Abstract
The vaccinia virus double-stranded RNA binding protein E3 has been demonstrated to inhibit the expression of cytokines, including beta interferon (IFN-β) and tumor necrosis factor alpha (TNF-α). However, few details regarding the molecular mechanisms of this inhibition have been described. Using real-time PCR arrays, we found that E3 suppressed the induction of a diverse array of cytokines representing members of the IFN, interleukin (IL), TNF, and transforming growth factor cytokine families. We discovered that the factor(s) responsible for the induction of IL-6, TNF-α, and inhibin beta A (INHBA) was associated with the early and late phases of virus infection. In contrast, the factor(s) which regulates IFN-β induction was associated with the late phase of replication. We have found that expression of these cytokines can be induced by transfection of cells with RNA isolated from vaccinia virus-infected cells. Moreover, we provide evidence that E3 antagonizes both PKR-dependent and PKR-independent pathways to regulate cytokine expression. PKR-dependent activation of p38 and NF-κB was required for vaccinia virus-induced INHBA expression, whereas induction of TNF-α required only PKR-dependent NF-κB activation. In contrast, induction of IL-6 and IFN-β was largely PKR independent. IL-6 induction is regulated by NF-κB, while IFN-β induction is mediated by IFN-β promoter stimulator 1 and IFN regulatory factor 3/NF-κB. Collectively, these results indicate that E3 suppresses distinct but interlinked host signaling pathways to inhibit the expression of a diverse array of cytokines.
Vaccinia virus is the prototypic member of the Orthopoxvirus genus of the Poxviridae. Vaccinia virus has been used as a laboratory model to study more-pathogenic poxviruses, as it shares greater than 90% genome sequence similarity with variola virus, the causative agent of smallpox (46). Vaccinia virus, like variola virus, encodes a plethora of host immune response antagonists, including secreted viral homologues of the alpha/beta interferon (IFN-α/β) and IFN-γ receptors (B18R and B8R, respectively) (35). The vaccinia virus double-stranded RNA (dsRNA) binding protein, E3, is another such IFN antagonist, conferring resistance to the antiviral effects of human type I and II IFNs in human cells (1).
The E3 protein is composed of a C-terminal dsRNA binding domain and an N-terminal Z-DNA binding domain. Mutant vaccinia viruses lacking E3 (vvΔE3L) display a replication-defective phenotype in many human cell lines, including HeLa cells (2, 8). While only the C-terminal domain appears essential for virus replication in cell culture, both domains are required for pathogenesis in vivo (5). The major function of E3 in cell culture has been shown to be the inhibition of dsRNA-dependent protein kinase (PKR) activity (9, 27, 40, 42). Upon activation through dsRNA binding, PKR phosphorylates the Ser51 residue of the α subunit of the translation initiation factor eukaryotic initiation factor 2 (17). This leads to the inhibition of both host and viral protein synthesis, thereby inhibiting viral replication. The replication of vvΔE3L can be partially rescued in HeLa cells in which PKR expression is suppressed (53).
PKR has also been identified as a regulator of cytokine expression. In human and mouse cells, PKR regulates cytokine expression following virus infection or treatment of cells with the synthetic dsRNA mimic poly(I) (pI)-poly(C) (pIC) (7, 19, 33, 44). PKR can activate signaling by both p38 and nuclear factor κB (NF-κB) (17), known mediators of the cytokine response. p38, a member of the mitogen-activated protein kinase (MAPK) family, can regulate IFN signaling and the cytokine response to adenovirus infection (30, 33, 37). Upon treatment of cells with pIC, PKR specifically interacts with MAPK kinase 6 (MKK6), resulting in MKK6 phosphorylation and downstream activation of p38 (44). E3 has been reported to suppress p38 and MKK3/6 phosphorylation in vaccinia virus-infected cells via an unknown mechanism (28). In addition to p38, PKR functions in NF-κB activation (17). However, the roles of PKR, p38, and NF-κB in mediating vaccinia virus-induced cytokine expression have not been reported.
NF-κB can be activated by the cytosolic dsRNA sensor retinoic acid-inducible gene I (RIG-I) (50). Upon dsRNA binding, RIG-I is activated and interacts with IFN-β promoter stimulator 1 (IPS-1, also known as MAVS, VISA, and Cardif) present in the mitochondrial membrane (24). This interaction coordinates the formation of a signaling complex resulting in downstream activation of NF-κB, interferon regulatory factor 3 (IRF3), and IRF7. Activation of NF-κB and IRF pathways by IPS-1 requires IKK-γ, a regulatory component of the IKK complex (55). In this manner, the signaling downstream of IPS-1 to both NF-κB and IRF transcription factors is linked. Knowledge of the interaction between poxviruses and IPS-1-dependent signaling is limited. RIG-I can regulate the production of type I IFN and tumor necrosis factor alpha (TNF-α) in myxoma virus-infected primary human macrophages (47). Recently, IPS-1 was shown to be required for IFN-β and interleukin 6 (IL-6) expression following infection of mouse cells with a vaccinia virus E3 deletion mutant virus (14).
To date, the molecular mechanism(s) by which the vaccinia virus E3 protein inhibits cytokine expression remains largely unknown. In this study, we investigated the global effect of E3 on cytokine profiles and related signaling pathways in vaccinia virus-infected cells. Using cytokine real-time PCR arrays, we found that E3 suppresses expression of a diverse array of cytokines, including members of the IFN, IL, TNF, and transforming growth factor (TGF) cytokine families. To our knowledge, we provide the first direct evidence to demonstrate that RNA isolated from vaccinia virus-infected cells, likely viral dsRNA, can induce cytokine expression when introduced into cells. We identified a novel role for PKR in mediating vaccinia virus-induced p38 phosphorylation and NF-κB activation to coordinate the expression of the cytokine inhibin beta A (INHBA). PKR-dependent NF-κB activation was also found to regulate virus-induced TNF-α expression, while IL-6 induction requires NF-κB but is largely PKR independent. Furthermore, we describe a PKR-independent pathway regulated by IPS-1, NF-κB, and IRF3 as the major signaling cascade mediating vaccinia virus-induced IFN-β expression. Our data clearly demonstrate that the vaccinia virus E3 protein can antagonize induction of multiple cytokines through several distinct but overlapping pathways.
MATERIALS AND METHODS
Cell culture and viruses.
HeLa and BHK21 cells were maintained in Dulbecco's modified Eagle's medium (Gibco), supplemented with 10% fetal calf serum and 1% penicillin-streptomycin, at 37°C, 5% CO2. The revertant (vvΔE3L-Rev) and E3L deletion mutant (vvΔE3L) viruses (Copenhagen strain) were constructed as previously described (1). Based on vvΔE3L, recombinant vaccinia viruses expressing E3 with deletion of either the N-terminal domain (amino acids 1 to 80) (vvE3LΔN) or the last 25 amino acids of the C-terminal domain (vvE3LΔC25) were also constructed, according to previously described protocols (1). For infections, confluent cell monolayers in six-well plates were infected at a multiplicity of infection (MOI) of 5, in a 300-μl inoculum. After a 1-h incubation at 37°C, the inoculum was removed, and 1 ml of fresh Dulbecco's modified Eagle's medium containing 2% fetal calf serum was added to the cells.
Antibodies and reagents.
Antibodies for phosphorylated p38 (Thr180/Tyr182) and MK2 (Thr334) and total PKR, p38, and MK2 were purchased from Cell Signaling Technologies. Phosphospecific PKR (Thr446) and antibodies for total IRF3, NF-κB p65, and NF-κB p50 were from Epitomics. The β-actin antibody was from Sigma. The E3 monoclonal antibody was a generous gift from Stuart Isaacs, University of Pennsylvania (48). pI and pIC were purchased from GE Healthcare.
Transfections—siRNA.
HeLa cells (1.5 × 105) were seeded in six-well plates. Cells were transfected with 100 nM of small interfering RNA (siRNA; Dharmacon) using HiPerFect (Qiagen). In all experiments, cells were used 72 h posttransfection for experiments. The PKR siRNA target sequence was 5′-GCGAGAAACUAGACAAAGU-3′. The p38 siRNA target sequence was 5′-GGAAUUCAAUGAUGUGUAU-3′. The IPS-1 siRNA target sequence was 5′-CAUCCAAAGUGCCUACUAG-3′. The IRF3 siRNA target sequence was 5′-AGACAUUCUGGAUGAGUUA-3′. The NF-κB p65 siRNA target sequence was 5′-GGAUUGAGGAGAAACGUAA-3′. The NF-κB p50 siRNA target sequence was 5′-GAUGGGAUCUGCACUGUAA-3′. Cells were also transfected with 100 nM scrambled siRNA (Ambion) as a negative control.
