Abstract
Hepatitis E virus (HEV) is the causative agent of hepatitis E, a major form of viral hepatitis in developing countries. The open reading frame 3 (ORF3) of HEV encodes a phosphoprotein with a molecular mass of approximately 13 kDa (hereinafter called vp13). vp13 is essential for establishing HEV infections in animals, yet its exact functions are still obscure. Our current study found evidence showing interaction between vp13 and microtubules. Live-cell confocal fluorescence microscopy revealed both filamentous and punctate distribution patterns of vp13 in cells transfected with recombinant ORF3 reporter plasmids. The filamentous pattern of vp13 was altered by a microtubule-destabilizing drug. The vp13 expression led to elevation of acetylated α-tubulin, indicating increased microtubule stability. Its association with microtubules was further supported by its presence in microtubule-containing pellets in microtubule isolation assays. Exposure of these pellets to a high-salt buffer caused release of the vp13 to the supernatant, suggesting an electrostatic interaction. Inclusion of ATP and GTP in the lysis buffer during microtubule isolation also disrupted the interaction, indicating its sensitivity to the nucleotides. Further assays showed that motor proteins are needed for the vp13 association with the microtubules because disruption of dynein function abolished the vp13 filamentous pattern. Analysis of ORF3 deletion constructs found that both of the N-terminal hydrophobic domains of vp13 are needed for the interaction. Thus, our findings suggest that the vp13 interaction with microtubules might be needed for establishment of an HEV infection.
The hepatitis E virus (HEV), the sole member of the genus Hepevirus, is a single-strand positive-sense RNA virus that is the causative agent in endemics and epidemics of acute human hepatitis in many parts of the world (5). Transmitted mainly from contaminated water through the fecal-oral route, HEV infection causes a fulminant form of hepatitis that has a mortality rate of up to 20% in pregnant women (28). HEV infection is considered zoonotic. Swine and chicken HEV strains have been found in the United States (11, 23). A swine strain can infect chimpanzees under experimental conditions, and a human strain that is genetically similar to the swine strain can experimentally infect pigs (22). Direct evidence of the zoonotic nature of HEV infection has been provided in reports of a series of cases of HEV infection in people who ate undercooked deer meat 6 to 7 weeks before the onset of the disease (19, 33, 39). HEV RNA recovered from the leftover deer meat was found to be identical in nucleotide sequence to the HEV RNA recovered from the individuals who became ill (31).
The HEV genome is approximately 7.2 kb in length and consists of three open reading frames (ORFs) (32). ORF1 encodes a nonstructural polyprotein that includes the RNA-dependent RNA polymerase. ORF2 encodes the capsid protein, the major structural protein in virion. ORF3 encodes a phosphoprotein that was found to be essential for establishing an HEV infection in macaques and pigs under experimental conditions (9, 12). It has been reported that ORF3 translation initiates at the third in-frame AUG codon, which lies 23 bases downstream of the ORF1 termination codon (10, 12). Propagation of HEV and studies of virus replication still rely upon nonhuman primates due to the lack of an effective cell culture system. As a result, functional study of the ORF3 product in HEV biology and infection is limited.
The phosphoprotein encoded by HEV ORF3 has a molecular mass of approximately 13 kDa (hereinafter called vp13) (32). The exact functions of vp13 in HEV infection remain unknown although the findings of a number of studies have shown that it plays a role in cellular signaling pathways (13, 17, 24, 34-36, 40). During subcellular fractionation of COS-7 cells transfected with a vp13-expressing plasmid, vp13 was found to partition with the cytoskeletal fraction (40). Deletion of the N-terminal hydrophobic domain of vp13 abolished the association with the cytoskeleton fraction. The vp13-binding proteins in the cytoskeleton and the nature of this interaction are not known.
In this study, we found that the HEV ORF3 product localizes to microtubules and interferes with their dynamics. The filamentous pattern of vp13 distribution in the cell was abolished by a microtubule-destabilizing drug. vp13 led to elevation of acetylated α-tubulin. These results suggested that vp13 interaction with the microtubules might facilitate HEV infection. We further studied the nature of the vp13-microtubule interaction.
MATERIALS AND METHODS
Cells, transfection, and chemicals.
Cells of HeLa, COS-7, and Huh-7 cell lines were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum. Transfection of the cells with various vectors was performed using FuGeneHD (Roche Diagnostics, Indianapolis, IN), according to the instructions of the manufacturer.
The microtubule-stabilizing drug paclitaxel (Taxol), the microtubule-destabilizing drug nocodazole, and the dynein inhibitor sodium vanadate (Na3VO4) were purchased from Sigma Aldrich (St. Louis, MO).
Plasmids.
HEV ORF3 was amplified from a pSK-E2 plasmid that contains the full-length cDNA of the HEV genome (GenBank accession no. AF444002) (6). PCR was conducted using the primers H3F4 and H3R2 (Table 1), which contain restriction sites for EcoRI or BamHI to facilitate directional cloning. The forward primer was designed to begin at the third in-frame AUG, which was recently found to be an authentic translation initiation codon for ORF3 (12).
TABLE 1.
Primers used for cloning ORF3 and truncation variants
| Namea | Sequence (5′ to 3′)b | Plasmidc |
|---|---|---|
| H3F4 | GCGAATTCATGGGTTCGCGACCATGCG | VenusN1-H3 |
| H3R2 | CCGGATCCTTGCGGCGCGGCCCCAGCTGTG | |
| H3F9 | GCGAATTCAATGGGTTCGCGACCATGC | VenusC1-H3 |
| H3R2 | CCGGATCCTTGCGGCGCGGCCCCAGCTGTG | |
| H3F2 | GCGGATCCAATGGGTTCGCGACCATGC | pCMV-H3 |
| H3R4 | CCGGATCCTTAGCGGCGCGGCCCCAGCTGTG | |
| H3F4 | GCGAATTCATGGGTTCGCGACCATGCG | VenusN1-H3D1 |
| H3R8 | CAGGATCCGTTGGTTGGATGAATATAG | |
| H3F4 | GCGAATTCATGGGTTCGCGACCATGCG | VenusN1-H3D2 |
| H3R9 | CTGGATCCCTGGTCACGCCAAGCGGA | |
| H3F7 | GCGAATTCATGCGCCACCGCCCGGTCAG | VenusN1-H3D3 |
| H3R2 | CCGGATCCTTGCGGCGCGGCCCCAGCTGTG | |
| H3F8 | GCGAATTCATGATTCATCCAACCAACCC | VenusN1-H3D4 |
| H3R2 | CCGGATCCTTGCGGCGCGGCCCCAGCTGTG | |
| H3F9 | GCGAATTCAATGGGTTCGCGACCATGC | VenusC1-H3NH |
| H3R10 | CTGGATCCTTAGTGGCGCGGGCAGCATAG |
F, forward; R, reverse.
