Abstract
Human noroviruses in the Caliciviridae family are the major cause of nonbacterial epidemic gastroenteritis worldwide. Primary human norovirus infection does not elicit lasting protective immunity, a fact that could greatly affect the efficacy of vaccination strategies. Little is known regarding the pathogenesis of human noroviruses or the immune responses that control them because there has previously been no small-animal model or cell culture system of infection. Using the only available small-animal model of norovirus infection, we found that primary high-dose murine norovirus 1 (MNV-1) infection fails to afford protection against a rechallenge with a homologous virus. Thus, MNV-1 represents a valuable model with which to dissect the pathophysiological basis for the lack of lasting protection against human norovirus infection. Interestingly, the magnitude of protection afforded by a primary MNV-1 infection inversely correlates with the inoculum dose. Future studies will elucidate the mechanisms by which noroviruses avoid the induction of protective immunity and the role played by the inoculum dose in this process, ultimately translating this knowledge into successful vaccination approaches.
Human noroviruses (NVs) are estimated to be responsible for >95% of the nonbacterial epidemic gastroenteritis that occurs worldwide. The course of the disease is rapid, with symptoms including vomiting, diarrhea, and nausea arising approximately 24 h following infection and typically resolving 24 to 48 h later. NV outbreaks occur most commonly in semiclosed communities such as nursing homes, schools, hospitals, cruise ships, and military settings (11, 24, 31). Persons of all ages are susceptible to NV infection. Human NVs are thus associated with considerable morbidity and have a significant economic impact. Numerous human volunteer challenge studies have demonstrated that long-term immunity is not induced following primary NV infection of some volunteers (13, 20, 26). The pathophysiological basis for this lack of protection is unclear, since virus-specific adaptive immune responses are generated (1, 7, 9, 10). A similar lack of immunity has been observed in some individuals for a number of other viral pathogens that infect at mucosal surfaces, such as rhinoviruses (32) and respiratory syncytial virus (RSV) (16). Importantly, typical vaccination strategies have been unsuccessful at eliciting protective anti-RSV immunity and studies with animal models to understand the lack of immunity to either natural or vaccinating virus have been uninformative because protection is induced in animals (27). Extrapolating from RSV studies, it may be difficult to vaccinate against NVs and it will be important to understand the underlying cause in order to design more efficacious treatment regimens. Studies with a small-animal model recapitulating this atypical immune outcome would be extremely valuable.
Murine norovirus 1 (MNV-1) infection of immunocompetent mice causes measurable enteric disease.
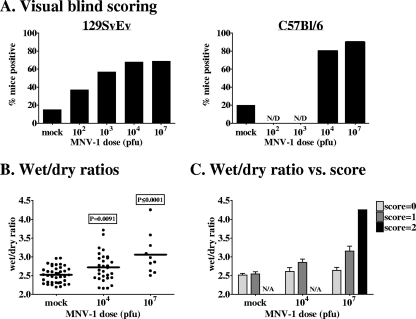
Despite the impact of human NV-induced disease, the pathogenic features of infection are not well understood due to the previous lack of cell culture and small-animal model systems (8). In 2003, we discovered the first murine NV, MNV-1 (21), and have subsequently determined that this virus replicates in macrophages and dendritic cells in vitro (35) and most likely in vivo (25, 35). Like the human NVs, MNV-1 is infectious orally (21) and is spread naturally between hosts (19). While previous studies have failed to observe disease in MNV-1-infected immunocompetent mice, these studies monitored infected mice only for overt external indicators of gastroenteritis (18, 19, 21, 30). In a more rigorous examination of putative gastroenteritis induction using internal and histopathological indicators, we observed that peroral inoculation of 129SvEv mice with 107 PFU MNV-1.CW3 results in decreased fecal contents, mild intestinal inflammation, and mild although not statistically significant diarrhea at 3 days postinfection (dpi) (25). All of the experiments in the