Abstract
Elite controllers or suppressors (ES) are human immunodeficiency virus type 1 (HIV-1)-infected patients who control viral replication to <50 copies/ml without antiretroviral therapy. Downregulation of HLA class I molecules is an important mechanism used by HIV-1 to evade the immune system. In this study, we showed that primary isolates from ES are as effective as isolates obtained from patients with progressive HIV-1 disease at downregulating HLA-A*2 and HLA-B*57 molecules on primary CD4+ T cells. Thus, a diminished ability of viral isolates from ES to evade HIV-specific immune responses probably does not contribute to the control of viral replication in these patients.
Long-term nonprogressors (LTNP) are human immunodeficiency virus type 1 (HIV-1)-infected individuals who maintain normal CD4+ T-cell counts and remain asymptomatic for longer than 10 years without therapy (7). Although many LTNP have detectable levels of HIV-1 RNA in their plasma, patients known as elite suppressors (ES) have viral loads of <50 RNA copies/ml. Understanding the factors involved in the maintenance of LTNP and ES statuses may be critical for the development of effective vaccines and immunotherapeutic treatments. One such factor under investigation is the role of cytotoxic-T-lymphocyte (CTL) responses. Several studies have shown that the HLA-B*27 and -B*57 alleles are overrepresented in cohorts of ES (13, 16, 19, 28, 29, 34). These findings suggest important roles for major histocompatibility complex class I (MHC-I) restriction and CD8+ T-cell responses in the control of viremia. Indeed, multiple studies have documented qualitatively superior CD8+ T cell function in ES compared to that in chronic HIV progressors (CP) (2, 5, 12, 27, 28, 37, 47).
Other studies suggest that some ES and LTNP are infected with attenuated viruses. One illustrative example comes from studies done on the Sydney Blood Bank Cohort, in which an LTNP donor transmitted an HIV-1 isolate with a large deletion in nef and the U3 region of the long terminal repeat to multiple recipients, all of whom became LTNP (11, 21). As in the Sydney Blood Bank Cohort studies, several other investigators have detected viruses with defective nef genes in LTNP and ES (1, 8, 18, 23, 25, 35, 36, 38, 43). In contrast, other studies showed that CD4+ T cells from ES could produce Gag when they were stimulated in vitro (20, 26), and full-length sequence analyses of plasma and proviral genomes revealed no evidence of significant deletions (30). Recent studies have suggested that plasma isolates (31) and replication-competent viruses (32) from HLA-B*57/B58*01 ES and LTNP, respectively, are less fit than isolates from B*57/B*5801 CP, but the difference in fitness observed is unlikely to fully explain the control of viral replication in these patients. Furthermore, we recently performed detailed genotypic and phenotypic analyses of replication-competent viruses isolated from ES and showed that these viruses were fully replication competent (6) Although nef is not required for viral replication in vitro, it has been strongly associated with pathogenesis in vivo (reviewed in reference 14). It is thus possible that some ES isolates are replication competent but have mutations in nef that result in diminished pathogenesis.
nef has been shown to be involved in the downregulation of both CD4 (15) and MHC-I (41). Several studies have shown that nef-induced MHC-I downregulation has a major impact on CTL function. In a seminal study, a dramatic reduction in HLA-A*2 expression by CD4+ T cells infected with wild-type virus but not by those infected with a virus carrying a defective nef gene was demonstrated. This downregulation resulted in diminished killing of HIV-1-infected cells by CTL clones specific for an HLA-A*2-restricted HIV-1 Gag epitope (10). Similarly, nef-mediated MHC-I downregulation was shown to impair the ability of HIV-1-specific CTL clones to suppress viral replication (42, 44). While these findings strongly suggest that HIV-1 partially evades the immune response by inducing MHC-I downregulation, other studies have demonstrated that primary CD8+ T cells from some ES and CP could effectively respond to autologous viral replication in autologous CD4+ T cells (26).