Restriction sites of BamHI and EcoRI included in the primers are in boldface.
D, deletion variant; NH, N-terminal hydrophobic domain.
ORF3 was cloned within three vectors. Two of the vectors contained Venus, which encodes a monomeric and photostable version of yellow fluorescent protein (29). In VenusN1, ORF3 was cloned upstream of Venus; in VenusC1, it was cloned downstream of Venus to confirm the vp13 expression pattern. This resulted in two recombinant plasmids that expressed the vp13-Venus fusion protein after transfection. ORF3 was also cloned within a pCMV (where CMV is cytomegalovirus) vector using PCR primers with selected restriction enzyme sites (Table 1). The resulting recombinant plasmid pCMV-H3 produces an untagged vp13 after transfection. In each plasmid, cloning was confirmed by restriction enzyme digestion and DNA sequencing.
We also constructed ORF3 deletion variants by cloning fragments of ORF3 into the VenusN1 vector using primers designed to amplify the ORF3 fragments during PCR (Table 1). These fragments were cloned using a procedure similar to that used to clone the full-length ORF3 into the VenusN1 vector.
A Myc-tagged p50/dynamitin construct (4) was kindly donated by Richard Vallee of Columbia University in New York, NY.
Confocal fluorescence microscopy.
Cells were seeded directly onto Lab-Tek chambered coverglass, incubated overnight, and transfected the next day. We used confocal fluorescence microscopy at 24 h after transfection to observe live cells on the coverglass or cells that had been fixed with 1% paraformaldehyde and mounted onto slides using SlowFade Gold antifade reagent containing 4′6′-diamidino-2-phenylinodole (DAPI) (Invitrogen, Carlsbad, CA). To determine the effects of nocodazole, we replaced the original medium with a medium containing 10 μM nocodazole and incubated the cells for 2 h. We then removed the nocodazole by washing the culture twice with plain DMEM. These cells were either observed using confocal fluorescence microscopy or incubated for an additional 4 h to recover from drug exposure.
An immunofluorescence assay (IFA) was carried out as reported previously (41) using rabbit anti-vp13 antibody (12). Specific antibody-vp13 reactions were detected using Alexa Fluor 488 goat anti-rabbit immunoglobulin G conjugate (Invitrogen) and observed using confocal fluorescence microscopy.
Western blot analysis.
Cells were transfected with a vp13-expressing plasmid or an empty vector and at 24 h after transfection lysed in Laemmli buffer. The samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting, as described previously (42). Briefly, the cell lysate samples were resolved in a 12% polyacrylamide gel. The separated proteins were then transferred to a nitrocellulose membrane and probed with rabbit anti-vp13 antibody. Specific reactions were detected using goat anti-rabbit immunoglobulin G conjugated with horseradish peroxidase (Sigma-Aldrich) and revealed using a chemiluminescence substrate. The chemiluminescence signal was recorded digitally using a ChemiDoc XRS imaging system (Bio-Rad Laboratories, Hercules, CA). β-Tubulin was detected on the same blotting membrane to normalize protein loading. Digital signal acquisition and densitometry analyses were conducted using the Quantity One Program, version 4.6 (Bio-Rad Laboratories). The expression of other proteins was detected using corresponding antibodies to β-tubulin, green fluorescent protein (GFP), and FLAG (Sigma-Aldrich), as well as acetylated α-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA).
Microtubule isolation and salt extraction assays.
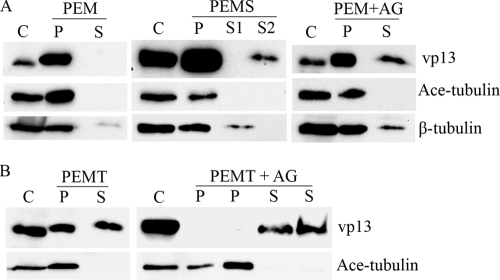
A microtubule isolation assay and a salt extraction assay were done as described previously (7, 26), with a few modifications. Briefly, HeLa cells were transfected with the VenusC1-H3 plasmid, and the lysates were collected in the microtubule-stabilizing buffer PEM (100 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], pH 6.9, 5 mM MgCl2, 1 mM EGTA) supplemented with 40 μM paclitaxel. In one experiment, ATP and GTP were added to the PEM buffer to a final concentration of 10 mM to release motor proteins from the microtubules. The cell lysates were collected with a cell scraper, transferred to a microcentrifuge tube, homogenized with a syringe and 25-gauge needle, and incubated for 10 min at 37°C. A 2-μl sample was mixed with trypan blue and checked for evidence of cell lysis to ensure that more than 95% cells had ruptured. The sample was then centrifuged at 100,000 × g for 30 min at 37°C using a buffer and rotor that had been warmed to 37°C. After centrifugation, the supernatant was transferred to labeled and chilled tubes. The pellet was resuspended in the PEM buffer (before adding the Laemmli buffer for SDS-PAGE) or in the PEMS buffer (PEM plus 500 mM KCl) for salt extraction. Paclitaxel was added to the buffer to a final concentration of 40 μM to stabilize the microtubules. The sample was centrifuged again at 100,000 × g for 30 min at 37°C. After centrifugation, the supernatant was transferred to a chilled tube, and the pellet was resuspended in the PEM buffer and then denatured in 1× Laemmli buffer at 95°C for 5 min. Proteins in the supernatant were precipitated using 10% trichloroacetic acid and resuspended in the PEM buffer before denaturing in Laemmli buffer and pH adjustment. The samples were then analyzed by SDS-PAGE and Western blotting using antibodies against vp13, acetylated α-tubulin, and β-tubulin. For experiments requiring detergent activity, cells were lysed in PEMT buffer (PEM buffer with 0.1% Triton X-100, 0.1% Tween 20, and 0.001% antifoam added), and cell homogenization and centrifugation were done as just described.
Cell viability assay.