present study were performed with the same MNV-1.CW3 isolate used in our previous analysis of 129SvEv mice (25); we will refer to it as MNV-1 hereafter. We have now scored increased numbers of mock-infected and MNV-1-infected mice for stool consistency and report that MNV-1 infection does indeed induce statistically significant mild diarrhea, as defined by a visible increase in fecal inconsistency, in immunocompetent 129SvEv mice in a dose-dependent manner (Table 1 and Fig. 1A). While such a visual scoring system is inherently subjective and the phenotype observed in MNV-1-infected mice is mild, the dose dependence and the statistical significance of data comparing infected and mock-infected control mice obtained upon blind scoring validate this method of disease assessment in our system. Moreover, there is a direct correlation between the visual assessment of fecal samples and their wet/dry ratios (an objective measure of fecal consistency). Specifically, fecal samples from MNV-1-infected mice displayed an increased wet/dry ratio compared to that of samples from control mice (Fig. 1B), with those samples scoring positive by visual assessment displaying the highest wet/dry ratios (Fig. 1C). These data were obtained from mice infected for 24 h; a similar, but not statistically significant, trend was observed in mice infected for 72 h (data not shown). A majority of C57BL/6 mice inoculated with either 104 or 107 PFU MNV-1 also scored positive in the visual scoring assay (Table 1 and Fig. 1A), demonstrating that MNV-1 infection induces fecal inconsistency in at least two wild-type mouse strains. Importantly, this scoring system provides a readout for disease in MNV-1-infected immunocompetent mice, facilitating an examination of whether primary infection elicits protection against a rechallenge.
TABLE 1.
Raw fecal consistency scores following primary MNV-1 infection
| Treatment (no. of PFU) | 129SvEv
|
C57BL/6
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequency positive | No. positive/total
|
P valuea | Frequency positive | No. positive/total
|
P valuea | |||||
| Score 1 | Score 2 | Both | Score 1 | Score 2 | Both | |||||
| Mock infection | 0.15 | 9/62 | 0/62 | 9/62 | 0.20 | 5/25 | 0/25 | 5/25 | ||
| 102 | 0.37 | 11/30 | 0/30 | 11/30 | 0.016 | NDb | ND | ND | ND | ND |
| 103 | 0.57 | 17/30 | 0/30 | 17/30 | <0.0001 | ND | ND | ND | ND | ND |
| 104 | 0.68 | 22/38 | 4/38 | 26/38 | <0.0001 | 0.80 | 19/25 | 1/25 | 20/25 | <0.0001 |
| 107 | 0.69 | 9/16 | 2/16 | 11/16 | <0.0001 | 0.90 | 8/10 | 1/10 | 9/10 | <0.0001 |
P values were determined by unpaired two-tailed t tests comparing the raw diarrheal scores of infected groups to those of mock-infected controls.
ND, not done.
FIG. 1.
MNV-1 causes dose-dependent mild diarrhea in wild-type mice. (A) 129SvEv or C57BL/6 mice were inoculated perorally with the indicated doses of MNV-1 at 5 to 6 weeks of age. All mice were fasted for 18 to 20 h prior to sample collection. At 72 hpi, mice were sacrificed and all feces below the cecum was collected and scored (0, normal feces; 1, mixed stool samples containing both solid and pasty feces; 2, pasty feces; 3, semiliquid feces; 4, liquid feces) independently by two investigators. At least one investigator scored the samples blindly. The data are presented as the frequency of mice receiving a score of 1 or greater from both investigators. Raw diarrheal scores are presented in Table 1. (B and C) Groups of 129SvEv mice (10 to 40 mice per group) were inoculated perorally with the indicated doses of MNV-1 at 5 to 6 weeks of age and fasted for 18 to 20 h prior to sample collection. At 24 hpi, mice were sacrificed and all feces below the cecum was collected, scored as described above, and immediately weighed. The feces was then dried at 70°C overnight and reweighed. Wet/dry ratios were determined by dividing the initial weight by the dried weight. The P values comparing MNV-1-infected mice to mock-infected mice are 0.0091 for 104-PFU infections and ≤0.0001 for 107-PFU infections (B). In panel C, wet/dry ratios are separated based on their score by visual assessment of fecal consistency.
