Abstract
Foot-and-mouth disease virus (FMDV) produces one of the most infectious of all livestock diseases, causing extensive economic loss in areas of breakout. Like other viral pathogens, FMDV recruits proteins encoded by host cell genes to accomplish the entry, replication, and release of infectious viral particles. To identify such host-encoded proteins, we employed an antisense RNA strategy and a lentivirus-based library containing approximately 40,000 human expressed sequence tags (ESTs) to randomly inactivate chromosomal genes in a bovine kidney cell line (LF-BK) that is highly susceptible to FMDV infection and then isolated clones that survived multiple rounds of exposure to the virus. Here, we report the identification of ESTs whose expression in antisense orientation limited host cell killing by FMDV and restricted viral propagation. The role of one such EST, that of ectonucleoside triphosphate diphosphohydrolase 6 (NTPDase6; also known as CD39L2), a membrane-associated ectonucleoside triphosphate diphosphohydrolase that previously was not suspected of involvement in the propagation of viral pathogens and which we now show is required for normal synthesis of FMDV RNA and proteins, is described in this report.
Foot-and-mouth disease is a highly contagious viral disease of cloven-hoofed livestock. Its causative agent, foot-and-mouth disease virus (FMDV), is a member of the Picornaviridae family and contains an unpaired positive-sense-strand RNA genome of about 8,300 nucleotides (7, 8, 16, 33). Although FMDV vaccines are available for livestock, the virus is antigenically variable, and there is no cross-protection among the seven known serotypes and only limited protection between the numerous subtypes that have evolved (for a review, see reference 42).
The RNA contained in FMDV particles encodes the viral proteins in addition to serving as a template for the synthesis of a complementary full-length negative RNA strand that in turn serves as a template for the synthesis of new viral genomes. Notwithstanding the simplicity of the mechanisms of genome replication and expression used by picornaviruses, knowledge about the functions of cellular genes affecting viral replication and propagation is limited. A small number of cellular genes—mostly involved in host immunity—are known to be activated by FMDV infection (4, 58). Additionally, genes encoding proteins that carry out normal host cell functions can be recruited to facilitate the entry or release of infecting viruses (for example, see reference 17, a recent review), and cell surface proteins that function as receptors for FMDV have been identified (5, 15, 26, 40). However, a systematic approach aimed at the direct discovery of cellular genes required for viral propagation, i.e., cellular genes exploited by pathogens, has not previously been applied to FMDV.
Several methods that enable homozygous functional inactivation of chromosomal genes in mammalian cells have been reported (6, 30, 34, 35, 43). In general, these strategies use RNA complementary to genomically encoded transcripts (antisense RNA [35] or small interfering RNA [31]) to interfere randomly with chromosomal gene expression. We employed one such gene inactivation strategy, which uses RNA encoded by a library of ∼40,000 expressed sequence tags (ESTs) (35) to functionally inactivate cellular genes in LF-BK cells, and identified cell clones that limit the propagation of FMDV.
MATERIALS AND METHODS
Cell culture and virus.
Bovine kidney (LF-BK) cells and resistant derivative cells were maintained in Dulbecco's modified Eagle medium (Gibco) supplemented with 10% fetal bovine serum. LF-BK cells were originally obtained from the Foreign Animal Disease Diagnostic Laboratory at the Plum Island Animal Disease Center (4, 54). The FMDV strain O/United Kingdom/2001 (O/UK/2001), porcine enterovirus, swine vesicular disease virus, and encephalomyocarditis virus were provided by Fred Brown. Procedures for infections and plaque assay with FMDV were described previously (12). The number of cells and multiplicity of infection (MOI) were calculated in each experiment.
Construction of LF-BK EST library.
A library of LF-BK cells expressing ESTs was generated as previously described (35). Briefly, an LF-BK cell line containing a tetracycline-activated promoter was made by infection of LF-BK cells with a retrovirus carrying pBabetTaPuro, in which the tetracycline-dependent transcriptional activator (tTA) is under the control of the cytomegalovirus (CMV) promoter (i.e., LF-BK EST tTA cells). The infected cells were then subjected to selection for growth in the presence of puromycin (2 μg/ml Sigma) for 2 to 3 weeks. The tTA activity of puromycin-resistant cell clones was tested by using a luciferase reporter gene under the control of a promoter activated by a Tet response element (21). A cell clone that showed high-level luciferase activity and at least a 50-fold induction of this activity after tetracycline treatment was selected, and a culture of these cells containing a collection of approximately 40,000 human ESTs under the control of the Tet response element-linked CMV promoter was infected with lentivirus (pLEST) (35). LF-BK EST tTA cells were selected by growth in the presence of 1 mg/ml of G418 (Gibco) for 3 to 4 weeks.
FMDV growth assay.
Cell monolayers were infected with FMDV O/UK/2001 at an MOI of 10 or 0.1. After 1 h, the cells were treated with wash buffer (150 mM NaCl and 20 mM morpholineethanesulfonic acid, pH 6.0) to inactivate unabsorbed viruses and then incubated in Dulbecco's modified Eagle medium at 37°C. Supernatant samples were collected at intervals and titrated on BHK-21 cells. The 50% tissue culture infective doses were determined as described previously (44).
Infectious-center assay.
Wild-type or mutant LF-BK tTA cells were infected with FMDV O/UK/2001 at an MOI of 10. After 1 h of adsorption, the cells were trypsinized and washed once with growth medium, once with wash buffer to inactivate residual virus, and once more with growth medium. These cells were diluted 10-fold, mixed with 6 × 105 LF-BK cells per sample, and seeded into six-well plates. At 48 h postinfection (hpi), plates were fixed and stained. The percentage of infected cells was determined from the number of plaques obtained in the plaque assay relative to the cell number before infection.
RNA extraction and PCR amplification of viral RNA (vRNA).