Cell viability was determined with a CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI). Briefly, cells were cultured in 96-well plates and treated as indicated. CellTiter-Glo reagent was added on the following days, and the cells were incubated for 10 min at room temperature. The luminescence signal was measured with a Victor3 Multilabel Counter (Perkin-Elmer, Waltham, MA). Relative percentages of luminescence intensity were calculated by comparison with a mock-treated control.
RESULTS
Filamentous pattern of vp13 expression.
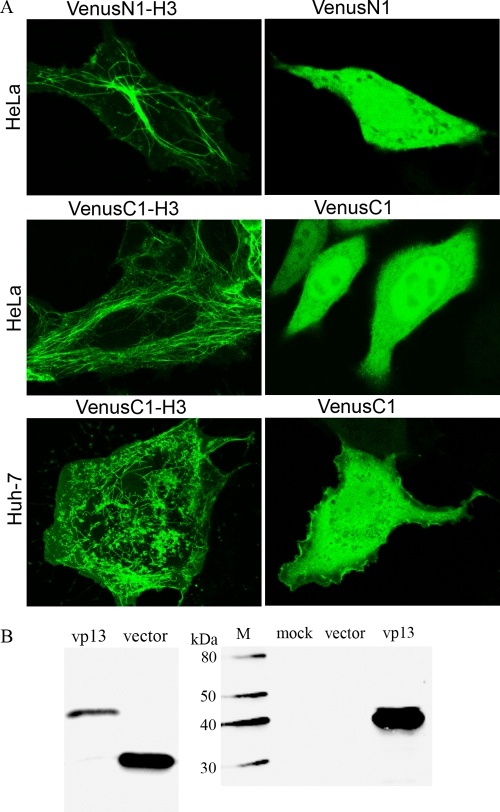
Using confocal fluorescence microscopy (magnification, ×63) of live HeLa cells 24 h after transfection with a VenusN1-H3 plasmid, we observed filamentous structures (Fig. 1A), indicating expression of the vp13-Venus fusion protein. The same structures were observed in cells that had been fixed with paraformaldehyde. The microtubule organizing center (MTOC) appeared bright green in some of the Venus-positive cells, and a punctate distribution was observed in the cytoplasm of many of these cells. By contrast, bright green fluorescence appeared throughout the cytoplasm and nucleus of cells that had been transfected with the empty vector (Fig. 1A).
FIG. 1.
Filamentous pattern of vp13 distribution observed using live-cell confocal fluorescence microscopy. (A) HeLa and Huh-7 cells were transfected with vp13 constructs or empty vectors. The VenusN1-H3 construct was for expression of the vp13 at the N terminus of the vp13-Venus fusion protein. The VenusC1-H3 construct was for expression of the vp13 at the C terminus of the fusion protein. Note the filamentous pattern of the vp13-Venus fusion protein in the left panel of the images. (B) Detection of fusion protein expression in HeLa cells transfected with VenusN1-H3 by Western blotting with mouse anti-GFP antibody (left panel) or rabbit anti-vp13 antibody (right panel). Cells transfected with the empty vector or mock transfected were included as controls. M, MagicMark XP Western Protein Standard (Invitrogen).
The same patterns were observed in HeLa cells that had been transfected with the VenusC1-H3 plasmid (Fig. 1A). By contrast, cells that had been transfected with an empty vector demonstrated the same homogeneous distribution of bright green fluorescence in both the cytoplasm and nucleus that was seen with the empty vector of VenusN1. These results confirmed that the vp13-Venus fusion protein produces the same spatial pattern whether vp13 is at the N or C terminus of the fusion protein. The observation of the vp13 filamentous pattern in the cell suggests that it localizes to microtubules.
The vp13 filamentous pattern seen in Huh-7 cells (Fig. 1A) was similar to that seen in the HeLa cells but formed at a lower rate and resulted in a somewhat different linear array. For this reason, we used HeLa cells to examine vp13 expression and its potential association with microtubules.
Using Western blotting with an antibody against GFP to confirm the expression and size of the vp13-Venus fusion protein, we found that the molecular mass of the fusion protein (approximately 40 kDa) was, as expected, greater than that of the Venus protein alone (27 kDa) (Fig. 1B). To confirm the expression of the vp13 fusion protein, we carried out Western blotting using a rabbit antibody against HEV vp13. We detected a band of the size expected for the fusion protein, but no bands were visible in lanes loaded with lysates from the cells that had received an empty vector or that underwent mock transfection (Fig. 1B). These results confirmed that the vp13-Venus fusion protein was expressed in transiently transfected cells.
Filamentous pattern of untagged vp13.
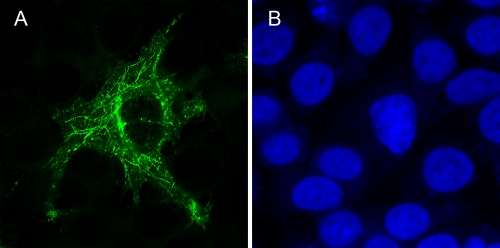
To determine whether the expression of untagged vp13 protein results in the same distribution pattern seen following expression of the vp13-Venus fusion protein, we transfected HeLa cells with the pCMV-H3 plasmid, which expresses untagged vp13. These cells were fixed for an IFA with a rabbit anti-vp13 antibody. We observed a linear arrangement of vp13 (Fig. 2) that was consistent with the pattern exhibited by the vp13-Venus fusion protein. Expression of vp13 in the cells was confirmed by Western blot analysis using rabbit anti-vp13 antibody (data not shown).
FIG. 2.
Filamentous pattern of untagged vp13 detected by IFA using rabbit anti-vp13 antibody. HeLa cells were transfected with the pCMV-H3 plasmid. Untagged vp13 was detected by IFA and then observed using confocal microscopy (A). Nuclear DNA was counterstained using DAPI (B).
Effect of microtubule depolymerization on vp13 expression pattern.
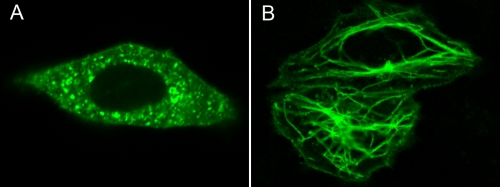
To investigate our speculation that vp13 localizes to microtubules, we transfected HeLa cells with the VenusN1-H3 plasmid and then exposed them to a nontoxic concentration of the microtubule-destabilizing drug nocodazole (10 μM) for 4 h on the next day after transfection. Using live-cell confocal fluorescence microscopy, we found that the filamentous pattern associated with vp13 had disappeared (Fig. 3A) and saw green punctate fluorescence in the cytoplasm. To determine whether removal of nocodazole from these cells would restore the vp13 filamentous pattern, we washed the cells twice with plain DMEM and observed them 4 h later. At that time, we saw the same filamentous structures that were observed before they were exposed to nocodazole (Fig. 3B). This finding demonstrated that the spatial arrangement of the vp13-Venus fusion protein is microtubule dependent.