MNV-1 is infectious at low doses.
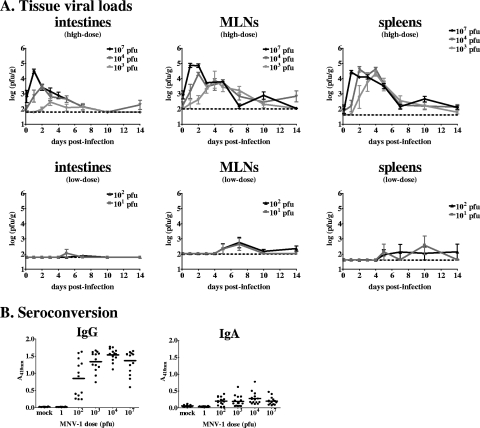
The minimal infectious dose of human NVs is thought to be quite low (29), but such studies are complicated by the absence of a cell culture system for virus quantification. To examine the minimal infectious dose of MNV-1, we infected groups of 129SvEv mice with doses of 101 to 107 PFU and determined the virus loads in their intestines, mesenteric lymph nodes (MLNs), and spleens at various times postinfection by using a standard virus plaque assay protocol (25, 35) (Fig. 2A). We noted that doses of ≥1,000 PFU initiated infection in a large majority, if not all, of the inoculated mice, while 10 and 100 PFU initiated infection in only a minority of the inoculated mice. All of the doses initiated an acute infection in at least a proportion of the inoculated mice, albeit with a dose-dependent delay in the kinetics of in vivo acute virus replication. There was a direct correlation between the doses and peak virus loads in all tissues. Using the Reed-Muench equation, we calculated the minimum infectious dose of MNV-1 to be 800 PFU for intestinal infection, 250 PFU for MLN infection, and 400 PFU for splenic infection. We also assessed the minimum MNV-1 dose required for seroconversion by using a previously published MNV-1-specific enzyme-linked immunosorbent assay (25) and found it to be between 1 and 100 PFU (Fig. 2B). Overall, these data represent the first unequivocal determination of minimum infectious NV doses.
FIG. 2.
MNV-1 is infectious at low doses. (A) 129SvEv mice were inoculated perorally with the indicated doses of MNV-1 at 5 to 6 weeks of age. At the postinfection times indicated, animals were perfused, organs were harvested, and viral burdens were determined by plaque assay. A minimum of three mice were analyzed for each dose and each time point. For clarity, high-dose (103 to 107 PFU) and low-dose (10 and 100 PFU) groups are shown in separate graphs. (B) 129SvEv mice were infected with the indicated doses of MNV-1 or mock infected at 5 to 6 weeks of age. At 6 weeks postinfection, serum was collected and analyzed for virus-specific IgG or IgA by enzyme-linked immunosorbent assay.
Primary high-dose MNV-1 infection does not induce protection against a secondary challenge.
