Abstract
A recent clinical trial in patients with hemophilia B has suggested that adeno-associated virus (AAV) capsid-specific cytotoxic T lymphocytes (CTLs) eliminated AAV-transduced hepatocytes and resulted in therapeutic failure. AAV capsids elicit a CTL response in animal models; however, these capsid-specific CTLs fail to kill AAV-transduced target cells in mice. To better model the human clinical trial data in mice, we introduced an immunodominant epitope derived from ovalbumin (OVA; SIINFEKL) into the AAV capsid and tested CTL-mediated killing of AAV2-transduced target tissues in vivo. Initially, in vitro experiments demonstrated both classical class I and cross-presentation of the OVA antigen, following endogenous expression or AAV2-OVA vector transduction, respectively. Furthermore, an OVA-specific CTL response was elicited after muscular or systemic injection of the AAV2-OVA vector. Finally, CTL reactivity was enhanced in mice with established SIINFEKL-specific immunity after AAV2-OVA/α1 anti-trypsin (AAT) administration. Most importantly, these OVA-specific CTLs decreased AAT expression in mice treated with AAV2-OVA/AAT vector that followed a time course mimicking uncoating kinetics of AAV2 transduction in OVA-immunized mice. These results demonstrate that AAV capsid-derived antigens elicit CD8+ CTL reactivity, and these CTLs eliminated AAV-transduced target cells in mice. Notably, this model system can be exploited to study the kinetics of capsid presentation from different serotypes of AAV and permit the design of novel strategies to block CTL-mediated killing of AAV-transduced cells.
Adeno-associated virus (AAV) is a single-stranded DNA parvovirus. Its replication relies on coinfection of a helper virus such as adenovirus or herpesvirus. In the absence of a helper virus, AAV establishes latency to integrate into the AAVS1 site of host chromosome 19 (11). The genome of AAV is ∼4.7 kb and contains two open reading frames encoding replication proteins and structural capsid proteins (21). The capsid proteins (VP) are composed of VP1, VP2, and VP3. The VP3 protein is the major structural component and constitutes nearly 80% of the virion shell with an overall ratio of 1:1:8 for VP1, VP2, VP3, respectively. While VP2 is thought to be nonessential for AAV transduction (30), the VP1 subunit contains a phospholipase A2 domain required for infectivity (9). Recombinant AAV (rAAV) vectors require only the 145-bp terminal repeats of the AAV genome in cis and all other viral factors supplied in trans for production (18). rAAV vectors have rapidly gained popularity in gene therapy applications and have proven effective in preclinical studies/clinical trials for a number of diseases (20, 31, 33).
AAV vectors mount a potent humoral immune response against capsid in animals and human. However, AAV vectors only contain the therapeutic gene flanked by two 145-bp AAV terminal repeats devoid of any AAV genes(23). In addition, AAV initiates long-term stable therapeutic gene expression in animal models (3-5, 17, 31). Based on these observations AAV has been thought to be relatively nonimmunogenic regarding the induction of cytotoxic T lymphocytes (CTLs) specific for capsid proteins. In spite of all of these observations, the recent clinical trial for hemophilia B (F9) gene therapy has otherwise suggested that AAV2 capsid initiates cell-mediated immunity that eliminates the AAV2 encoding F9 (AAV2/F9) vector transduced liver cells (15). Against this backdrop, numerous attempts to replicate aforementioned observations in animal models have been made. Preliminary results from these studies support direct presentation and cross-presentation of the AAV2 capsid in animal models (6, 12, 13, 22, 29). However, capsid-specific CTLs did not eliminate AAV2-transduced target cells in mice (12, 13, 29), inconsistent with observations made in a clinical trial for hemophilia B with AAV2/F9 gene therapy. A potential explanation for this discrepancy is the weak immunogenicity of the AAV2 capsid in mice. Accordingly, we hypothesized that incorporation of a peptide epitope into the AAV2 capsid would increase immunogenicity of the rAAV and therefore could be exploited to mimic events ongoing in humans and study approaches to block capsid-specific CTL reactivity in mice.
We chose to introduce the MHC-H2Kb-restricted SIINFEKL peptide derived from ovalbumin (OVA) into AAV2 capsid. Integration of the OVA epitope into AAV capsids elicited a specific CTL response. Most importantly, after administration of genetically engineered AAV2 vectors into OVA peptide-immunized mice, OVA-specific CTL reactivity was further enhanced, thereby limiting transgene expression in vivo. The modified vector described herein is a potentially valuable tool for future studies focused on developing strategies to evade capsid-specific CTL-mediated elimination of AAV-transduced target cells in animal models.
MATERIALS AND METHODS
Cells and virus.
AAV virus production was previously described (34). The virus titer was determined by Southern dot blot. To rule out the possibility of minimal Cap-OVA plasmid construct in the virus preps, we have tested for AAV-OVA virus using an alkaline gel and did not find the Cap2-OVA plasmid contamination in the virus preps after blotting with transgene-specific probe and pCap2-OVA backbone probe.
Mice.
C57BL/6 mice and BALB/c mice were purchased from Jackson Laboratory (Bar Harbor, ME). The OT-1/Rag-1 mouse is transgenic for an H2Kb-restricted T-cell receptor that recognizes the OVA-derived SIINFEKL peptide and is deficient in the recombinase reactivating gene 1 (Rag-1 gene); therefore, this mouse does not develop mature T or B cells expressing endogenous receptors (Taconic Farms, Germantown, NY). All mice were maintained in a specific pathogen-free facility at the University of North Carolina at Chapel Hill. The University of North Carolina Institutional Animal Care and Use Committee approved all procedures.
