Abstract
Prion neuroinvasion from peripheral tissues involves agent replication in the lymphoreticular system (LRS) prior to entry into the nervous system. This study investigated the role of the LRS in prion neuroinvasion from the oral and nasal mucosa in wild-type and immunodeficient mice and in hamsters infected with the HY and DY strains of the transmissible mink encephalopathy (TME) agent. Following inoculation at neural sites, all hosts were susceptible to prion disease and had evidence of prion infection in the brain, but infection of the LRS was found only in scrapie-infected wild-type mice and HY TME-infected hamsters. In the LRS replication-deficient models, prion neuroinvasion was not observed following intraperitoneal or oral inoculation. However, immunodeficient mice, which have impaired follicular dendritic cells, were susceptible to scrapie following intratongue and intranasal inoculation despite the absence of PrPSc in the tongue or the nasal cavity. For DY TME, hamsters were susceptible following intratongue but not intranasal inoculation and PrPSc was limited to nerve fibers of the tongue. These findings indicate that neuroinvasion from the tongue and nasal cavity can be independent of LRS infection but neuroinvasion was partially dependent on the strain of the prion agent and/or the host species. The paucity of PrPSc deposition in the oral and nasal mucosa from LRS replication-deficient hosts following neuroinvasion from these tissues suggests an infection of nerve fibers that is below the threshold of PrPSc detection and/or the transport of the prion agent along cranial nerves without agent replication.
In natural and experimental prion infections originating in the periphery, prion agent replication in the lymphoreticular system (LRS) precedes agent entry and spread in the peripheral nervous system. In the LRS, follicular dendritic cells (FDCs) are the major target of prion infection, and blocking or reversing FDC maturation can prevent scrapie agent replication in the LRS (25, 26, 28, 30, 32). Other migrating cell populations may also influence the progression of experimental prion disease (27, 36). From the LRS, centripetal spread of the prion agent to the spinal cord or brain occurs by spread along nerve fibers of the peripheral nervous system. In the central nervous system, prion agent replication can induce neurodegeneration and disease after an incubation period that can last from weeks to years. For example, in lambs from flocks with endemic scrapie, agent replication is initially detected in the gut-associated lymphoid tissues prior to proximal and distal spread in the LRS, infection of peripheral nerves that innervate the LRS, and subsequent spread to the spinal cord (19, 42). In addition, scrapie agent infection of the vagal nerve, which innervates many peripheral organs including the digestive tract, results in axonal transport directly to the dorsal motor nucleus of the vagus in the brain stem (29, 41). The role of scrapie infection in the LRS in the latter pathway of neuroinvasion is unknown. A similar pathway of prion neuroinvasion occurs in mule deer experimentally infected with the chronic wasting disease agent with the exception that early infection is also established in the lymph nodes of the upper gastrointestinal tract (37, 38). Recent studies indicate that a similar pathway of neuroinvasion occurs in natural and experimental bovine spongiform encephalopathy (BSE) following oral exposure except that agent replication in the LRS is greatly reduced and appears restricted to portions of the gut-associated lymphoid tissues (13, 20, 39).
There are natural prion diseases in sheep and cattle that do not exhibit the typical distribution of the prion agent in the brain and LRS that are presumably acquired via oral prion exposure (5, 33). The absence of the abnormal isoform of the prion protein, PrPSc, in the LRS and dorsal motor nucleus of the vagus in atypical scrapie and the H type or L type of BSE raises the question as to whether these cases are due to infection by an alternate route(s) other than ingestion or whether these cases have an etiology that is distinct from that of acquired prion diseases. Direct prion infection of nerve fibers or terminals in highly innervated tissues, such as the mucosa in the head, has been suggested to represent potential sites of prion agent entry that would not require prior agent replication in the LRS (4, 12, 31). The presence of scrapie or BSE infection in the retina, sensory fibers of the tongue, and nasal mucosa of sheep, goat, and/or cattle suggests that the eye, tongue, or nasal cavity could be alternate sites of prion agent entry into hosts (8, 11, 15, 16, 40). Experimental prion inoculation at these mucosal sites can cause prion disease and in some cases rapid neuroinvasion (4, 9, 17, 18). Another explanation for this distribution of infection is that centrifugal spread of the prion agent away from the brain and along cranial nerves could serve as a pathway for prion infection and accumulation in these mucosal tissues (4, 10, 43).