We tested the hypothesis that ES are infected with HIV-1 isolates that are less capable of downregulating MHC-I molecules. This could potentially cause the isolates to be more susceptible to CD8+ T-cell suppression of replication and may explain the superior CD8+ T-cell responses reported in prior ES studies (2, 5, 12, 27, 28, 37, 47). To date, fully characterized replication-competent isolates have been reported from just six ES subjects (1, 3, 6). We compared the MHC-I downregulation capacity of isolates from five of these ES to that of isolates obtained from resting CD4+ T cells of eight patients with progressive disease (viral load, >10,000 copies/ml). In order to develop a physiological model for HIV-1-induced MHC-I downregulation, we enriched primary CD4+ T cells from peripheral blood mononuclear cells (PBMC) from donors who were HLA-A*2 and/or HLA-B*57 positive by CD8+ T cell depletion with magnetic beads (Dynal), followed by activation in vitro with phytohemagglutinin for 3 days. For evaluation of HLA-A*2 downregulation, CD4+ T cells were obtained from HIV-seronegative donors. CD4+ T cells from ES were used for the evaluation of HLA-B*57 downregulation. This allele was as effectively downregulated in these ES as it was in multiple HLA-B*57 CP (data not shown). Following activation, the cells were infected with primary HIV-1 isolates from ES or CP by spinoculation (33). The primary isolates were obtained as previously described from latently infected CD4+ T cells (9). The median peak viral load and CD4+ T-cell nadir of the CP from whom viral isolates were obtained was 81,000 copies/ml and 279 cells/μl, respectively, and thus these isolates should be effective at HLA downregulation (22).
At different time points, the cells were harvested and stained with either fluorescein isothiocyanate (FITC)-conjugated anti-HLA-A*2 (Becton Dickinson) and tricolor-conjugated anti-CD4 antibodies (Caltag) or biotinylated anti-HLA-B*57 antibody (One Lambda) followed by FITC-conjugated streptavidin, peridinin chlorophyll protein-Cy5.5-conjugated anti-CD4 antibody (Becton Dickenson), and allophycocyanin-conjugated anti-CD3 antibody. The cells were fixed and permeabilized with Cytofix/Cytoperm solution (Becton Dickenson). Intracellular staining was then performed with the phycoerythrin-conjugated Gag-specific monoclonal antibody Kc57 or an immunoglobulin G1 mouse isotype control (Beckman Coulter). A total of 100,000 to 500,000 events were analyzed for each sample. HLA typing of ES was performed as previously described (4). The HLA-specific antibodies were tested on cells from a panel of ES with known HLA types to confirm specificity.
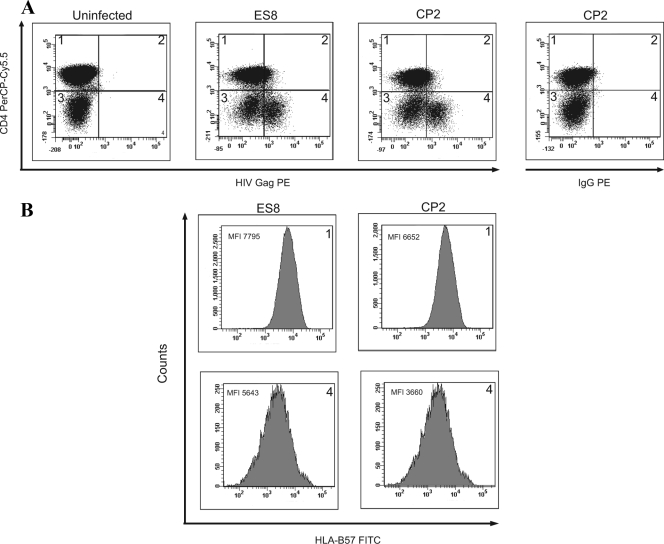
MHC-I downregulation was measured by comparing the mean fluorescence intensities (MFI) of HLA-A*2 and HLA-B*57 on HIV-1-infected versus noninfected CD4+ T cells. Infected cells were defined as cells that stained positive for intracellular Gag and had downregulated CD4 (Fig. 1). Uninfected CD4+ T cells were defined as cells that expressed high levels of CD4 and were negative for intracellular Gag protein. In order to standardize values, we determined relative MFI by dividing the MFI of the infected population by that of the CD4-positive, uninfected population. The Wilcoxon Mann-Whitney test was used to analyze the data.