Cytoplasmic RNA was extracted from cells according to the RNeasy protocol (Qiagen). Reverse transcriptions were done with SuperScript II reverse transcriptase (Invitrogen) with random hexamers. PCR amplification of FMDV RNA was carried out using the antisense 5′TCAGGGTTGCAACCGACCGC3′ and sense 5′TTCGAGAACGGCACGGTCGG3′ primers corresponding to genomic positions 3940 to 3959 and 3762 to 3781, respectively, of FMDV O1 Campos/Bra/58 (GenBank accession number AJ320488) (G. A. König and M. E. Piccone, unpublished).
DNA extraction and PCR amplification of EST insert.
Genomic DNA was isolated from cells by the Red Extract-N-Amp tissue PCR kit (Sigma) following the manufacturer's instructions. Primers to amplify the EST insert were Lenti3′ (5′CATAGCGTAAAAGGAGCAACA3′), which is located 3′ to the EST inserts in the pLEST vector, and either ESTR_NheI (5′TCTGCTAGCCACACAGGAAACAGCTATG3′) or ESTF_NheI (5′TCTGCTAGCTTGTAAAACGACGGCCAGTG3′), depending on the orientation of the EST insertion into the pLEST vector. Use of these primers enabled rapid determination of the orientation of the ESTs and consequently indicated whether they were transcribed in the sense or antisense direction relative to the CMV promoter. The EST sequences and orientations were confirmed by DNA sequencing of the PCR products, and their corresponding genes were identified by BLAST searching (University of California—Santa Cruz Genome Bioinformatics).
Northern blot analysis.
LF-BK tTA and resistant cell clones were infected with FMDV O/UK/2001 for 1 h at 37°C. Cytoplasmic RNA was extracted at various times postinfection according to the RNeasy protocol (Qiagen). Equal amounts of total RNA were separated on a 1% denaturing formaldehyde gel, blotted onto a nylon membrane, and hybridized with a 32P-labeled antisense RNA corresponding to the 3D viral RNA polymerase region of the FMDV genome. The same blots were stripped and reprobed for the ß-actin gene as a loading control.
Protein overexpression, Western blot analysis, and immunofluorescence microscopy.
To overexpress ectonucleoside triphosphate diphosphohydrolase 6 (NTPDase6), NTPDase cDNA cut out from the pCMV Sport vector (a gift from Terence L. Kirley [23]) was inserted into pLentiCMVBSD for a selection marker. Cells transfected with the plasmid mentioned above were selected with 10 μg/ml blasticidin (Invitrogen) for 5 days. For Western blotting of NTPDase6 protein, cell lysates separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were transferred to a nitrocellulose membrane, and the protein was detected by polyclonal antibody (a gift from Terence L. Kirley [23]). For the detection of viral proteins, cells were infected at an MOI of 10, and at 4 hpi, mock-infected and infected cell lysates were fractionated on a 4 to 12% SDS-PAGE gel and blotted onto polyvinylidene difluoride membranes; Western blot analyses were performed using antiserum prepared against FMDV 3D polymerase (kindly provided by He Wang, APHIS) and visualized by chemiluminescence (Invitrogen). An antitubulin monoclonal antibody (MAb) was purchased from Lab Vision. Immunofluorescence was performed as described by O'Donnell et al. in 2001 (41). Briefly, LF-BK tTA or NTPDase6-expressing cell clones grown on coverslips (EMS) were infected as described above. At 4 hpi, cells were fixed, permeabilized, and immunostained using a pool of MAbs 10GA4.2.2 and 12FE9.2.1 (kindly provided by Marvin J. Grubman) against structural proteins of FMDV type O (51). A fluorescence-labeled goat anti-mouse immunoglobulin G was used as the secondary antibody (Molecular Probes). Cells were imaged in a Leica TCS SP2 confocal microscope.
RESULTS
Construction of the EST library in the LF-BK cell line and the isolation of FMDV-resistant cell clones.
A variety of cell lines previously have been used for FMDV infection, including BHK-21 (baby hamster kidney), LF-BK (bovine kidney) (4, 50), and IB-RS-2 (pig kidney) cells. However, as our experimental protocol employed a screen for cell clones that survive FMDV infection, and survival of cells independently of EST effects represents an undesirable background, we carried out an initial search for a cell line that consistently showed a survival frequency of less than 10−6 following infection by FMDV under the experimental conditions that we used. The LF-BK cell line was chosen for our experiments because of its high susceptibility to FMDV infection and the absence of surviving cells after four rounds of FMDV infection.
To isolate FMDV-resistant clones, 2 × 106 of both naïve LF-BK tTA cells and LF-BK tTA cells containing the EST library were seeded in a T25 flask and infected two hours later with FMDV O/UK/2001 at an MOI of 10. After 2 days, cells were placed in growth media and incubation was continued at 37°C. The culture media were changed every 2 days and monitored for virus-induced cytopathology. Surviving cells were recovered and challenged with FMDV again as described above. After four rounds of FMDV infection, the surviving cells were cloned and expanded for further study. No survivors were obtained from cultures of parental LF-BK cells uninfected with the pLEST-EST library.
About 180 surviving cell clones were isolated, and each was tested for the ability to produce FMDV in a virus plaque assay, which showed different extents of limitation of virus production among the different clones. The EST inserts in these clones were identified by sequencing genomic PCR-amplified DNA. Table 1 shows a list of these ESTs. Some clones contained the same EST, and these may be siblings. We noted that certain ESTs correspond to genes that have been implicated in other viral infections, e.g., the IRF7 (interferon-regulatory factor 7) (for recent reviews, see references 25 and 36) and SPP (signal peptide peptidase) (1, 22; for a review, see reference 38) genes.
TABLE 1.
