressing cells, CD4 accumulated within large vesicles that were often decorated by the ESCRT-0 component HRS (Fig. 3E to G), a phenotype that is characteristic of the enlarged endosomes that form upon perturbation of the ESCRT machinery (40, 46).
FIG. 3.
Depletion of TSG101 inhibits Nef-induced CD4 degradation. (A) Cells were either mock transfected or transfected with siRNA for TSG101 (a component of ESCRT-I) twice at 48-h intervals. After the second round of siRNA transfection, cells were transfected with pCMV-CD4 together with pCIneo-Nef-wt or pCIneo-Nef-LL/AA. After 16 h, equivalent amounts of cell lysates were subjected to SDS-PAGE and immunoblotting with antibodies to CD4, Nef, TSG101, and actin (loading control). (B) The CD4 signal intensity for each condition shown in panel A was determined and used to calculate the relative amount of CD4 in either mock- or siRNA-treated cells. Bars represent the means ± standard deviations from three independent experiments. (C) Cells treated in the presence or absence of siRNA for TSG101 as in panel A were subsequently transfected with pCMV-CD4 together with pNef-wt.IRES.GFP or pNef-LL/AA.IRES.GFP and analyzed for cell surface CD4 by FACS, as described in Materials and Methods. (D) Bar graphs represent surface CD4 levels (median values from FACS histogram plots) in cells transfected with pNef-wt.IRES.GFP relative to levels in cells transfected with pNef-LL/AA.IRES.GFP (100%). Results are shown for either mock- or TSG101 siRNA-treated cells as in panel A and represent the means ± standard deviations (n = 3). (E to G) Cells depleted of TSG101 as in panel A were cotransfected with pCMV-CD4 and pCIneo-Nef-wt, fixed, permeabilized, and costained with mouse monoclonal antibody to CD4 and rabbit polyclonal antibody to HRS, followed by incubation with secondary antibodies as described in the legends of Fig. 2A to F. Cells were imaged by confocal laser scanning microscopy. Cell outlines are indicated by dashed lines. The insets show the boxed areas at a magnification of ×3. Yellow in the merged images indicates colocalization. Bar, 10 μm.
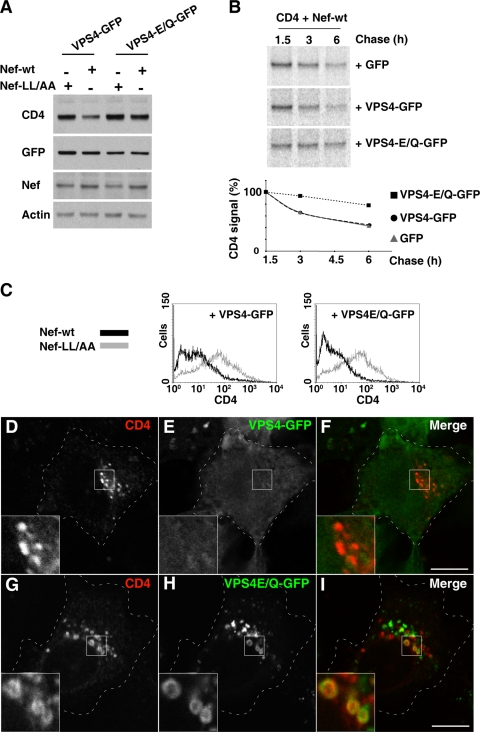
Further evidence for the involvement of the ESCRT machinery was obtained by expression of a dominant-negative VPS4 mutant (VPS4-E/Q). VPS4 is an AAA ATPase that recycles ESCRT machinery components by mediating their dissociation from endosomes to the cytosol (6). Mutation of VPS4 E223 to Q blocks ATP hydrolysis and renders VPS4 a dominant-negative inhibitor of ESCRT recycling (6, 10). We observed that expression of VPS4-E/Q, but not wild-type VPS4, protected CD4 from Nef-induced degradation, as shown by immunoblot analysis of total CD4 levels (Fig. 4A) and pulse-chase analysis of CD4 turnover (Fig. 4B). In contrast, VPS4-E/Q expression did not prevent CD4 disappearance from the cell surface (Fig. 4C). The internalized CD4 accumulated within enlarged vesicles decorated with the “locked” VPS4-E/Q protein (Fig. 4G to I). Taken together, these experiments involving TSG101 depletion and dominant-negative VPS4-E/Q expression demonstrated that the ESCRT machinery is required for Nef-induced targeting of CD4 to the MVB pathway en route to lysosomes. In addition, they showed that, even when targeting to the MVB pathway is blocked, CD4 is incapable of returning to the cell surface. Thus, Nef not only targets CD4 to the MVB pathway but also prevents its recycling to the plasma membrane.
FIG. 4.
Expression of dominant-negative VPS4 inhibits Nef-induced CD4 degradation. (A) HeLa cells were transfected with pCMV-CD4 and pCIneo-Nef-wt or pCIneo-Nef-LL/AA in combination with pGFP-based constructs encoding VPS4 or VPS4-E/Q. After 16 h, cell lysates were prepared, and equivalent amounts were analyzed by SDS-PAGE and immunoblotting with antibodies to CD4, GFP, Nef, and actin (loading control), as shown at the left of each panel. (B) HeLa cells were transfected with the above indicated plasmids encoding CD4, Nef-wt, and either GFP, VPS4-GFP, or VPS4-E/Q-GFP, as shown at the right of each panel. After 16 h cells were pulse-labeled with [35S]methionine-cysteine for 15 min, followed by incubation in chase buffer for the times indicated above each lane. Cell extracts were used for immunoprecipitation with antibody to CD4, and the resulting immunoprecipitates were analyzed by SDS-PAGE. CD4 signal intensity was quantified using the ImageQuant software and is expressed as a percentage of the total amount of CD4 immunoprecipitated at time 1.5 h. (C) HeLa cells transfected as in panel A were analyzed for surface CD4 by FACS, as described in the Materials and Methods section. (D to I) HeLa cells grown on coverslips were transfected with the above indicated plasmids encoding CD4 and Nef-wt, plus either VPS4-GFP (D to F) or VPS4-E/Q-GFP (G to I). After 16 h, cells were fixed, permeabilized, and stained with mouse monoclonal antibody to CD4 followed by Alexa-594-conjugated donkey antibody to mouse IgG (red channel). Cells were imaged by confocal laser scanning microscopy. Cell outlines are indicated by dashed lines. The insets show the boxed areas at a magnification of ×3. Bar, 10 μm.