FIG. 1.
Analysis of HLA-B*57 downregulation on HIV-1-infected cells. (A) CD8+ T-cell-depleted PBMC were stained with anti-HLA-B*57 and anti-CD4 monoclonal antibodies 3 days after infection with primary isolates from an ES (ES8) or a CP (CP2). Cells in quadrant 1 are uninfected CD4+ T cells, and cells in quadrant 4 (Gag-positive, low levels of CD4) are infected cells that have downregulated CD4. (PE, phycoerythrin; IgG, immunoglobulin G.) (B) The MFI of HLA-B*57 were compared for uninfected (quadrant 1) and infected (quadrant 4) cells from each sample.
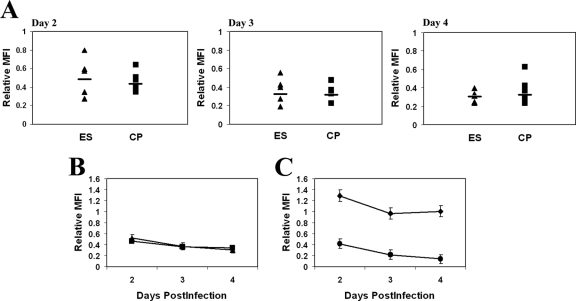
To determine if there was a difference in the ability of HIV-1 isolates cultured from ES versus CP to downregulate HLA-A*2, we measured the MFI of this molecule on infected CD4+ T cells that had downregulated CD4. On average, primary CD4+ T cells infected by ES viruses had levels of MHC-I downregulation of about two- to threefold, with relative MFI of 0.51, 0.37, and 0.30 on days 2, 3, and 4, respectively. Similarly, cells infected by isolates cultured from CP had relative MFI of 0.46, 0.36, and 0.33 on days 2, 3, and 4, respectively (Fig. 2B). These differences were not significantly different at any time point.
FIG. 2.
(A) Relative MFIs of HLA-A*02 on cells infected with isolates from five ES (triangles) and eight CP (squares) on days 2 to 4 postinfection. The relative MFI is defined as the MFI of the infected cells divided by the MFI of the uninfected CD4+ T cells in each sample. The horizontal bars represent the median for each group. (B) Average relative MFI of HLA-A*02 for cells infected with isolates from ES and CP on each day. (C) Average relative MFI of HLA-A*02 for cells infected with the wild-type NL4-3-green fluorescent protein virus (diamonds) or the Nef− Vpr− mutant virus (circles).
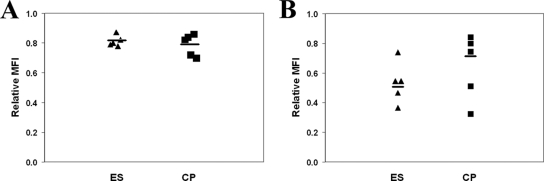
In order to rule out nonspecific downregulation of MHC-I on infected cells, we determined the MFI of HLA-DR and CD45 RO on cells infected with isolates from two subjects. The average relative MFI of the two proteins were 1.28 and 1.48, respectively, indicating that the MHC-I was in fact specifically downregulated. Since mutations in Nef have been shown to abrograte HLA downregulation, we also compared HLA-A2 downregulation by the HIV-1-based reporter construct NL4-3-green fluorescent protein and a Nef− Vpr− mutant vector (45, 46). As shown in Fig. 2C, no downregulation of HLA-A2 was seen at any point after infection with the Nef− Vpr− mutant virus, whereas infection with wild-type virus caused a degree of downregulation that was similar to that seen with primary isolates from ES and CP. Finally, we also looked at CD3 downregulation, as this molecule has been shown to be downregulated by Nef from HIV-2 and many simian immunodeficiency virus (SIV) isolates but not from HIV-1 (39). Furthermore, since SIVsmm nef isolated from sooty mangabeys with preserved CD4+ T-cell counts causes significantly more downregulation than SIVsmm nef from sooty mangabeys with CD4+ T-cell depletion (40), we determined whether isolates from ES also selectively downregulated this molecule. As shown in Fig. 3A, there was no significant downmodulation of CD3 after infection of cells with isolates from ES or CP.