Isolation and transfection of viral RNA.
Confluent HeLa cells were infected with vvΔE3L-Rev or vvΔE3L at an MOI of 5 and collected at 2 (early) and 8 (late) h postinfection (hpi). Cells were also pretreated with 50 μg/ml of cytosine arabinoside (araC), infected with vvΔE3L-Rev or vvΔE3L, and collected at 8 (late) hpi. Total RNA was isolated by TRIzol (Invitrogen) extraction, and residual genomic DNA contamination was removed using the Turbo DNA-free kit (Ambion). Duplicate samples of HeLa cell RNA and RNA from late-stage infections with vvΔE3L-Rev and vvΔE3L were also digested with RNase III (Ambion). HeLa cells were then either mock transfected or transfected with 5 μg of the TRIzol-extracted total RNA (described above) using Attractene (Qiagen), according to the manufacturer's protocol. Cells were also transfected with 1 μg of pI or pIC. Cells were collected 6 h posttransfection.
Western blotting.
Ten minutes before collection, cells were treated with 5 μl of Phosphatase Inhibitor Cocktail III (Calbiochem). Growth medium was then removed, and cells were collected in 1 ml of phosphate-buffered saline and pelleted by centrifugation at 6,000 rpm for 2 min. Cell pellets were lysed in 200 μl of 1× Laemmli buffer. Protein samples were separated on Criterion XT precast gels (Bio-Rad) and transferred to Hybond-C nitrocellulose membranes (Amersham Bioscience). Membranes were developed with Western Lightning Chemiluminescence Reagent Plus (Perkin-Elmer). Cellular and nuclear fractionation was performed using the NE-PER kit (Pierce) according to the manufacturer's protocol.
RT-PCR.
RNA was extracted using the RNeasy minikit (Qiagen). Reverse transcription-PCR (RT-PCR) was performed on 1.5 μg of RNA using the Advantage RT-For-PCR kit (Clontech). PCR was performed using GoTaq Green (Promega). The INHBA forward primer sequence was 5′-ATGCCCTTGCTTTGGCTGAGAGGAT-3′, and the reverse primer sequence was 5′-TGCTGGAAGAGGCGGATGGT-3′. The IL-6 forward primer sequence was 5′-ATGAACTCCTTCTCCACAAGCGCCT-3′, and the reverse primer sequence was 5′-ATTTGTGGTTGGGTCAGG-3′. The TNF-α forward primer sequence was 5′-ATGAGCACTGAAAGCATGATCCGGG-3′, and the reverse primer sequence was 5′-TGGTAGGAGACGGCGATGC-3′. The IFN-β forward primer sequence was 5′-TGTCTCCTCCAAATTGCTCTC-3′, and the reverse primer sequence was 5′-TCCTTGGCCTTCAGGTAATG-3′. The A17 forward primer sequence was 5′-ATGAGTTATTTAAGATATTACAATATGCTT-3′, and the reverse primer sequence was 5′-TCGTCAGTATTTAAACTGTTAAATGTTGGT-3′. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward primer sequence was 5′-AAGGTGAAGGTCGGAGTCAA-3′, and the reverse primer sequence was 5′-TTACTCCTTGGAGGCCATGT-3′.
Real-time PCR arrays.
The RT2 Profiler PCR Array System/Common Human Cytokines (SABiosciences) was used to quantify cytokine mRNA expression. Confluent HeLa cells in six-well plates were infected with vvΔE3L-Rev or vvΔE3L at an MOI of 5 and collected 12 hpi. RNA was extracted using the RNeasy kit (Qiagen). Residual genomic DNA contamination was removed using the Turbo DNA-free kit (Ambion). RNA from each sample (0.75 μg) was reverse transcribed into cDNA using the RT2 First Strand kit (SABiosciences). Real-time PCR was performed using the RT2 SYBR green qPCR Master Mix (SABiosciences) on an ABI 7500 Fast 96-well real-time PCR machine (Applied Biosystems). Results were analyzed using the PCR Array Data Analysis Web Portal (SABiosciences). GAPDH and β-actin were chosen as internal loading controls for standardization between samples.
RESULTS
E3 suppresses expression of a diverse array of cytokines.
The effect of E3 on cytokine expression was investigated using real-time PCR arrays. The relative expression of 84 cytokines, including IFNs; ILs; and members of the TNF, TGF, and platelet-derived growth factor/vascular endothelial growth factor superfamilies, was determined. HeLa cells were mock infected or infected with a vaccinia E3L deletion virus (vvΔE3L) or a revertant virus based on vvΔE3L with the E3L gene reinserted into the viral genome (vvΔE3L-Rev) (1). Cytokines detected with a threshold cycle value of ≥35 were scored as not significantly expressed and excluded from the analysis. Infection of cells with both viruses induced detectable expression of the majority of cytokines (Table 1). Many cytokines were regulated to similar degrees by the two viruses. For example, IFN-α8 was upregulated 8.7-fold (vvΔE3L-Rev) and 7.4-fold (vvΔE3L) compared to mock-infected cells. The expression of several cytokines was unaffected by infection with either virus, including IL-1β, colony-stimulating factors 1 and 2, and BMP8B. While cellular stress due to the cytopathic effect of infection could explain the increased cytokine expression to some degree, infection of cells with vvΔE3L, compared to vvΔE3L-Rev, resulted in significant upregulation of several proinflammatory and antiviral cytokines. IFN-β, TNF-α, and INHBA were all upregulated ∼10-fold in vvΔE3L-infected cells compared to cells infected with vvΔE3L-Rev. IL-6, IL-8, IL-20, and lymphotoxin A were also upregulated by 4.2-, 4.1-, 8.3-, and 4.5-fold, respectively, in vvΔE3L-infected cells. IL-12A, ILIF7, and MSTN were more strongly induced by infection with vvΔE3L-Rev than by infection with vvΔE3L.
TABLE 1.
E3 inhibits cytokine gene transcription in vaccinia virus-infected cellsa
| Cytokine | Fold change in expression of cells infected with virus:
|
|
|---|---|---|
| vvΔE3L-Rev | vvΔE3L | |
| IL family | ||
| IL-20 | 16.9692 | 141.1612 |
| IL-8 | 5.382 | 21.8961 |
| IL-6 | 5.1563 | 21.6997 |
| IL-13 | 26.1847 | 20.21 |
| IL-11 | 6.3685 | 17.7556 |
| IL-21 | 12.6674 | 14.0734 |
| IL-12B | 8.2132 | 13.6943 |
| IL-12A | 67.2448 | 10.6634 |
| IL-24 | 3.2867 | 9.9894 |
| IL-1α | 2.9279 | 8.2867 |
| IL-22 | 13.086 | 7.3554 |
| IL-1F7 | 24.1199 | 6.3939 |
| IL-15 | 4.8566 | 6.2441 |
| IL-19 | 1.2502 | 5.8288 |
| IL-17C | 6.3575 | 5.6934 |
| IL-18 | 3.157 | 5.0919 |
| TXLNA | 4.7878 | 3.223 |
| IL-7 | 1.9657 | 2.6799 |
| IL-1β | 2.0006 | −1.0476 |
| IFN family | ||
| IFN-β1 | 8.1181 | 84.4134 |
| IFN-α5 | 5.8801 | 10.0763 |
| IFN-α8 | 8.7171 | 7.4276 |
| IFN-α2 | 13.7175 | 7.3554 |
| IFN-α4 | 15.3275 | 7.3554 |
| IFN-κ | 12.3612 | 7.2627 |
| IFN-α1 | 4.2923 | 2.8206 |
| Platelet-derived/vascular endothelial growth factor family | ||
| FIGF | 8.5923 | 3.3792 |
| Platelet-derived growth factor A | 1.5096 | −1.1755 |
| TNF family | ||
| TNF | 12.9542 | 123.7856 |
| Lymphotoxin A | 8.2132 | 37.2173 |
| TNFSF14 | 10.2351 | 14.0802 |
| Lymphotoxin B | 8.2132 | 11.3806 |
| TNFSF13 | 8.2132 | 9.4342 |
| TNFSF11 | 8.9952 | 6.602 |
| TNFSF13B | 7.7235 | 6.4773 |
| TNFRSF11B | −1.1347 | 3.8552 |
| TNFSF12 | −1.1514 | 2.1245 |
| CD70 | 2.0485 | 1.5409 |
| TNFSF10 | −2.5634 | −4.2167 |
| TGF family | ||
| INHBA | 3.3791 | 36.4311 |
| GDF5 | 8.2132 | 26.2819 |
| BMP5 | 13.619 | 15.9358 |
| GDF9 | 7.9142 | 12.1291 |
| GDF10 | 1.8031 | 11.1232 |
| BMP2 | 7.2927 | 10.1675 |
| TGF-β2 | 3.5065 | 7.4648 |
| MSTN | 21.1129 | 7.3554 |
| BMP6 | 6.0241 | 5.1798 |
| BMP4 | 3.3497 | 4.57 |
| INHA | 4.7689 | 4.2708 |
| NODAL | 2.8135 | 2.5196 |
| BMP1 | 3.7465 | 2.0967 |
| TGF-β3 | 5.2204 | 1.9836 |
| GDF11 | 1.6646 | 1.5154 |
| TGF-α | 3.1955 | 1.3995 |
| BMP8B | 1.5024 | −1.1093 |
| TGF-β1 | 1.5958 | −1.3357 |
| Other | ||
| Colony-stimulating factor 2 | 1.9693 | −1.0382 |
| Colony-stimulating factor 1 | −1.2033 | −1.7845 |
Confluent HeLa cells in six-well plates were either mock infected or infected with vvΔE3L-Rev or vvΔE3L at an MOI of 5. Cells were collected at 12 hpi for analysis. Extracted RNA (0.75 μg) was reverse transcribed from each sample, and real-time PCR arrays to detect common human cytokines were used. GAPDH and β-actin were used to normalize the mRNA levels between samples. Cytokines displaying a threshold cycle value of ≥35 were defined as not significantly detected and excluded from the analysis. Results are shown as the change in expression of infected cells in comparison to mock-infected cells and are representative of three independent experiments.