CD4 ubiquitination on lysine residues is not required for its downregulation by Nef.
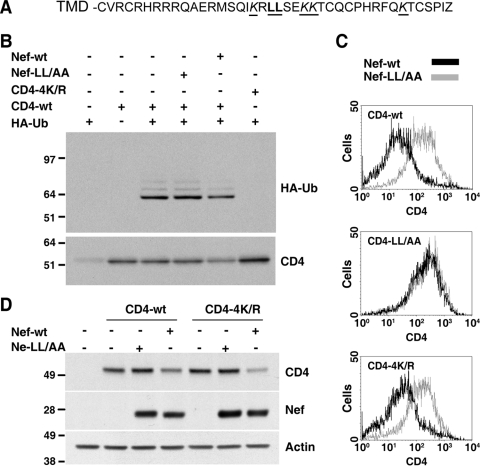
Targeting of most transmembrane proteins to the MVB pathway is dependent on ubiquitination of lysine residues in the cytosolic domains of these proteins (40, 46). The cytosolic tail of CD4 contains four lysine residues that are potential targets for ubiquitination (Fig. 5A). To test whether CD4 is ubiquitinated and whether Nef affects CD4 ubiquitination, we cotransfected cells with different combinations of plasmids encoding CD4, Nef, and HA-tagged ubiquitin (HA-Ub). Cell extracts were subjected to immunoprecipitation with anti-CD4, followed by immunoblotting with anti-HA (Fig. 5B). Using this protocol for cells cotransfected with CD4 and HA-Ub, we observed a prominent band with a size corresponding to monoubiquitinated CD4 and fainter, more slowly migrating species corresponding to multiple ubiquitinated species (Fig. 5B, upper panel). Mutation of the four cytosolic lysine residues in CD4 to arginine (CD4-4K/R) abrogated all detectable CD4 ubiquitination at steady state (Fig. 5B, upper panel). Interestingly, expression of Nef did not increase but, rather, decreased the amount of ubiquitinated CD4 (Fig. 5B, upper panel). This decrease, however, paralleled the reduction of total CD4 levels (Fig. 5B, lower panel) so that the ratio of ubiquitinated to total CD4 did not change. The Nef dileucine mutant Nef-LL/AA, which is unable to downregulate CD4 from the cell surface, had no effect on either CD4 ubiquitination (Fig. 5B, upper panel) or total levels (Fig. 5B, lower panel). Thus, CD4 undergoes constitutive ubiquitination on cytosolic tail lysine residues irrespective of the presence of Nef. The ability of Nef to target CD4 to the MVB pathway is therefore not due to enhancement of CD4 lysine ubiquitination.
FIG. 5.
Nef-induced downregulation does not require CD4 ubiquitination. (A) Sequence of the CD4 C-terminal cytosolic tail with key residues underlined. The dileucine motif involved in CD4 internalization is highlighted in bold, and the four lysine residues are indicated in italics. (B) HeLa cells were cotransfected with pCMV-based vectors encoding either CD4-wt or CD4-4K/R and pCIneo-based plasmids encoding HA-tagged ubiquitin, Nef-wt, or Nef-LL/AA, as indicated on top. After 16 h, equivalent amounts of cell lysates were subjected to immunoprecipitation with antibody to CD4. The resulting immunoprecipitates were analyzed by SDS-PAGE, followed by immunoblotting with antibodies to HA (upper panel) or CD4 (lower panel). (C) FACS analysis of HeLa cells transfected with pCMV-CD4 (CD4-wt, CD4-4K/R, or CD4-LL/AA) and pNef.IRES.GFP (Nef-wt or Nef-LL/AA). After 16 h, cell surface CD4 was labeled, and cells were analyzed by FACS, as described in Materials and Methods. (D) HeLa cells were transfected with pCMV-CD4 (CD4-wt or CD4-4K/R) and pCIneo-Nef (Nef-wt or Nef-LL/AA). After 16 h, equivalent amounts of cell lysates were subjected to SDS-PAGE and immunoblotting with antibodies to CD4, Nef, and actin (loading control). Molecular mass (in kDa) markers are indicated on the left.