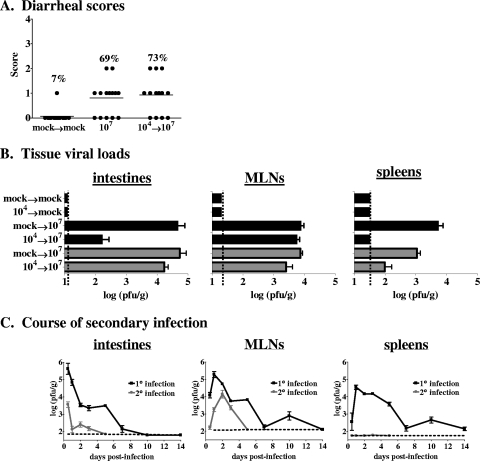
To determine whether a primary MNV-1 infection induces protection against a secondary challenge, we infected 129SvEv mice with 104 PFU MNV-1 and then rechallenged them with 107 PFU MNV-1 6 weeks later. The incidence of fecal inconsistency in mice receiving a secondary 107-PFU infection was similar to that in mice receiving a primary 107-PFU infection (Fig. 3A). We conclude that a primary high-dose MNV-1 infection does not elicit protection against disease upon rechallenge with a homologous virus. To begin dissecting the underlying cause of the lack of protection against MNV-1-induced disease, we tested whether virus loads in rechallenged mice differed from those in mice receiving a primary infection at 48 hpi, a time at which high levels of virus were observed during primary infection. There was a significant decrease in virus loads in the intestines and a complete absence of infectious virions in the spleens of previously exposed 129SvEv mice (Fig. 3B, black bars). Surprisingly, though (based on the expected effect of a memory immune response), there was no reduction in the peak level of virus detected in MLNs of previously exposed 129SvEv mice compared to mice exposed to MNV-1 only once. The virions detected in mucosal sites of rechallenged mice were not indicative of persistent virus remaining from the primary infection, since mice receiving a primary MNV-1 infection and a mock challenge 6 weeks later had no detectable virus in any tissue (Fig. 3B). Thus, memory immune responses to a high dose of MNV-1 do not induce sterilizing immunity. Similar results were observed in the C57BL/6 strain of mice, except that the virus loads in both intestines and MLNs were statistically similar between the primary and secondary challenge groups and a proportion of the rechallenged mice even had detectable virus in their spleens (Fig. 3B, gray bars), demonstrating that protective immunity is not induced in either 129SvEv or C57BL/6 mice following a primary high-dose MNV-1 infection. These data also suggest that the C57BL/6 model is a more robust one for determining the underlying basis of immune failure against secondary infections, a point that should be considered in future mechanistic studies. While mucosal infection and disease were not prevented in rechallenged mice, the virus was cleared more rapidly from their mucosal tissues as no virus could be detected in their intestines or MLNs at 5 dpi, in contrast to those of mice receiving a primary exposure (Fig. 3C). Moreover, the amount of virus detected in mucosal tissues was reduced during a secondary challenge even as early as 12 hpi. It should be noted that there were statistically similar levels of virus in the MLNs of primary and secondary challenge groups between 2 and 3 dpi. Collectively, these data indicate that memory immune responses to MNV-1 are elicited during a primary high-dose exposure but they are not effective at preventing disease in rechallenged hosts and they are particularly ineffective in MLNs early after infection. Overall, these data are consistent with the lack of protection against disease observed in NV-infected humans.
FIG. 3.
Primary MNV-1 infection does not protect from a secondary challenge. (A) Groups of 129SvEv mice (14 or 15 per group, 5 to 6 weeks of age) were inoculated perorally with 104 PFU MNV-1 or mock infected. Six weeks later, the mock-infected mice were rechallenged with a mock inoculum (mock→mock) while the MNV-1-infected mice were challenged with 107 PFU MNV-1 (104→107). For stool consistency measurement, all feces below the cecum was collected at 72 hpi and scored as described in the legend to Fig. 1. Data from 107-PFU primary infections from Fig. 1 are included for clarity (107). The frequency of mice receiving a score of 1 or greater is listed above each data set. The P value comparing MNV-1-rechallenged mice to mock-infected mice is 0.0002. (B) Groups of 129SvEv (black bars) or C57BL/6 (gray bars) mice were mock infected or inoculated perorally with 104 PFU MNV-1 (listed first on the y axis for each bar) at 5 to 6 weeks of age. Six weeks later, these same mice were either mock infected or infected with 107 PFU MNV-1 (listed second on the y axis for each bar). Two days after the secondary challenge, animals were perfused, organs were harvested, and viral burdens were determined by plaque assay. Each group contained 3 to 10 mice, and the data for all of the mice in each group were averaged. Limits of detection are indicated by dashed lines. (C) 129SvEv mice were inoculated perorally with a mock inoculum (1° infection) or 104 PFU MNV-1 (2° infection). Six weeks later, both groups of mice were inoculated perorally with 107 PFU MNV-1. At the postinfection times indicated, a minimum of three mice from each group were perfused, organs were harvested, and viral burdens were determined by plaque assay.