FIG. 3.
Exposure to the microtubule-destabilizing drug nocodazole abolished the filamentous vp13 pattern. HeLa cells were transiently transfected with the VenusC1-H3 plasmid and exposed to nocodazole on the next day after transfection. Using confocal microscopy, we found that microtubule depolymerization abolished the filamentous pattern of the vp13-Venus fusion protein (A), but it was recovered in culture 4 h after the drug was removed (B).
Effect of vp13 on microtubule stability.
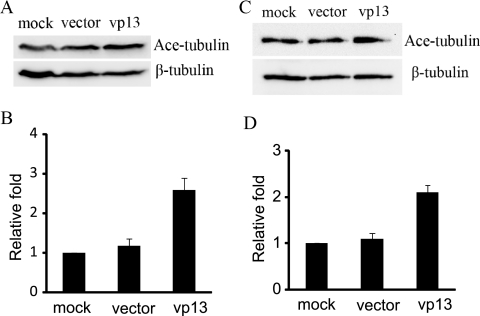
Tubulin acetylation is a well-established marker of microtubule stability (38). We investigated the level of tubulin acetylation in HeLa cells expressing vp13. Western blotting revealed that acetylated α-tubulin levels were elevated in HeLa cells transfected with pCMV-H3 compared with controls (Fig. 4A). Densitometry analysis of digital images of Western blots from three separate experiments revealed that acetylated α-tubulin levels were 2.6-fold higher in HeLa cells expressing vp13 than in cells that underwent mock transfection and that no change occurred in cells transfected with an empty vector (Fig. 4B). α-Tubulin acetylation was also investigated in Huh-7 cells expressing vp13, with similar results (Fig. 4C). Densitometry analysis revealed that acetylated α-tubulin levels were 2.1-fold higher in Huh-7 cells expressing vp13 than in those that underwent mock transfection, and no change was observed in cells transfected with an empty vector (Fig. 4D). These findings suggest that microtubule stability is enhanced in HeLa and Huh-7 cells that express vp13.
FIG. 4.
Elevation of acetylated α-tubulin levels in vp13-positive cells. (A) Western blot detection of acetylated α-tubulin (Ace-tubulin) in HeLa cells transiently transfected with the pCMV-H3 plasmid (vp13 lane) or an empty vector. Detection of β-tubulin in the same blot was conducted for normalization. (B) Graphic illustration of densitometry analysis of the digital images of Western blots from three separate experiments. The acetylated α-tubulin level is presented as the relative increase in expression in comparison with mock-transfected controls. Error bars indicate variations in the three experiments. (C) Western blot detection of acetylated α-tubulin in Huh-7 cells transfected with the vp13 expression plasmid. (D) Graphic illustration of densitometry analysis of the digital images of Western blots from three separate experiments as the relative increase in expression.
Factors affecting vp13-microtubule interaction.
Finding that vp13 localizes to the microtubules, we speculated that vp13 would coprecipitate with the microtubules during centrifugation. We investigated this possibility using a microtubule isolation assay, in which HeLa cells transfected with a vp13 expression plasmid were homogenized in the microtubule stabilization buffer PEM, and cell lysate samples were centrifuged to separate the microtubules from free tubulin and other soluble cytoplasmic materials. Microtubule-associated proteins (MAPs) are expected to form pellets with the microtubules during centrifugation. Western blot analysis showed that vp13 was present in the pellet fraction, as expected, and not in the supernatant (Fig. 5A, left panel). Similarly, acetylated α-tubulin was detected only in the pellet fraction, as expected. Resuspension of the pellet in a high-salt (500 mM KCl) PEMS buffer, followed by centrifugation, is expected to result in the release of MAPs and MAP-like proteins from the pellets, as the binding of these proteins to the microtubules is charge dependent (see review in reference 21). When this procedure was performed, we found vp13 in both the pellet and the supernatant (Fig. 5A, middle panel). This result indicates that vp13 behaves like MAPs and MAP-like proteins in that it associates with microtubules through electrostatic interactions. The presence of vp13 in the pellet after salt extraction, however, indicates that it is also present in subcellular organelles; this is consistent with the punctate distribution of vp13 that accompanied the filamentous arrangement observed under fluorescence microscopy. Acetylated α-tubulin was, once again, found only in the pellet fraction, as expected. Its absence from the supernatant after salt extraction confirmed the stability of tubulin polymerization and, therefore, the stability of the resulting microtubules.
FIG. 5.
The electrostatic interaction between vp13 and microtubules was detected by microtubule isolation and salt extraction assays. (A) Microtubule isolation with PEM buffer and salt extraction. The vp13 protein was detected by Western blotting in the pellet (P lanes) fraction only when the cells were homogenized in PEM buffer and the microtubule-containing pellets formed (left panel). A portion of the vp13 protein shifted to the supernatant fraction (S lanes) after extraction of microtubule pellet with a high-salt PEMS buffer (S2 lane in middle panel). S1, the supernatant after the first round of centrifugation; S2, the supernatant after salt extraction of the pellet and the second round of centrifugation. Some of the vp13 protein was also detected in the supernatant fraction when the cells were homogenized in PEM buffer supplemented with ATP and GTP (AG) (right panel). Acetylated α-tubulin (Ace-tubulin) was detected in only the pellet fraction in all of the experiments. β-Tubulin was detected to indicate protein loading. The supernatant fraction (S2 lane) after extraction of the microtubule pellet with PEMS buffer does not contain free tubulin. Total cell lysate was included as a control (C lanes). (B) Microtubule isolation with PEMT buffer and the release of vp13 in the presence of ATP and GTP. The vp13 protein was detected in both the pellet (P) and supernatant (S) fractions when the cells were homogenized in PEMT buffer (left panel). All of the vp13 shifted to the supernatant fraction when ATP and GTP were included in the PEMT lysis buffer (right panel). Cells in duplicate wells that were transfected and processed in this experiment are shown as two lanes for the pellet and supernatant fractions in the right panel.