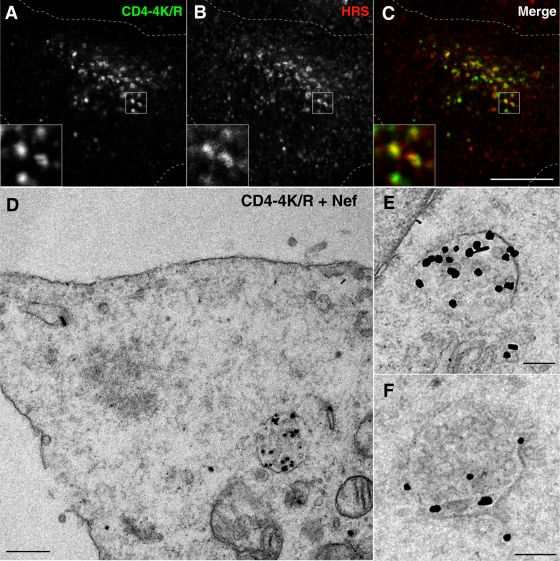
Despite the lack of a detectable effect of Nef on CD4 ubiquitination, we hypothesized that constitutive ubiquitination of CD4 could nevertheless be a requisite for downregulation. To examine this possibility, we compared the Nef-induced downregulation of wild-type CD4 and CD4-4K/R using various assays (Fig. 5 and 6). FACS analysis showed that CD4 downregulation from the cell surface was not dependent on the four lysine residues in the CD4 tail (Fig. 5C, lower panel) although it required the dileucine motifs in Nef (Fig. 5C, upper panel) and in the CD4 tail (Fig. 5C, middle panel). In addition, immunoblot analysis showed that the four lysine residues in the CD4 tail were not required for the reduction of total CD4 levels elicited by Nef (Fig. 5D). Furthermore, Nef caused redistribution of the CD4-4K/R mutant to cytoplasmic vesicles that colocalized with endogenous HRS, as observed by immunofluorescence microscopy (Fig. 6A to C). Finally, immunoelectron microscopy revealed the presence of downregulated CD4-4K/R within MVBs, including intraluminal vesicles (Fig. 6D to F). Taken together, these results indicated that ubiquitination of CD4 on lysine residues is dispensable for both its internalization and targeting to the MVB pathway induced by Nef.
FIG. 6.
CD4 ubiquitination is not required for delivery to MVBs. (A to C) HeLa cells transfected with pCMV-CD4-4K/R mutant and pCIneo-Nef-wt were fixed, permeabilized, and double labeled with mouse monoclonal antibody to CD4 and rabbit polyclonal antibody to HRS, followed by incubation with secondary antibodies as described in the legends of Fig. 2D to F. Cells were imaged by confocal laser scanning microscopy. Cell outlines are indicated by dashed lines. The insets show the boxed areas at a magnification of ×3. Bar, 10 μm. (D to F) HeLa cells were transfected with the above indicated plasmids encoding CD4-4K/R and Nef-wt and, 16 h later, were fixed and prepared for immunoelectron microscopy as described in Materials and Methods. Higher magnifications of MVBs labeled for CD4 are shown in panels E and F. Bars, 0.5 μm (D) and 0.2 μm (E and F).
Nef ubiquitination on lysine residues is also dispensable for CD4 downregulation.
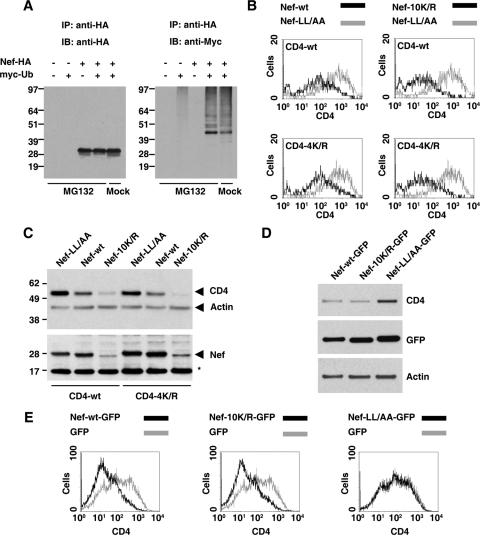
Since Nef binds to the tail of CD4, we thought that perhaps Nef itself became ubiquitinated, thus conferring on CD4 the ability to be sorted in a Ub-dependent manner. To investigate this possibility, we coexpressed HA-tagged Nef with myc-tagged Ub in HeLa cells, incubated cells in the absence or presence of the proteasomal inhibitor MG132, and then examined Nef ubiquitination by immunoprecipitation with anti-HA and immunoblotting with anti-myc. Using this protocol, we observed a ladder of ubiquitinated Nef species, of which monoubiquitinated Nef was by far the most prominent, particularly in MG132-treated cells (Fig. 7A, right panel). Several negative controls confirmed the specificity of ubiquitinated Nef detection (Fig. 7A). Mutation of all 10 lysines in Nef to arginine resulted in a construct (Nef-10K/R) that was expressed at much lower levels in the cells, perhaps due to conformational instability (Fig. 7C). Even on long exposures, however, this mutant was found not to be detectably ubiquitinated (data not shown). Strikingly, despite its lower expression levels, this Nef mutant downregulated both wild-type CD4 and the CD4-4K/R mutant from the cell surface, as determined by FACS analysis (Fig. 7B). Moreover, the Nef-10K/R mutant was active in decreasing the total levels of both wild-type CD4 and CD4-4K/R, as assessed by immunoblot analysis (Fig. 7C). Nef-10K/R, like wild-type Nef, also redistributed CD4 from the cell surface to intracellular vesicles (see Fig. S2 in the supplemental material). Thus, both the removal of CD4 from the cell surface and its targeting to degradation by the MVB pathway in HeLa cells are independent of lysine ubiquitination of not only CD4 but also Nef.
FIG. 7.