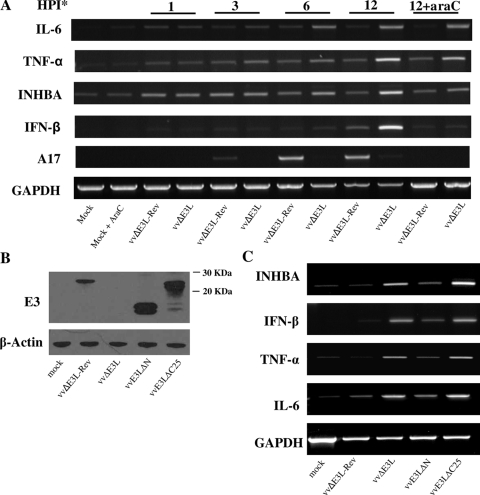
We then sought to determine whether the observed cytokine induction requires viral DNA replication by pretreating cells with araC, an inhibitor of DNA replication. Cells were infected with vvΔE3L-Rev or vvΔE3L and collected at several time points postinfection. The expression of IFN-β, TNF-α, IL-6, and INHBA was determined by RT-PCR (Fig. 1A). A slight upregulation of cytokine expression was observed by 1 hpi. At late times postinfection (12 hpi), we observed significant induction of TNF-α, IFN-β, and INHBA expression in cells infected with vvΔE3L but not vvΔE3L-Rev. IL-6 expression was induced by vvΔE3L at 6 hpi and sustained until 12 hpi. Pretreatment of cells with araC abolished the induction of IFN-β in cells infected with vvΔE3L. In contrast, induction of IL-6 was unaffected by araC treatment. Induction of INHBA was greatly reduced by araC, whereas TNF-α induction was only slightly reduced by araC. To ensure that araC treatment had blocked viral DNA replication, we determined the expression level of the viral A17 gene, which is a late gene and requires viral DNA replication to be transcribed. We found that A17 expression was completely blocked in araC-treated cells (Fig. 1A). The replication of vvΔE3L is inhibited at the stage of intermediate protein synthesis (31, 53), and thus, A17 is not expressed by vvΔE3L. The expression of GAPDH was measured as a loading control. The level of GAPDH remained constant except at late times (6 and 12 hpi), when both viruses induced a shutoff in host gene expression, as previously described (34). Pretreatment of cells with araC was able to restore GAPDH transcription to levels comparable to those in mock-infected cells.
FIG. 1.
Induction of cytokines by virus infection. (A) Confluent HeLa cells in six-well plates were either mock infected or infected with vvΔE3L-Rev or vvΔE3L at an MOI of 5 and collected at the times indicated. Select wells were also treated with 50 μg/ml of araC 1 h prior to infection. Cytokine expression was analyzed by RT-PCR. (B) Confluent BHK21 cells were infected with vvΔE3L-Rev, vvΔE3L, vvE3LΔN, or vvE3LΔC25 at an MOI of 5 and collected at 6 hpi. The expression of E3 and β-actin was determined by Western blotting. (C) Confluent HeLa cells were mock infected or infected with vvΔE3L-Rev, vvΔE3L, vvE3LΔN, or vvE3LΔC25 at an MOI of 5. Cells were collected at 12 hpi, and RNA was extracted for RT-PCR.
To determine the role of the N-terminal Z-DNA binding and C-terminal dsRNA binding domains of E3 in the inhibition of cytokine expression, HeLa cells were infected with vvΔE3L-Rev, vvΔE3L, or vaccinia virus recombinants expressing E3 with deletions of the last 25 amino acids of the C-terminal domain (vvE3LΔC25) or the entire N-terminal domain (vvE3LΔN). Previously, it has been shown that mutation of several residues in the terminal 25 amino acids of E3 abolishes its dsRNA binding function (21). To verify the expression of E3 in the recombinant viruses, BHK21 cells were infected at an MOI of 5 and collected at 6 hpi. We observed the correct size of the E3 truncations expressed by the recombinant viruses (Fig. 1B). Next, HeLa cells were infected with the recombinant vaccinia viruses and collected at 12 hpi. Cells infected with vvΔE3L and vvE3LΔC25 expressed significantly higher levels of IFN-β, TNF-α, IL-6, and INHBA than did cells infected with vvΔE3L-Rev or vvE3LΔN (Fig. 1C). Expression of IFN-β, TNF-α, and INHBA was also slightly higher in cells infected with vvE3LΔN than in vvΔE3L-Rev-infected cells.
PKR, p38, and IPS-1 mediate vvΔE3L-induced cytokine expression.
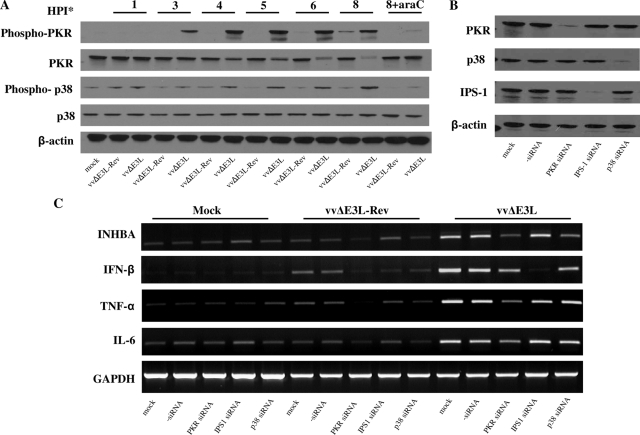
E3 has been demonstrated to inhibit PKR and p38 activation (28). However, the mechanism by which E3 inhibits p38 phosphorylation is not known. Moreover, both PKR and p38 are known to regulate the cytokine response to virus infection (7, 19, 37), but it remains to be determined whether these proteins play a role in vaccinia virus-induced cytokine expression. Therefore, to study the signaling pathways which may regulate cytokine expression in vaccinia virus-infected cells, we investigated the roles of PKR and p38 activation, as measured by phosphorylation, following infection of HeLa cells with vvΔE3L-Rev or vvΔE3L. The levels of total and phosphorylated p38 and PKR were determined by Western blotting, and the level of β-actin was determined as a loading control. Both viruses induced a minimal, transient increase in phosphorylated p38 by 1 hpi which returned to basal levels by 3 hpi (Fig. 2A). A sustained increase in PKR and p38 phosphorylation was detected in vvΔE3L-infected cells as early as 3 and 4 hpi, respectively. At 8 hpi, the difference in phosphorylation of p38 was most dramatic. The total level of p38 and β-actin remained constant. The total PKR band appeared as a double band, was less intense in vvΔE3L-infected cells after 3 hpi, and correlated with increased phosphorylation of PKR. Infection of cells with vvΔE3L-Rev resulted in minimal PKR phosphorylation at late times postinfection. The phosphorylation of p38 and PKR during vvΔE3L infection could be blocked by pretreatment of cells with araC.