In this work, we investigated the role of the LRS in prion neuroinvasion from the oral and nasal cavities. In order to investigate neuroinvasion following neural and extraneural routes of inoculation in which prion replication is blocked in the LRS, we used two rodent models for prion infection. In muMT mice, which lack mature B cells, and in lymphotoxin-α (LTα) null mice, FDCs do not undergo maturation, and as a result, these mice do not develop clinical disease following intraperitoneal inoculation of the scrapie agent but are susceptible following direct inoculation into the brain (23, 30). In a second model, the HY and DY strains of the transmissible mink encephalopathy (TME) agent were used to investigate neuroinvasion in Syrian hamsters. The HY and DY TME agents can replicate in the nervous system, but the DY TME agent does not replicate in the LRS, and therefore, the DY TME agent is not pathogenic following intraperitoneal (i.p.) inoculation (2, 3). Following intratongue (i.t.) or intranasal (i.n.) inoculation, prion neuroinvasion was independent of scrapie agent replication in the LRS of immunodeficient mice, but evidence for scrapie infection of peripheral nerve fibers or olfactory neurons at these mucosa was lacking. In hamsters, i.t. inoculation of the HY or DY TME agent resulted in PrPSc deposition in nerve fibers and prion disease, but only the HY TME agent caused disease following i.n. inoculation. These findings suggest that neuroinvasion from the oral and nasal mucosa in LRS replication-deficient rodents can be independent of LRS infection, but the paucity of PrPSc at these mucosal sites of exposure in immunodeficient mice and DY TME-infected hamsters suggests that neuroinvasion is due to either a low-level prion infection of the nervous system at the site of inoculation or transport of the prion agent in axons in the absence of agent replication at the site of prion entry. These findings indicate that these mucosal tissues may not exhibit early evidence of infection and therefore will prove difficult to identify as a portal for agent entry.
MATERIALS AND METHODS
Animal inoculations and tissue collection.
All procedures involving animals were approved by the Institutional Animal Care and Use Committee and comply with the Guide for the Care and Use of Laboratory Animals. For the murine studies, weanling male or female muMT null (Jackson Laboratories, Bar Harbor, ME), LTα null mice (Jackson Laboratories, Bar Harbor, ME), and C57BL/6J wild-type controls were inoculated with a brain homogenate from an RML strain-infected mouse containing 106.9 median lethal doses (LD50) per gram. This prion titer is based on endpoint titration following intracerebral (i.c.) inoculation as described previously (6). In muMT null and LTα null mice, there are defects in secondary lymphoid organs, including incomplete development of the germinal center and a disorganization of the cytoarchitecture of secondary lymphoid tissues (1, 22). These species of immunodeficient mice do not support replication of the scrapie agent in secondary lymphoid organs, possibly due to impaired FDC maturation (23, 30, 34). Mice were inoculated by the i.c. (102.2 LD50 or 103.2 LD50), i.p. (102.6 LD50 or 103.6 LD50), i.t. (102.9 LD50), or i.n. (102.9 LD50 or 103.9 LD50) route. For hamster studies, weanling (4- to 5-week-old) outbred male golden Syrian hamsters (Harlan-Sprague-Dawley, Indiana) were inoculated with a 1% or 10% (wt/vol) brain homogenate from a HY TME agent-infected (107.5 or 108.5 LD50 per ml) or DY TME agent-infected (105.4 or 106.4 LD50 per ml) hamster. Hamsters were inoculated by the i.c. (50 μl), intra-sciatic-nerve (2 μl), i.t. (20 μl), i.p. (100 μl), per os (100 μl), or i.n. (20 μl) route. For i.t. inoculation, rodents were lightly anesthetized and 20 μl of brain homogenate was bilaterally inoculated into the lingual muscles on the underside of the tongue using a 30-gauge needle. For i.n. inoculations, rodents were lightly anesthetized and a narrow pipette tip was used to inoculated 10 μl of brain homogenate into each nostril. Between three and eight rodents were inoculated for each mouse species or TME strain for each route of inoculation as illustrated in Table 1 or 2, respectively. Animals were observed three times per week for the onset of neurological disease as described previously (7). Following euthanasia, frozen tissues were collected for Western blot analysis or the animals were perfused with fixative and tissues were prepared for PrPSc immunohistochemistry.