ESTs isolated from the FMDV-resistant cell clones
| Genea | EST orientation | GenBank accession no. |
|---|---|---|
| Solute carrier family 36 | Sense | AA417270 |
| Amino acid transporter system A1 | AA740867 | |
| Homo sapiens RIMS binding protein 3B | AI014598 | |
| Homo sapiens BAC clone GS1-99H8 | AC004010 | |
| Density-regulated protein | Sense | AI131374 |
| Unknown EST; clone IMAGE:1845584 | AI219496 | |
| Phosphorylase kinase, beta isoform b | Sense | AI241470 |
| Forkhead box K2 | Antisense | AI953229 |
| Unknown EST | Sense | AV714462 |
| Zinc fingers and homeoboxes 2 | Sense | AV722233 |
| Hypothetical protein LOC284058 | Antisense | AW960454 |
| Unknown EST | Sense | AW968629 |
| Unknown EST | Sense | AW970795 |
| Unknown EST | Antisense | AW978415 |
| Histocompatibility (minor) 13, transcript variant 1 | Antisense | AA504780 |
| Hypothetical protein MGC26847 | Antisense | BC014601 |
| Cleavage- and polyadenylation-specific factor 6 | BE868574 | |
| Uncharacterized protein CHCHD1 | Antisense | BF791947 |
| Unknown EST; clone IMAGE:4824178 | Sense | BG720581 |
| Glutaredoxin 5 | AA677563 | |
| Homo sapiens zinc finger protein 10 | Antisense | N53840 |
| Angiotensinogen (erpin peptidase inhibitor, cladeA, member 8) | AI359985 | |
| Dopa decarboxylase | Antisense | AA702640 |
| NTPDase6 | Antisense | AA45523 |
| Interferon regulatory factor 7 transcript variant d | Antisense | AA877255 |
| Kinesin family member 5A | Sense | AA984728 |
| Myosin IXA | AI382029 | |
| Homo sapiens transmembrane protein 115 | NM_007024 | |
| ATPase, (Na+/K+) transporting, beta 4 polypeptide | NM_012069 | |
| Zinc finger protein | NM_014480 | |
| G-protein-coupled receptor 48 | NM_018490 | |
| Hypothetical protein FLJ21918 | NM_024939 | |
| Homo sapiens EH domain binding protein 1 | Antisense | R88919 |
The gene name refers to the GenBank entry. BAC, bacterial artificial chromosome.
Among the virus-resistant clones, one that contained the NTPDase6 EST insert showed especially prominent resistance to FMDV production. NTPDase6 belongs to a family of enzymes known to modulate a variety of important cellular responses by controlling extracellular nucleotide concentrations; these include blood clotting and neural signaling transmission (29, 55, 56; for a review, see reference 27). However, NTPDase6, unlike other members of the NTPDase family, exists as both a soluble form and a membrane-bound form, raising the possibility that this enzyme may be implicated in membrane rearrangement events that occur during normal FMDV replication (32, 37, 39). The cell clone containing an antisense NTPDase6 EST was further characterized.
Reconstitution of the FMDV resistance phenotype by antisense expression of an NTPDase6 EST.
In order to confirm that the FMDV resistance phenotype exhibited by the NTPDase6-expressing clone resulted from expression of an antisense EST vector rather than from spontaneous mutation in the virus-resistant cell clone or from the cis-acting effects of the EST insertion on adjacent chromosomal genes, independent LF-BK cell clones expressing the NTPDase6 EST in the antisense orientation were made by the same procedure as the one used in constructing the EST library by using a lentivirus vector contained just the NTPDase6 EST.
Approximately 30 reconstituted LF-BK tTA cell clones expressing the antisense RNA of NTPDase6 EST had an altered FMDV resistance phenotype. These clones showed variation in their ability to limit virus production, possibly due to differences in the expression of the vector/EST depending on their insertion sites in the chromosome. Compared to the LF-BK tTA cell line in a plaque assay using FMDV O/UK/2001, the reconstituted clones that showed the most prominent limitation in virus production, clones 8 and 30, also produced fewer and smaller plaques (Fig. 1A). The EST effects were not unique for FMDV O/UK/2001, as similar results were obtained with FMDV isolates from serotypes A (A24) and C (C3 Resende) (data not shown). However, challenge of the reconstituted clones with other picornaviruses, including porcine enterovirus, swine vesicular disease virus, and encephalomyocarditis virus, showed that the clones transcribing NTPDase6 in the antisense direction are as susceptible to these viruses as the naïve cell lines (Fig. 1B), suggesting that the viral resistance of the reconstituted clones is specific to FMDV.
FIG. 1.
Plaque titration assay on LF-BK tTA and NTPDase6-expressing cell clones. Cells were infected with FMDV O/UK/2001 at MOIs of 10−3 to 10−4 (A) or different picornaviruses (e.g., encephalomyocarditis virus [EMCV] and swine vesicular disease virus [SVDV]) (B) for 1 h at 37°C and cultured with gum tragacanth. Cells were fixed and stained at 48 hpi. (C) FMDV resistance can be suppressed in the NTPDase6-expressing cells in response to the addition of doxycycline. Before virus infection, cells were cultured in the absence (−D) or presence (+D) of 2 μg/ml doxycycline, and viral resistance was examined by plaque assay with virus titers ranging from an MOI of 10−3 to an MOI of 10−5.
Clones 8 and 30 were examined for dependence of the FMDV resistance phenotype on transcription from the tetracycline-regulated promoter in the pLEST vector by adding doxycycline to reduce transcription of the EST sequence. Plaque assays showed that both clones acquired the ability to produce control levels of FMDV with the addition of doxycycline (Fig. 1C), confirming that the virus resistance phenotype is dependent on the expression of the EST.