FIG. 2.
PKR, p38, and IPS-1 mediate the cytokine response to vvΔE3L. (A) Confluent HeLa cells in six-well plates were either mock infected or infected with vvΔE3L-Rev or vvΔE3L at an MOI of 5. Cells were collected at the times indicated. Selected wells were also treated with 50 μg/ml of araC 1 h prior to infection. Western blotting was performed to detect total and phosphorylated PKR and p38. (B) HeLa cells (1.5 × 105) were seeded in six-well plates. Cells were then transfected with 100 nM PKR-, p38-, or IPS-1-specific siRNAs for 72 h. Western blotting was performed to determine the efficiency of the siRNA knockdown. (C) Cells were transfected as described above and then either mock infected or infected with vvΔE3L-Rev or vvΔE3L at an MOI of 5. Cells were collected at 12 hpi, and RNA was extracted for RT-PCR. Results are representative of three independent experiments.
We next sought to directly determine whether PKR and p38 play a role in vaccinia virus-induced cytokine expression. We also chose to study IPS-1, which has recently been reported to mediate vaccinia virus-induced IFN-β and IL-6 in mouse cells (14). Four different siRNAs targeting PKR, p38, or IPS-1 were tested for their ability to suppress target expression (data not shown). The most effective siRNA was used in all subsequent experiments. HeLa cells were transfected with PKR-, p38-, or IPS-1-specific siRNAs and then infected with either vvΔE3L-Rev or vvΔE3L. The siRNA knockdown efficiency was determined by Western blotting (Fig. 2B). Expression of cytokines in infected cells was analyzed by RT-PCR (Fig. 2C). The level of GAPDH was determined as a loading control. Ablation of PKR expression resulted in a significant decrease in TNF-α, INHBA, and, to a much lesser degree, IL-6 and IFN-β induction by vvΔE3L. Suppression of p38 expression reduced the induction of INHBA but did not affect the other cytokines tested. In contrast, transfection with IPS-1 siRNAs abolished IFN-β induction but did not affect the induction of TNF-α, INHBA, or IL-6. It should be noted from Fig. 2C that none of the siRNAs used activated the IFN response as measured by IFN-β expression.
PKR is required for p38 activation during vvΔE3L infection.
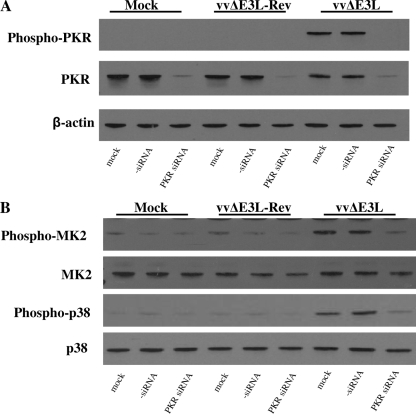
PKR has been identified as an upstream mediator of p38 activation and cytokine expression (44). Both PKR and p38 were observed to play a role in INHBA expression (Fig. 2C). Given that E3 is a known PKR inhibitor, we hypothesized that E3 inhibits p38 activation through a PKR-dependent mechanism. To address this possibility, we suppressed PKR expression in HeLa cells by using PKR-specific siRNAs and infected the cells with either vvΔE3L-Rev or vvΔE3L. PKR expression was significantly abrogated in cells transfected with PKR-specific siRNAs, and the phosphorylation of PKR was undetectable (Fig. 3A). vvΔE3L-induced phosphorylation of p38 was blocked when PKR expression was suppressed (Fig. 3B). Furthermore, in cells in which PKR expression was suppressed, the phosphorylation of MK2, a substrate of activated p38, was abolished.
FIG. 3.
E3 inhibits p38 activation through a PKR-dependent mechanism. (A) HeLa cells (1.5 × 105) were seeded in six-well plates. Cells were then transfected with 100 nM PKR-specific siRNAs or control siRNAs for 72 h. Cells were then either mock infected or infected with vvΔE3L-Rev or vvΔE3L at an MOI of 5 and collected at 6 hpi for Western blot analysis of the knockdown efficiency. (B) Cells were treated as described above, and the effect of PKR knockdown on p38 signal transduction was determined by Western blotting. Results are representative of three independent experiments.
NF-κB and IRF3 are required for vvΔE3L-induced cytokine expression.
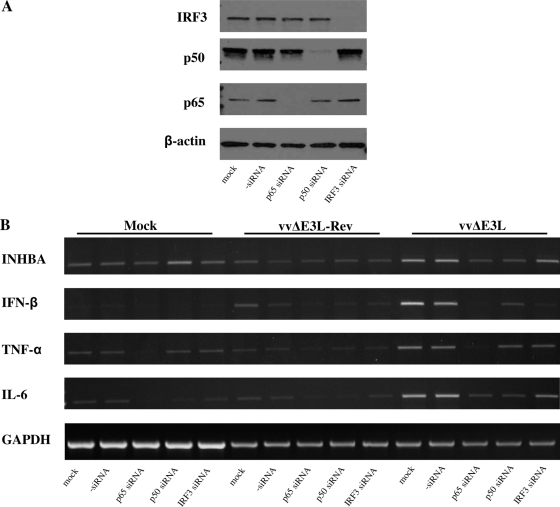
IPS-1 mediates IFN-β induction through activation of NF-κB and IRF3 (24). As shown in Fig. 2C, IPS-1 mediates vvΔE3L-induced IFN-β expression. Therefore, we attempted to determine the role of NF-κB and IRF3 in the cytokine response to vaccinia virus. HeLa cells were transfected with NF-κB p65, NF-κB p50, or IRF3 siRNAs and infected with vvΔE3L-Rev or vvΔE3L. The siRNA knockdown efficiency was determined by Western blotting (Fig. 4A). The expression of INHBA, IFN-β, TNF-α, and IL-6 was determined by RT-PCR (Fig. 4B). The expression of INHBA, IFN-β, TNF-α, and IL-6 was significantly reduced in vvΔE3L-infected cells treated with p65 siRNAs, while the expression of INHBA, IFN-β, and IL-6, but not TNF-α, was reduced in cells treated with NF-κB p50 siRNAs. In contrast, the expression of IFN-β, but not INHBA, TNF-α, or IL-6, was significantly reduced in vvΔE3L-infected cells treated with IRF3 siRNAs.
FIG. 4.
E3 suppresses cytokine expression through NF-κB- and IRF3-mediated pathways. (A) HeLa cells (1.5 × 105) were seeded in six-well plates. Cells were then transfected with 100 nM NF-κB p65-, NF-κB p50-, or IRF3-specific siRNAs for 72 h. Western blotting was performed to determine the efficiency of the siRNA knockdown. (B) Cells were transfected as described above and then either mock infected or infected with vvΔE3L-Rev or vvΔE3L at an MOI of 5. Cells were collected at 12 hpi, and RNA was extracted for RT-PCR. Results are representative of three independent experiments.