Nef ubiquitination is not required for CD4 downregulation in HeLa or T cells. (A) HeLa cells were transfected with pCIneo-based plasmids encoding the proteins shown on top. Cells were incubated for 16 h and then for an additional 6 h in either the presence or absence of 10 μM MG132, as indicated below the lanes. After incubation, equivalent amounts of cell lysates were subjected to immunoprecipitation with antibody to HA. The resulting immunoprecipitates were analyzed by SDS-PAGE followed by immunoblotting with antibodies to HA (left panel) or myc (right panel). (B) FACS analysis of HeLa cells transfected with pCMV-CD4 (CD4-wt or CD4-4K/R) and pNef.IRES.GFP (Nef-wt, Nef-LL/AA, or Nef-10K/R). After 16 h, cells were analyzed for surface CD4 by FACS, as described in the Materials and Methods section. (C) HeLa cells were transfected with pCMV-CD4 (CD4-wt or CD4-4K/R) and pCIneo-Nef (Nef-wt, Nef-LL/AA, or Nef-10K/R). After 16 h, equivalent amounts of cell lysates were subjected to SDS-PAGE and immunoblotting with antibodies to CD4, Nef, and actin (loading control). A nonspecific band detected by the Nef antibody is indicated by an asterisk. Molecular mass (in kDa) markers are indicated on the left. (D) Human JM T cells endogenously expressing CD4 were transfected with pNef-GFP (Nef-wt-GFP, Nef-LL/AA-GFP, or Nef-10K/R-GFP). After 16 h, equivalent amounts of cell lysates were subjected to SDS-PAGE and immunoblotting with antibodies to CD4, GFP, and actin (loading control). (E) JM T-cells were transfected with pGFP-based plasmids encoding either GFP or one of the Nef-GFP fusion proteins indicated above each panel. After 16 h, cells were stained with APC-conjugated antibody to CD4 and analyzed by FACS as described in Materials and Methods.
Lysine ubiquitination-independent downregulation of CD4 in T cells.
Although it is well established that Nef can downregulate CD4 in a wide variety of cell types, we sought to confirm our key findings in a more natural host cell type, human JM T cells, which express CD4 endogenously. These analyses were performed using Nef plasmids that were tagged at the C terminus with GFP because we found that this form of Nef-10K/R was expressed at levels similar to those of wild-type Nef in T cells (Fig. 7D). FACS analyses showed that Nef-10K/R-GFP downregulated endogenous CD4 from the surface of JM cells just as efficiently as Nef-GFP (Fig. 7E). This was in contrast to Nef-LL/AA-GFP that, as expected, did not decrease surface CD4 levels (Fig. 7E). Similar results were obtained with human A3.01 T cells (see Fig. S3 in the supplemental material). Nef-10K/R-GFP also decreased total CD4 levels in JM cells to the same extent as wild-type Nef (Fig. 7D). These results indicated that the lysine ubiquitination independence of Nef-induced CD4 downregulation is not limited to HeLa cells but can also be observed in T cells.
DISCUSSION
The results of our experiments indicate that the downregulation of CD4 by Nef involves two sequential processes. One is the well-known acceleration of CD4 internalization (2, 64) through an AP-2/clathrin pathway (17, 25, 45). This process depends on a dileucine motif in the CD4 tail (2, 31, 37), as well as a dileucine motif (14, 17, 22, 25, 32) and a diacidic motif (3, 51) in Nef. The other process, demonstrated here, is the targeting of internalized CD4 to the MVB pathway for eventual degradation in lysosomes. This second process prevents retrieval of internalized CD4 to the plasma membrane, thereby ensuring sustained attenuation of CD4 expression at the cell surface.
As is the case for most other transmembrane proteins that are targeted to the MVB pathway (40, 46), CD4 targeting depends on the ESCRT machinery. Interference with this machinery does not prevent endocytic removal of CD4 from the cell surface but blocks its subsequent delivery to lysosomes (Fig. 3 and 4). This results in accumulation of CD4 within aberrantly enlarged endosomes, from where recycling to the cell surface is apparently impeded (Fig. 3 and 4). Targeting of most cargoes to the MVB pathway is mediated by ubiquitination, which functions as a signal for recognition by components of the ESCRT machinery (40, 46). We found that CD4 is indeed multiply ubiquitinated on lysine residues at steady state, but the extent of this modification is independent of the presence of Nef (Fig. 5). This is in contrast to the effect of the HIV-1 Vpu protein, which enhances ubiquitination and degradation of CD4 through recruitment of the β-TrCP Ub ligase (9, 56). It also differs from the mechanism of the myxomavirus M153R protein, which itself is a Ub ligase that ubiquitinates CD4 and induces its destruction in lysosomes (54). Our observations thus make it unlikely that Nef acts like these proteins to promote CD4 ubiquitination. We also found that mutation of the four lysine residues in the CD4 tail, which abolished all detectable ubiquitination, does not preclude its Nef-induced targeting to the MVB pathway (Fig. 5 and 6). Thus, downregulated CD4 might be another example of a minority of cargoes such as the delta opioid receptor (36) and the interleukin-2 receptor β chain (79) in mammals and Sna3p (58, 60, 63, 78) and Ath1p (38) in yeast, which are sorted to the MVB pathway in an ESCRT-dependent but apparently ubiquitination-independent manner.
Does CD4 lysine ubiquitination then have any physiological role? We have found that mutation of the four lysine residues in the CD4 tail decreases both the basal and phorbol ester-induced (1) rates of CD4 turnover (L. daSilva, K. Janvier, and J. Bonifacino, unpublished observations), implying that these processes do follow the canonical MVB pathway. We surmise that expression of Nef overrides this mechanism by accelerating the turnover and making it independent of lysine ubiquitination.
We considered the possibility that Nef itself could be ubiquitinated and, through binding to the CD4 tail, could confer in trans the ability for CD4 to be sorted in a Ub-dependent manner. We found that Nef is indeed ubiquitinated on lysine residues (Fig. 7), but, strikingly, this modification is also dispensable for CD4 removal from the cell surface and targeting to the MVB pathway (Fig. 7). The independence of CD4 downregulation on lysine ubiquitination of Nef demonstrated here is at variance with the results of a study published while our manuscript was in preparation (44). This study showed that ubiquitination of Nef, particularly at lysine-144, is required for CD4 downregulation from the cell surface in the murine T-cell hybridoma BYCD4. The reasons for this discrepancy are not apparent as we find that none of the 10 lysine residues in Nef, including lysine-144, is required for downregulation of cell surface CD4 in various human nonlymphoid and T cells (Fig. 7; see also Fig. S2 and S3 in the supplemental material).