The supernatant after PEMS resuspension of the microtubule pellet and centrifugation also contained motor proteins, which led us to speculate that vp13 interacts with motor proteins. Indeed, many viruses sequester the dynein machinery of infected cells in order to move the microtubules along to the site of viral replication (1, 20, 27, 30). The motor proteins dynein and kinesin can be separated from microtubules by the addition of the nucleotides ATP and GTP to the microtubule pellet (25). To investigate the possible relationship between vp13 and motor proteins, we carried out another microtubule isolation assay using PEM buffer supplemented with ATP and GTP. Western blot analysis revealed that vp13 was present in both the pellet and supernatant (Fig. 5A, right panel), whereas dynein was found only in the supernatant (data not shown). This indicated that the nucleotides reduced the ability of dynein to interact with the microtubules and, in the process, released some of the vp13 to the supernatant.
The vp13 remaining in the pellet had survived PEM lysis and salt extraction. This led us to speculate that the residual vp13 represented the protein in subcellular organelles that the lysis buffer PEM was unable to extract. To investigate this, we used PEMT lysis buffer, which includes the detergent Triton X-100, to disrupt the subcellular membranes and, thus, facilitate extraction of vp13 from the subcellular organelles. The pellet fraction after this lysis and centrifugation procedure is expected to contain only the vp13 associated with the microtubules, and the supernatant fraction is expected to contain the vp13 extracted from the subcellular organelles. As expected, both the pellet and supernatant contained vp13 after PEMT lysis and centrifugation (Fig. 5B, left panel). When ATP and GTP were added to the PEMT lysis buffer, all of the vp13 shifted from the pellet to the supernatant, and acetylated α-tubulin again remained in the pellet (Fig. 5B, right panel). This result confirmed that the interaction between vp13 and the microtubules—like the interaction of dynein with the microtubules—was nucleotide sensitive.
Role of dynein in the vp13 association with the microtubules.
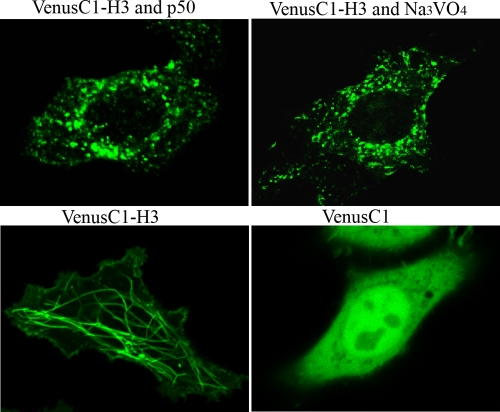
Dynein is a large protein complex that functions as a molecular motor to transport various cellular elements toward the minus end of the microtubules (15). Realizing that vp13 associates with microtubules, we decided to investigate the possibility that dynein plays a role in the spatial distribution of vp13 in the cell. Dynein binds to membranous organelles through another large protein complex called dynactin (15). Overexpression of a dynactin subunit, p50/dynamitin, disrupts the dynactin complex and, thus, blocks dynein activity (4). To determine whether dynein contributes to the interaction of vp13 and microtubules, we cotransfected HeLa cells with a p50/dynamitin expression plasmid and VenusC1-H3. Using live-cell confocal fluorescence microscopy, we found that the vp13 linear array had been abolished (Fig. 6), and we saw many punctate structures in the cytoplasm. To confirm the observation, we used sodium vanadate, a well-known inhibitor of dynein activity (15). Incubation of the HeLa cells transfected with VenusC1-H3 in a nontoxic concentration (100 μM) for 2 h abolished the linear arrangement of vp13 (Fig. 6). These findings suggest that dynein plays a role in the interaction between vp13 and microtubules.
FIG. 6.
Disruption of dynein motor activity abolishes the vp13 interaction with microtubules. Expression of p50/dynamitin in HeLa cells abolished the filamentous vp13 pattern. HeLa cells were cotransfected with VenusC1-H3 and p50/dynamitin constructs. Live-cell confocal fluorescence microscopy was conducted on the next day after transfection. Exposure of HeLa cells to sodium vanadate (Na3VO4) abolished the filamentous vp13 pattern. Twenty-four hours after being transfected with VenusC1-H3, HeLa cells were exposed to Na3VO4 for 2 h. Untreated HeLa cells transfected with VenusC1-H3 or an empty vector were included as controls.
The microtubule-binding region of vp13.
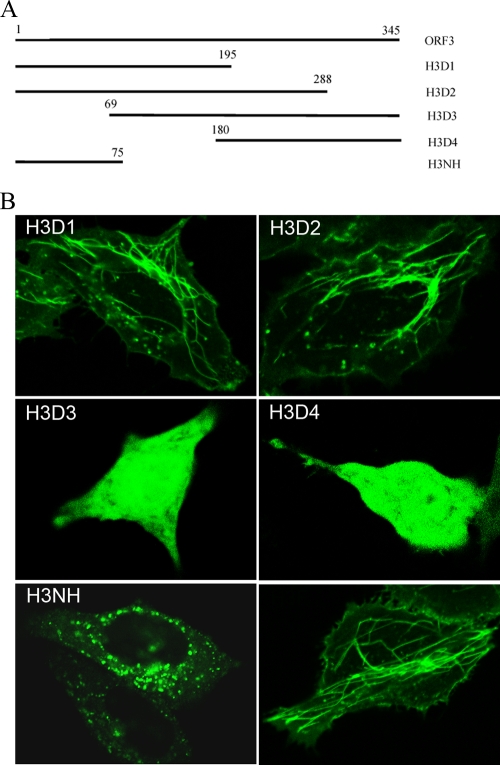
To identify the microtubule-binding region of vp13, we created four truncated versions of ORF3 in the VenusN1 vector (Fig. 7A). Transfection of HeLa cells with the four truncation variants of vp13 plasmids showed that VenusN1-H3D1 and VenusN1-H3D2 had filamentous expression patterns similar to the full-length vp13-Venus fusion protein though at lower fluorescence intensity (Fig. 7B). These two plasmids contained the hydrophobic N-terminal domains of vp13. In cells that had been transfected with VenusN1-H3D3 and VenusN1-H3D4 containing truncated ORF3 fragments, the fusion protein had a homogenous distribution, similar to that seen in cells that received an empty vector (Fig. 7B). These findings indicate that the N terminus of vp13 is needed to produce the filamentous arrangement of this protein.