Reduction of gene expression by antisense RNA commonly is not accompanied by decreased abundance of the RNA target (for reviews, see references 2 and 13), and consistent with such findings, Northern blot analysis of mRNA isolated from the parental cells and the clone 30 showed no significant difference in NPTDase6 mRNA levels (data not shown). In Western blotting experiments, rabbit polyclonal antibody directed against human NTPDase6 protein failed to detect endogenous levels of the bovine NTPDase6 protein (data not shown), and this antibody also did not detect endogenous levels of simian NTPDase6 protein in a previous study by the antibody's producers (22). To confirm that the decreased FMDV production by clone 30 resulted from the effects of the antisense EST on NPTDase6 expression, we therefore introduced a human NTPDase6 overexpression construct into clone 30 and tested for the ability of this construct to reverse the virus resistance phenotype. As seen in Fig. 2A, Western blotting showed elevated NTPDase6 expression, and this was accompanied by reversal of the virus resistance phenotype. Whereas overexpression of human NPTDase6 protein reversed the virus resistance phenotype of bovine cells containing the NPTDase6 antisense EST, such overexpression did not increase virus sensitivity to a higher-than-normal level in either these cells or the parental LF-BK cells (Fig. 2B).
FIG. 2.
Overexpression of NTPDase6 in clone 30 reversed the FMDV resistance phenotype. (A) Western blotting was used for the detection of the overexpression of NTPDase6 in LF-BK tTA and clone 30 cells. Lane 1, LF-BK tTA cells; lane 2, clone 30 cells; lane 3, LF-BK tTA cells with overexpressed NTPDase6; lane 4, clone 30 cells with overexpressed NTPDase6. Molecular masses are given to the left of the gel. (B) Plaque titration assay on LF-BK tTA and clone 30 cells. The virus titers used in this assay ranged from an MOI of 10−2 to an MOI of 10−6. The overexpression of NTPDase6 in clone 30 decreased the resistance to FMDV infection.
Characterization of cells showing NTPDase6-mediated resistance to FMDV.
Having confirmed that the expression of antisense NTPDase6 can limit the ability of LF-BK cells to produce FMDV, we further analyzed the growth of viruses in the reconstituted NTPDase6-expressing clones compared to viral growth in the wild-type cell line. Cells were infected with FMDV O/UK/2001 at high and low MOIs (10 and 0.1, respectively), and samples were titrated in BHK-21 cells (Fig. 3A and B, respectively). At an MOI of 10, one-step growth curves showed that the virus replicated more slowly in the reconstituted clones, and a 90% decrease in virus production was observed. The decrease in viral production was even more prominent at an MOI of 0.1, when the decrease in virus production in cells containing the antisense NPTDase6 EST showed a decrease in virus production to 1 to 2% of the normal amount.
FIG. 3.
Growth curves for FMDV in LF-BK tTA and NTPDase6-expressing cell clones. Cells were infected with FMDV O/UK/2001 at an MOI of 10 (A) or an MOI of 0.1 (B) for 1 h at 37°C, as described in Materials and Methods. Supernatant fluids were removed and titrated at the times indicated. Each datum is the mean value for at least three independent experiments. TCID50, 50% tissue culture infective doses.
We investigated the stage of the viral life cycle at which blockage of virus production occurred in antisense NTPDase6-expressing cells. Viral replication was evaluated by Northern blot analysis. At an MOI of 10, extensive degradation of host cell RNA was observed (10), and a comparison between the vRNA levels of the cell clones could not be assessed (data not shown). However, at an MOI of 0.1, vRNA accumulations in both of the reconstitution clones we examined (i.e., clones 8 and 30) were markedly reduced compared to vRNA accumulation of the parental LF-BK tTa cells (Fig. 4A), suggesting an impairment of the vRNA replication in the resistant cells. In addition, when the cells were cultivated in the presence of doxycycline, the impairment in vRNA production was diminished (clone 30; Fig. 4B), confirming that the decrease in vRNA accumulation is controlled by expression of the antisense NTPDase6 EST. Similar doxycycline-dependent regulation in vRNA production was also observed for clone 8 (data not shown).
FIG. 4.
FMDV RNA synthesis in infected LF-BK tTA and NTPDase6-expressing cell clones. Equal numbers of cells were infected with FMDV O/UK/2001 and at the times postinfection indicated in the figure; total RNA was extracted and quantified by use of a spectrophotometer. Equal amounts of RNA were loaded in each lane lane, and Northern blotting was performed by using the 32P-labeled 3D antisense RNA probe as described in Materials and Methods. Infection of cell clone 8 and clone 30 at an MOI of 0.1 in the absence of doxycycline (A) and infection of cell clone 30 at an MOI of 0.1 in the absence or presence of 2 μg/ml doxycycline (B). The lower panels show corresponding levels of ß-actin mRNA as a control.
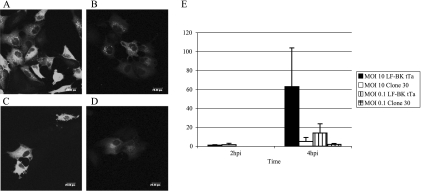
The expression of viral proteins in NTPDase6-expressing cells was compared to expression in LF-BK tTa cells by using Western blot analysis and immunofluorescence microscopy. The production levels of both the vRNA polymerase (3D) (Fig. 5A) and the viral structural protein VP1 (Fig. 5B) in clone 30 were reduced compared to those in LF-BK tTA cells at an MOI of 10. We then checked the viral structural proteins using immunofluorescence microscopy. Both clone 30 and LF-BK tTA cells were infected with FMDV at an MOI of 10, and the cells were stained with structural-protein-specific MAbs 10GA4.2.2 and 12FE9.2.1. At 2 hpi, almost 100% of both naïve cells and resistant cells had punctate staining that was homogeneously distributed in the cytoplasm and showed similar intensities of fluorescence. At 4 hpi, although equivalent percentages (higher that 95%) of cells expressing viral proteins were detected, the immunofluorescence intensity was much lower in the cells of clone 30 (Fig. 6A and B). Similar results were obtained when cells were infected with a virus at an MOI of 0.1 (Fig. 6C and D). NTPDase6-expressing cells showed about five to nine times lower fluorescent intensity/area when we randomly quantitated these values from 20 cells from each condition (Fig. 6E).