It remains to be determined how Nef can target CD4 to the MVB pathway without lysine ubiquitination in either cis or trans. One possibility is that either Nef or CD4 undergoes transient ubiquitination on residues other than lysine (e.g., cysteine, serine, threonine, or the protein's N terminus), as previously shown for the ubiquitination-dependent downregulation of major histocompatibility class I (MHC-I) molecules by K3 Ub ligases encoded by some herpes viruses (16, 77). Nef might also be able to interact with the ESCRT machinery in a Ub-independent manner, as it does with AP-2 at the plasma membrane (17, 18, 25, 51). In this regard, Nef has been shown to bind directly to the ESCRT protein AIP1/Alix (21). This interaction was found to promote proliferation of MVBs and budding of viral particles from infected cells (21), but we speculate that it might also mediate targeting of CD4 to the MVB pathway. In agreement with a role for Nef in endosomes, Nef-GFP localizes to not only the plasma membrane but also a population of cytoplasmic vesicles that contain downregulated CD4 (see Fig. S4A to C in the supplemental material) and other endosomal markers (data not shown).
In addition to AP-2, three other structurally related complexes, AP-1 (14, 20, 25, 32, 41, 43, 51, 66), AP-3 (20, 41, 43, 51), and COPI (7, 42, 61), have been shown to interact with Nef. The interaction of Nef with COPI, in particular, has been correlated with lysosomal targeting of CD4 (61, 69) although these findings have been contested (42). In any event, none of these complexes is known to interact with the ESCRT machinery, so it is unclear what role, if any, they might play in targeting CD4 to the MVB pathway. Nef could also exert its effects on CD4 indirectly through activation of a signal transduction pathway. Such a mechanism, involving a kinase cascade, has been postulated to mediate the downregulation of MHC-I molecules by Nef (5, 11, 39) as an alternative to the interaction of Nef with AP-1 (53, 66). In addition to CD4 and MHC-I, Nef downregulates other cell surface proteins, including CD8 (72), CD28 (73), CXCR4 (76), and CCR5 (26). It will now be of interest to determine whether Nef also targets these proteins to the MVB pathway and whether the ESCRT machinery and ubiquitination are involved in this targeting.
Supplementary Material
Acknowledgments
We thank C. Haft, S. Urbé, P. Woodman, and the NIH AIDS Research and Reference Reagent Program for kind gifts of reagents; X. Zhu and N. Tsai for excellent technical assistance; W. Sundquist for helpful discussions; and R. Chaudhuri and J. Magadán for critical review of the manuscript.
This work was supported by the NIH Intramural AIDS Targeted Antiviral Program and the Intramural Program of the National Institute of Child Health and Human Development.
Footnotes
Published ahead of print on 29 April 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Acres, R. B., P. J. Conlon, D. Y. Mochizuki, and B. Gallis. 1986. Rapid phosphorylation and modulation of the T4 antigen on cloned helper T cells induced by phorbol myristate acetate or antigen. J. Biol. Chem. 26116210-16214. [PubMed] [Google Scholar]
- 2.Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76853-864. [DOI] [PubMed] [Google Scholar]
- 3.Aiken, C., L. Krause, Y. L. Chen, and D. Trono. 1996. Mutational analysis of HIV-1 Nef: identification of two mutants that are temperature-sensitive for CD4 downregulation. Virology 217293-300. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, S. J., M. Lenburg, N. R. Landau, and J. V. Garcia. 1994. The cytoplasmic domain of CD4 is sufficient for its down-regulation from the cell surface by human immunodeficiency virus type 1 Nef. J. Virol. 683092-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkins, K. M., L. Thomas, R. T. Youker, M. J. Harriff, F. Pissani, H. You, and G. Thomas. 2008. HIV-1 Nef binds PACS-2 to assemble a multikinase cascade that triggers major histocompatibility complex class I (MHC-I) down-regulation: analysis using short interfering RNA and knock-out mice. J. Biol. Chem. 28311772-11784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babst, M., B. Wendland, E. J. Estepa, and S. D. Emr. 1998. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 172982-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benichou, S., M. Bomsel, M. Bodeus, H. Durand, M. Doute, F. Letourneur, J. Camonis, and R. Benarous. 1994. Physical interaction of the HIV-1 Nef protein with beta-COP, a component of non-clathrin-coated vesicles essential for membrane traffic. J. Biol. Chem. 26930073-30076. [PubMed] [Google Scholar]
- 8.Benson, R. E., A. Sanfridson, J. S. Ottinger, C. Doyle, and B. R. Cullen. 1993. Downregulation of cell-surface CD4 expression by simian immunodeficiency virus Nef prevents viral super infection. J. Exp. Med. 1771561-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binette, J., M. Dube, J. Mercier, D. Halawani, M. Latterich, and E. A. Cohen. 2007. Requirements for the selective degradation of CD4 receptor molecules by the human immunodeficiency virus type 1 Vpu protein in the endoplasmic reticulum. Retrovirology 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bishop, N., and P. Woodman. 2000. ATPase-defective mammalian VPS4 localizes to aberrant endosomes and impairs cholesterol trafficking. Mol. Biol. Cell 11227-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blagoveshchenskaya, A. D., L. Thomas, S. F. Feliciangeli, C. H. Hung, and G. Thomas. 2002. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell 111853-866. [DOI] [PubMed] [Google Scholar]
- 12.Bonifacino, J. S. 1998. Protein labeling and immunoprecipitation. In J. S. Bonifacino, M. Dasso, J. B. Harford, J. Lippincott-Schwartz, and K. Yamada (ed.), Current protocols in cell biology. John Wiley & Sons, New York, NY.