TABLE 1.
Incubation period following inoculation of wild-type and immunodeficient mice with the RML scrapie agent
| Route of inoculation | Expt 1a
|
Expt 2
|
||||
|---|---|---|---|---|---|---|
| Murine strain | Incubation periodb | No. affected/no. inoculated | Murine strain | Incubation periodb | No. affected/no. inoculated | |
| Cerebral | C57BL/6c | 127 ± 1.4 | 5/5 | C57BL/6d | 123 ± 7.0 | 2/2 |
| LTα nullc | 136 ± 3.1 | 5/5 | muMTd | 134 ± 1.0 | 3/3 | |
| Peritoneal | C57BL/6c | 184 ± 9.0 | 5/5 | C57BL/6d | 168 ± 4.3 | 4/4 |
| LTα nullc | >330 | 0/5 | muMTd | >345 | 0/3 | |
| Tongue | C57BL/6d | 167 ± 0.6 | 5/5 | C57BL/6d | 146 ± 7.8 | 4/4 |
| LTα nulld | 161 ± 8.6 | 5/5 | muMTd | 144 ± 13.4 | 4/4 | |
| Nasal | C57BL/6d | 235 | 1/4 | C57BL/6e | 236 ± 3.5 | 5/8 |
| LTα nulld | >365 | 0/4 | muMTe | 226 ± 0 | 2/8 | |
Experiments 1 and 2 differ in the strain of immunodeficient mice and the dose of the RML scrapie agent inoculated.
Mean days ± standard error of the mean.
The concentration of inoculum was 103.9 LD50 per ml of RML scrapie, but the volume inoculated varies among the routes of inoculation as stated in Materials and Methods.
The concentration of inoculum was 104.9 LD50 per ml of RML scrapie agent, but the volume inoculated varies among the routes of inoculation as stated in Materials and Methods.
The concentration of inoculum was 105.9 LD50 per ml of RML scrapie, but the volume inoculated varies among the routes of inoculation as stated in Materials and Methods.
TABLE 2.
Incubation periods of HY TME and DY TME in hamsters following inoculation by neural or extraneural routes
| Routea | HY TME agent
|
DY TME agent
|
||
|---|---|---|---|---|
| Incubation period, mean days ± SEM | No. affected/no. inoculated | Incubation period, mean days ± SEM | No. affected/no. inoculated | |
| Cerebral | 62 ± 2.0 | 4/4 | 173 ± 0.0 | 3/3 |
| Sciatic nerve | 64 ± 1.0 | 6/6 | 211 ± 3.5 | 8/8 |
| Tongue | 82 ± 1.0 | 3/3 | 245 ± 5.9 | 5/8 |
| Peritoneal | 112 ± 9.7 | 4/4 | >500 | 0/4 |
| Oral ingestion | 122 ± 1.1 | 6/6 | >600 | 0/6 |
| Nasal | 130 ± 5.5 | 4/4 | >500 | 0/4 |
Hamsters were inoculated with either a 1% (wt/vol) or 10% (wt/vol) brain homogenate from a HY TME-infected (109.5 LD50/g) or DY TME-infected (107.4 LD50/g) hamster.
Rodent bioassay for prion infectivity.
Brains and spleens were collected under sterile conditions from symptomatic or asymptomatic mice following mock inoculation or inoculation with the RML scrapie agent by various routes. In hamsters orally inoculated with the DY TME agent, brains, Peyer's patches, spleens, and submandibular lymph nodes were collected from asymptomatic animals after 500 days postinoculation. The tissues were minced with disposable razor blades, sterile saline was added, and the tissue was triturated using successively smaller-gauge needles and a 1-ml syringe. One percent (wt/vol) murine or hamster tissue homogenates were i.c. inoculated into either C57BL/6J mice or Syrian hamsters, respectively, and the time to onset of clinical symptoms was recorded. At least two mice or hamsters per treatment group were analyzed by bioassay, shown in Table 3 and Fig. 2.
TABLE 3.