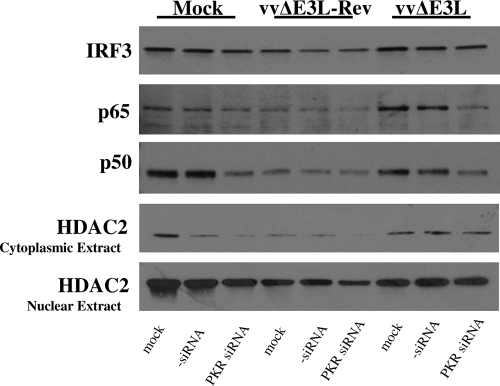
Previous work has demonstrated that E3 can inhibit NF-κB nuclear translocation, but the role of PKR in this process was not determined (28). Given that both PKR and NF-κB mediated vvΔE3L-induced TNF-α and INHBA expression, we investigated the role of PKR in regulating NF-κB nuclear translocation, a hallmark of NF-κB activation. HeLa cells were transfected with PKR-specific siRNAs and infected with vvΔE3L-Rev or vvΔE3L. Nuclear extracts were prepared at 8 hpi for Western blotting. As a control to determine fractionation efficiency, the level of histone deacetylase 2, an enzyme localized in the nucleus, was determined in both cytoplasmic and nuclear fractions. High levels of histone deacetylase 2 were detected in the nuclear fraction, while only a weak signal was observed in the cytoplasmic fraction (Fig. 5). Significant nuclear translocation of p65 was observed in vvΔE3L-infected cells. However, when PKR expression was suppressed in vvΔE3L-infected cells, p65 nuclear translocation was blocked. In comparison to vvΔE3L-Rev-infected cells, more NF-κB p50 was present in the nucleus of vvΔE3L-infected cells. Suppression of PKR expression led to a decrease in nuclear p50 in vvΔE3L- and mock-infected cells, to a level comparable with that in vvΔE3L-Rev-infected cells. In contrast, suppression of PKR expression did not affect the level of IRF3 in the nucleus. Suppression of PKR expression also had no effect on the level of p65, p50, or IRF3 in cytoplasmic protein fractions (data not shown).
FIG. 5.
E3 antagonizes PKR-dependent NF-κB nuclear translocation. HeLa cells (1.5 × 105) were seeded in six-well plates. Cells were then transfected with 100 nM PKR siRNA for 48 h. Cells were then mock infected or infected with vvΔE3L-Rev or vvΔE3L at an MOI of 5, and nuclear extracts were prepared at 8 hpi for Western blotting. Results are representative of three independent experiments. HDAC2, histone deacetylase 2.
Vaccinia virus-infected cells contain RNA species capable of inducing cytokine expression.
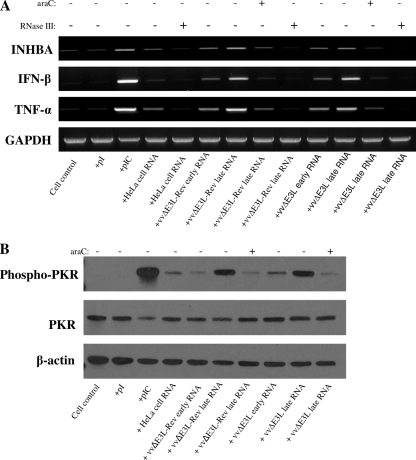
Since it is generally believed that transcription of poxvirus intermediate and late genes can produce viral dsRNA species (4, 12, 31), we sought to determine whether transfection of cells with total RNA isolated from vaccinia virus-infected HeLa cells could induce cytokine expression. HeLa cells were mock infected or infected with vvΔE3L-Rev or vvΔE3L at an MOI of 5 and collected at 2 (early) and 8 (late) hpi. Cells were also treated with araC to inhibit viral DNA replication and intermediate/late gene transcription and collected at 8 hpi. TRIzol-extracted total RNA was digested with DNase to remove residual genomic DNA contamination, and 5 μg of each sample was transfected into HeLa cells. Prior to transfection, a set of RNA samples was also digested with RNase III, a nuclease specific for dsRNA. Cells were also transfected with 1 μg of pI or pIC. The expression of IFN-β, TNF-α, INHBA, and GAPDH was determined by RT-PCR (Fig. 6A). Transfection of HeLa cells with pIC, but not pI, resulted in IFN-β, TNF-α, and INHBA induction. Transfection of HeLa cells with RNA from mock-infected HeLa cells was found to induce minimal cytokine expression. RNA from HeLa cells collected at early times after infection with vvΔE3L-Rev and vvΔE3L induced cytokine expression to a degree comparable to that induced by the cellular RNA alone. In contrast, when RNA collected at late times postinfection was introduced into cells, we observed a significant induction of IFN-β and TNF-α. INHBA expression was induced to a lesser degree, in comparison to IFN-β and TNF-α, by both pIC and late-stage RNA. However, transfection of RNA from cells treated with araC and collected at late times postinfection induced cytokine expression to levels comparable to that observed for transfection of cellular RNA or RNA collected at early times postinfection. Pretreatment of RNA samples with RNase III abolished the observed induction of cytokines. We also attempted to determine whether IL-6 expression could be induced by transfection of cells with late-stage RNA. However, our results were inconclusive, as IL-6 was induced more strongly by late RNA but we were unable to completely abolish this induction by RNase III or RNase A digestion (data not shown).
FIG. 6.
Induction of cytokine expression and PKR phosphorylation by RNA associated with vaccinia virus infection. (A) Confluent HeLa cells were infected with vvΔE3L-Rev or vvΔE3L at an MOI of 5 and collected at early (2 hpi) or late (8 hpi) times postinfection. Cells were also pretreated with 50 μg/ml of araC 1 h prior to infection, infected with vvΔE3L-Rev or vvΔE3L, and collected at 8 hpi. Total RNA samples were extracted with TRIzol and digested with DNase. Five micrograms of each RNA sample or 1 μg pI or pIC was then transfected into HeLa cells. RNA samples were also pretreated prior to transfection with RNase III. Cells were collected at 6 h posttransfection, and cytokine expression was examined by RT-PCR. (B) HeLa cells were transfected with the RNA samples described for panel A and collected at 6 h posttransfection. The levels of β-actin, PKR, and phosphorylated PKR were examined by Western blotting.
The activation of PKR was also examined following transfection of the above DNase-digested RNA samples into HeLa cells (Fig. 6B). We observed minimal PKR activation in cells transfected with cellular RNA, RNA from early-stage infections, or RNA from late-stage infections treated with araC. In contrast, robust PKR activation was observed in cells transfected with RNA from late-stage infections.
DISCUSSION
Modulation of cellular signaling cascades is a common mechanism by which viruses suppress the host immune response. Although E3 has been reported to suppress cytokine expression, the signaling pathways regulating vaccinia virus-induced cytokine expression remain largely unknown. In this report, we investigated the molecular mechanisms by which the vaccinia virus E3 protein inhibits cytokine expression. We found that E3 suppresses cytokine induction by antagonizing several distinct but interlinked signaling cascades. The primary role of PKR in the response to vaccinia virus has to date centered on the regulation of translation through phosphorylation of eukaryotic initiation factor 2α. Although E3 has been previously shown to inhibit both p38 and NF-κB (28), as well as cytokine expression (13), no direct data have been provided to link these processes to PKR. Here we detail, in the context of vaccinia virus infection, the role of PKR in regulating cytokine expression through activation of p38 and NF-κB activity. Therefore, our results expand upon the current knowledge of the role of PKR in the antiviral response to vaccinia virus and help to explain how p38 and NF-κB are activated following vaccinia virus infection.
Using real-time PCR arrays, we identified a subset of cytokines whose expression is inhibited by E3 and which includes members of the IFN, IL, TNF, and TGF cytokine families (Table 1). The Z-DNA binding domain of E3 appears to be largely dispensable for the inhibition of cytokine expression. However, cells infected with vvE3LΔN expressed slightly higher levels of IFN-β, TNF-α, and INHBA than did cells infected with vvΔE3L-Rev. Although the C-terminal domain of E3 likely mediates most of the PKR-inhibitory effect, the N-terminal domain of E3 has also been demonstrated to play a role in PKR inhibition at late times postinfection (28). Therefore, the limited induction of these cytokines by the vvE3LΔN virus may result from incomplete inhibition of PKR or other host antiviral factors.