Antigen dose influences the mucosal immune response to MNV-1.
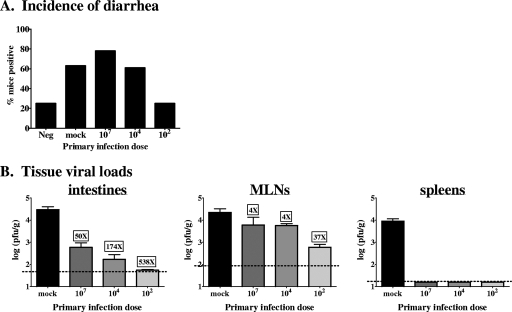
We next tested whether the primary inoculum dose influences the level of protection induced by MNV-1 infection. Lower primary inoculum doses reduced the incidence of fecal inconsistency during secondary MNV-1 infection to the levels observed in mock-infected control mice, in contrast to higher primary infection doses, which offered no protection against disease (Table 2 and Fig. 4A). Supporting this dose effect, there was a clear inverse correlation between the inoculum dose at primary exposure and the reduction in intestinal virus titers upon rechallenge (Fig. 4B). We also observed a more subtle dose-dependent effect on MLN virus loads. These data indicate that mucosal memory immune responses to MNV-1 are more effective when primed with lower doses of virus. These data also demonstrate that mucosal memory immune responses to MNV-1 are effective at very early times postchallenge under certain conditions (i.e., when primed with low doses of virus). Thus, the lack of protection against disease observed in mice receiving high primary infection doses (Fig. 3A and 4A) cannot be solely explained with the kinetic argument that disease occurs before there has been sufficient time for memory immune cells to proliferate and respond.
TABLE 2.
Raw fecal consistency scores following secondary MNV-1 infection
| Treatment | Frequency positive | No. positive/total
|
P valuea | ||
|---|---|---|---|---|---|
| Score 1 | Score 2 | Both | |||
| Mock infection→mock infection | 0.25 | 5/20 | 0/20 | 5/20 | |
| Mock infection→107 | 0.63 | 12/19 | 0/19 | 12/19 | 0.016 |
| 102→107 | 0.25 | 5/20 | 0/20 | 5/20 | 1.0 |
| 104→107 | 0.61 | 10/18 | 1/18 | 11/18 | 0.019 |
| 107→107 | 0.78 | 13/18 | 1/18 | 14/18 | 0.0006 |
P values were determined by unpaired two-tailed t tests comparing the raw diarrheal scores of infected groups to those of mock-infected controls (Mock infection →mock infection).
FIG. 4.
Protective MNV-1 immunity is influenced by the primary inoculum dose. Groups of 129SvEv mice were mock infected or inoculated perorally with the indicated doses of MNV-1 at 5 to 6 weeks of age. Six weeks later, all groups were infected with 107 PFU MNV-1. (A) Three days after the secondary challenge, stool samples were collected and scored as described in the legend to Fig. 1, except that these fecal samples were scored independently and blindly by three investigators. The majority score was assigned to each mouse. An additional group of mice received a mock inoculum at both the primary and secondary challenges as a negative control (Neg). Data are presented as the frequency of mice receiving a score of 1 or greater. Raw diarrheal scores are presented in Table 2. (B) Two days after the secondary challenge, animals were perfused, organs were harvested, and viral burdens were determined by plaque assay. Each group contained 5 to 15 mice, and the data for all of the mice in each group were averaged. Limits of detection are indicated by dashed lines. The n-fold difference between each secondary challenge group and the primary infection group is shown above each bar for intestines and MLNs.
Conclusions.