FIG. 3.
(A) Relative MFI of CD3 on cells infected with isolates from five ES (triangles) and five CP (squares) on day 3 postinfection. The horizontal bars represent the median for each group. (B) Relative MFI of HLA-B*57 on cells infected with isolates from ES and CP.
Epidemiologic studies have suggested that HLA-B alleles play a larger role than HLA-A alleles in determining the outcome of infection (17). Furthermore, while HLA-B*57 is the most overrepresented allele seen in ES, there have not been any studies looking at downregulation of this MHC-I protein. Activated CD4+ T cells from an HLA-B*5703-positive ES were infected with isolates from five ES and five CP, and the degree of HLA-B*57 downregulation was measured on day 3. As shown in Fig. 3B, the average relative MFI of cells infected with isolates from five ES was 0.53, which was not significantly different from the average relative MFI of 0.64 that was seen in cells infected with isolates from five progressors.
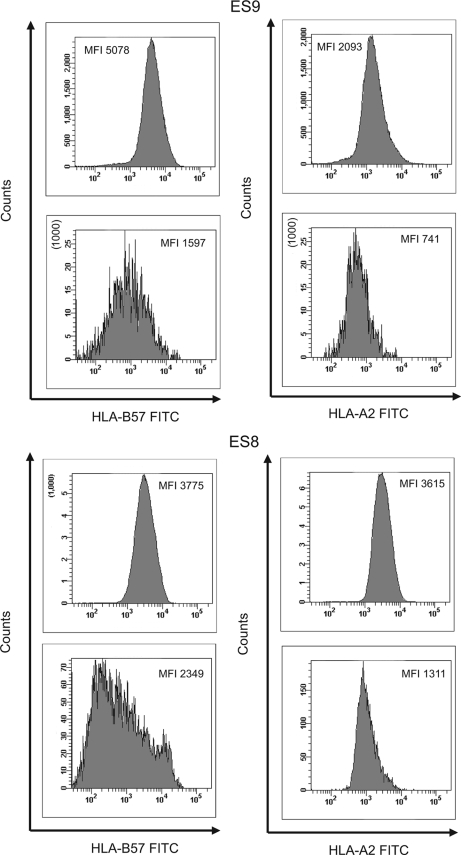
While it appeared that there was generally more downregulation of HLA-A*2 than HLA-B*57, the studies were performed in cells from different donors, and this precluded a direct comparison of the MFI of the two MHC-I alleles. Two ES in our cohort were positive for both HLA alleles, and the degrees of downregulation of these proteins could thus be compared. CD4+ T cells from ES8 were infected with autologous virus (6), and cells from ES9 were infected with a primary isolate from the CP who transmitted virus to her (3). For patient ES8, HLA-A2 showed a greater degree of downregulation than HLA-B57 at day 3 (a relative MFI of 0.36 versus 0.62) (Fig. 4). In contrast, in ES9 the degrees of downregulation of the two proteins were nearly identical (a relative MFI of 0.35 for HLA-A2 versus 0.31 for HLA-B*57).
FIG. 4.