Construction of recombinant plasmids.
The SIINFEKL epitope was cloned into AAV2's HI loop by substitution of amino acids (aa) 658 to 665 of capsid by PCR with two pairs of primers, i.e. the primers F1 (5′-CACGTGACTGGCAAAGACTCATC) and R1 (5′-GTTGATTATACTAGGATTCGCAGGTACCGGGG) and the primers F2 (5-TTTGAAAAACTGTTTGCTTCCTTCATCACACAGTACTC) and R2 (5′-GACCATGATTACGCCAAGCTCG); boldface represents the OVA SIINFEKL nucleotide sequence. The PCR products from primers F1R1 and F2R2 were digested with BsiWI and NotI, respectively. pXR2-OVA was generated by insertion of digested PCR products into pXR2. The Cap2-OVA fragment from pXR2-OVA with SwaI and NotI cohesive ends was cloned into the pTR/CBA-α1 anti-trypsin (AAT) backbone digested with EcoRI (blunted) and NotI to form pTR/CBA-Cap2-OVA.
Peptides.
AMQMLKETI (human immunodeficiency virus type 1 [HIV-1] gag24 199-207) and SIINFEKL (OVA 257-264) were synthesized in the University of North Carolina at Chapel Hill Microprotein Sequencing and Peptide Synthesis Facility and were >98% pure. Peptides were dissolved in dimethyl sulfoxide at a concentration of 20 mg/ml and stored at −20°C.
Transfection.
Nearly confluent 293 cells in a six-well plate were transfected with 10 μg of the respective plasmids using Lipofectamine 2000 according to manufacturer's instruction (Invitrogen). After 24 h, cells were used for interaction with spleen cells from OT-1 mice.
Flow cytometry.
293 cells transfected with plasmids encoding H2Kb and pAAV2-OVA were cocultured with 106 spleen cells were costained with a fluorescein isothiocyanate (FITC)-labeled rabbit anti-mouse CD69 antibody (BD Pharmingen) and an anti-CD8-phycoerythrin (PE) antibody (BD Pharmingen) for 1 h at 4°C on ice. Cells were then washed and analyzed by flow cytometry. The antibody isotype control was also included for each experiment.
Tetramer staining.
OVA-specific tetramers were prepared as described by Altman et al. (1). Briefly, H2Kb monomers were folded by the addition of the OVA (SIINFEKL) peptide and β-microglobulin subunit overnight in folding buffer. Folded monomers were purified by using size exclusion and then biotinylated using biotin and BirA enzyme (Avidity). Tetramers were assembled by adding biotin-conjugating H2Kb monomers with streptavidin-PE (eBioscience) 24 h before use.
Spleen cells were cultured with 10 μg of SIINFEKL/ml overnight. After washing, spleen cells were then incubated with a PE-labeled H2Kb-SIINFEKL tetramers for 30 min at room temperature and then washed three additional times. Spleen cells were incubated with FITC-conjugated rat anti-mouse CD8 antibody for 30 min at 4°C. After washing, cells were analyzed by flow cytometry.
Intracellular IFN-γ staining.
OT-1 spleen cells (106) were cocultured with 293/Kb cells overnight. Cells were incubated with 5 μM/ml brefeldin A (BD Pharmingen) for 5 h, stained with a FITC-conjugated anti-CD8 antibody, and then fixed with Cytofix/Cytoperm (BD Pharmingen). Cells were then incubated with a PE-conjugated anti-gamma interferon (IFN-γ) antibody, washed, and analyzed by flow cytometry.
Immunization.
Dendritic cells (DCs) were generated as described before (12). For immunization, mature DCs (106/ml) were pulsed with SIINFEKL or HIV gag 24 (AMQMLKETI) peptides at 10 μg/ml for 2 h at 37°C. A total of 5 × 105 DCs were then injected into C57BL/6 mice via tail vein three times at weekly intervals (24).
For AAV2 immunization, 2 × 1011 particles of AAV2-OVA vector were injected into C57BL/6 mice intramuscularly (i.m.) or intravenously (i.v.). Six weeks later in vitro (tetramer staining for spleen cells) or in vivo CTL assays were carried out.
In vivo CTL assay.
An in vivo CTL assay was carried out as described by Chen et al. with minor modifications (6). A total of 107 spleen cells from naive syngeneic mice were incubated with either high (5 μM) or low (0.5 μM) concentrations of carboxyfluorescein succinimidyl ester (CFSE) in phosphate-buffered saline at room temperature for 12 min. CFSE labeling was stopped by the addition of fetal bovine serum to a final concentration of 20% (vol/vol). After the cells were washed, CFSEhigh cells were incubated with 10 μg of peptide/ml at 37°C for 1 h, whereas CFSElow cells were incubated in medium only. The AMQMLKETI peptide was used as a negative control to pulse a separate population of CFSEhi cells. After labeling and peptide pulsing, both populations of target cells were washed and mixed together in ice-cold phosphate-buffered saline, 107 cells of each population i.v. injected into mice. Spleen cells were prepared 24 h later and analyzed by flow cytometry. The percent specific lysis was determined by the ratio of recovered non-peptide-treated control spleen cells to peptide-sensitized spleen cells (e.g., percentage of CFSElow cells/percentage of CFSEhigh cells). The percent specific lysis (%) equaled 100 × (1 − [ratio of cells recovered from naive mice/ratio of cells recovered from infected mice]).