Poxvirus genomes are transcribed via a cascade-like mechanism, with the transcription of early genes occurring prior to viral DNA replication, while intermediate and late genes are transcribed only after viral DNA replication (6). It is generally believed that poxvirus intermediate and late gene transcripts are the major source of viral dsRNA (4, 12, 31). Vaccinia virus-induced cytokine expression was differentially sensitive to treatment with araC, a potent inhibitor of DNA replication (Fig. 1A). araC treatment abolished vvΔE3L-induced IFN-β expression, suggesting that a pathogen-associated molecular pattern (PAMP) associated with the intermediate/late phase of virus infection (“late” PAMP) is essential for the induction of IFN-β. In contrast, IL-6 induction is not sensitive to araC treatment, while TNF-α and INHBA are partially sensitive. Thus, a PAMP(s) associated with the early phase of replication (“early” PAMP) may be capable of inducing IL-6, TNF-α, and INHBA expression. However, araC blocked PKR activation, which may be required for efficient expression of these cytokines. Thus, our results also suggest the role of “late” PAMPs in induction of IL-6, TNF-α, and INHBA. Furthermore, although araC inhibits PKR phosphorylation, araC is unlikely to affect any function of PKR in which it plays a purely structural role. It has been reported that catalytically inactive PKR mutants can still activate NF-κB under some conditions (11, 22). This may explain why IL-6 induction, which is minimally PKR dependent, is unaffected by araC. Taken together, our data suggest that E3 is capable of suppressing expression of cytokines induced by both “early” and “late” PAMPs.
dsRNA species associated with vaccinia virus intermediate and late gene transcription have been suggested to be one of the major PAMPs responsible for inducing cellular innate immune responses. However, no study has been reported to directly confirm the role of RNA species associated with vaccinia virus replication in induction of such innate immune responses. In this report, we demonstrate that a robust cytokine response can be induced in HeLa cells transfected with total RNA collected from late-stage vaccinia virus infections (Fig. 6A). Further, transfection of these late-stage RNA samples into cells results in robust PKR activation (Fig. 6B). It might be expected that vvΔE3L-Rev-infected cells would contain more dsRNA than would vvΔE3L-infected cells given that vvΔE3L-Rev produces both intermediate and late viral transcripts associated with the ability to form dsRNA, while vvΔE3L produces only intermediate transcripts. However, we found that cytokine induction and PKR activation were equally induced by late-stage RNA from vvΔE3L-Rev- and vvΔE3L-infected cells. It is possible that the vvΔE3L virus produces dsRNA quantities above a threshold of dsRNA required for maximal induction of these responses and therefore that RNAs from cells infected with either virus induce cytokine expression and PKR activation to comparable degrees. Interestingly, we found that total RNAs isolated from uninfected HeLa cells or cells with early infections or late infections treated with araC induced slight cytokine expression and PKR phosphorylation. dsRNA species such as double-stranded primary microRNAs (which naturally exist in the nucleus) are isolated in total RNA extracts. Therefore, transfection of these RNA samples into HeLa cells is likely to introduce dsRNA into the cytoplasm. This may account for the observed induction of cytokines and PKR activation even in cells transfected with cellular HeLa RNA.
The enhanced cytokine response in cells transfected with RNA from late-stage infected cells suggests that RNA species associated with virus infection can induce cytokine expression and activate PKR. Given that araC treatment blocked the upregulation of cytokines and PKR activation induced by RNA collected at late times postinfection, we propose that vaccinia virus RNA, most likely dsRNA, is recognized by host cells, resulting in cytokine expression. That pretreating this RNA prior to transfection with RNase III, a nuclease specific for dsRNA, abolished the ability of the preparation to induce cytokine expression further supports the role of dsRNA as the activating molecule. In addition, we found that transfection of HeLa cells with single-stranded RNA molecules such as pI (Fig. 6A) or in vitro-transcribed single-stranded enhanced green fluorescent protein RNA does not induce cytokine expression or PKR phosphorylation (data not shown). Collectively, these results suggest that dsRNA and not single-stranded RNA is the activator of PKR and cytokine expression. We cannot rule out the possibility that other PAMPs activate IL-6, INHBA, and TNF-α expression, as induction of these cytokines is not completely blocked by araC. It remains possible that a virally induced cellular RNA could also function as an activator of cytokine expression. To our knowledge, this is the first direct evidence to demonstrate that RNA species produced within vaccinia virus-infected cells are capable of initiating an innate immune response including PKR activation and cytokine induction.
The signaling pathways which may recognize these RNA species to mediate cytokine induction were investigated. PKR contributes to vvΔE3L-induced TNF-α, INHBA, and, to a much lesser degree, IL-6 and IFN-β gene transcription (Fig. 2C). We provide direct evidence that PKR is required for p38 activation in response to vvΔE3L (Fig. 3B). Although E3 has been reported to inhibit p38 activation, we demonstrate conclusively that E3 blocks INHBA induction by antagonizing PKR-dependent p38 activation. Efficient induction of INHBA requires both PKR and p38, and thus, PKR-mediated p38 activation likely plays a key role in the regulation of this cytokine.
In addition to PKR and p38, INHBA expression also requires NF-κB. We found that E3 suppresses PKR-dependent NF-κB nuclear translocation (Fig. 5). p38 is known to regulate NF-κB phosphorylation and transcriptional activity (23, 52). Infection of cells with vvΔE3L, but not vvΔE3L-Rev, resulted in PKR-dependent activation of p38 (Fig. 3B). Thus, the induction of INHBA in vvΔE3L-infected cells may potentially be explained by the activation of the p38 signaling and its regulation of NF-κB function. Moreover, MK2, a substrate of activated p38, was phosphorylated to its active state in a PKR-dependent manner in cells infected with vvΔE3L. Activation of the p38-MK2 pathway can enhance the stability of cytokine mRNAs harboring A/U-rich elements within the 3′ untranslated region (26, 29). Therefore, it is also possible that an MK2-mediated enhancement of INHBA mRNA stability is responsible for the increase in INHBA mRNA levels observed. Collectively, our results are the first to provide direct evidence of the mechanism by which E3 suppresses p38 activation and NF-κB nuclear translocation.
Efficient induction of TNF-α expression requires PKR and NF-κB but not p38. Therefore, the pathway(s) regulating TNF-α expression is distinct from that for INHBA. PKR has been demonstrated to activate the IKK complex and NF-κB-dependent transcription (51). The N-terminal domain of PKR was identified as the region responsible for the interaction with, and activation of, IKKβ and NF-κB (3). We demonstrate that E3 suppresses TNF-α and INHBA expression by inhibiting PKR-dependent NF-κB activation. The fact that TNF-α induction requires only p65 while INHBA requires both p65 and p50 supports the concept that several distinct but interlinked pathways regulate the cytokine response to vaccinia virus. Several other vaccinia virus proteins capable of inhibiting NF-κB have been identified (10, 15, 18, 20, 43). Even though the vvΔE3L virus expresses these NF-κB inhibitors, we still observed NF-κB nuclear translocation, in agreement with previous reports (28). Therefore, these proteins are unable to fully inhibit the activation of NF-κB following infection of cells with vvΔE3L. It is possible that PKR activation in the absence of E3 signals to NF-κB through a mechanism not targeted by the described vaccinia virus NF-κB inhibitors.
The pathway mediating IL-6 and IFN-β expression differs from the TNF-α and INHBA pathways in several ways. First, induction of IL-6 and IFN-β gene transcription is largely PKR independent. Second, activation of the IFN-β pathway is highly sensitive to inhibition of viral DNA replication, whereas activation of the IL-6 pathway is unaffected by inhibition of viral DNA replication. We found that PKR does not regulate IRF3 nuclear translocation or induction of IFN-β gene transcription. It was recently reported that PKR does mediate IRF3 nuclear translocation in response to vvΔE3L (54). The explanation for this conflict remains unclear and could be due to experimental conditions. However, our results are supported by the finding that loss of PKR from mouse cells does not affect the induction of IFN-β by vvΔE3L (49). Moreover, E3 inhibits Newcastle disease virus-induced IRF3 phosphorylation in a PKR-independent manner (45). Similarly to a recent report for murine cells (14), we have also identified IPS-1 as a major regulator of IFN-β gene transcription following infection of human HeLa cells with vvΔE3L and found this process to require viral DNA replication (Fig. 1A and 2C). In mouse keratinocytes, E3 inhibited IL-6 expression through an IPS-1-dependent mechanism (14). In our study, we found that IPS-1 was dispensable for IL-6 expression. This discrepancy may be due to differences in species- and tissue-specific immune responses and/or the method of gene silencing.
Downstream of IPS-1, activation of both IRF3 and NF-κB has been shown to regulate IFN-β expression (24). IRF3 activity is thought to be essential for IFN-β expression, while in some systems, NF-κB may be dispensable for IFN-β induction (38). In the context of vvΔE3L infection, we found that the combined function of NF-κB and IRF3 is essential in activation of IFN-β gene transcription (Fig. 4B). IFN-β was the only cytokine tested which required IRF3 expression, again demonstrating that unique pathways mediate vaccinia virus-induced cytokine expression.