Comparison of the relative MFI of HLA-A*02 and HLA-B*57 on CD8+ T-cell-depleted PBMC from ES8 and ES9 that were infected with autologous virus (ES8) or with the primary isolate from the CP who transmitted the virus to ES9. The MFI of HLA-A*2 or HLA-B*57 on uninfected CD4+ T cells (top panels) and infected cells that had downregulated CD4 (bottom panels) are shown.
This is the first study to look at downregulation of MHC-I proteins on CD4+ T cells infected with HIV-1 isolates cultured from ES CD4+ T cells. We used a physiological model where primary CD4+ T cells were infected with primary HIV-1 isolates. One advantage of this approach is that it accounts for HLA downregulation mediated by viral proteins such as Tat (24), as well as Nef. Similar amounts of MHC-I downregulation were seen for cells infected with replication-competent isolates cultured from ES and progressors. These results demonstrate that most ES are not infected by HIV-1 virions that are deficient in downregulating MHC-I compared to those of CP. Thus, it is likely that other factors enable ES to control viremia. The identification of these factors will have implications for the design of HIV-1 vaccines.
Acknowledgments
This work was supported by NIH grants R56 AI73185-01A1 and R01AI080328.
We thank Robert Siliciano and Thomas Williams for helpful discussions and Ferdynand Kos of the Johns Hopkins University School of Medicine Human Immunology Core for flow cytometry analysis of the HLA-B*57 data. We thank Hung-Chih Yang and Jason Dinoso for assistance with the graphics.
Footnotes
Published ahead of print on 22 April 2009.
REFERENCES
- 1.Alexander, L., E. Weiskopf, T. C. Greenough, N. C. Gaddis, M. R. Auerbach, M. H. Malim, S. J. O'Brien, B. D. Walker, J. L. Sullivan, and R. C. Desrosiers. 2000. Unusual polymorphisms in human immunodeficiency virus type 1 associated with nonprogressive infection. J. Virol. 744361-4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida, J. R., D. A. Price, L. Papagno, Z. A. Arkoub, D. Sauce, E. Bornstein, T. E. Asher, A. Samri, A. Schnuriger, I. Theodorou, D. Costagliola, C. Rouzioux, H. Agut, A. G. Marcelin, D. Douek, B. Autran, and V. Appay. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 2042473-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey, J. R., K. O'Connell, H. C. Yang, Y. Han, J. Xu, B. Jilek, T. M. Williams, S. C. Ray, R. F. Siliciano, and J. N. Blankson. 2008. Transmission of human immunodeficiency virus type 1 from a patient who developed AIDS to an elite suppressor. J. Virol. 827395-7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey, J. R., T. M. Williams, R. F. Siliciano, and J. N. Blankson. 2006. Maintenance of viral suppression in HIV-1-infected HLA-B*57+ elite suppressors despite CTL escape mutations. J. Exp. Med. 2031357-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 1074781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blankson, J. N., J. R. Bailey, S. Thayil, H. C. Yang, K. Lassen, J. Lai, S. K. Gandhi, J. D. Siliciano, T. M. Williams, and R. F. Siliciano. 2007. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J. Virol. 812508-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchbinder, S. P., M. H. Katz, N. A. Hessol, P. M. O'Malley, and S. D. Holmberg. 1994. Long-term HIV-1 infection without immunologic progression. AIDS 81123-1128. [DOI] [PubMed] [Google Scholar]