- 13.Bonifacino, J. S., and J. H. Hurley. 2008. Retromer. Curr. Opin. Cell Biol. 20427-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bresnahan, P. A., W. Yonemoto, S. Ferrell, D. Williams-Herman, R. Geleziunas, and W. C. Greene. 1998. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr. Biol. 81235-1238. [DOI] [PubMed] [Google Scholar]
- 15.Burtey, A., J. Z. Rappoport, J. Bouchet, S. Basmaciogullari, J. Guatelli, S. M. Simon, S. Benichou, and A. Benmerah. 2007. Dynamic interaction of HIV-1 Nef with the clathrin-mediated endocytic pathway at the plasma membrane. Traffic 861-76. [DOI] [PubMed] [Google Scholar]
- 16.Cadwell, K., and L. Coscoy. 2005. Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science 309127-130. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhuri, R., O. W. Lindwasser, W. J. Smith, J. H. Hurley, and J. S. Bonifacino. 2007. Downregulation of CD4 by human immunodeficiency virus type 1 Nef is dependent on clathrin and involves direct interaction of Nef with the AP2 clathrin adaptor. J. Virol. 813877-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaudhuri, R., R. Mattera, O. W. Lindwasser, M. S. Robinson, and J. S. Bonifacino. 2009. A basic patch on α-adaptin is required for binding of human immunodeficiency virus type 1 Nef and cooperative assembly of a CD4-Nef-AP-2 complex. J. Virol. 832518-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chowers, M. Y., C. A. Spina, T. J. Kwoh, N. J. Fitch, D. D. Richman, and J. C. Guatelli. 1994. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J. Virol. 682906-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coleman, S. H., N. Van Damme, J. R. Day, C. M. Noviello, D. Hitchin, R. Madrid, S. Benichou, and J. C. Guatelli. 2005. Leucine-specific, functional interactions between human immunodeficiency virus type 1 Nef and adaptor protein complexes. J. Virol. 792066-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa, L. J., N. Chen, A. Lopes, R. S. Aguiar, A. Tanuri, A. Plemenitas, and B. M. Peterlin. 2006. Interactions between Nef and AIP1 proliferate multivesicular bodies and facilitate egress of HIV-1. Retrovirology 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craig, H. M., M. W. Pandori, and J. C. Guatelli. 1998. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc. Natl. Acad. Sci. USA 9511229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, V. A. Lawson, S. Crowe, A. Maerz, S. Sonza, J. Learmont, J. S. Sullivan, A. Cunningham, D. Dwyer, D. Dowton, and J. Mills. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270988-991. [DOI] [PubMed] [Google Scholar]
- 24.de Ronde, A., B. Klaver, W. Keulen, L. Smit, and J. Goudsmit. 1992. Natural HIV-1 NEF accelerates virus replication in primary human lymphocytes. Virology 188391-395. [DOI] [PubMed] [Google Scholar]
- 25.Doray, B., I. Lee, J. Knisely, G. Bu, and S. Kornfeld. 2007. The γ/σ1 and α/σ2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol. Biol. Cell 181887-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubey, S., M. Khalid, C. Wesley, S. A. Khan, A. Wanchu, and S. Jameel. 2008. Downregulation of CCR5 on activated CD4 T cells in HIV-infected Indians. J. Clin. Virol. 4325-31. [DOI] [PubMed] [Google Scholar]
- 27.Foster, J. L., and J. V. Garcia. 2008. HIV-1 Nef: at the crossroads. Retrovirology 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia, J. V., and A. D. Miller. 1991. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 350508-511. [DOI] [PubMed] [Google Scholar]
- 29.Geyer, M., O. T. Fackler, and B. M. Peterlin. 2001. Structure-function relationships in HIV-1 Nef. EMBO Rep. 2580-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glushakova, S., J. Munch, S. Carl, T. C. Greenough, J. L. Sullivan, L. Margolis, and F. Kirchhoff. 2001. CD4 down-modulation by human immunodeficiency virus type 1 Nef correlates with the efficiency of viral replication and with CD4+ T-cell depletion in human lymphoid tissue ex vivo. J. Virol. 7510113-10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gratton, S., X. J. Yao, S. Venkatesan, E. A. Cohen, and R. P. Sekaly. 1996. Molecular analysis of the cytoplasmic domain of CD4: overlapping but noncompetitive requirement for lck association and down-regulation by Nef. J. Immunol. 1573305-3311. [PubMed] [Google Scholar]
- 32.Greenberg, M., L. DeTulleo, I. Rapoport, J. Skowronski, and T. Kirchhausen. 1998. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr. Biol. 81239-1242. [DOI] [PubMed] [Google Scholar]
- 33.Greenberg, M. E., S. Bronson, M. Lock, M. Neumann, G. N. Pavlakis, and J. Skowronski. 1997. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 166964-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grzesiek, S., S. J. Stahl, P. T. Wingfield, and A. Bax. 1996. The CD4 determinant for downregulation by HIV-1 Nef directly binds to Nef. Mapping of the Nef binding surface by NMR. Biochemistry 3510256-10261. [DOI] [PubMed] [Google Scholar]
- 35.Guy, B., M. P. Kieny, Y. Riviere, C. Le Peuch, K. Dott, M. Girard, L. Montagnier, and J. P. Lecocq. 1987. HIV F/3′ orf encodes a phosphorylated GTP-binding protein resembling an oncogene product. Nature 330266-269. [DOI] [PubMed] [Google Scholar]