FIG. 7.
Identification of the microtubule-interacting domain in vp13. (A) Schematic of the ORF3 truncation constructs. The numbers above each line indicate the starting and ending nucleotides of ORF3 or its truncated constructs. H3D1 and H3D2 contain both of the hydrophobic domains in the N terminus of vp13. H3D3 contains only the second hydrophobic domain in the N terminus of vp13. H3NH contains only the first hydrophobic domain in the N terminus of vp13. (B) Confocal fluorescence images of live HeLa cells transfected with these constructs. The same codes are used in the image labeling. H3F, VenusC1-H3 containing full-length ORF3.
To determine whether the two hydrophobic domains in the N terminus of vp13 are responsible for the interaction between vp13 and microtubules, we cloned the fragment corresponding to the first hydrophobic domain into the VenusC1 vector and transfected the resulting recombinant plasmid, VenusC1-H3NH, into HeLa cells. Using live-cell confocal fluorescence microscopy, we found punctate green fluorescence (Fig. 7B) in these cells but no filamentous fluorescent patterns. This finding suggests that the first hydrophobic domain in the N terminus of vp13 alone cannot support the association of vp13 with microtubules. Cells transfected with VenusN1-H3D3, which contained sequences of the second hydrophobic domain, demonstrated a homogeneous distribution of vp13 (Fig. 7B), indicating that it cannot, on its own, support the vp13-microtubule interaction. These findings suggest that both hydrophobic domains in the N terminus of vp13 are necessary for vp13 to associate with microtubules.
The consequence of interfering with microtubule dynamics is apoptosis. We conducted a cell viability assay to determine whether vp13 affected cell growth. We did not detect any change in growth rate for cells expressing vp13 compared with normal cells (data not shown). This suggests that vp13 has no detectable adverse effect on cell growth.
DISCUSSION
Viral capsids cannot move freely through the cytoplasm because of a high concentration of cytoplasmic proteins and organelles and because of the cytoskeleton (37). Much of the cytoskeleton consists of microtubules, which are polarized filaments that play an important role in the transport of vesicles and organelles throughout the cytoplasm. Several viruses, including adenovirus and herpes simplex virus, are known to use microtubules to move throughout the cell (2, 18). For example, VP26—a herpes simplex virus capsid surface protein—interacts with dynein light chains to mediate retrograde transport of the capsid (3). The adenovirus capsid also binds to dynein for microtubule-associated transport of the viral genome to the nucleus of the cell (14).
We investigated vp13 function and found the interaction between vp13 and microtubules. Our findings advance our understanding of vp13 and suggest that it uses the microtubule network to facilitate HEV infection.
Filamentous pattern of the vp13.
The linear array pattern of vp13 molecules in cells expressing a vp13-Venus fusion protein suggested the existence of a relationship between vp13 and microtubules. This arrangement had not been observed previously by other investigators (36, 40), possibly because of their choice of expression vectors and cell lines.
Venus reporter vectors were used in this study to express the vp13-Venus fusion protein and to study the vp13 relationship with microtubules. One major advantage of the Venus vectors is that they express monomeric reporter proteins and, thus, avoid overexpression and protein aggregation. We studied the expression of a vp13-Venus fusion protein in HeLa and Huh-7 cells. We observed a filamentous pattern in both types of cells although the filaments were shorter and developed at a lower rate in the Huh-7 cells than in the HeLa cells. The difference in filament length may reflect differences in cell-type-specific mechanisms of microtubule organization.
Confirmation of a vp13-microtubule relationship.
We were able to confirm that vp13 associates with microtubules by exposing transfected cells to nocodazole (a microtubule-destabilizing drug), which abolished the filamentous vp13 pattern. Removal of the drug restored α-tubulin polymerization and the filamentous arrangement of vp13.
A microtubule isolation assay provided further evidence of vp13-microtubule interactions by demonstrating that vp13 is found in microtubule-containing pellets after cell homogenization in PEM buffer and centrifugation. Extraction of the pellet with a high-salt PEMS buffer led to a shift of some of the vp13 from the pellet to the supernatant because the salt disrupted ionic bonds, thus allowing vp13 to dissociate from the microtubules. This finding indicates that the vp13-microtubule interaction is electrostatic.
In a Western blot analysis carried out after exposure to a PEMS buffer or a PEM buffer supplemented with ATP and GTP, the vp13 bands in the supernatant lanes appeared weak (Fig. 5). This was probably because the proteins in the supernatant had been precipitated with trichloroacetic acid and were diluted during adjustment of the sample pH after the pellet was resuspended and denatured in the Laemmli buffer. However, this experiment was conducted to show the presence of vp13, not the total amount of protein. The location of vp13 during microtubule isolation assays provided critical information about the vp13-microtubule relationship. After PEM lysis, its absence in the supernatant indicated that it is not a soluble cytosol protein. After PEMS lysis, its presence in the pellet indicated that it was associated with subcellular organelles. The latter was confirmed using PEMT lysis buffer, which included detergent to facilitate extraction of vp13 from the membranous organelles.
We speculated that vp13 interacts with microtubules through other proteins, because (i) salt extraction suggested that the vp13-microtubule interaction is electrostatic, (ii) the two hydrophobic domains in the N terminus of vp13 are needed for the interaction, and (iii) vp13 was found in the cytoskeleton fraction but did not coprecipitate with tubulin in the presence of an anti-tubulin antibody (40). Its presence in the supernatant after exposure to ATP and GTP pointed strongly toward a motor protein as the link between vp13 and microtubules because it suggested that the vp13 interaction with microtubules, like dynein, is sensitive to nucleotides.
Role of dynein in the vp13-microtubule interaction.
Dynein motor activity was determined to be critical for vp13 to localize to microtubules since the vp13 filamentous pattern was abolished after (i) overexpression of p50/dynamitin (a subunit of dynactin), which disrupts dynein motor activity; or (ii) exposure to the dynein inhibitor sodium vanadate. This drug does not disturb tubulin polymerization (16), and inhibition of dynein motor function does not affect microtubule assembly (8). Therefore, it would not have disrupted the vp13 pattern if vp13 interacted directly with microtubules. The loss of the filamentous vp13 pattern with the loss of dynein activity suggests that vp13 might associate with the microtubules through the dynein motor complex. This is consistent with our observation that vp13 shifts from the pellet to the supernatant fraction when exposed to ATP and GTP, given that the binding of dynein to microtubules is nucleotide sensitive.