FIG. 5.
Western blot analysis of the FMDV 3D protein expressed in LF-BK tTA cells and NTPDase6-expressing cell clone 30. Equal numbers of cells were infected at an MOI of 10, and cytoplasmic cell extracts were prepared after 4 hpi. Equal volumes of cell extracts were separated by SDS-PAGE and blotted, and viral proteins were identified by reactivity with anti-3D (A) or anti-VP1 (B) antiserum (upper panel) as described in Materials and Methods. We noticed that the loadings of lysate for mock-infected and infected cells may not be equal, judging from the loading control antitubulin (α-tubulin; lower panel). However, the loadings of the infected LF-BK tTA and NTPDase6-expressing cell lysate are comparable (lanes 2 and 4 for both panels A and B), and the lower production of viral proteins in the NTPDase6-expressing cell is apparent. 3CD is the precursor of the 3C viral proteinase and the 3D viral DNA polymerase.
FIG. 6.
Immunofluorescence assay of LF-BK tTA cells and NTPDase6-expressing cell clone 30. Both cell types were infected with FMDV O/UK/2001 at an MOI of 10 (panels A and B, respectively) or at an MOI of 0.1 (panels C and D, respectively). After 4 hpi, cells were fixed for immunofluorescence staining using MAbs against the structural viral proteins and the secondary antibody goat anti-mouse immunoglobulin G labeled with Alexa Fluor 594 (Molecular Probes). The average fluorescence intensities of virus-infected cells (listed on the y axis) are shown in panel E. The scale shown in panels A through D is 20 μm.
The immunofluorescence microscopy results described above imply that the entry of FMDVs is not impaired in cells expressing antisense NTPDase6, as the numbers of infected cells and the concentrations of viral proteins are about the same for both antisense NTPDase6 EST-expressing and naïve cells at early time points. To test directly whether FMDV can efficiently bind to and infect the mutant cells, we carried out infectious-center assays for clone 30. Both clone 30 and LF-BK tTA cells were infected with O/UK/2001 at an MOI of 10 and treated with a low-pH saline buffer to inactivate residual virus. Cells were trypsinized and seeded with either LF-BK tTA cells or clone 30. After a 4-h incubation to allow cell attachment, the cultures were incubated under gum tragacanth for 48 h. The percentages of infected cells were approximately 69.75% (standard deviation, 22.12%) for LF-BK tTA cells and 33.5% (standard deviation, 18.04%) for clone 30 cells, demonstrating comparable virus-binding efficiencies in both cells. We also observed that when FMDV-infected cells were seeded on an indicator lawn of LF-BK cells, the size of the plaques in the indicator lawn was undiminished by the expression of antisense NTPDase6 RNA in the initially infected cells, consistent with the view that NTPDase6 is not required for viral entry. However, direct infection by FMDV at an MOI of 10 of cells expressing antisense NTPDase6 RNA resulted in very small plaques. As an MOI of 10 is expected to lead to simultaneous infection of all cells in the population, these results argue that cells expressing antisense NTPDase6 RNA generate fewer viruses than the wild-type LF-BK tTA cell. Together with the results of vRNA replication, protein synthesis, and infectious-center assays, these data support the view that impaired expression of NTPDase6 affects virus production rather than entry.
DISCUSSION
Virus infections require the participation of viral and cellular factors. A powerful approach for the discovery of host factors required in viral infections involves the identification of host cells that do not support normal infection. For many viruses, including picornaviruses, resistant cell lines have been described (19, 20, 28, 46). Cell lines naturally resistant to FMDV have been established by growing the cells that survived standard cytolytic infections (14). However, no cellular genes have been linked to such resistance.
The application of an EST-based gene inactivation approach (35) has enabled the isolation of FMDV-resistant cells that result from inhibition of the actions of specific cellular genes. A library of cells that normally are susceptible to FMDV infection was infected by FMDV, the surviving cells were isolated and cloned, and the ESTs present in the survivors were analyzed. Among such isolates, one clone expressing an EST of the NTPDase6 gene in an antisense orientation was chosen for further studies because of the strong viral resistance phenotype and the previously unexpected involvement of NTPDases in viral production.
With the parental LF-BK tTA cell line as a control, the resistance phenotype of cell clones expressing antisense NTPDase6 RNA was more prominent at a low MOI (0.1), producing a virus yield that was 50- to 200-fold lower than that at a high MOI (10). Results of infectious-center assays indicated that the blockage in viral infection occurred after the initial virus-host interaction, as virus binding levels were similar in resistant and susceptible cells. In addition, both structural and nonstructural proteins encoded by the virus were reduced in the NTPDase6-expressing cells, suggesting that the effects of the MOI on the resistance phenotype may result from a lower frequency of reinfection as a consequence of decreased production of infectious viral particles.
NTPDase6, also known as CD39L2, belongs to a family of ectonucleoside triphosphate diphosphohydrolases (E- NTPDase family). It is an integral plasma membrane protein that hydrolyzes extracellular nucleotides and is expressed in the heart, brain, placenta, lung, liver, kidney, pancreas, skeletal muscle, and endothelial tissues (9). Previous evidence that NTPDase6 is associated with the Golgi apparatus and preferentially mediates the hydrolysis of GDP has led to the hypothesis that the enzyme facilitates protein glycosylation in the Golgi apparatus (9, 24, 57). Currently, only information about the cellular functions carried out by this enzyme family is available, and the involvement of NTPDase6 in virus production is unanticipated (for a review, see reference 45). However, a recent study indicates that NTPDase1/CD39, a plasma membrane enzyme of the NTPDase family, can be incorporated into human immunodeficiency virus type 1 particles and still remain active (3).