- 36.Hislop, J. N., A. Marley, and M. Von Zastrow. 2004. Role of mammalian vacuolar protein-sorting proteins in endocytic trafficking of a non-ubiquitinated G protein-coupled receptor to lysosomes. J. Biol. Chem. 27922522-22531. [DOI] [PubMed] [Google Scholar]
- 37.Hua, J., W. Blair, R. Truant, and B. R. Cullen. 1997. Identification of regions in HIV-1 Nef required for efficient downregulation of cell surface CD4. Virology 231231-238. [DOI] [PubMed] [Google Scholar]
- 38.Huang, J., F. Reggiori, and D. J. Klionsky. 2007. The transmembrane domain of acid trehalase mediates ubiquitin-independent multivesicular body pathway sorting. Mol. Biol. Cell 182511-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hung, C. H., L. Thomas, C. E. Ruby, K. M. Atkins, N. P. Morris, Z. A. Knight, I. Scholz, E. Barklis, A. D. Weinberg, K. M. Shokat, and G. Thomas. 2007. HIV-1 Nef assembles a Src family kinase-ZAP-70/Syk-PI3K cascade to downregulate cell-surface MHC-I. Cell Host Microbe 1121-133. [DOI] [PubMed] [Google Scholar]
- 40.Hurley, J. H. 2008. ESCRT complexes and the biogenesis of multivesicular bodies. Curr. Opin. Cell Biol. 204-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janvier, K., H. Craig, D. Hitchin, R. Madrid, N. Sol-Foulon, L. Renault, J. Cherfils, D. Cassel, S. Benichou, and J. Guatelli. 2003. HIV-1 Nef stabilizes the association of adaptor protein complexes with membranes. J. Biol. Chem. 2788725-8732. [DOI] [PubMed] [Google Scholar]
- 42.Janvier, K., H. Craig, S. Le Gall, R. Benarous, J. Guatelli, O. Schwartz, and S. Benichou. 2001. Nef-induced CD4 downregulation: a diacidic sequence in human immunodeficiency virus type 1 Nef does not function as a protein sorting motif through direct binding to β-COP. J. Virol. 753971-3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janvier, K., Y. Kato, M. Boehm, J. R. Rose, J. A. Martina, B. Y. Kim, S. Venkatesan, and J. S. Bonifacino. 2003. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 γ-σ1 and AP-3 δ-σ3 hemicomplexes. J. Cell Biol. 1631281-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin, Y. J., C. Y. Cai, X. Zhang, and S. J. Burakoff. 2008. Lysine 144, a ubiquitin attachment site in HIV-1 Nef, is required for Nef-mediated CD4 down-regulation. J. Immunol. 1807878-7886. [DOI] [PubMed] [Google Scholar]
- 45.Jin, Y. J., C. Y. Cai, X. Zhang, H. T. Zhang, J. A. Hirst, and S. J. Burakoff. 2005. HIV Nef-mediated CD4 down-regulation is adaptor protein complex 2 dependent. J. Immunol. 1753157-3164. [DOI] [PubMed] [Google Scholar]
- 46.Katzmann, D. J., G. Odorizzi, and S. D. Emr. 2002. Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol. 3893-905. [DOI] [PubMed] [Google Scholar]
- 47.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65651-662. [DOI] [PubMed] [Google Scholar]
- 48.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332228-232. [DOI] [PubMed] [Google Scholar]
- 49.Lama, J., A. Mangasarian, and D. Trono. 1999. Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr. Biol. 9622-631. [DOI] [PubMed] [Google Scholar]
- 50.Lindwasser, O. W., R. Chaudhuri, and J. S. Bonifacino. 2007. Mechanisms of CD4 downregulation by the Nef and Vpu proteins of primate immunodeficiency viruses. Curr. Mol. Med. 7171-184. [DOI] [PubMed] [Google Scholar]
- 51.Lindwasser, O. W., W. J. Smith, R. Chaudhuri, P. Yang, J. H. Hurley, and J. S. Bonifacino. 2008. A diacidic motif in human immunodeficiency virus type 1 Nef is a novel determinant of binding to AP-2. J. Virol. 821166-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Little, S. J., N. L. Riggs, M. Y. Chowers, N. J. Fitch, D. D. Richman, C. A. Spina, and J. C. Guatelli. 1994. Cell surface CD4 downregulation and resistance to superinfection induced by a defective provirus of HIV-1. Virology 205578-582. [DOI] [PubMed] [Google Scholar]
- 53.Lubben, N. B., D. A. Sahlender, A. M. Motley, P. J. Lehner, P. Benaroch, and M. S. Robinson. 2007. HIV-1 Nef-induced down-regulation of MHC class I requires AP-1 and clathrin but not PACS-1 and is impeded by AP-2. Mol. Biol. Cell 183351-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mansouri, M., E. Bartee, K. Gouveia, B. T. Hovey Nerenberg, J. Barrett, L. Thomas, G. Thomas, G. McFadden, and K. Fruh. 2003. The PHD/LAP-domain protein M153R of myxomavirus is a ubiquitin ligase that induces the rapid internalization and lysosomal destruction of CD4. J. Virol. 771427-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mardones, G. A., P. V. Burgos, D. A. Brooks, E. Parkinson-Lawrence, R. Mattera, and J. S. Bonifacino. 2007. The trans-Golgi network accessory protein p56 promotes long-range movement of GGA/clathrin-containing transport carriers and lysosomal enzyme sorting. Mol. Biol. Cell 183486-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Margottin, F., S. P. Bour, H. Durand, L. Selig, S. Benichou, V. Richard, D. Thomas, K. Strebel, and R. Benarous. 1998. A novel human WD protein, h-βTrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol. Cell 1565-574. [DOI] [PubMed] [Google Scholar]