Luciferase imaging.
Six weeks after intramuscular (i.m.) injection of AAV2-OVA/luc into BALB/c mice, the imaging was performed by using Xenogen system.
ELISA for human AAT.
Details for AAT detection by enzyme-linked immunosorbent assay (ELISA) were described as previously (12).
Statistical analysis.
The Student t test was used to perform statistical analysis. Differences were considered statistically significant at P < 0.05.
RESULTS
H2Kb-SIINFEKL complexes are detected on the surfaces of cells transfected with the Cap2-OVA plasmid construct.
The OVA epitope SIINFEKL induces a strong CTL response in C57BL/6 mice and is commonly used to investigate CTL-mediated immune responses. Accordingly, this immunodominant epitope was inserted into a tolerant position of the AAV2 capsid: the HI loop. The HI loop has been shown to tolerate insertions or substitution without a loss of function including AAV virion assembly, receptor binding, and host range (7, 19). Our previous work has demonstrated that a 4-aa linker insertion at residue 649 just before the HI loop does not affect virus yield and infectivity compared to wild-type AAV2 vectors (19). To further characterize the HI loop for gene targeting purposes, we swapped the HI loop of AAV2 with HI loops from serotypes 1 and 8. A similar virus yield was achieved. All viruses from wild-type AAV2 and mutants with AAV1 and eight HI loops substitution had similar infectivities and heparin-binding capacities (7). Oligonucleotides encoding the SIINFEKL epitope were cloned into the AAV2 HI loop by substitution of aa 658 to 665 of capsid to generate the construct pCap2-OVA. To determine whether the OVA epitope in the context of Cap2-OVA was synthesized, processed, and presented, splenic SIINFEKL-specific OT-1 CD8+ T cells were cocultured with H2Kb-expressing human embryonic kidney cells (293 cells) transfected with the pCap2-OVA plasmid construct. After coculture with pCap2-OVA-transfected 293 cells, 9.52% and 2.57% of OT-1 CD8+ T cells expressed the activation marker CD69 and intracellular IFN-γ, respectively (Fig. 1). In contrast, no significant increase in the frequency of OT-1 CD8+ T cells expressing CD69 or intracellular IFN-γ was detected upon coculture with 293 cells transfected with Cap2 compared to wild-type 293 cells (Fig. 1). These results demonstrate that the SIINFEKL peptide in the context of Cap2-OVA is processed and presented to T cells.
FIG. 1.

OVA epitope presentation in cells transfected with the chimeric AAV2-OVA capsid gene (pAAV2-OVA). 293 cells with 80% to 90% confluent in a six-well plate were transfected with 5 μg of a plasmid expressing H2Kb and 5 μg of the genes encoding the AAV2 capsid or the chimeric AAV2 capsid harboring the OVA epitope (pCap2-OVA). The next day, transfected 293 cells were incubated with spleen cells from OT-1 mice for 24 h. Then, OT-1 spleen cells were stained for CD8 and either CD69 or intracellular IFN-γ and analyzed by flow cytometry. The data represent the results of one of three different experiments.
H2Kb-SIINFEKL complexes are detected on the surface of cells transduced with AAV2-OVA in vitro.
Next, we tested whether an infectious AAV virion could be assembled using the Cap2-OVA gene using a standard triple transfection method. AAV2 particles were packaged with either green fluorescent protein (GFP), AAT or firefly luciferase (Luc) cDNA. Compared to wild-type AAV2 capsid, similar titers of packaged Cap2-OVA were obtained as determined by Southern dot blot analysis (data not shown). In addition, a similar frequency of transduced 293 cells was detected for the respective packaged AAV recombinants regardless of the encoded transgenes (Fig. 2).
FIG. 2.
Transduction efficiency of AAV2-OVA vector in vitro. 293 cells in a 24-well plate were transduced with different AAV2-OVA vector. After 24 h, 293 cells were analyzed for transgene expression. (A) The image was taken under a fluorescence microscopy for dsAAV2/GFP (A1) or dsAAV2-OVA/GFP (A2) transduction at 103 particles per cell. (B) Luciferase activity was measured after cells was harvested and lysed with passive buffer for AAV2-OVA/luc transduction at 103 or 104 particles per cell. (C) AAT expression was detected with ELISA in supernatant from AAV2-OVA/AAT transduction at 103 particles per cell. The results represent the means of three individual experiment ± the standard derivations.
To determine whether the OVA epitope expressed by the AAV virion was efficiently processed and presented, H2Kb-expressing 293 cells were transduced with AAV2-OVA/AAT at 105 particles/cell and subsequently cocultured with splenic OT-1 CD8+ T cells. The frequency of OT-1 CD8+ T cells expressing CD69 and intracellular IFN-γ was increased ∼8-fold upon coculture with 293 cells transduced with AAV2-OVA/AAT relative to cultures containing 293 cells transduced with AAV2/AAT or mock-transfected cells (Fig. 3). This result further demonstrates that the SIINFEKL epitope in the context of AAV2 capsid protein is processed and presented to SIINFEKL-specific CD8+ T cells.