- 8.Calugi, G., F. Montella, C. Favalli, and A. Benedetto. 2006. Entire genome of a strain of human immunodeficiency virus type 1 with a deletion of nef that was recovered 20 years after primary infection: large pool of proviruses with deletions of env. J. Virol. 8011892-11896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chun, T. W., L. Carruth, D. Finzi, X. Shen, J. A. DiGiuseppe, H. Taylor, M. Hermankova, K. Chadwick, J. Margolick, T. C. Quinn, Y. H. Kuo, R. Brookmeyer, M. A. Zeiger, P. Barditch-Crovo, and R. F. Siliciano. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387183-188. [DOI] [PubMed] [Google Scholar]
- 10.Collins, K. L., B. K. Chen, S. A. Kalams, B. D. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391397-401. [DOI] [PubMed] [Google Scholar]
- 11.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, V. A. Lawson, S. Crowe, A. Maerz, S. Sonza, J. Learmont, J. S. Sullivan, A. Cunningham, D. Dwyer, D. Dowton, and J. Mills. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270988-991. [DOI] [PubMed] [Google Scholar]
- 12.Emu, B., E. Sinclair, D. Favre, W. J. Moretto, P. Hsue, R. Hoh, J. N. Martin, D. F. Nixon, J. M. McCune, and S. G. Deeks. 2005. Phenotypic, functional, and kinetic parameters associated with apparent T-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment. J. Virol. 7914169-14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emu, B., E. Sinclair, H. Hatano, A. Ferre, B. Shacklett, J. N. Martin, J. M. McCune, and S. G. Deeks. 2008. HLA class I-restricted T-cell responses may contribute to the control of HIV infection, but such responses are not always necessary for long-term virus control. J. Virol. 825398-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster, J. L., and J. V. Garcia. 2008. HIV-1 Nef: at the crossroads. Retrovirology 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia, J. V., and A. D. Miller. 1991. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 350508-511. [DOI] [PubMed] [Google Scholar]
- 16.Han, Y., J. Lai, P. Barditch-Crovo, J. E. Gallant, T. M. Williams, R. F. Siliciano, and J. N. Blankson. 2008. The role of protective HCP5 and HLA-C associated polymorphisms in the control of HIV-1 replication in a subset of elite suppressors. AIDS 22541-544. [DOI] [PubMed] [Google Scholar]
- 17.Kiepiela, P., A. J. Leslie, I. Honeyborne, D. Ramduth, C. Thobakgale, S. Chetty, P. Rathnavalu, C. Moore, K. J. Pfafferott, L. Hilton, P. Zimbwa, S. Moore, T. Allen, C. Brander, M. M. Addo, M. Altfeld, I. James, S. Mallal, M. Bunce, L. D. Barber, J. Szinger, C. Day, P. Klenerman, J. Mullins, B. Korber, H. M. Coovadia, B. D. Walker, and P. J. Goulder. 2004. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature 432769-775. [DOI] [PubMed] [Google Scholar]
- 18.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332228-232. [DOI] [PubMed] [Google Scholar]
- 19.Lambotte, O., F. Boufassa, Y. Madec, A. Nguyen, C. Goujard, L. Meyer, C. Rouzioux, A. Venet, J. F. Delfraissy, et al. 2005. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin. Infect. Dis. 411053-1056. [DOI] [PubMed] [Google Scholar]
- 20.Lamine, A., A. Caumont-Sarcos, M. L. Chaix, A. Saez-Cirion, C. Rouzioux, J. F. Delfraissy, G. Pancino, and O. Lambotte. 2007. Replication-competent HIV strains infect HIV controllers despite undetectable viremia (ANRS EP36 study). AIDS 211043-1045. [DOI] [PubMed] [Google Scholar]
- 21.Learmont, J. C., A. F. Geczy, J. Mills, L. J. Ashton, C. H. Raynes-Greenow, R. J. Garsia, W. B. Dyer, L. McIntyre, R. B. Oelrichs, D. I. Rhodes, N. J. Deacon, and J. S. Sullivan. 1999. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N. Engl. J. Med. 3401715-1722. [DOI] [PubMed] [Google Scholar]
- 22.Lewis, M. J., A. Balamurugan, A. Ohno, S. Kilpatrick, H. L. Ng, and O. O. Yang. 2008. Functional adaptation of Nef to the immune milieu of HIV-1 infection in vivo. J. Immunol. 1804075-4081. [DOI] [PubMed] [Google Scholar]