The potential interaction of vp13 with dynein suggests that vp13 may be transported to minus ends of microtubules toward MTOC. This point is consistent with our observation that MTOCs are bright green in some vp13-expressing cells. However, the dynein interaction might be one of the mechanisms for the vp13 association with microtubules since the filamentous pattern showed vp13 distribution along microtubules.
Both N-terminal hydrophobic domains of vp13 are needed for the interaction.
An analysis of truncated ORF3 constructs revealed that both hydrophobic N-terminal domains in vp13 are necessary for vp13-microtubule interactions to take place. One individual domain was not adequate for the association with microtubules. A punctate pattern was observed in cells transfected with a construct containing only the first hydrophobic domain, and the homogenous pattern was observed in cells transfected with a construct containing only the second hydrophobic domain. The punctate pattern indicates that the protein is expressed in subcellular organelles. The homogenous expression indicates that the protein is soluble in the cytosol.
Enhanced microtubule stability.
Microtubule stability was enhanced in cells expressing vp13, as indicated by an increase in acetylated α-tubulin levels in these cells. Under normal physiological conditions, α-tubulin acetylation and deacetylation provide a powerful and dynamic mechanism for controlling microtubule assembly. Interference with these dynamics can have adverse consequences, including apoptosis (29). We did not observe apoptosis or any change in viability in cells expressing vp13, possibly because vp13 blocks the release of cytochrome c (24), thereby protecting the cell from programmed death.
Overall, our data demonstrate that vp13 interacts with microtubules and interferes with their dynamics, which could create an intracellular environment conducive to the establishment of a successful HEV infection.
Acknowledgments
This work was supported by an institutional start-up fund from the University of Maryland.
We thank Xiang-Jin Meng at Virginia Polytechnic and Science University for his gift of the rabbit antibody against HEV ORF3 and Suzanne Emerson at the National Institutes for Health for her gift of the pSK-E2 construct.
Footnotes
Published ahead of print on 15 April 2009.
REFERENCES
- 1.Alonso, C., J. Miskin, B. Hernaez, P. Fernandez-Zapatero, L. Soto, C. Canto, I. Rodriguez-Crespo, L. Dixon, and J. M. Escribano. 2001. African swine fever virus protein p54 interacts with the microtubular motor complex through direct binding to light-chain dynein. J. Virol. 759819-9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dales, S., and Y. Chardonnet. 1973. Early events in the interaction of adenoviruses with HeLa cells. IV. Association with microtubules and the nuclear pore complex during vectorial movement of the inoculum. Virology 56465-483. [DOI] [PubMed] [Google Scholar]
- 3.Douglas, M. W., R. J. Diefenbach, F. L. Homa, M. Miranda-Saksena, F. J. Rixon, V. Vittone, K. Byth, and A. L. Cunningham. 2004. Herpes simplex virus type 1 capsid protein VP26 interacts with dynein light chains RP3 and Tctex1 and plays a role in retrograde cellular transport. J. Biol. Chem. 27928522-28530. [DOI] [PubMed] [Google Scholar]
- 4.Echeverri, C. J., B. M. Paschal, K. T. Vaughan, and R. B. Vallee. 1996. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 132617-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emerson, S. U., H. Nguyen, J. Graff, D. A. Stephany, A. Brockington, and R. H. Purcell. 2004. In vitro replication of hepatitis E virus (HEV) genomes and of an HEV replicon expressing green fluorescent protein. J. Virol. 784838-4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emerson, S. U., M. Zhang, X. J. Meng, H. Nguyen, M. St Claire, S. Govindarajan, Y. K. Huang, and R. H. Purcell. 2001. Recombinant hepatitis E virus genomes infectious for primates: importance of capping and discovery of a cis-reactive element. Proc. Natl. Acad. Sci. USA 9815270-15275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goode, B. L., and S. C. Feinstein. 1994. Identification of a novel microtubule binding and assembly domain in the developmentally regulated inter-repeat region of tau. J. Cell Biol. 124769-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grabham, P. W., G. E. Seale, M. Bennecib, D. J. Goldberg, and R. B. Vallee. 2007. Cytoplasmic dynein and LIS1 are required for microtubule advance during growth cone remodeling and fast axonal outgrowth. J. Neurosci. 275823-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graff, J., H. Nguyen, C. Yu, W. R. Elkins, M. St Claire, R. H. Purcell, and S. U. Emerson. 2005. The open reading frame 3 gene of hepatitis E virus contains a cis-reactive element and encodes a protein required for infection of macaques. J. Virol. 796680-6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graff, J., U. Torian, H. Nguyen, and S. U. Emerson. 2006. A bicistronic subgenomic mRNA encodes both the ORF2 and ORF3 proteins of hepatitis E virus. J. Virol. 805919-5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haqshenas, G., H. L. Shivaprasad, P. R. Woolcock, D. H. Read, and X. J. Meng. 2001. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J. Gen. Virol. 822449-2462. [DOI] [PubMed] [Google Scholar]
- 12.Huang, Y. W., T. Opriessnig, P. G. Halbur, and X. J. Meng. 2007. Initiation at the third in-frame AUG codon of open reading frame 3 of the hepatitis E virus is essential for viral infectivity in vivo. J. Virol. 813018-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kar-Roy, A., H. Korkaya, R. Oberoi, S. K. Lal, and S. Jameel. 2004. The hepatitis E virus open reading frame 3 protein activates ERK through binding and inhibition of the MAPK phosphatase. J. Biol. Chem. 27928345-28357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelkar, S. A., K. K. Pfister, R. G. Crystal, and P. L. Leopold. 2004. Cytoplasmic dynein mediates adenovirus binding to microtubules. J. Virol. 7810122-10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King, S. M. 2000. The dynein microtubule motor. Biochim. Biophys. Acta 149660-75. [DOI] [PubMed] [Google Scholar]
- 16.Kirazov, E. P., and D. G. Weiss. 1986. Effects of vanadate on the assembly and disassembly of purified tubulin. Cell Motil. Cytoskeleton 6314-323. [DOI] [PubMed] [Google Scholar]