FIG. 3.

OVA epitope presentation in cells transduced with the AAV2-OVA vector. 293 cells were transfected with 5 μg of the H2Kb plasmid and, 24 h later, the cells were incubated with AAV2-OVA/AAT vector or AAV2/AAT vector at 105 particles/cell for 2 h. Then, spleen cells harvested from OT-1 mice were added, followed by coculture for 24 h. CD8/CD69 or CD8/IFN-γ expression was investigated by flow cytometry. The data represent from the results of one of three independent experiments.
Immunization with AAV2-OVA vector induces an OVA-specific CTL response in vivo.
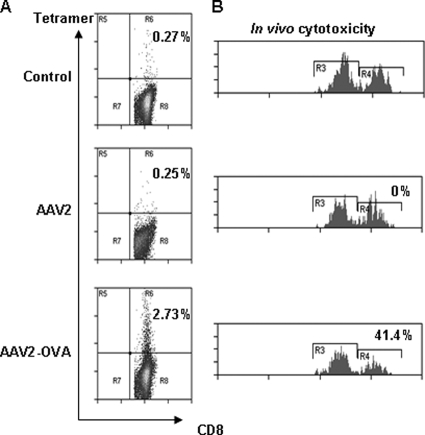
It has been reported that i.m. injection of AAV2 vector elicits a capsid-specific CTL response in mice (14, 29). To test this in our model system, 2 × 1011 particles of AAV2-OVA/AAT were injected i.m. into C57BL/6 mice, and 6 weeks later the induction of SIINFEKL-specific CD8+ T cells was examined via tetramer staining. In AAV2-OVA/AAT-immunized mice, 2.73% of splenic CD8+ T cells stained with H2Kb-OVA tetramer, which in turn was ∼10-fold increased relative mice injected with AAV2 (0.25%) or left untreated (0.27%) (Fig. 4). Consistent with this observation, CTL-mediated killing of SIINFEKL-pulsed target cells was increased in mice immunized with AAV2-OVA but not AAV2 or in animals left untreated (Fig. 4B). This result demonstrates that administration of AAV2-OVA induces a CTL response specific for the SIINFEKL peptide.
FIG. 4.
OVA-specific CTL response was elicited with muscular injection of AAV2-OVA vector. (A) A total of 2 × 1011 particles of AAV2-OVA/AAT vectors were injected into muscle of C57BL mice, and 6 weeks later the spleen cells were harvested for OVA-specific CTL tetramer staining analyzed by flow cytometry. (B) In vivo CTL function analysis. Spleen cells from C57BL/C mouse were either labeled with a high concentration of CFSE (CFSEH) and pulsed with SIINFEKL peptide or labeled with a low concentration of CFSE (CFSEL). The mixture of both populations of target cells were injected i.v. into AAV-OVA vector receiving mice after 6 weeks. After 24 h, single cells were isolated from the spleens and analyzed by flow cytometry to determine the percentage of remaining target cells that are either CFSEH or CFSEL, and the in vivo CTL-mediated killing was calculated (see Materials and Methods for more details). The number represents the in vivo CTL-specific killing from one of four mice.
Injection of AAV2-OVA i.v. also elicited a CTL response specific for the SIINFEKL peptide. C57BL/6 mice received 2 × 1011 particles of AAV2-OVA/AAT i.v., and 6 weeks later the frequency of H2Kb-OVA tetramer binding CD8+ T cells measured in the liver and spleen. As demonstrated in Fig. 5, a marked increase in the frequency of H2Kb-OVA tetramer binding CD8+ T cells was detected in both spleen and liver of AAV2-OVA treated mice versus animals injected with AAV2 or left untreated (Fig. 5A and B). In vivo CTL assay further demonstrated increased CTL function in AAV2-OVA-immunized mice compared to the control and AAV2-immunized groups (Fig. 5C).
FIG. 5.
OVA-specific CTL response was elicited with systemic injection of AAV2-OVA vector. A total of 2 × 1011 particles of AAV2-OVA/AAT vectors were injected into C57BL mice via tail vein, 6 weeks later spleen cells (A) or liver cells (B) were harvested for OVA-specific CTL tetramer staining. or an in vivo CTL function analysis was performed (C). All data represent the means from AAV vector-treated mice (n = 3 or 4) and the standard derivations. *, P < 0.05 versus control or AAV group.
AAV2-OVA administration enhances an established OVA-specific CTL response.
To examine the effect of AAV2-OVA administration in mice with preexisting immunity, C57BL/6 mice were treated with SIINFEKL-pulsed DCs twice but not three times at weekly intervals since the strongest CTL response can be induced with three peptide-pulsed DC immunizations (24). Ten days after the last treatment, 2 × 1011 particles of AAV2-OVA were injected i.v. Two weeks post-AAV vector injection, the frequency of H2Kb-OVA tetramer binding CD8+ T cells and in vivo CTL function were measured. No CTL response was observed in AAV2-OVA treated mice without prior OVA peptide immunization (Fig. 6), perhaps the period after administration of vector was too short to elicit an immune response. In established OVA-immunized mice, 0.73% and 3.45% of CD8+ T cells bound H2Kb-OVA tetramer in the spleens and the livers of AAV2-treated mice, respectively. Although the administration of AAV2-OVA vector increased tetramer-positive cells to 1.53% and 4.9% in the spleen and liver, respectively (Fig. 6A and B, P < 0.05 compared to AAV-OVA vector application). Only 0.32% and 1.63% CD8+ T cells stained with H2Kb-OVA tetramer in untreated mice (Fig. 6A and B). In vivo CTL function assay demonstrated that CTLs exerted more killing of target cells in DC-immunized mice with AAV2-OVA vector than with AAV2 vector (87% killing versus 64%, respectively, Fig. 6C, P < 0.05). These data indicate that the administration of AAV2-OVA vector further enhances an established CTL response and provides additional evidence that OVA epitope can be presented on the cell surface and induce a CTL response after AAV2-OVA administration.