- 23.Mariani, R., F. Kirchhoff, T. C. Greenough, J. L. Sullivan, R. C. Desrosiers, and J. Skowronski. 1996. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J. Virol. 707752-7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsui, M., R. J. Warburton, P. C. Cogswell, A. S. Baldwin, Jr., and J. A. Frelinger. 1996. Effects of HIV-1 Tat on expression of HLA class I molecules. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 11233-240. [DOI] [PubMed] [Google Scholar]
- 25.Michael, N. L., G. Chang, L. A. d'Arcy, P. K. Ehrenberg, R. Mariani, M. P. Busch, D. L. Birx, and D. H. Schwartz. 1995. Defective accessory genes in a human immunodeficiency virus type 1-infected long-term survivor lacking recoverable virus. J. Virol. 694228-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Migueles, S. A., A. C. Laborico, H. Imamichi, W. L. Shupert, C. Royce, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, C. W. Hallahan, and M. Connors. 2003. The differential ability of HLA B*5701+ long-term nonprogressors and progressors to restrict human immunodeficiency virus replication is not caused by loss of recognition of autologous viral gag sequences. J. Virol. 776889-6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Migueles, S. A., A. C. Laborico, W. L. Shupert, M. S. Sabbaghian, R. Rabin, C. W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, and M. Connors. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 31061-1068. [DOI] [PubMed] [Google Scholar]
- 28.Migueles, S. A., C. M. Osborne, C. Royce, A. A. Compton, R. P. Joshi, K. A. Weeks, J. E. Rood, A. M. Berkley, J. B. Sacha, N. A. Cogliano-Shutta, M. Lloyd, G. Roby, R. Kwan, M. McLaughlin, S. Stallings, C. Rehm, M. A. O'Shea, J. Mican, B. Z. Packard, A. Komoriya, S. Palmer, A. P. Wiegand, F. Maldarelli, J. M. Coffin, J. W. Mellors, C. W. Hallahan, D. A. Follman, and M. Connors. 2008. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 291009-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Migueles, S. A., M. S. Sabbaghian, W. L. Shupert, M. P. Bettinotti, F. M. Marincola, L. Martino, C. W. Hallahan, S. M. Selig, D. Schwartz, J. Sullivan, and M. Connors. 2000. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. USA 972709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miura, T., M. A. Brockman, C. J. Brumme, Z. L. Brumme, J. M. Carlson, F. Pereyra, A. Trocha, M. M. Addo, B. L. Block, A. C. Rothchild, B. M. Baker, T. Flynn, A. Schneidewind, B. Li, Y. E. Wang, D. Heckerman, T. M. Allen, and B. D. Walker. 2008. Genetic characterization of human immunodeficiency virus type 1 in elite controllers: lack of gross genetic defects or common amino acid changes. J. Virol. 828422-8430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miura, T., M. A. Brockman, Z. L. Brumme, C. J. Brumme, F. Pereyra, A. Trocha, B. L. Block, A. Schneidewind, T. M. Allen, D. Heckerman, and B. D. Walker. 2009. HLA-associated alterations in replication capacity of chimeric NL4-3 viruses carrying gag-protease from elite controllers of human immunodeficiency virus type 1. J. Virol. 83140-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navis, M., I. Schellens, D. van Baarle, J. Borghans, P. van Swieten, F. Miedema, N. Kootstra, and H. Schuitemaker. 2007. Viral replication capacity as a correlate of HLA B57/B5801-associated nonprogressive HIV-1 infection. J. Immunol. 1793133-3143. [DOI] [PubMed] [Google Scholar]
- 33.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 7410074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereyra, F., M. M. Addo, D. E. Kaufmann, Y. Liu, T. Miura, A. Rathod, B. Baker, A. Trocha, R. Rosenberg, E. Mackey, P. Ueda, Z. Lu, D. Cohen, T. Wrin, C. J. Petropoulos, E. S. Rosenberg, and B. D. Walker. 2008. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 197563-571. [DOI] [PubMed] [Google Scholar]