Our results indicate that cells expressing antisense NTPDase6 RNA show resistance to FMDV infection but not to infection by other picornaviruses. Replication of picornaviruses occurs in the cytoplasm and induces a rearrangement of intracellular membranes that results in the formation of virus replication complexes. Picornaviruses from different genera utilize different cellular membranes and ways to assemble their replication complexes (18, 47), and this may account for distinct actions of NPTDase on FMDV. Potentially, NTPDase6 may affect the quantities of nucleosides required for glycosylation of host proteins essential for FMDV replication or another essential step in virus production.
For poliovirus, the most widely studied picornavirus, the membranes utilized for replication are derived from the endoplasmic reticulum and Golgi apparatus (48, 49, 52, 53). In the case of FMDV, the replication complex recently has been described, and the involvement of the Golgi apparatus or the endoplasmic reticulum has been reported (32, 37, 39, 41). Preliminary electromicroscopy analysis of NTPDase6-expressing cell clone 30 infected with FMDV at an MOI of 10 showed a reduced number of replication complexes at 4 hpi compared to infected LF-BK tTA cells. In addition, in about half of the NTPDase6-expressing infected cells observed, the replication vesicles have fewer strands or the fibrillar material is absent from the interior of the vesicle (T. G. Burrage and M. E. Piccone, unpublished observations). The results reported here, together with analogous studies related to African swine fever virus (11), suggest that modification of genes of FMDV-susceptible cells can render cells genetically resistant to infection by economically important livestock virus. The identification of additional cellular genes essential for virus propagation and subsequent inactivation of these genes in transgenic livestock may provide a practical approach to the control of livestock infectious diseases.
Acknowledgments
This paper is dedicated to the memory of Fred Brown.
We thank Terence L. Kirley for the generous gift of the NTPDase6 plasmid and anti-NTPDase6 antibody.
This work was supported by the Defense Advance Research Projects Agency (DARPA) of the U.S. Department of Defense.
We have no financial or other relationships relevant to the research reported.
Footnotes
Published ahead of print on 15 April 2009.
REFERENCES
- 1.Ait-Goughoulte, M., C. Hourioux, R. Patient, S. Trassard, D. Brand, and P. Roingeard. 2006. Core protein cleavage by signal peptide peptidase is required for hepatitis C virus-like particle assembly. J. Gen. Virol. 87855-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, B. F., and B. P. Monia. 1999. Novel mechanisms for antisense-mediated regulation of gene expression. Biochim. Biophys. Acta 14893-18. [DOI] [PubMed] [Google Scholar]
- 3.Barat, C., G. Martin, A. R. Beaudoin, J. Sevigny, and M. J. Tremblay. 2007. The nucleoside triphosphate diphosphohydrolase-1/CD39 is incorporated into human immunodeficiency type 1 particles, where it remains biologically active. J. Mol. Biol. 371269-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baxt, B. 1987. Effect of lysosomotropic compounds on early events in foot-and-mouth disease virus replication. Virus Res. 7257-271. [DOI] [PubMed] [Google Scholar]
- 5.Berinstein, A., M. Roivainen, T. Hovi, P. W. Mason, and B. Baxt. 1995. Antibodies to the vitronectin receptor (integrin alpha V beta 3) inhibit binding and infection of foot-and-mouth disease virus to cultured cells. J. Virol. 692664-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berns, K., E. M. Hijmans, J. Mullenders, T. R. Brummelkamp, A. Velds, M. Heimerikx, R. M. Kerkhoven, M. Madiredjo, W. Nijkamp, B. Weigelt, R. Agami, W. Ge, G. Cavet, P. S. Linsley, R. L. Beijersbergen, and R. Bernards. 2004. A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature 428431-437. [DOI] [PubMed] [Google Scholar]
- 7.Boothroyd, J. C., T. J. Harris, D. J. Rowlands, and P. A. Lowe. 1982. The nucleotide sequence of cDNA coding for the structural proteins of foot-and-mouth disease virus. Gene 17153-161. [DOI] [PubMed] [Google Scholar]
- 8.Boothroyd, J. C., P. E. Highfield, G. A. Cross, D. J. Rowlands, P. A. Lowe, F. Brown, and T. J. Harris. 1981. Molecular cloning of foot and mouth disease virus genome and nucleotide sequences in the structural protein genes. Nature 290800-802. [DOI] [PubMed] [Google Scholar]
- 9.Braun, N., S. Fengler, C. Ebeling, J. Servos, and H. Zimmermann. 2000. Sequencing, functional expression and characterization of rat NTPDase6, a nucleoside diphosphatase and novel member of the ecto-nucleoside triphosphate diphosphohydrolase family. Biochem. J. 351639-647. [PMC free article] [PubMed] [Google Scholar]
- 10.Capozzo, A. V., D. J. Burke, J. W. Fox, I. E. Bergmann, J. L. La Torre, and P. R. Grigera. 2002. Expression of foot and mouth disease virus non-structural polypeptide 3ABC induces histone H3 cleavage in BHK21 cells. Virus Res. 9091-99. [DOI] [PubMed] [Google Scholar]
- 11.Chang, A. C., L. Zsak, Y. Feng, R. Mosseri, Q. Lu, P. Kowalski, A. Zsak, T. G. Burrage, J. G. Neilan, G. F. Kutish, Z. Lu, W. Laegreid, D. L. Rock, and S. N. Cohen. 2006. Phenotype-based identification of host genes required for replication of African swine fever virus. J. Virol. 808705-8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinsangaram, J., M. E. Piccone, and M. J. Grubman. 1999. Ability of foot-and-mouth disease virus to form plaques in cell culture is associated with suppression of alpha/beta interferon. J. Virol. 739891-9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crooke, S. T. 1999. Molecular mechanisms of action of antisense drugs. Biochim. Biophys. Acta 148931-44. [DOI] [PubMed] [Google Scholar]