FIG. 6.
The administration of AAV2-OVA vector further enhanced the preexisting CTL response. C57BL mice were immunized with OVA peptide SIINFEKL-pulsed DCs twice weekly. Ten days after last DC vaccination, 2 × 1011 particles of AAV2-OVA/AAT vectors were injected via tail vein. At 2 weeks after vector injection, the spleen and the liver were harvested for tetramer-specific CTL analysis (panels A and B, respectively) or in vivo CTL assay was carried out (C). All data represent the mean of four mice and the standard derivation. *, P < 0.05 or **, P < 0.01 (versus control or AAV2-OVA); #, P < 0.05 (versus CTL+AAV2).
AAV2-OVA/AAT vector-transduced target cells are eliminated in vivo by OVA-specific CTLs.
In previous animal studies, the AAV2 capsid induced a specific CTL response, and yet these CTLs did not eliminate AAV2-transduced cells. In contrast, cells with endogenous AAV2 capsid expression were killed in vivo (12). To determine whether the increased immunogenicity of the AAV2-OVA capsid resulted in killing of transduced cells, C57BL/6 mice were treated with SIINFEKL-pulsed DCs as described above. Ten days after the last immunization, mice received i.v. 2 × 1011 particles of AAV2-OVA/AAT. Significantly less circulating AAT was detected in mice injected with AAV2-OVA/AAT and receiving SIINFEKL-pulsed DCs relative to animals injected only with AAV2-OVA/AAT (Fig. 7A). Notably, a 25% decrease of AAT was observed in DC-treated mice one week after virus administration (P < 0.05), while a fourfold decrease was observed at week 5, compared to the mice not receiving the DC pretreatment (P < 0.01). Thereafter, AAT level remained stable, suggesting that in mice with established SIINFEKL-specific immunity the reduction of AAT levels was not due to immunity to AAT per se but likely due to CTL targeting of AAV2-OVA transduced cells. Also, we observed that the liver enzyme AST was elevated in OVA-immunized mice after administration of AAV2-OVA/AAT vector (Fig. 7B). To rule out the possibility of CTL nonspecific mediated killing of AAV2-OVA-transduced liver cells, mice received DCs pulsed with the HIV gag p24 peptide. The AAT levels in blood after AAV2-OVA/AAT injection were comparable to mice not receiving the DC transfers (Fig. 7C). These results suggest that the SIINFEKL epitope is processed and presented on the surface of liver cells transduced by AAV2-OVA, which in turn are killed by SIINFEKL-specific CTLs.
FIG. 7.
Cytotoxicity of OVA-specific CTLs on AAV2-OVA transduced cells in vivo. Mice were injected intravascularly with peptide-pulsed DCs three times on a weekly basis. Ten days after the last immunization, 2 × 1011 particles of AAV2/OVA-AAT vectors were administered by tail vein injection. The AAT level in the blood was detected at the indicated time points after AAV administration by ELISA. The AST in the blood was also measured in mice. (A) The kinetics of AAT expression in the blood in OVA peptide-immunized mice with AAV2-OVA vector administration. (B) The kinetic change of the AST in the blood in OVA peptide-immunized mice with AAV2-OVA vector administration. (C) The AAT expression in the blood at week 6 in p24 peptide-immunized mice after AAV2-OVA vector administration. All data represent the mean results from four mice and the standard derivation. *, P < 0.05 or **, P < 0.01 (versus AAV2-OVA/AAT).
DISCUSSION
In this study we have demonstrated that a CTL response was elicited from an engineered AAV vector containing the OVA-derived SIINFEKL peptide in the AAV2 capsid HI loop. OVA-specific CTLs were further enhanced by administration of AAV2-OVA vector and eliminated AAV2-OVA vector transduced target cells in vivo. These laboratory-derived AAV mutants will help us investigate the mechanism of AAV capsid presentation to induce a CTL response after in vivo application and explore novel approaches to evade immune-response-mediated elimination of AAV transduced target cells.
Concerns over the AAV capsid-induced CTL response have been brought to the forefront by results from a recent clinical trial in patients with hemophilia B after treatment with AAV2/F9 vectors. In one patient, the therapeutic level of F9 was achieved in the blood at week 2 after injection of AAV2/F9. Several weeks later, the F9 level plummeted to baseline values. It was suggested that the elimination of AAV2-transduced liver cells was mediated by memory CTLs against the AAV2 capsid since the patient had low titer of neutralizing antibodies against AAV2 in his blood before treatment and AAV2 capsid peptide stimulated lymphocytes to secrete IFN-γ (15). Although a CTL response to AAV2 capsid has also been confirmed in animal models, these CTLs did not clear AAV2-transduced target cells in mice, which is inconsistent with the interpretation of the clinical data (12, 14, 29). Attempts to replicate these findings in transgenic mice with human HLA gene knock-in were disappointing, since AAV2 administration via muscular injection did not elicit a capsid specific CTL response, which is different from what was observed in wild-type mice (BALB/c and C57BL/c) after injection of AAV vector (13, 16, 22, 29). One possible explanation is that the AAV2 capsid is a weak antigen in animal models. In order to recreate the observed results from the clinical trial is to enhance AAV2 capsid immunogenicity through mutagenesis, while ensuring minimal effect on packaging capacity, infectivity, or tropism of AAV virions.