TME bioassay of tissue from hamsters following oral inoculation of the DY TME agent
| Tissuea | Incubation period, mean days ± SEMb | No. affected/no. inoculated |
|---|---|---|
| Hamster #1 | ||
| Brain | >407 | 0/3 |
| Peyer's patch | >407 | 0/3 |
| Spleen | >407 | 0/3 |
| Submandibular lymph node | >407 | 0/3 |
| Hamster #2 | ||
| Brain | >390 | 0/3 |
| Peyer's patch | >390 | 0/2 |
| Spleen | >390 | 0/3 |
| Submandibular lymph node | >306 | 0/3 |
| DY TME brain controlc | 237 ± 1.7 | 3/3 |
Hamsters were fed a food pellet containing 105.4 LD50 of the DY TME agent brain homogenate. Tissues were collected from clinically normal DY TME agent-inoculated hamsters after 600 days postinoculation, homogenized in sterile saline, and i.c. inoculated into recipient hamsters for the animal infectivity bioassay.
Aged asymptomatic hamsters were culled after the indicated day postinoculation.
Dosage was 103.1 LD50 of the DY TME agent.
FIG. 2.
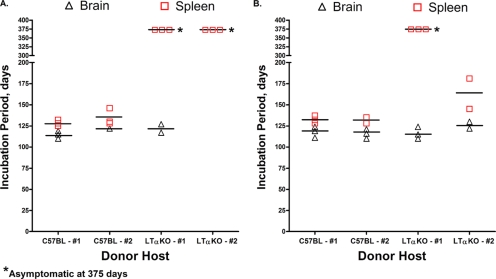
Incubation period for C57BL/6J wild-type mice following i.c. inoculation of brain or spleen homogenates from C57BL/6J or LTα null mice. C57BL/6J mice or LTα null mice were either i.c. (A), i.t. (B), or i.p. (data not shown) inoculated with the RML scrapie agent as described in Table 1. At clinical disease or in asymptomatic aged mice, the brain and spleen were collected, homogenized, and i.c. inoculated into recipient C57BL/6J mice as a 1% or 10% (wt/vol) homogenate. Symbols (▵ and □) represent the incubation periods of individual animals, and the horizontal bars are the mean incubation period for a particular tissue and route of inoculation. i.c. inoculated C57BL/6J mice surviving to 375 days postinoculation were asymptomatic, and PrPSc was not found in the brains of these aged mice by Western blot (data not shown). The brains and spleens collected at 375 days postinoculation from LTα null mice that were i.p. inoculated with a mock or RML scrapie brain homogenate also did not cause scrapie when inoculated into recipient C57BL/6J mice (data not shown). In most cases, detection of scrapie infection in tissues from at least two mice (#1, #2) was assayed for each route of inoculation.
Tissue enrichment for PrPSc.
For PrPSc analysis of brain from clinically ill or asymptomatic rodents, a 5% (wt/vol) homogenate in Dulbecco's phosphate-buffered saline was digested with 0.4 U per ml of proteinase K (Roche Diagnostics Corporation, Indianapolis, IN). Homogenates were incubated at 37°C for 1 h with constant agitation, followed by the addition of 1 mM PefaBloc (Roche Diagnostics Corporation, Indianapolis, IN.). PrPSc enrichment from tongue, spleen, and submandibular lymph node was performed by detergent extraction, differential ultracentrifugation, and proteinase K digestion as described previously (3, 4). For spleen, tissue was homogenized using a Ten Broeck grinder in 10 mM Tris-HCl (pH 7.5) containing 5 mM MgCl2 to produce a 20% (wt/vol) homogenate. Tissue homogenates were incubated with 100 U per ml of Benzonase nuclease (Novagen, Inc., Madison, WI) at 37°C for 1 h with constant agitation. Tongue and submandibular lymph node were dispersed following incubation in Liberase Blendzyme 2 (Hoffmann-La Roche Ltd, Basel, Switzerland) at 55 μg/ml in 25 mM HEPES, pH 7.4, containing 3% sucrose for 30 min to 60 min at 37°C. Protease digestion was stopped by the addition of complete mini-protease inhibitor (Hoffmann-La Roche Ltd., Basel, Switzerland). Following enzymatic dissociation, tongue and spleen homogenates were diluted with buffer to make a 5% or 10% (wt/vol) tissue homogenate containing buffer A (10% [wt/vol] N-lauroylsarcosine in 10 mM Tris-HCl [pH 7.5], 1 mM EDTA, 100 mM NaCl, and 1 mM dithiothreitol), and further enrichment for PrPSc was performed as described previously (3). A minimum of three mice or hamsters from each group were analyzed by Western blotting (see Fig. 1, 3, and 4), although fewer animals were included for illustrative purposes.