Overall, the data presented here strengthen the utility of MNV-1 as a model for human NVs. Specifically, we have demonstrated that (i) MNV-1 causes mild clinical disease in wild-type hosts, (ii) MNV-1 is infectious at very low doses, and (iii) primary MNV-1 infection fails to elicit protective immunity, at least under specific infection conditions. One possible explanation for the lack of protection elicited by primary MNV-1 infection is an inappropriate tolerogenic response by the mucosal immune system similar to its response to commensal gut flora. In support of this possibility, the antigen dose can influence the oral induction of tolerance to model antigens (5, 6, 12, 14, 28, 34). Why, then, did we observe peripheral protection irrespective of the primary infection dose? While commensal bacteria are recognized by the mucosal immune system and induce regulatory T cells and immunoglobulin A (IgA)-secreting B cells locally, they are prevented from disseminating past the MLNs and the peripheral immune system thus remains ignorant of them (23). In contrast, MNV-1 reaches peripheral tissues such as the spleen within 24 h during a primary infection (25) (Fig. 2A) and induces peripheral immune responses, including serum IgG (21) and IgA (Fig. 2B). Thus, a breach of the mucosal system may permit the generation of peripheral MNV-1-specific immune responses that prevent virus dissemination. Importantly, this peripheral response is insufficient to protect the host from a rechallenge. Another potential explanation to explain the lack of protective immunity is that MNV-1 infection of mucosal antigen-presenting cells directly impairs the functioning of these cells such that they are incapable of stimulating appropriate memory immune responses. In support of this possibility, the antigen dose can influence immune responses to pathogens (2, 15, 22, 33). We are currently exploring these possible mechanisms.
In contrast to our studies analyzing protection induced by a single exposure to MNV-1, it has recently been reported that repeated exposure to high doses of MNV-1 elicits protection against a rechallenge, as measured by virus loads at 3 dpi (4). Although one possible explanation for this discrepancy between studies is that repeated exposure to NVs elicits full protection against viral replication and disease, an equally plausible explanation is that repeated exposure to NVs alters the kinetics of infection or reduces the peak levels of virus but does not affect the disease outcome. This latter explanation is consistent with our observations following a single exposure to MNV-1. Because the repeated-exposure studies only analyzed virus loads at 3 dpi (4), it will be critical in future studies to take into account protection against the full course of infection and the development of disease, as the interpretation of these studies has important implications for candidate NV vaccine regimens.
Multiple mucosal pathogens fail to elicit protective immunity (16, 20, 26), and many are difficult to vaccinate against (3, 17). A failure to induce robust and lasting mucosal immunity is the likely explanation for both. Dissecting the mechanism responsible for the lack of protection against MNV-1 may offer global insight into the pathways used by pathogens to impair and/or evade immunostimulatory immune responses in the gut. This may have important general implications for mucosal vaccine design.
Acknowledgments
The project described here was supported by grant P20-RR018724, entitled Center for Molecular and Tumor Virology, from the National Center for Research Resources, a component of the National Institutes of Health.
Footnotes
Published ahead of print on 29 April 2009.