- 35.Premkumar, D. R., X. Z. Ma, R. K. Maitra, B. K. Chakrabarti, J. Salkowitz, B. Yen-Lieberman, M. S. Hirsch, and H. W. Kestler. 1996. The nef gene from a long-term HIV type 1 nonprogressor. AIDS Res. Hum. Retrovir. 12337-345. [DOI] [PubMed] [Google Scholar]
- 36.Rhodes, D. I., L. Ashton, A. Solomon, A. Carr, D. Cooper, J. Kaldor, and N. Deacon. 2000. Characterization of three nef-defective human immunodeficiency virus type 1 strains associated with long-term nonprogression. J. Virol. 7410581-10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saez-Cirión, A., C. Lacabaratz, O. Lambotte, P. Versmisse, A. Urrutia, F. Boufassa, F. Barre-Sinoussi, J. F. Delfraissy, M. Sinet, G. Pancino, A. Venet, et al. 2007. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc. Natl. Acad. Sci. USA 1046776-6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salvi, R., A. R. Garbuglia, A. Di Caro, S. Pulciani, F. Montella, and A. Benedetto. 1998. Grossly defective nef gene sequences in a human immunodeficiency virus type 1-seropositive long-term nonprogressor. J. Virol. 723646-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schindler, M., J. Munch, O. Kutsch, H. Li, M. L. Santiago, F. Bibollet-Ruche, M. C. Muller-Trutwin, F. J. Novembre, M. Peeters, V. Courgnaud, E. Bailes, P. Roques, D. L. Sodora, G. Silvestri, P. M. Sharp, B. H. Hahn, and F. Kirchhoff. 2006. Nef-mediated suppression of T cell activation was lost in a lentiviral lineage that gave rise to HIV-1. Cell 1251055-1067. [DOI] [PubMed] [Google Scholar]
- 40.Schindler, M., S. Wildum, N. Casartelli, M. Doria, and F. Kirchhoff. 2007. Nef alleles from children with non-progressive HIV-1 infection modulate MHC-II expression more efficiently than those from rapid progressors. AIDS 211103-1107. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2338-342. [DOI] [PubMed] [Google Scholar]
- 42.Tomiyama, H., H. Akari, A. Adachi, and M. Takiguchi. 2002. Different effects of Nef-mediated HLA class I down-regulation on human immunodeficiency virus type 1-specific CD8+ T-cell cytolytic activity and cytokine production. J. Virol. 767535-7543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamada, T., and A. Iwamoto. 2000. Comparison of proviral accessory genes between long-term nonprogressors and progressors of human immunodeficiency virus type 1 infection. Arch. Virol. 1451021-1027. [DOI] [PubMed] [Google Scholar]
- 44.Yang, O. O., P. T. Nguyen, S. A. Kalams, T. Dorfman, H. G. Gottlinger, S. Stewart, I. S. Chen, S. Threlkeld, and B. D. Walker. 2002. Nef-mediated resistance of human immunodeficiency virus type 1 to antiviral cytotoxic T lymphocytes. J. Virol. 761626-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, H., Y. Zhou, C. Alcock, T. Kiefer, D. Monie, J. Siliciano, Q. Li, P. Pham, J. Cofrancesco, D. Persaud, and R. F. Siliciano. 2004. Novel single-cell-level phenotypic assay for residual drug susceptibility and reduced replication capacity of drug-resistant human immunodeficiency virus type 1. J. Virol. 781718-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou, Y., L. Shen, H. C. Yang, and R. F. Siliciano. 2008. Preferential cytolysis of peripheral memory CD4+ T cells by in vitro X4-tropic human immunodeficiency virus type 1 infection before the completion of reverse transcription. J. Virol. 829154-9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zimmerli, S. C., A. Harari, C. Cellerai, F. Vallelian, P. A. Bart, and G. Pantaleo. 2005. HIV-1-specific IFN-γ/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. USA 1027239-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]