- 14.de la Torre, J. C., E. Martinez-Salas, J. Diez, A. Villaverde, F. Gebauer, E. Rocha, M. Davila, and E. Domingo. 1988. Coevolution of cells and viruses in a persistent infection of foot-and-mouth disease virus in cell culture. J. Virol. 622050-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duque, H., M. LaRocco, W. T. Golde, and B. Baxt. 2004. Interactions of foot-and-mouth disease virus with soluble bovine αVβ3 and αVβ6 integrins. J. Virol. 789773-9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forss, S., K. Strebel, E. Beck, and H. Schaller. 1984. Nucleotide sequence and genome organization of foot-and-mouth disease virus. Nucleic Acids Res. 126587-6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujii, K., J. H. Hurley, and E. O. Freed. 2007. Beyond Tsg101: the role of Alix in ‘ESCRTing’ HIV-1. Nat. Rev. Microbiol. 5912-916. [DOI] [PubMed] [Google Scholar]
- 18.Gazina, E. V., J. M. Mackenzie, R. J. Gorrell, and D. A. Anderson. 2002. Differential requirements for COPI coats in formation of replication complexes among three genera of Picornaviridae. J. Virol. 7611113-11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gee, G. V., K. Manley, and W. J. Atwood. 2003. Derivation of a JC virus-resistant human glial cell line: implications for the identification of host cell factors that determine viral tropism. Virology 314101-109. [DOI] [PubMed] [Google Scholar]
- 20.Geenen, K., H. W. Favoreel, and H. J. Nauwynck. 2005. Higher resistance of porcine trigeminal ganglion neurons towards pseudorabies virus-induced cell death compared with other porcine cell types in vitro. J. Gen. Virol. 861251-1260. [DOI] [PubMed] [Google Scholar]
- 21.Gossen, M., and H. Bujard. 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 895547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heimann, M., G. Roman-Sosa, B. Martoglio, H. J. Thiel, and T. Rumenapf. 2006. Core protein of pestiviruses is processed at the C terminus by signal peptide peptidase. J. Virol. 801915-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hicks-Berger, C. A., B. P. Chadwick, A. M. Frischauf, and T. L. Kirley. 2000. Expression and characterization of soluble and membrane-bound human nucleoside triphosphate diphosphohydrolase 6 (CD39L2). J. Biol. Chem. 27534041-34045. [DOI] [PubMed] [Google Scholar]
- 24.Hirschberg, C. B., and M. D. Snider. 1987. Topography of glycosylation in the rough endoplasmic reticulum and Golgi apparatus. Annu. Rev. Biochem. 5663-87. [DOI] [PubMed] [Google Scholar]
- 25.Hiscott, J. 2007. Convergence of the NF-kappaB and IRF pathways in the regulation of the innate antiviral response. Cytokine Growth Factor Rev. 18483-490. [DOI] [PubMed] [Google Scholar]
- 26.Jackson, T., S. Clark, S. Berryman, A. Burman, S. Cambier, D. Mu, S. Nishimura, and A. M. King. 2004. Integrin αvβ8 functions as a receptor for foot-and-mouth disease virus: role of the beta-chain cytodomain in integrin-mediated infection. J. Virol. 784533-4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin, R. C., B. Voetsch, and J. Loscalzo. 2005. Endogenous mechanisms of inhibition of platelet function. Microcirculation 12247-258. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan, G., A. Levy, and V. R. Racaniello. 1989. Isolation and characterization of HeLa cell lines blocked at different steps in the poliovirus life cycle. J. Virol. 6343-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy, C., L. D. Todorov, S. Mihaylova-Todorova, and P. Sneddon. 1997. Release of soluble nucleotidases: a novel mechanism for neurotransmitter inactivation? Trends Pharmacol. Sci. 18263-266. [DOI] [PubMed] [Google Scholar]
- 30.Kimchi, A. 2003. Antisense libraries to isolate tumor suppressor genes. Methods Mol. Biol. 222399-412. [DOI] [PubMed] [Google Scholar]
- 31.Kittler, R., G. Putz, L. Pelletier, I. Poser, A. K. Heninger, D. Drechsel, S. Fischer, I. Konstantinova, B. Habermann, H. Grabner, M. L. Yaspo, H. Himmelbauer, B. Korn, K. Neugebauer, M. T. Pisabarro, and F. Buchholz. 2004. An endoribonuclease-prepared siRNA screen in human cells identifies genes essential for cell division. Nature 4321036-1040. [DOI] [PubMed] [Google Scholar]
- 32.Knox, C., K. Moffat, S. Ali, M. Ryan, and T. Wileman. 2005. Foot-and-mouth disease virus replication sites form next to the nucleus and close to the Golgi apparatus, but exclude marker proteins associated with host membrane compartments. J. Gen. Virol. 86687-696. [DOI] [PubMed] [Google Scholar]
- 33.Kurz, C., S. Forss, H. Kupper, K. Strohmaier, and H. Schaller. 1981. Nucleotide sequence and corresponding amino acid sequence of the gene for the major antigen of foot and mouth disease virus. Nucleic Acids Res. 91919-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, L., and S. N. Cohen. 1996. Tsg101: a novel tumor susceptibility gene isolated by controlled homozygous functional knockout of allelic loci in mammalian cells. Cell 85319-329. [DOI] [PubMed] [Google Scholar]
- 35.Lu, Q., W. Wei, P. E. Kowalski, A. C. Chang, and S. N. Cohen. 2004. EST-based genome-wide gene inactivation identifies ARAP3 as a host protein affecting cellular susceptibility to anthrax toxin. Proc. Natl. Acad. Sci. USA 10117246-17251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malmgaard, L. 2004. Induction and regulation of IFNs during viral infections. J. Interferon Cytokine Res. 24439-454. [DOI] [PubMed] [Google Scholar]