REFERENCES
- 1.Agus, S. G., R. Dolin, R. G. Wyatt, A. J. Tousimis, and R. S. Northrup. 1973. Acute infectious nonbacterial gastroenteritis: intestinal histopathology. Histologic and enzymatic alterations during illness produced by the Norwalk agent in man. Ann. Intern. Med. 7918-25. [DOI] [PubMed] [Google Scholar]
- 2.Boonstra, A., C. Asselin-Paturel, M. Gilliet, C. Crain, G. Trinchieri, Y. J. Liu, and A. O'Garra. 2003. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential Toll-like receptor ligation. J. Exp. Med. 197101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandtzaeg, P. 2007. Induction of secretory immunity and memory at mucosal surfaces. Vaccine 255467-5484. [DOI] [PubMed] [Google Scholar]
- 4.Chachu, K. A., A. D. LoBue, D. W. Strong, R. S. Baric, and H. W. Virgin. 2008. Immune mechanisms responsible for vaccination against and clearance of mucosal and lymphatic norovirus infection. PLoS Pathog. 4e1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, Y., J. Inobe, R. Marks, P. Gonnella, V. K. Kuchroo, and H. L. Weiner. 1995. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature 376177-180. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Y., V. K. Kuchroo, J. Inobe, D. A. Hafler, and H. L. Weiner. 1994. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science 2651237-1240. [DOI] [PubMed] [Google Scholar]
- 7.Cukor, G., N. A. Nowak, and N. R. Blacklow. 1982. Immunoglobulin M responses to the Norwalk virus of gastroenteritis. Infect. Immun. 37463-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duizer, E., K. J. Schwab, F. H. Neill, R. L. Atmar, M. P. Koopmans, and M. K. Estes. 2004. Laboratory efforts to cultivate noroviruses. J. Gen. Virol. 8579-87. [DOI] [PubMed] [Google Scholar]
- 9.Erdman, D. D., G. W. Gary, and L. J. Anderson. 1989. Development and evaluation of an IgM capture enzyme immunoassay for diagnosis of recent Norwalk virus infection. J. Virol. Methods 2457-66. [DOI] [PubMed] [Google Scholar]
- 10.Erdman, D. D., G. W. Gary, and L. J. Anderson. 1989. Serum immunoglobulin A response to Norwalk virus infection. J. Clin. Microbiol. 271417-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fankhauser, R. L., J. S. Noel, S. S. Monroe, T. Ando, and R. I. Glass. 1998. Molecular epidemiology of “Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J. Infect. Dis. 1781571-1578. [DOI] [PubMed] [Google Scholar]
- 12.Friedman, A., and H. L. Weiner. 1994. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc. Natl. Acad. Sci. USA 916688-6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green, K. Y. 2006. Caliciviridae: the noroviruses, p. 949-980. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 5th ed., vol. 1. Lippincott, Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 14.Gregerson, D. S., W. F. Obritsch, and L. A. Donoso. 1993. Oral tolerance in experimental autoimmune uveoretinitis. Distinct mechanisms of resistance are induced by low dose vs high dose feeding protocols. J. Immunol. 1515751-5761. [PubMed] [Google Scholar]
- 15.Hao, X., T. S. Kim, and T. J. Braciale. 2008. Differential response of respiratory dendritic cell subsets to influenza virus infection. J. Virol. 824908-4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson, F. W., A. M. Collier, W. A. Clyde, Jr., and F. W. Denny. 1979. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N. Engl. J. Med. 300530-534. [DOI] [PubMed] [Google Scholar]
- 17.Holmgren, J., and C. Czerkinsky. 2005. Mucosal immunity and vaccines. Nat. Med. 11S45-S53. [DOI] [PubMed] [Google Scholar]
- 18.Hsu, C. C., L. K. Riley, H. M. Wills, and R. S. Livingston. 2006. Persistent infection with and serologic cross-reactivity of three novel murine noroviruses. Comp. Med. 56247-251. [PubMed] [Google Scholar]