- 17.Korkaya, H., S. Jameel, D. Gupta, S. Tyagi, R. Kumar, M. Zafrullah, M. Mazumdar, S. K. Lal, L. Xiaofang, D. Sehgal, S. R. Das, and D. Sahal. 2001. The ORF3 protein of hepatitis E virus binds to Src homology 3 domains and activates MAPK. J. Biol. Chem. 27642389-42400. [DOI] [PubMed] [Google Scholar]
- 18.Kristensson, K., E. Lycke, M. Roytta, B. Svennerholm, and A. Vahlne. 1986. Neuritic transport of herpes simplex virus in rat sensory neurons in vitro. Effects of substances interacting with microtubular function and axonal flow [nocodazole, taxol and erythro-9-3-(2-hydroxynonyl)adenine]. J. Gen. Virol. 672023-2028. [DOI] [PubMed] [Google Scholar]
- 19.Li, T. C., K. Chijiwa, N. Sera, T. Ishibashi, Y. Etoh, Y. Shinohara, Y. Kurata, M. Ishida, S. Sakamoto, N. Takeda, and T. Miyamura. 2005. Hepatitis E virus transmission from wild boar meat. Emerg. Infect. Dis. 111958-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mabit, H., M. Y. Nakano, U. Prank, B. Saam, K. Dohner, B. Sodeik, and U. F. Greber. 2002. Intact microtubules support adenovirus and herpes simplex virus infections. J. Virol. 769962-9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maccioni, R. B., and V. Cambiazo. 1995. Role of microtubule-associated proteins in the control of microtubule assembly. Physiol. Rev. 75835-864. [DOI] [PubMed] [Google Scholar]
- 22.Meng, X. J., P. G. Halbur, M. S. Shapiro, S. Govindarajan, J. D. Bruna, I. K. Mushahwar, R. H. Purcell, and S. U. Emerson. 1998. Genetic and experimental evidence for cross-species infection by swine hepatitis E virus. J. Virol. 729714-9721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng, X. J., R. H. Purcell, P. G. Halbur, J. R. Lehman, D. M. Webb, T. S. Tsareva, J. S. Haynes, B. J. Thacker, and S. U. Emerson. 1997. A novel virus in swine is closely related to the human hepatitis E virus. Proc. Natl. Acad. Sci. USA 949860-9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moin, S. M., M. Panteva, and S. Jameel. 2007. The hepatitis E virus (HEV) ORF3 protein protects cells from mitochondrial depolarization and death. J. Biol. Chem. 28221124-21133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paschal, B. M., H. S. Shpetner, and R. B. Vallee. 1987. MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J. Cell Biol. 1051273-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pipeleers, D. G., M. A. Pipeleers-Marichal, P. Sherline, and D. M. Kipnis. 1977. A sensitive method for measuring polymerized and depolymerized forms of tubulin in tissues. J. Cell Biol. 74341-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ploubidou, A., V. Moreau, K. Ashman, I. Reckmann, C. Gonzalez, and M. Way. 2000. Vaccinia virus infection disrupts microtubule organization and centrosome function. EMBO J. 193932-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purcell, R. H., and S. U. Emerson. 2001. Hepatitis E virus, p. 3051-3061. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, vol. 2, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 29.Sorger, P. K., M. Dobles, R. Tournebize, and A. A. Hyman. 1997. Coupling cell division and cell death to microtubule dynamics. Curr. Opin. Cell Biol. 9807-814. [DOI] [PubMed] [Google Scholar]
- 30.Suomalainen, M., M. Y. Nakano, S. Keller, K. Boucke, R. P. Stidwill, and U. F. Greber. 1999. Microtubule-dependent plus- and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J. Cell Biol. 144657-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi, K., N. Kitajima, N. Abe, and S. Mishiro. 2004. Complete or near-complete nucleotide sequences of hepatitis E virus genome recovered from a wild boar, a deer, and four patients who ate the deer. Virology 330501-505. [DOI] [PubMed] [Google Scholar]
- 32.Tam, A. W., M. M. Smith, M. E. Guerra, C. C. Huang, D. W. Bradley, K. E. Fry, and G. R. Reyes. 1991. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology 185120-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tei, S., N. Kitajima, K. Takahashi, and S. Mishiro. 2003. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 362371-373. [DOI] [PubMed] [Google Scholar]
- 34.Tyagi, S., H. Korkaya, M. Zafrullah, S. Jameel, and S. K. Lal. 2002. The phosphorylated form of the ORF3 protein of hepatitis E virus interacts with its non-glycosylated form of the major capsid protein, ORF2. J. Biol. Chem. 27722759-22767. [DOI] [PubMed] [Google Scholar]
- 35.Tyagi, S., M. Surjit, and S. K. Lal. 2005. The 41-amino-acid C-terminal region of the hepatitis E virus ORF3 protein interacts with bikunin, a Kunitz-type serine protease inhibitor. J. Virol. 7912081-12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyagi, S., M. Surjit, A. K. Roy, S. Jameel, and S. K. Lal. 2004. The ORF3 protein of hepatitis E virus interacts with liver-specific α1-microglobulin and its precursor α1-microglobulin/bikunin precursor (AMBP) and expedites their export from the hepatocyte. J. Biol. Chem. 27929308-29319. [DOI] [PubMed] [Google Scholar]
- 37.Verkman, A. S. 2002. Solute and macromolecule diffusion in cellular aqueous compartments. Trends Biochem. Sci. 2727-33. [DOI] [PubMed] [Google Scholar]
- 38.Westermann, S., and K. Weber. 2003. Post-translational modifications regulate microtubule function. Nat. Rev. Mol. Cell Biol. 4938-947. [DOI] [PubMed] [Google Scholar]
- 39.Yazaki, Y., H. Mizuo, M. Takahashi, T. Nishizawa, N. Sasaki, Y. Gotanda, and H. Okamoto. 2003. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J. Gen. Virol. 842351-2357. [DOI] [PubMed] [Google Scholar]
- 40.Zafrullah, M., M. H. Ozdener, S. K. Panda, and S. Jameel. 1997. The ORF3 protein of hepatitis E virus is a phosphoprotein that associates with the cytoskeleton. J. Virol. 719045-9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang, Y., R. D. Sharma, and P. S. Paul. 1998. Monoclonal antibodies against conformationally dependent epitopes on porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 63125-136. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, Y. J., K. Y. Wang, D. A. Stein, D. Patel, R. Watkins, H. M. Moulton, P. L. Iversen, and D. O. Matson. 2007. Inhibition of replication and transcription activator and latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus by morpholino oligomers. Antivir. Res. 7312-23. [DOI] [PMC free article] [PubMed] [Google Scholar]