- 57.Mariani, R., and J. Skowronski. 1993. CD4 down-regulation by nef alleles isolated from human immunodeficiency virus type 1-infected individuals. Proc. Natl. Acad. Sci. USA 905549-5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McNatt, M. W., I. McKittrick, M. West, and G. Odorizzi. 2007. Direct binding to Rsp5 mediates ubiquitin-independent sorting of Sna3 via the multivesicular body pathway. Mol. Biol. Cell 18697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller, M. D., M. T. Warmerdam, I. Gaston, W. C. Greene, and M. B. Feinberg. 1994. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J. Exp. Med. 179101-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oestreich, A. J., M. Aboian, J. Lee, I. Azmi, J. Payne, R. Issaka, B. A. Davies, and D. J. Katzmann. 2007. Characterization of multiple multivesicular body sorting determinants within Sna3: a role for the ubiquitin ligase Rsp5. Mol. Biol. Cell 18707-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piguet, V., F. Gu, M. Foti, N. Demaurex, J. Gruenberg, J. L. Carpentier, and D. Trono. 1999. Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of β-COP in endosomes. Cell 9763-73. [DOI] [PubMed] [Google Scholar]
- 62.Ray, N., and R. W. Doms. 2006. HIV-1 coreceptors and their inhibitors. Curr. Top. Microbiol. Immunol. 30397-120. [DOI] [PubMed] [Google Scholar]
- 63.Reggiori, F., and H. R. Pelham. 2001. Sorting of proteins into multivesicular bodies: ubiquitin-dependent and -independent targeting. EMBO J. 205176-5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rhee, S. S., and J. W. Marsh. 1994. Human immunodeficiency virus type 1 Nef-induced down-modulation of CD4 is due to rapid internalization and degradation of surface CD4. J. Virol. 685156-5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roeth, J. F., and K. L. Collins. 2006. Human immunodeficiency virus type 1 Nef: adapting to intracellular trafficking pathways. Microbiol. Mol. Biol. Rev. 70548-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roeth, J. F., M. Williams, M. R. Kasper, T. M. Filzen, and K. L. Collins. 2004. HIV-1 Nef disrupts MHC-I trafficking by recruiting AP-1 to the MHC-I cytoplasmic tail. J. Cell Biol. 167903-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rossi, F., A. Gallina, and G. Milanesi. 1996. Nef-CD4 physical interaction sensed with the yeast two-hybrid system. Virology 217397-403. [DOI] [PubMed] [Google Scholar]
- 68.Sanfridson, A., B. R. Cullen, and C. Doyle. 1994. The simian immunodeficiency virus Nef protein promotes degradation of CD4 in human T cells. J. Biol. Chem. 2693917-3920. [PubMed] [Google Scholar]
- 69.Schaefer, M. R., E. R. Wonderlich, J. F. Roeth, J. A. Leonard, and K. L. Collins. 2008. HIV-1 Nef targets MHC-I and CD4 for degradation via a final common β-COP-dependent pathway in T cells. PLoS Pathog. 4e1000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shugars, D. C., M. S. Smith, D. H. Glueck, P. V. Nantermet, F. Seillier-Moiseiwitsch, and R. Swanstrom. 1993. Analysis of human immunodeficiency virus type 1 nef gene sequences present in vivo. J. Virol. 674639-4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stoddart, C. A., R. Geleziunas, S. Ferrell, V. Linquist-Stepps, M. E. Moreno, C. Bare, W. Xu, W. Yonemoto, P. A. Bresnahan, J. M. McCune, and W. C. Greene. 2003. Human immunodeficiency virus type 1 Nef-mediated downregulation of CD4 correlates with Nef enhancement of viral pathogenesis. J. Virol. 772124-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stove, V., I. Van de Walle, E. Naessens, E. Coene, C. Stove, J. Plum, and B. Verhasselt. 2005. Human immunodeficiency virus Nef induces rapid internalization of the T-cell coreceptor CD8αβ. J. Virol. 7911422-11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Swigut, T., N. Shohdy, and J. Skowronski. 2001. Mechanism for down-regulation of CD28 by Nef. EMBO J. 201593-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Terwilliger, E. F., E. Langhoff, D. Gabuzda, E. Zazopoulos, and W. A. Haseltine. 1991. Allelic variation in the effects of the nef gene on replication of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 8810971-10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Traub, L. M. 2005. Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim. Biophys. Acta 1744415-437. [DOI] [PubMed] [Google Scholar]
- 76.Venzke, S., N. Michel, I. Allespach, O. T. Fackler, and O. T. Keppler. 2006. Expression of Nef downregulates CXCR4, the major coreceptor of human immunodeficiency virus, from the surfaces of target cells and thereby enhances resistance to superinfection. J. Virol. 8011141-11152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang, X., R. Herr, W. Chua, L. Lybarger, E. Wiertz, and T. Hansen. 2007. Ubiquitination of serine, threonine, or lysine residues on the cytoplasmic tail can induce ERAD of MHC-I by viral E3 ligase mK3. J. Cell Biol. 177613-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Watson, H., and J. S. Bonifacino. 2007. Direct binding to Rsp5p regulates ubiquitination-independent vacuolar transport of Sna3p. Mol. Biol. Cell 181781-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamashita, Y., K. Kojima, T. Tsukahara, H. Agawa, K. Yamada, Y. Amano, N. Kurotori, N. Tanaka, K. Sugamura, and T. Takeshita. 2008. Ubiquitin-independent binding of Hrs mediates endosomal sorting of the interleukin-2 receptor beta-chain. J. Cell Sci. 1211727-1738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.