Mutagenesis of the AAV2 capsid has been widely studied and has provided valuable information to understand AAV2 biology and structure. Specific ligands have been incorporated into the AAV2 virion surface via insertion or substitution to enhance AAV2's transduction in specific tissues. At residue 138, the N terminus of VP-2, the insertion of an expanded serpin receptor ligand (KFNKPFVFLI) or hemagglutinin epitope (YPYDVPDYA) or gaussia luciferase does not influence AAV2 biological characteristics including virus packaging, transduction efficiency, and heparin-binding capacity (2, 32). However, insertions at residue 587 or 521 abolish the AAV2 heparin-binding ability and changes the tissue tropism (8, 25, 26). The HI loop of AAV2 is localized between residues 653 and 669 (VP1 numbering) based on the crystal structure and surrounds the fivefold pore, extending from each subunit and overlapping the underlying subunit (35). The HI loop is present in autonomous parvoviruses, including canine parvovirus, porcine parvovirus, and minute virus of mice. The amino acid sequence of the HI loop varies among different serotypes of AAV. We inserted 4 aa into the 649 residue just before the HI loop using linker insertion mutagenesis and demonstrated that this mutant maintained virus morphology, stability, and infectivity comparable to the wild-type virion (19). Recently, we swapped the AAV2 HI loop with HI loops from other serotypes, none of these seemed to influence the AAV2 biological profile (7). This information implicates that we can swap AAV HI loop with a strong immunogenic domain. After OVA epitope SIINFEKL peptide swapping, similar virus yield and tropism were observed in vitro and in vivo compared to wtAAV2 vector.
Several lines of evidence confirmed that OVA antigen presentation occurred after AAV2-OVA vector transduction in vitro and in vivo. (i) AAV2-OVA vector transduced H-2Kb cells activated spleen cells from OT-1 mice. (ii) OVA-specific CTL reactivity was elicited after i.v. or i.m. administration of AAV2-OVA vector into C57BL/6 mice. (iii) In preimmunized mice with OVA SIINFEKL peptide, OVA-specific CTL function was further enhanced after injection of AAV2-OVA vector. (iv) OVA-specific CTLs eliminated AAV2-OVA transduced liver cells and resulted in decreased transgene expression.
It is interesting to point out that OVA-specific CTLs were observed in the liver at a higher level than in the spleen. This observation is consistent to Walk's finding after muscular injection of AAV2/LacZ vector, i.e., higher number of LacZ-specific CTLs were demonstrated in the liver than in the spleen and muscle (28). The reason for this is that the liver is a graveyard and holds CTLs regardless of whether the liver is the target or not (10). It is worth noting that OVA-specific CTLs in the liver kill AAV-OVA vector-transduced liver cells, which mimics observations in the clinical trial. Our previous data demonstrated that AAV capsid-specific CTLs did not kill AAV2-transduced liver cells but that these CTLs eliminated AAV2-transduced liver cells when the AAV2 capsid genome was included in AAV vector transgene (12), a finding which is consistent with the observation when OVA antigen is expressed as a vector cotransgene, killing is 100% (data not shown). In the present study the inclusion of OVA CTL immunodomain SIINFEKL into AAV2 virion resulted in OVA-specific CTL-mediated elimination of AAV2-OVA-transduced liver cells. Again, this result further supports weak immunogenicity of AAV capsid to induce a CTL response in mice.
Recently, 12 serotypes and over 100 variants of AAV have been isolated and used as gene therapy vectors, in addition to different tropism in vivo, the kinetics of transgene expression delivered by different serotype vectors is different, probably arising from differential vector trafficking and uncoating (27). Transgene expression reaches the peak and remains stable much earlier in vivo with AAV8 than with AAV2, possibly due to faster uncoating of AAV8 virions than of AAV2 (27). Despite low neutralizing antibody cross-reactivity among AAV serotypes, the high homology of capsid protein sequences and observed cross-reactivity of CTL response against capsid among AAV serotypes and variants have been documented (14, 16). Integration of a strong immunodomain into AAV capsid, as reported here, will permit studies focused on better understanding of the kinetics of capsid cross-presentation for other serotypes.