FIG. 1.
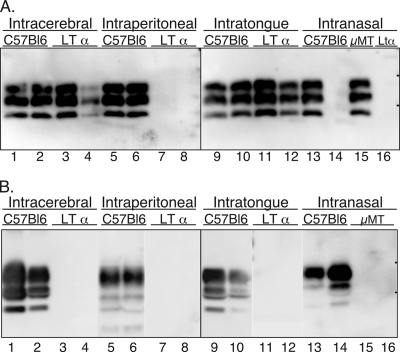
Distribution of PrPSc in wild-type and immunodeficient mice following neural and extraneural routes of inoculation. C57BL/6J wild-type, LTα null, and muMT (μMT) mice were inoculated by the i.c., i.p., i.t., or i.n. route with the RML scrapie agent, and PrPSc was examined in brain homogenates (0.4 mg) (A) or enriched from spleen tissue (25 mg) (B) of terminally ill or aged asymptomatic mice. Tissues from two representative mice from each inoculation group are illustrated in each panel, except for the i.n. route, for which different mice are illustrated in lanes 14 and 16 in panels A and B. In panel A, lanes 14 and 16 were from mice that did not develop clinical disease, whereas in panel B, these lanes contain preparations from clinically ill mice. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot with anti-PrP 6H4 monoclonal antibody were performed as described in Materials and Methods. The short horizontal bars on the right edge of each panel indicate the 20- and 30-kDa markers.
FIG. 3.
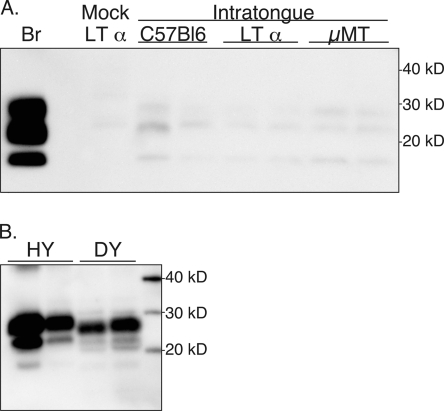
PrPSc deposition in tongues of mice and hamsters following i.t. inoculation of the scrapie or TME agent. In panel A, C57BL/6J wild-type, LTα null, and muMT (μMT) mice were inoculated in the tongue with the RML scrapie agent, and PrPSc was enriched from tongue tissue (100 mg) of clinically ill mice. Brain (Br) was used as a positive control. For panel B, tongue tissue (50 mg) was enriched for PrPSc from clinically ill hamsters following i.t. inoculation with the HY TME or DY TME agent. Samples were analyzed by SDS-PAGE and Western blot with anti-PrP 6H4 (A) or 3F4 (B) monoclonal antibody as described in Materials and Methods. Molecular mass markers in kilodaltons are indicated to the right of the panel.
FIG. 4.
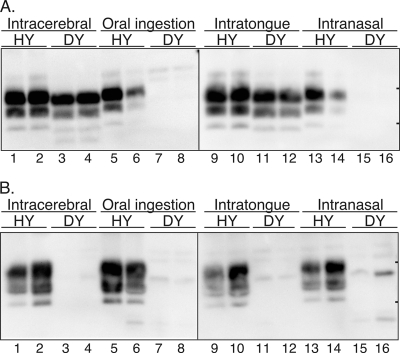
Distribution of PrPSc in hamsters infected with the HY and DY strains of the TME agent following neural and extraneural routes of inoculation. Syrian golden hamsters were inoculated by the i.c., oral ingestion, i.t., or i.n. route, and in terminally ill or aged hamsters, PrPSc was examined in brain homogenates (0.25 mg) (A) or enriched from spleen (25 mg) (B). Tissues from two representative hamsters from each inoculation group are illustrated in each panel. SDS-PAGE and Western blot with anti-PrP 3F4 antibody were performed as described in Materials and Methods. In the lanes with spleen tissue of hamsters infected with the DY TME agent, weak immunoreactive polypeptides of various molecular masses were sometimes found, but these were considered to represent nonspecific PrP immunoreactivity based on repeated analysis of samples. The short horizontal bars on the right edge of each panel indicate the 20- and 30-kDa markers.
