Abstract
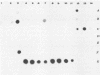
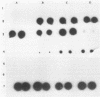
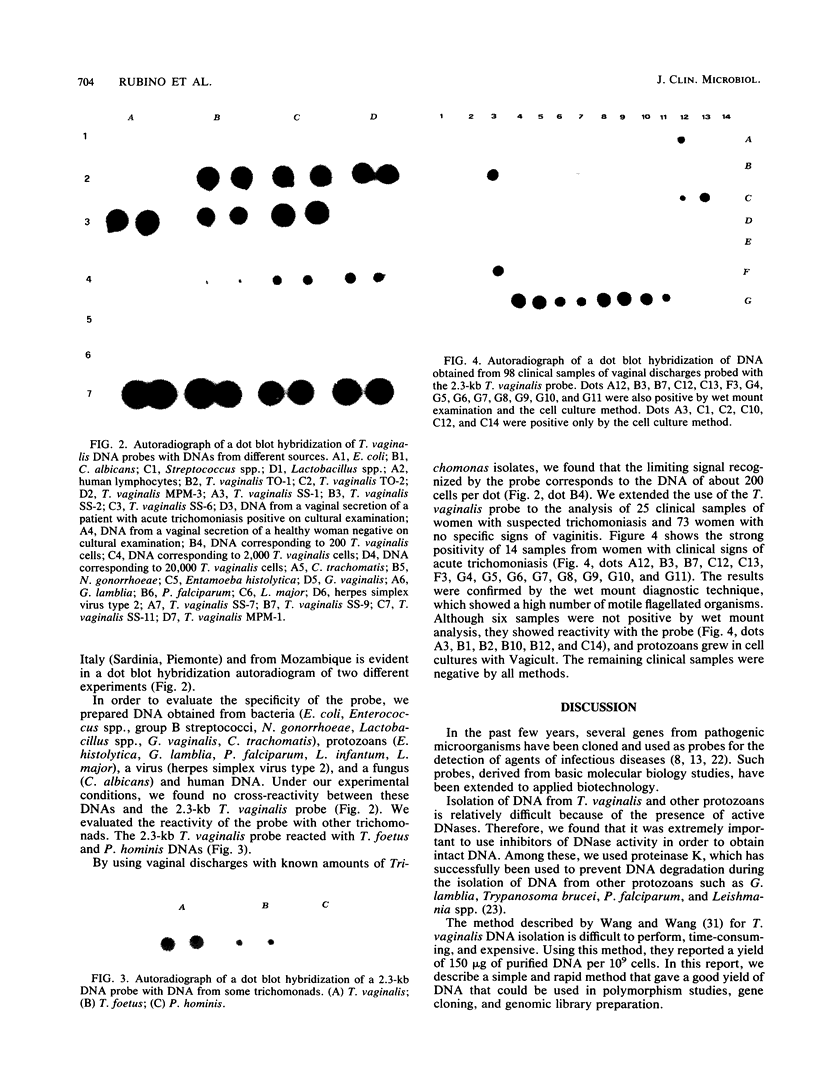
Trichomoniasis is one of the most widespread sexually transmitted diseases in the world. Diagnosis can be achieved by several methods, such as direct microscopic observation of vaginal discharge, cell culture, and immunological techniques. A 2.3-kb Trichomonas vaginalis DNA fragment present in strains from diverse geographic areas was cloned and used as a probe to detect T. vaginalis DNA in vaginal discharge by a dot blot hybridization technique. This probe was specific for T. vaginalis DNA. It recognized strains from two regions in Italy (Sardinia, Piemonte) and from Mozambique (Africa). In addition, our probe did not cross-react with bacterial (Escherichia coli, Enterococcus spp., group B streptococci, Gardnerella vaginalis, Neisseria gonorrhoeae, Chlamydia trachomatis, and Lactobacillus spp.), viral (herpes simplex virus type 2), fungal (Candida albicans), protozoan (Entamoeba histolytica, Giardia lamblia, Plasmodium falciparum, Leishmania major, and Leishmania infantum), or human nucleic acids. The probe reacted with Pentatrichomonas hominis and Trichomonas foetus. The limit signal recognized by our probe corresponded to the DNA of 200 T. vaginalis isolates. The 2.3-kb probe was used in a clinical analysis of 98 samples. Of these, 20 samples were found to be positive both with the probe and by cell culture, and only 14 of these were positive by a standard wet mount method.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F. Enzyme linked immunosorbent assay for detecting antibody to Trichomonas vaginalis: use of whole cells and aqueous extract as antigen. Br J Vener Dis. 1984 Jun;60(3):164–170. doi: 10.1136/sti.60.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Kasmala L. Monoclonal antibody to a major glycoprotein immunogen mediates differential complement-independent lysis of Trichomonas vaginalis. Infect Immun. 1986 Sep;53(3):697–699. doi: 10.1128/iai.53.3.697-699.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Suprun-Brown L., Kasmala L., Smith J., Spence M. Heterogeneity of Trichomonas vaginalis and discrimination among trichomonal isolates and subpopulations with sera of patients and experimentally infected mice. Infect Immun. 1985 Sep;49(3):463–468. doi: 10.1128/iai.49.3.463-468.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney J. A., Unadkat P., Yule A., Rajakumar R., Lacey C. J., Ackers J. P. New rapid latex agglutination test for diagnosing Trichomonas vaginalis infection. J Clin Pathol. 1988 Jul;41(7):806–808. doi: 10.1136/jcp.41.7.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND L. S. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957 Aug;43(4):488–490. [PubMed] [Google Scholar]
- Greenwood J. R., Kirk-Hillaire K. Evaluation of acridine orange stain for detection of Trichomonas vaginalis in vaginal specimens. J Clin Microbiol. 1981 Dec;14(6):699–699. doi: 10.1128/jcm.14.6.699-.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jírovec O., Petrů M. Trichomonas vaginalis and trichomoniasis. Adv Parasitol. 1968;6:117–188. doi: 10.1016/s0065-308x(08)60473-x. [DOI] [PubMed] [Google Scholar]