In summary, substitution of the AAV2 virion HI loop with the OVA epitope SIINFEKL results in (i) an OVA/H2Kb complex presented on the cell surface, (ii) OVA-specific CTL activation, and (iii) CTL killing of AAV-OVA vector transduced target cells in vivo. These data support a mechanism of T-cell killing that follows the kinetics of capsid uncoating as primary reason for target cell elimination. Also, the data further confirmed our assumption that AAV capsid possesses poor antigenicity in mice. It remains to be determined if this weak antigenicity is or is not seen in AAV clinical studies as observed in rodent studies. Our study demonstrates that the utilization of an AAV-OVA vector in C57BL mice is an ideal model developed to mimic immune responses that may also be observed in human clinical trials. However, it should be noted that OVA is a “super” antigen that does not exist naturally in AAV capsid and that presentation of capsid antigen in the setting of the present study may not represent all of the immune response results observed in patients with different HLA class I alleles. More importantly from the present study, the application of this engineered AAV-OVA capsid now allows preclinical studies of the kinetics of capsid antigen cross-presentation at different vector doses (as well as different serotypes) in animal models, which should better predict the likelihood of safety concerns in current and future AAV clinical trials. Not only will work from the present study directly lead to a better understanding of the results obtained from the human trials, but it will also create a context in which novel approaches can be tested to evade host immune response-mediated elimination of AAV transduced target cells.
Acknowledgments
This study was supported in part by NIH research grants 5P01GM059299, R01AI072176, and 1U54AR056953 to R.J.S. and grant R01AI058014 to R.T. and an Alpha-1 Foundation grant to C.L.
We thank M. Bevan at the University of Washington for providing the pCBA-OVA construct and R. Flavell at Yale University providing the H2-Kb plasmid.
Footnotes
Published ahead of print on 15 April 2009.
REFERENCES
- 1.Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 27494-96. [DOI] [PubMed] [Google Scholar]
- 2.Asokan, A., J. S. Johnson, C. Li, and R. J. Samulski. 2008. Bioluminescent virion shells: new tools for quantitation of AAV vector dynamics in cells and live animals. Gene Ther. 151618-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao, H., Y. Liu, J. Rabinowitz, C. Li, R. J. Samulski, and C. E. Walsh. 2000. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol. Ther. 2619-623. [DOI] [PubMed] [Google Scholar]
- 4.Chao, H., P. E. Monahan, Y. Liu, R. J. Samulski, and C. E. Walsh. 2001. Sustained and complete phenotype correction of hemophilia B mice following intramuscular injection of AAV1 serotype vectors. Mol. Ther. 4217-222. [DOI] [PubMed] [Google Scholar]
- 5.Chao, H., R. Samulski, D. Bellinger, P. Monahan, T. Nichols, and C. Walsh. 1999. Persistent expression of canine factor IX in hemophilia B canines. Gene Ther. 61695-1704. [DOI] [PubMed] [Google Scholar]
- 6.Chen, J., Q. Wu, P. Yang, H. C. Hsu, and J. D. Mountz. 2006. Determination of specific CD4 and CD8 T-cell epitopes after AAV2- and AAV8-hF. IX gene therapy. Mol. Ther. 13260-269. [DOI] [PubMed] [Google Scholar]
- 7.DiPrimio, N., A. Asokan, L. Govindasamy, M. Agbandje-McKenna, and R. J. Samulski. 2008. Surface loop dynamics in adeno-associated virus capsid assembly. J. Virol. 825178-5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girod, A., M. Ried, C. Wobus, H. Lahm, K. Leike, J. Kleinschmidt, G. Deleage, and M. Hallek. 1999. Genetic capsid modifications allow efficient re-targeting of adeno-associated virus type 2. Nat. Med. 51438. [DOI] [PubMed] [Google Scholar]
- 9.Girod, A., C. E. Wobus, Z. Zadori, M. Ried, K. Leike, P. Tijssen, J. A. Kleinschmidt, and M. Hallek. 2002. The VP1 capsid protein of adeno-associated virus type 2 is carrying a phospholipase A2 domain required for virus infectivity. J. Gen. Virol. 83973-978. [DOI] [PubMed] [Google Scholar]
- 10.Huang, L., G. Soldevila, M. Leeker, R. Flavell, and I. N. Crispe. 1994. The liver eliminates T cells undergoing antigen-triggered apoptosis in vivo. Immunity 1741-749. [DOI] [PubMed] [Google Scholar]
- 11.Kotin, R. M., R. M. Linden, and K. I. Berns. 1992. Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. EMBO J. 115071-5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li, C., M. Hirsch, A. Asokan, B. Zeithaml, H. Ma, T. Kafri, and R. J. Samulski. 2007. Adeno-associated virus type 2 (AAV2) capsid-specific cytotoxic T lymphocytes eliminate only vector-transduced cells coexpressing the AAV2 capsid in vivo. J. Virol. 817540-7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li, H., S. L. Murphy, W. Giles-Davis, S. Edmonson, Z. Xiang, Y. Li, M. O. Lasaro, K. A. High, and H. C. Ertl. 2007. Pre-existing AAV capsid-specific CD8+ T cells are unable to eliminate AAV-transduced hepatocytes. Mol. Ther. 15792-800. [DOI] [PubMed] [Google Scholar]