Western blot.
Samples were analyzed on a 12% morpholinepropanesulfonic acid NuPAGE gel (Invitrogen, Carlsbad, CA), and proteins were transferred to a polyvinylidene difluoride membrane and incubated with monoclonal anti-PrP 3F4 antibody (a gift of V. Lawson, National Institute of Allergy and Infectious Diseases, Rocky Mountain Laboratories, Hamilton, MT) at a 1:5,000 (spleen and submandibular lymph node) or 1:40,000 dilution for detection of hamster PrPSc or anti-PrP 6H4 antibody (Prionics, Switzerland) at a 1:10,000 dilution or anti-PrP SAF 70 monoclonal antibody (Cayman Chemical, Ann Arbor, MI) at 3 μg/ml for detection of murine PrPSc. The detection system included incubation with anti-mouse immunoglobulin G (IgG) alkaline phosphatase conjugate (Promega, Madison, WI) at a dilution of 1:20,000. Blots were developed using the CDP-Star substrate (Applied Biosystems, Foster City, CA) and imaged with a Kodak Image Station 2000MM imaging system (Eastman Kodak Company, Rochester, NY).
Immunohistochemistry.
Immunostaining for hamster PrPSc was performed as described previously (4, 12). Briefly, animals were intracardially perfused with paraformaldehye-lysine-periodate fixative, followed by postfixation in paraformaldehye-lysine-periodate for 5 to 7 h. Paraffin-embedded tissue sections (5 μm) were subjected to antigen retrieval by treatment with formic acid for 20 min, followed by a streptavidin-biotin blocking step. Hamster PrPSc was detected by successive incubation with monoclonal anti-PrP 3F4 antibody, horse anti-mouse IgG biotin conjugate (Vector Laboratories, Burlingame, CA.), and streptavidin conjugated to horseradish peroxidase. For immunodetection of murine PrPSc, tissue sections were subjected to hydrated autoclaving following formic acid treatment. Monoclonal anti-PrP 6H4 antibody (1:2,000), donkey anti-mouse Fab2 biotin conjugate, and streptavidin conjugated to horseradish peroxidase were used for PrPSc immunodetection in brain tissue. DakoCytomation ARK (DakoCytomation, Carpinteria, CA) was used for immunodetection of murine PrPSc in spleen, submandibular lymph node, tongue, and nasal mucosa tissue according to the manufacturer's instructions. Murine isotype IgG antibody was used as control for the immunodetection of murine or hamster PrPSc. Visualization of PrPSc staining was performed using either 3-amino-9-ethylcarbazole (0.5 mg per ml) in 100 mM sodium acetate (pH 5.0) and 0.01% H2O2 or DAB+ (DakoCytomation, Carpinteria, CA). Tissue was counterstained with hematoxylin. A minimum of 3 animals per group and 15 sections per tissue per animal were examined with a Nikon E600 microscope for each antibody immunostaining procedure.
Statistical analysis.
Incubation period data were expressed as mean days ± standard error of the mean and were analyzed by the Bonferroni t test using the Statistical Analysis System (v. 9.0.3; SAS, Cary, IN). In all comparisons, a P value less than 0.05 was used to determine whether two data sets were statistically different.
RESULTS
Pathogenicity of the RML scrapie agent in wild-type mice, LTα null and muMT null mice by intracerebral and intraperitoneal routes of inoculation.
The role of the LRS in prion neuroinvasion was investigated with wild-type C57BL/6J mice, as well as LTα and muMT null mice, by inoculation of the RML scrapie agent into neural and extraneural tissues. Prior studies demonstrated that LTα and muMT null mice do not support scrapie agent replication in the LRS following inoculation with the RML strain of the scrapie agent (23, 30). In the current study, all three murine hosts were susceptible to prion disease following intracerebral (i.c.) inoculation of an RML scrapie brain homogenate, and there was no statistical difference (P > 0.05, Bonferroni t test) in the incubation periods between the C57BL/6J control mice and the LTα null mice (127 ± 1.4 days versus 136 ± 3.1 days) or between the C57BL/6J mice and the muMT null mice (123 ± 7 days versus 134 ± 1 days) (Table 1). However, following intraperitoneal (i.p.) inoculation, the LTα and muMT null mice did not develop symptoms of scrapie by 330 days postinoculation while the C57BL/6J mice had an incubation period of 168 ± 4.3 days and 184 ± 9 days at the two doses of RML scrapie agent that were assayed (102.6 LD50 or 103.6 LD50) (Table 1). For the i.c. and i.p. inoculated C57BL/6J mice, PrPSc was detected in the brain and spleen by Western blot (Fig. 1A and B, lanes 1, 2, 5, and 6) and in the submandibular lymph node (SMLN) (data not shown) at clinical disease. In the LTα and muMT null mice, PrPSc was detected in the brain following i.c. inoculation, but it was not found in the spleen or SMLN of clinically ill mice, nor was it present in asymptomatic mice in the brain, spleen, or SMLN after 330 days following i.p. inoculation (Fig. 1A and B, lanes 3, 4, 7, 8; also data not shown).