- KOTT H., ADLER S. A serological study of Trichomonas sp. parasitic in man. Trans R Soc Trop Med Hyg. 1961 Jul;55:333–344. doi: 10.1016/0035-9203(61)90102-x. [DOI] [PubMed] [Google Scholar]
- Krieger J. N., Holmes K. K., Spence M. R., Rein M. F., McCormack W. M., Tam M. R. Geographic variation among isolates of Trichomonas vaginalis: demonstration of antigenic heterogeneity by using monoclonal antibodies and the indirect immunofluorescence technique. J Infect Dis. 1985 Nov;152(5):979–984. doi: 10.1093/infdis/152.5.979. [DOI] [PubMed] [Google Scholar]
- Krieger J. N. Urologic aspects of trichomoniasis. Invest Urol. 1981 May;18(8):411–417. [PubMed] [Google Scholar]
- Mason M. M., Lasker B. A., Riggsby W. S. Molecular probe for identification of medically important Candida species and Torulopsis glabrata. J Clin Microbiol. 1987 Mar;25(3):563–566. doi: 10.1128/jcm.25.3.563-566.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P. R. Serodiagnosis of Trichomonas vaginalis infection by the indirect fluorescent antibody test. J Clin Pathol. 1979 Dec;32(12):1211–1215. doi: 10.1136/jcp.32.12.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip A., Carter-Scott P., Rogers C. An agar culture technique to quantitate Trichomonas vaginalis from women. J Infect Dis. 1987 Feb;155(2):304–308. doi: 10.1093/infdis/155.2.304. [DOI] [PubMed] [Google Scholar]
- Rubino S., Fiori P. L., Cappuccinelli P. Biotechnological applications for the diagnosis and prevention of parasitic diseases. J Chemother. 1989 Jul;1(4 Suppl):951–953. [PubMed] [Google Scholar]
- Smith R. F. Detection of Trichomonas vaginalis in vaginal specimens by direct immunofluorescence assay. J Clin Microbiol. 1986 Dec;24(6):1107–1108. doi: 10.1128/jcm.24.6.1107-1108.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spence M. R., Hollander D. H., Smith J., McCaig L., Sewell D., Brockman M. The clinical and laboratory diagnosis of Trichomonas vaginalis infection. Sex Transm Dis. 1980 Oct-Dec;7(4):168–171. doi: 10.1097/00007435-198010000-00004. [DOI] [PubMed] [Google Scholar]
- Street D. A., Taylor-Robinson D., Ackers J. P., Hanna N. F., McMillan A. Evaluation of an enzyme-linked immunosorbent assay for the detection of antibody to Trichomonas vaginalis in sera and vaginal secretions. Br J Vener Dis. 1982 Oct;58(5):330–333. doi: 10.1136/sti.58.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su K. E. Antibody to Trichomonas vaginalis in human cervicovaginal secretions. Infect Immun. 1982 Sep;37(3):852–857. doi: 10.1128/iai.37.3.852-857.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Waters A. P., McCuthan T. F. Ribosomal RNA: nature's own polymerase-amplified target for diagnosis. Parasitol Today. 1990 Feb;6(2):56–59. doi: 10.1016/0169-4758(90)90071-b. [DOI] [PubMed] [Google Scholar]
- Watt R. M., Philip A., Wos S. M., Sam G. J. Rapid assay for immunological detection of Trichomonas vaginalis. J Clin Microbiol. 1986 Oct;24(4):551–555. doi: 10.1128/jcm.24.4.551-555.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]