Several of the cytokines suppressed by E3 have been demonstrated to play a pivotal role in host defense against vaccinia virus infection in vitro and in vivo. For example, IL-6-deficient mice display a defective cytotoxic T-cell response against vaccinia virus and support 1,000-fold-greater viral replication in the lungs (25). It has been reported that the vaccinia virus-specific T-cell response is diminished in transgenic mice expressing E3 (16). The cytokine activin A, a homodimer of INHBA subunits, can regulate the differentiation of monocytes into dendritic cells and enhance antibody production by B cells (36, 41). Moreover, natural killer cells are activated to proliferate and secrete effector molecules during vaccinia virus infection in a manner dependent on type I IFN receptor signaling (32). Thus, the enhanced innate cytokine response which occurs in the absence of E3 may help explain the replication-defective phenotype of the vvΔE3L virus in mice. While vaccinia virus replication has been shown to be sensitive to IFN treatment in mice (39), it would also be of interest to determine whether TNF-α, IL-6, or INHBA can limit vaccinia virus replication in vivo.
In summary, our data clearly demonstrate that the vaccinia virus E3 protein can suppress a diverse array of cytokines (including members of the IL, IFN, TNF, and TGF-β families) whose induction involves both “early” and “late” PAMPs associated with vaccinia virus infection. Although vaccinia virus dsRNA has long been hypothesized to be a PAMP, no direct evidence has been provided in support of this concept. We have found that RNA species, mainly dsRNA, present in vaccinia virus-infected cells at late times can be recognized by host cells to trigger PKR activation and cytokine gene transcription. Furthermore, we characterized the distinct but interlinked signaling pathways regulating the induction of these cytokines. We describe a requirement for PKR in the activation of p38 signal transduction and NF-κB nuclear translocation and highlight the function of E3 in suppressing these events to limit cytokine expression associated with both “early” and “late” PAMPs. We also identified a PKR-independent response mediated by IPS-1, NF-κB, and IRF3 to regulate vaccinia virus-induced IFN-β expression associated with a “late” PAMP. As vaccinia virus E3 and its homologues in other orthopoxviruses are highly conserved, our results should contribute to the understanding of the strategies used by orthopoxviruses to suppress the host immune response. In addition, the findings described in this communication may aid in the development of safer, more effective vaccinia virus-based recombinant vaccines.
Acknowledgments
This work was supported by the Public Health Agency of Canada and the Natural Sciences and Engineering Research Council of Canada.
Footnotes
Published ahead of print on 15 April 2009.
REFERENCES
- 1.Arsenio, J., Y. Deschambault, and J. Cao. 2008. Antagonizing activity of vaccinia virus E3L against human interferons in Huh7 cells. Virology 377124-132. [DOI] [PubMed] [Google Scholar]
- 2.Beattie, E., E. B. Kauffman, H. Martinez, M. E. Perkus, B. L. Jacobs, E. Paoletti, and J. Tartaglia. 1996. Host-range restriction of vaccinia virus E3L-specific deletion mutants. Virus Genes 1289-94. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet, M. C., C. Daurat, C. Ottone, and E. F. Meurs. 2006. The N-terminus of PKR is responsible for the activation of the NF-kappaB signaling pathway by interacting with the IKK complex. Cell. Signal. 181865-1875. [DOI] [PubMed] [Google Scholar]
- 4.Boone, R. F., R. P. Parr, and B. Moss. 1979. Intermolecular duplexes formed from polyadenylylated vaccinia virus RNA. J. Virol. 30365-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandt, T. A., and B. L. Jacobs. 2001. Both carboxy- and amino-terminal domains of the vaccinia virus interferon resistance gene, E3L, are required for pathogenesis in a mouse model. J. Virol. 75850-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broyles, S. S. 2003. Vaccinia virus transcription. J. Gen. Virol. 842293-2303. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti, A., A. J. Sadler, N. Kar, H. A. Young, R. H. Silverman, and B. R. Williams. 2008. Protein kinase R-dependent regulation of interleukin-10 in response to double-stranded RNA. J. Biol. Chem. 28325132-25139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, H. W., L. H. Uribe, and B. L. Jacobs. 1995. Rescue of vaccinia virus lacking the E3L gene by mutants of E3L. J. Virol. 696605-6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, H. W., J. C. Watson, and B. L. Jacobs. 1992. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 894825-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, R. A., G. Ryzhakov, S. Cooray, F. Randow, and G. L. Smith. 2008. Inhibition of IkappaB kinase by vaccinia virus virulence factor B14. PLoS Pathog. 4e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu, W. M., D. Ostertag, Z. W. Li, L. Chang, Y. Chen, Y. Hu, B. Williams, J. Perrault, and M. Karin. 1999. JNK2 and IKKbeta are required for activating the innate response to viral infection. Immunity 11721-731. [DOI] [PubMed] [Google Scholar]
- 12.Colby, C., C. Jurale, and J. R. Kates. 1971. Mechanism of synthesis of vaccinia virus double-stranded ribonucleic acid in vivo and in vitro. J. Virol. 771-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng, L., P. Dai, W. Ding, R. D. Granstein, and S. Shuman. 2006. Vaccinia virus infection attenuates innate immune responses and antigen presentation by epidermal dendritic cells. J. Virol. 809977-9987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng, L., P. Dai, T. Parikh, H. Cao, V. Bhoj, Q. Sun, Z. Chen, T. Merghoub, A. Houghton, and S. Shuman. 2008. Vaccinia virus subverts a mitochondrial antiviral signaling protein-dependent innate immune response in keratinocytes through its double-stranded RNA binding protein, E3. J. Virol. 8210735-10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiPerna, G., J. Stack, A. G. Bowie, A. Boyd, G. Kotwal, Z. Zhang, S. Arvikar, E. Latz, K. A. Fitzgerald, and W. L. Marshall. 2004. Poxvirus protein N1L targets the I-kappaB kinase complex, inhibits signaling to NF-kappaB by the tumor necrosis factor superfamily of receptors, and inhibits NF-kappaB and IRF3 signaling by toll-like receptors. J. Biol. Chem. 27936570-36578. [DOI] [PubMed] [Google Scholar]
- 16.Domingo-Gil, E., E. Perez-Jimenez, I. Ventoso, J. L. Najera, and M. Esteban. 2008. Expression of the E3L gene of vaccinia virus in transgenic mice decreases host resistance to vaccinia virus and Leishmania major infections. J. Virol. 82254-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia, M. A., J. Gil, I. Ventoso, S. Guerra, E. Domingo, C. Rivas, and M. Esteban. 2006. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol. Mol. Biol. Rev. 701032-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gedey, R., X. L. Jin, O. Hinthong, and J. L. Shisler. 2006. Poxviral regulation of the host NF-κB response: the vaccinia virus M2L protein inhibits induction of NF-κB activation via an ERK2 pathway in virus-infected human embryonic kidney cells. J. Virol. 808676-8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilfoy, F. D., and P. W. Mason. 2007. West Nile virus-induced interferon production is mediated by the double-stranded RNA-dependent protein kinase PKR. J. Virol. 8111148-11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham, S. C., M. W. Bahar, S. Cooray, R. A. Chen, D. M. Whalen, N. G. Abrescia, D. Alderton, R. J. Owens, D. I. Stuart, G. L. Smith, and J. M. Grimes. 2008. Vaccinia virus proteins A52 and B14 share a Bcl-2-like fold but have evolved to inhibit NF-kappaB rather than apoptosis. PLoS Pathog. 4e1000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho, C. K., and S. Shuman. 1996. Mutational analysis of the vaccinia virus E3 protein defines amino acid residues involved in E3 binding to double-stranded RNA. J. Virol. 702611-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishii, T., H. Kwon, J. Hiscott, G. Mosialos, and A. E. Koromilas. 2001. Activation of the I kappa B alpha kinase (IKK) complex by double-stranded RNA-binding defective and catalytic inactive mutants of the interferon-inducible protein kinase PKR. Oncogene 201900-1912. [DOI] [PubMed] [Google Scholar]