- 37.Martín-Acebes, M. A., M. Gonzalez-Magaldi, M. F. Rosas, B. Borrego, E. Brocchi, R. Armas-Portela, and F. Sobrino. 2008. Subcellular distribution of swine vesicular disease virus proteins and alterations induced in infected cells: a comparative study with foot-and-mouth disease virus and vesicular stomatitis virus. Virology 374432-443. [DOI] [PubMed] [Google Scholar]
- 38.Martoglio, B., and T. E. Golde. 2003. Intramembrane-cleaving aspartic proteases and disease: presenilins, signal peptide peptidase and their homologs. Hum. Mol. Genet. 12(special no. 2)R201-R206. [DOI] [PubMed] [Google Scholar]
- 39.Monaghan, P., H. Cook, T. Jackson, M. Ryan, and T. Wileman. 2004. The ultrastructure of the developing replication site in foot-and-mouth disease virus-infected BHK-38 cells. J. Gen. Virol. 85933-946. [DOI] [PubMed] [Google Scholar]
- 40.Neff, S., P. W. Mason, and B. Baxt. 2000. High-efficiency utilization of the bovine integrin αvβ3 as a receptor for foot-and-mouth disease virus is dependent on the bovine β3 subunit. J. Virol. 747298-7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Donnell, V. K., J. M. Pacheco, T. M. Henry, and P. W. Mason. 2001. Subcellular distribution of the foot-and-mouth disease virus 3A protein in cells infected with viruses encoding wild-type and bovine-attenuated forms of 3A. Virology 287151-162. [DOI] [PubMed] [Google Scholar]
- 42.Piatti, P., S. Hassard, J. F. Newman, and F. Brown. 1995. Antigenic variants in a plaque-isolate of foot-and-mouth disease virus: implications for vaccine production. Vaccine 13781-784. [DOI] [PubMed] [Google Scholar]
- 43.Primiano, T., M. Baig, A. Maliyekkel, B. D. Chang, S. Fellars, J. Sadhu, S. A. Axenovich, T. A. Holzmayer, and I. B. Roninson. 2003. Identification of potential anticancer drug targets through the selection of growth-inhibitory genetic suppressor elements. Cancer Cell 441-53. [DOI] [PubMed] [Google Scholar]
- 44.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27493-497. [Google Scholar]
- 45.Robson, S. C., J. Sevigny, and H. Zimmermann. 2006. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2409-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roller, R. J., and B. C. Herold. 1997. Characterization of a BHK(TK-) cell clone resistant to postattachment entry by herpes simplex virus types 1 and 2. J. Virol. 715805-5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rust, R. C., L. Landmann, R. Gosert, B. L. Tang, W. Hong, H. P. Hauri, D. Egger, and K. Bienz. 2001. Cellular COPII proteins are involved in production of the vesicles that form the poliovirus replication complex. J. Virol. 759808-9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sandoval, I. V., and L. Carrasco. 1997. Poliovirus infection and expression of the poliovirus protein 2B provoke the disassembly of the Golgi complex, the organelle target for the antipoliovirus drug Ro-090179. J. Virol. 714679-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlegel, A., T. H. Giddings, Jr., M. S. Ladinsky, and K. Kirkegaard. 1996. Cellular origin and ultrastructure of membranes induced during poliovirus infection. J. Virol. 706576-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stave, J. W., J. L. Card, and D. O. Morgan. 1986. Analysis of foot-and-mouth disease virus type O1 Brugge neutralization epitopes using monoclonal antibodies. J. Gen. Virol. 672083-2092. [DOI] [PubMed] [Google Scholar]
- 51.Stave, J. W., J. L. Card, D. O. Morgan, and V. N. Vakharia. 1988. Neutralization sites of type O1 foot-and-mouth disease virus defined by monoclonal antibodies and neutralization-escape virus variants. Virology 16221-29. [DOI] [PubMed] [Google Scholar]
- 52.Strauss, D. M., L. W. Glustrom, and D. S. Wuttke. 2003. Towards an understanding of the poliovirus replication complex: the solution structure of the soluble domain of the poliovirus 3A protein. J. Mol. Biol. 330225-234. [DOI] [PubMed] [Google Scholar]
- 53.Suhy, D. A., T. H. Giddings, Jr., and K. Kirkegaard. 2000. Remodeling the endoplasmic reticulum by poliovirus infection and by individual viral proteins: an autophagy-like origin for virus-induced vesicles. J. Virol. 748953-8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swaney, L. M. 1988. A continuous bovine kidney cell line for routine assays of foot-and-mouth disease virus. Vet. Microbiol. 181-14. [DOI] [PubMed] [Google Scholar]
- 55.Todorov, L. D., R. Clerkin, S. T. Mihaylova-Todorova, M. A. Khoyi, and D. P. Westfall. 2001. Beta2-adrenoceptor-mediated prejunctional facilitation and postjunctional inhibition of sympathetic neuroeffector transmission in the guinea pig vas deferens. J. Pharmacol. Exp. Ther. 298623-633. [PubMed] [Google Scholar]
- 56.Todorov, L. D., S. Mihaylova-Todorova, T. D. Westfall, P. Sneddon, C. Kennedy, R. A. Bjur, and D. P. Westfall. 1997. Neuronal release of soluble nucleotidases and their role in neurotransmitter inactivation. Nature 38776-79. [DOI] [PubMed] [Google Scholar]
- 57.Trombetta, E. S., and A. Helenius. 1999. Glycoprotein reglucosylation and nucleotide sugar utilization in the secretory pathway: identification of a nucleoside diphosphatase in the endoplasmic reticulum. EMBO J. 183282-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang, Z. D., G. Hutching, P. Kitching, and S. Alexandersen. 2002. The effects of gamma interferon on replication of foot-and-mouth disease virus in persistently infected bovine cells. Arch. Virol. 1472157-2167. [DOI] [PubMed] [Google Scholar]