A murine scrapie infectivity assay, which is a more sensitive method for detecting the scrapie agent, was used to measure scrapie agent replication in the brain and spleen tissue of C57BL/6J mice or LTα null mice following i.c. and i.p. inoculation. Brain and spleen homogenates from i.c. inoculated C57BL/6J mice at clinical disease were i.c. inoculated into recipient C57BL/6J mice, and these mice had incubation periods of 110 to 122 days and 121 to 146 days, respectively (Fig. 2A). Bioassay of brain homogenates from symptomatic LTα null mice following i.c. inoculation resulted in onset of scrapie at 117 to 127 days, which was similar to that found in brain tissue of i.c. inoculated C57BL/6J mice. However, bioassay of spleen homogenates from clinical LTα null mice did not lead to symptoms of scrapie after 375 days postinoculation in recipient mice, indicating that scrapie agent replication did not occur in the spleens of these null mice (Fig. 2A). Following i.p. inoculation of LTα null mice, neither brain nor spleen tissue from asymptomatic animals at 330 days postinoculation caused scrapie in recipient mice by 375 days after i.c. inoculation (data not shown). These findings confirm the results of the Western blot studies and demonstrate that the scrapie agent cannot replicate in the LRS of LTα null mice (Table 4).
TABLE 4.
Role of LRS in prion neuroinvasion from neural and extraneural tissues
| Host/straina | Prion strain | Route of inoculation | Presence of:
|
Neuroinvasion LRS independent | ||
|---|---|---|---|---|---|---|
| Clinical diseaseb | PrPSc in LRS | PrPSc at inoculationc | ||||
| C57BL/6J | RML scrapie | Cerebral | Yes | Yes | Yes | Yes |
| C57BL/6J | RML scrapie | Peritoneal | Yes | Yes | Unknown | Not likely |
| C57BL/6J | RML scrapie | Tongue | Yes | Yes | No | Possible |
| C57BL/6J | RML scrapie | Nasal | Yes | Yes | No | Possible |
| Hamster | HY TME | Cerebral | Yes | Yes | Yes | Yes |
| Hamster | HY TME | Peritoneal | Yes | Yes | Unknown | Not likely |
| Hamster | HY TME | Tongue | Yes | Yes | Yes | Yesd |
| Hamster | HY TME | Nasal | Yes | Yes | Yes | Possible |
| muMT mice | RML scrapie | Cerebral | Yes | No | Yes | Yes |
| muMT mice | RML scrapie | Peritoneal | No | No | Not likely | No |
| muMT mice | RML scrapie | Tongue | Yes | No | No | Yes |
| muMT mice | RML scrapie | Nasal | Yes | No | No | Yes |
| Hamster | DY TME | Cerebral | Yes | No | Yes | Yes |
| Hamster | DY TME | Peritoneal | No | No | Not likely | No |
| Hamster | DY TME | Tongue | Yes | No | Yes | Yes |
| Hamster | DY TME | Nasal | No | No | No | No |
Data from muMT mice were similar to findings observed for LTα null mice except where noted in the text; hamsters were Syrian golden hamsters.
Clinical disease also indicates prion infection in the brain.
At tissue site of prion inoculation.
See the work of Bartz et al. (2005) (3).
Pathogenicity of the RML scrapie agent in wild-type, LTα null, and muMT null mice following intratongue inoculation.