- 23.Jijon, H., B. Allard, and C. Jobin. 2004. NF-kappaB inducing kinase activates NF-kappaB transcriptional activity independently of IkappaB kinase gamma through a p38 MAPK-dependent RelA phosphorylation pathway. Cell. Signal. 161023-1032. [DOI] [PubMed] [Google Scholar]
- 24.Kawai, T., K. Takahashi, S. Sato, C. Coban, H. Kumar, H. Kato, K. J. Ishii, O. Takeuchi, and S. Akira. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6981-988. [DOI] [PubMed] [Google Scholar]
- 25.Kopf, M., H. Baumann, G. Freer, M. Freudenberg, M. Lamers, T. Kishimoto, R. Zinkernagel, H. Bluethmann, and G. Kohler. 1994. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368339-342. [DOI] [PubMed] [Google Scholar]
- 26.Kotlyarov, A., Y. Yannoni, S. Fritz, K. Laass, J. B. Telliez, D. Pitman, L. L. Lin, and M. Gaestel. 2002. Distinct cellular functions of MK2. Mol. Cell. Biol. 224827-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langland, J. O., and B. L. Jacobs. 2004. Inhibition of PKR by vaccinia virus: role of the N- and C-terminal domains of E3L. Virology 324419-429. [DOI] [PubMed] [Google Scholar]
- 28.Langland, J. O., J. C. Kash, V. Carter, M. J. Thomas, M. G. Katze, and B. L. Jacobs. 2006. Suppression of proinflammatory signal transduction and gene expression by the dual nucleic acid binding domains of the vaccinia virus E3L proteins. J. Virol. 8010083-10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lasa, M., K. R. Mahtani, A. Finch, G. Brewer, J. Saklatvala, and A. R. Clark. 2000. Regulation of cyclooxygenase 2 mRNA stability by the mitogen-activated protein kinase p38 signaling cascade. Mol. Cell. Biol. 204265-4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, Y., A. Sassano, B. Majchrzak, D. K. Deb, D. E. Levy, M. Gaestel, A. R. Nebreda, E. N. Fish, and L. C. Platanias. 2004. Role of p38alpha Map kinase in type I interferon signaling. J. Biol. Chem. 279970-979. [DOI] [PubMed] [Google Scholar]
- 31.Ludwig, H., Y. Suezer, Z. Waibler, U. Kalinke, B. S. Schnierle, and G. Sutter. 2006. Double-stranded RNA-binding protein E3 controls translation of viral intermediate RNA, marking an essential step in the life cycle of modified vaccinia virus Ankara. J. Gen. Virol. 871145-1155. [DOI] [PubMed] [Google Scholar]
- 32.Martinez, J., X. Huang, and Y. Yang. 2008. Direct action of type I IFN on NK cells is required for their activation in response to vaccinia viral infection in vivo. J. Immunol. 1801592-1597. [DOI] [PubMed] [Google Scholar]
- 33.Meusel, T. R., K. E. Kehoe, and F. Imani. 2002. Protein kinase R regulates double-stranded RNA induction of TNF-alpha but not IL-1 beta mRNA in human epithelial cells. J. Immunol. 1686429-6435. [DOI] [PubMed] [Google Scholar]
- 34.Moss, B. 1968. Inhibition of HeLa cell protein synthesis by the vaccinia virion. J. Virol. 21028-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moss, B., and J. L. Shisler. 2001. Immunology 101 at poxvirus U: immune evasion genes. Semin. Immunol. 1359-66. [DOI] [PubMed] [Google Scholar]
- 36.Ogawa, K., M. Funaba, and M. Tsujimoto. 2008. A dual role of activin A in regulating immunoglobulin production of B cells. J. Leukoc. Biol. 831451-1458. [DOI] [PubMed] [Google Scholar]
- 37.Rajaiya, J., J. Xiao, R. V. Rajala, and J. Chodosh. 2008. Human adenovirus type 19 infection of corneal cells induces p38 MAPK-dependent interleukin-8 expression. Virol. J. 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Randall, R. E., and S. Goodbourn. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 891-47. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez, J. R., D. Rodriguez, and M. Esteban. 1991. Interferon treatment inhibits early events in vaccinia virus gene expression in infected mice. Virology 185929-933. [DOI] [PubMed] [Google Scholar]
- 40.Romano, P. R., F. Zhang, S. L. Tan, M. T. Garcia-Barrio, M. G. Katze, T. E. Dever, and A. G. Hinnebusch. 1998. Inhibition of double-stranded RNA-dependent protein kinase PKR by vaccinia virus E3: role of complex formation and the E3 N-terminal domain. Mol. Cell. Biol. 187304-7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scutera, S., E. Riboldi, R. Daniele, A. R. Elia, T. Fraone, C. Castagnoli, M. Giovarelli, T. Musso, and S. Sozzani. 2008. Production and function of activin A in human dendritic cells. Eur. Cytokine Netw. 1960-68. [DOI] [PubMed] [Google Scholar]
- 42.Sharp, T. V., F. Moonan, A. Romashko, B. Joshi, G. N. Barber, and R. Jagus. 1998. The vaccinia virus E3L gene product interacts with both the regulatory and the substrate binding regions of PKR: implications for PKR autoregulation. Virology 250302-315. [DOI] [PubMed] [Google Scholar]
- 43.Shisler, J. L., and X. L. Jin. 2004. The vaccinia virus K1L gene product inhibits host NF-κB activation by preventing IκBα degradation. J. Virol. 783553-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silva, A. M., M. Whitmore, Z. Xu, Z. Jiang, X. Li, and B. R. Williams. 2004. Protein kinase R (PKR) interacts with and activates mitogen-activated protein kinase kinase 6 (MKK6) in response to double-stranded RNA stimulation. J. Biol. Chem. 27937670-37676. [DOI] [PubMed] [Google Scholar]
- 45.Smith, E. J., I. Marie, A. Prakash, A. Garcia-Sastre, and D. E. Levy. 2001. IRF3 and IRF7 phosphorylation in virus-infected cells does not require double-stranded RNA-dependent protein kinase R or Ikappa B kinase but is blocked by vaccinia virus E3L protein. J. Biol. Chem. 2768951-8957. [DOI] [PubMed] [Google Scholar]
- 46.Upton, C., S. Slack, A. L. Hunter, A. Ehlers, and R. L. Roper. 2003. Poxvirus orthologous clusters: toward defining the minimum essential poxvirus genome. J. Virol. 777590-7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, F., X. Gao, J. W. Barrett, Q. Shao, E. Bartee, M. R. Mohamed, M. Rahman, S. Werden, T. Irvine, J. Cao, G. A. Dekaban, and G. McFadden. 2008. RIG-I mediates the co-induction of tumor necrosis factor and type I interferon elicited by myxoma virus in primary human macrophages. PLoS Pathog. 4e1000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weaver, J. R., M. Shamim, E. Alexander, D. H. Davies, P. L. Felgner, and S. N. Isaacs. 2007. The identification and characterization of a monoclonal antibody to the vaccinia virus E3 protein. Virus Res. 130269-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiang, Y., R. C. Condit, S. Vijaysri, B. Jacobs, B. R. Williams, and R. H. Silverman. 2002. Blockade of interferon induction and action by the E3L double-stranded RNA binding proteins of vaccinia virus. J. Virol. 765251-5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5730-737. [DOI] [PubMed] [Google Scholar]
- 51.Zamanian-Daryoush, M., T. H. Mogensen, J. A. DiDonato, and B. R. Williams. 2000. NF-κB activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-κB-inducing kinase and IκB kinase. Mol. Cell. Biol. 201278-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zechner, D., R. Craig, D. S. Hanford, P. M. McDonough, R. A. Sabbadini, and C. C. Glembotski. 1998. MKK6 activates myocardial cell NF-kappaB and inhibits apoptosis in a p38 mitogen-activated protein kinase-dependent manner. J. Biol. Chem. 2738232-8239. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, P., B. L. Jacobs, and C. E. Samuel. 2008. Loss of protein kinase PKR expression in human HeLa cells complements the vaccinia virus E3L deletion mutant phenotype by restoration of viral protein synthesis. J. Virol. 82840-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang, P., and C. E. Samuel. 2008. Induction of protein kinase PKR-dependent activation of interferon regulatory factor 3 by vaccinia virus occurs through adapter IPS-1 signaling. J. Biol. Chem. 28334580-34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao, T., L. Yang, Q. Sun, M. Arguello, D. W. Ballard, J. Hiscott, and R. Lin. 2007. The NEMO adaptor bridges the nuclear factor-kappaB and interferon regulatory factor signaling pathways. Nat. Immunol. 8592-600. [DOI] [PubMed] [Google Scholar]