- 14.Li, H., S. L. Murphy, W. Giles-Davis, S. Edmonson, Z. Xiang, Y. Li, M. O. Lasaro, K. A. High, and H. C. Ertl. 2007. Pre-existing AAV capsid-specific CD8+ T cells are unable to eliminate AAV-transduced hepatocytes. Mol. Ther. 15792-800. [DOI] [PubMed] [Google Scholar]
- 15.Manno, C. S., G. F. Pierce, V. R. Arruda, B. Glader, M. Ragni, J. J. Rasko, M. C. Ozelo, K. Hoots, P. Blatt, B. Konkle, M. Dake, R. Kaye, M. Razavi, A. Zajko, J. Zehnder, P. K. Rustagi, H. Nakai, A. Chew, D. Leonard, J. F. Wright, R. R. Lessard, J. M. Sommer, M. Tigges, D. Sabatino, A. Luk, H. Jiang, F. Mingozzi, L. Couto, H. C. Ertl, K. A. High, and M. A. Kay. 2006. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat. Med. 12342-347. [DOI] [PubMed] [Google Scholar]
- 16.Mingozzi, F., M. V. Maus, D. J. Hui, D. E. Sabatino, S. L. Murphy, J. E. Rasko, M. V. Ragni, C. S. Manno, J. Sommer, H. Jiang, G. F. Pierce, H. C. Ertl, and K. A. High. 2007. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 13419-422. [DOI] [PubMed] [Google Scholar]
- 17.Monahan, P. E., R. J. Samulski, J. Tazelaar, X. Xiao, T. C. Nichols, D. A. Bellinger, M. S. Read, and C. E. Walsh. 1998. Direct intramuscular injection with recombinant AAV vectors results in sustained expression in a dog model of hemophilia. Gene Ther. 540-49. [DOI] [PubMed] [Google Scholar]
- 18.Rabinowitz, J. E., F. Rolling, C. Li, H. Conrath, W. Xiao, X. Xiao, and R. J. Samulski. 2002. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J. Virol. 76791-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabinowitz, J. E., W. Xiao, and R. J. Samulski. 1999. Insertional mutagenesis of AAV2 capsid and the production of recombinant virus. Virology 265274-285. [DOI] [PubMed] [Google Scholar]
- 20.Romano, G. 2005. Current development of adeno-associated viral vectors. Drug News Perspect. 18311-316. [DOI] [PubMed] [Google Scholar]
- 21.Rose, J. A., J. V. Maizel, Jr., J. K. Inman, and A. J. Shatkin. 1971. Structural proteins of adenovirus-associated viruses. J. Virol. 8766-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabatino, D. E., F. Mingozzi, D. J. Hui, H. Chen, P. Colosi, H. C. Ertl, and K. A. High. 2005. Identification of mouse AAV capsid-specific CD8+ T-cell epitopes. Mol. Ther. 121023-1033. [DOI] [PubMed] [Google Scholar]
- 23.Samulski, R. J., K. I. Berns, M. Tan, and N. Muzyczka. 1982. Cloning of adeno-associated virus into pBR322: rescue of intact virus from the recombinant plasmid in human cells. Proc. Natl. Acad. Sci. USA 792077-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serody, J. S., E. J. Collins, R. M. Tisch, J. J. Kuhns, and J. A. Frelinger. 2000. T-cell activity after dendritic cell vaccination is dependent on both the type of antigen and the mode of delivery. J. Immunol. 1644961-4967. [DOI] [PubMed] [Google Scholar]
- 25.Shi, W., and J. S. Bartlett. 2003. RGD inclusion in VP3 provides adeno-associated virus type 2 (AAV2)-based vectors with a heparan sulfate-independent cell entry mechanism. Mol. Ther. 7515-525. [DOI] [PubMed] [Google Scholar]
- 26.Shi, X., G. Fang, W. Shi, and J. S. Bartlett. 2006. Insertional mutagenesis at positions 520 and 584 of adeno-associated virus type 2 (AAV2) capsid gene and generation of AAV2 vectors with eliminated heparin-binding ability and introduced novel tropism. Hum. Gene Ther. 17353-361. [DOI] [PubMed] [Google Scholar]
- 27.Thomas, C. E., T. A. Storm, Z. Huang, and M. A. Kay. 2004. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J. Virol. 783110-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velazquez, V. M., D. G. Bowen, and C. M. Walker. 2009. Silencing of T lymphocytes by antigen-driven programmed death in recombinant adeno-associated virus vector (rAAV)-mediated gene therapy. Blood 113538-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, L., J. Figueredo, R. Calcedo, J. Lin, and J. M. Wilson. 2007. Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum. Gene Ther. 18185-194. [DOI] [PubMed] [Google Scholar]
- 30.Warrington, K. H., Jr., O. S. Gorbatyuk, J. K. Harrison, S. R. Opie, S. Zolotukhin, and N. Muzyczka. 2004. Adeno-associated virus type 2 VP2 capsid protein is nonessential and can tolerate large peptide insertions at its N terminus. J. Virol. 786595-6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warrington, K. H., Jr., and R. W. Herzog. 2006. Treatment of human disease by adeno-associated viral gene transfer. Hum. Genet. 119571-603. [DOI] [PubMed] [Google Scholar]
- 32.Wu, P., W. Xiao, T. Conlon, J. Hughes, M. Agbandje-McKenna, T. Ferkol, T. Flotte, and N. Muzyczka. 2000. Mutational analysis of the adeno-associated virus type 2 (AAV2) capsid gene and construction of AAV2 vectors with altered tropism. J. Virol. 748635-8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu, Z., A. Asokan, and R. J. Samulski. 2006. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol. Ther. 14316-327. [DOI] [PubMed] [Google Scholar]
- 34.Xiao, X., J. Li, and R. J. Samulski. 1998. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 722224-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie, Q., W. Bu, S. Bhatia, J. Hare, T. Somasundaram, A. Azzi, and M. S. Chapman. 2002. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc. Natl. Acad. Sci. USA 9910405-10410. [DOI] [PMC free article] [PubMed] [Google Scholar]