- 19.Hsu, C. C., C. E. Wobus, E. K. Steffen, L. K. Riley, and R. S. Livingston. 2005. Development of a microsphere-based serologic multiplexed fluorescent immunoassay and a reverse transcriptase PCR assay to detect murine norovirus 1 infection in mice. Clin. Diagn. Lab. Immunol. 121145-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, P. C., J. J. Mathewson, H. L. DuPont, and H. B. Greenberg. 1990. Multiple-challenge study of host susceptibility to Norwalk gastroenteritis in US adults. J. Infect. Dis. 16118-21. [DOI] [PubMed] [Google Scholar]
- 21.Karst, S. M., C. E. Wobus, M. Lay, J. Davidson, and H. W. Virgin IV. 2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science 2991575-1578. [DOI] [PubMed] [Google Scholar]
- 22.Legge, K. L., and T. J. Braciale. 2005. Lymph node dendritic cells control CD8+ T cell responses through regulated FasL expression. Immunity 23649-659. [DOI] [PubMed] [Google Scholar]
- 23.Macpherson, A. J., and E. Slack. 2007. The functional interactions of commensal bacteria with intestinal secretory IgA. Curr. Opin. Gastroenterol. 23673-678. [DOI] [PubMed] [Google Scholar]
- 24.McCarthy, M., M. K. Estes, and K. C. Hyams. 2000. Norwalk-like virus infection in military forces: epidemic potential, sporadic disease, and the future direction of prevention and control efforts. J. Infect. Dis. 181(Suppl. 2)S387-S391. [DOI] [PubMed] [Google Scholar]
- 25.Mumphrey, S. M., H. Changotra, T. N. Moore, E. R. Heimann-Nichols, C. E. Wobus, M. J. Reilly, M. Moghadamfalahi, D. Shukla, and S. M. Karst. 2007. Murine norovirus 1 infection is associated with histopathological changes in immunocompetent hosts but clinical disease is prevented by STAT1-dependent interferon responses. J. Virol. 813251-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parrino, T. A., D. S. Schreiber, J. S. Trier, A. Z. Kapikian, and N. R. Blacklow. 1977. Clinical immunity in acute gastroenteritis caused by Norwalk agent. N. Engl. J. Med. 29786-89. [DOI] [PubMed] [Google Scholar]
- 27.Power, U. F. 2008. Respiratory syncytial virus (RSV) vaccines—two steps back for one leap forward. J. Clin. Virol. 4138-44. [DOI] [PubMed] [Google Scholar]
- 28.Spahn, T. W., A. Fontana, A. M. Faria, A. J. Slavin, H. P. Eugster, X. Zhang, P. A. Koni, N. H. Ruddle, R. A. Flavell, P. D. Rennert, and H. L. Weiner. 2001. Induction of oral tolerance to cellular immune responses in the absence of Peyer's patches. Eur. J. Immunol. 311278-1287. [DOI] [PubMed] [Google Scholar]
- 29.Teunis, P. F., C. L. Moe, P. Liu, S. E. Miller, L. Lindesmith, R. S. Baric, P. J. Le, and R. L. Calderon. 2008. Norwalk virus: how infectious is it? J. Med. Virol. 801468-1476. [DOI] [PubMed] [Google Scholar]
- 30.Thackray, L. B., C. E. Wobus, K. A. Chachu, B. Liu, E. R. Alegre, K. S. Henderson, S. T. Kelley, and H. W. Virgin IV. 2007. Murine noroviruses comprising a single genogroup exhibit biological diversity despite limited sequence divergence. J. Virol. 8110460-10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thornton, S. A., S. S. Sherman, T. Farkas, W. Zhong, P. Torres, and X. Jiang. 2005. Gastroenteritis in US Marines during Operation Iraqi Freedom. Clin. Infect. Dis. 40519-525. [DOI] [PubMed] [Google Scholar]
- 32.Turner, R. B., and R. B. Couch. 2006. Rhinoviruses, p. 895-910. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 5th ed., vol. 1. Lippincott, Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 33.Wherry, E. J., V. Teichgraber, T. C. Becker, D. Masopust, S. M. Kaech, R. Antia, U. H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4225-234. [DOI] [PubMed] [Google Scholar]
- 34.Whitacre, C. C., I. E. Gienapp, C. G. Orosz, and D. M. Bitar. 1991. Oral tolerance in experimental autoimmune encephalomyelitis. III. Evidence for clonal anergy. J. Immunol. 1472155-2163. [PubMed] [Google Scholar]
- 35.Wobus, C. E., S. M. Karst, L. B. Thackray, K.-O. Chang, S. V. Sosnovtsev, G. Belliot, A. Krug, J. M. Mackenzie, K. Y. Green, and H. W. Virgin. 2004. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2e432. [DOI] [PMC free article] [PubMed] [Google Scholar]