To investigate scrapie agent neuroinvasion from the oral cavity, C57BL/6J, LTα, and muMT null mice were inoculated in the tongue with the RML scrapie agent at two different doses, and mice were monitored for the following: (i) the onset of clinical disease, (ii) PrPSc deposition in brain and spleen, and (iii) scrapie agent replication in brain and spleen. Following intratongue (i.t.) inoculation, all three strains of mice were susceptible to prion disease, but there was no difference in incubation periods (P > 0.05, Bonferroni t test) between the C57BL/6J mice and the LTα null mice (167 ± 0.6 days versus 161 ± 8.6 days) or between the C57BL/6J mice and the muMT null mice (146 ± 7.8 days versus 144 ± 13.4 days) (Table 1). The distribution of PrPSc in brain and spleen tissue in the i.t.-inoculated mice was similar to the tissue distribution described following i.c. inoculation into these three murine hosts. PrPSc was found in the brain and spleen tissue of C57BL/6J mice and in the brain tissue of LTα and muMT null mice but not in the spleen tissue of LTα and muMT null mice following i.t. inoculation (Fig. 1A and B, lanes 9 to 12; also data not shown).
Measurement of scrapie agent infectivity following i.t. inoculation of the RML scrapie agent from the same brain and spleen samples that were analyzed for PrPSc by Western blotting (Fig. 1) revealed that brain and spleen homogenates of symptomatic C57BL/6J mice induced onset of disease in recipient mice at 110 to 129 days and 128 to 135 days, respectively (Fig. 2B). In LTα null mice that developed scrapie following i.t. inoculation, brain homogenates induced onset of disease in recipient mice between 113 and 134 days, which was a level of scrapie infection similar to that found in the brain tissue of C57BL/6J mice. The spleen from one of the symptomatic LTα null mice following i.t. inoculation did not cause disease in recipient mice after 375 days postinoculation. However, the spleen from a second symptomatic mouse did cause clinical disease at 135 and 181 days postinoculation in the scrapie infectivity assay (Fig. 2B). PrPSc was not detected in the spleen from this second mouse (Fig. 1B, lane 11) even though spleen homogenates from C57BL/6J mice that also induced scrapie after 135 days postinoculation had evidence of PrPSc in the spleen (Fig. 1B, lane 9). The spleen from this LTα null mice was the only one out of a total of six spleens from LTα null inoculated mice that showed evidence of scrapie infection (Fig. 2; also data not shown from LTα null mice following i.p. inoculation), and PrPSc was not found in eight spleens from LTα null mice inoculated with the RML scrapie agent (Fig. 1B). We interpret these findings to indicate that in one LTα null mouse, there was either incomplete penetrance of the null mutation and subsequent scrapie agent replication or there was a low-level contamination of the spleen upon tissue collection. Overall, these studies suggest that prion neuroinvasion following i.t. inoculation of LTα and muMT null mice does not depend on scrapie agent replication in the LRS.
To investigate the route(s) of neuroinvasion following i.t. inoculation of mice with the RML scrapie agent, the distribution of PrPSc was measured in the tongue by Western blot and immunohistochemistry. PrPSc was enriched from tongues (100 mg, which represents 70% to 100% of the tongue) of mock- and RML scrapie-infected mice after i.t. inoculation and analyzed by Western blot. A weak PrPSc signal of three polypeptides between 18 kDa and 28 kDa was sometimes observed in tongues of C57BL/6J, LTα null, and muMT null mice, but there was also a weak signal observed in tongues of mock-infected LTα null mice (Fig. 3A). The molecular mass of the polypeptide fragments in this mock-infected animal was between 25 and 32 kDa. Based on analysis of additional tongues from these mice, we were unable to conclude that the immunoreactivity in the scrapie-infected mice was PrPSc specific despite the apparent correct molecular mass of these polypeptides. Using immunohistochemistry, tongues from symptomatic mice following i.t. inoculation did not reveal PrPSc deposition in nerve fibers, ganglion, skeletal muscle, muscle spindles, epithelium, or mucus and serous glands of C57BL/6 or LTα null mice (data not shown). Strong PrP immunostaining was found in the taste buds of the foliate and circumvallate papillae, but this pattern was found in both mock- and scrapie-infected mice, suggesting either nonspecific immunoreactivity or high levels of PrPC that are immunoreactive (data not shown). These findings are consistent with the Western blot data, and they indicate that PrPSc was not found in the tongues of scrapie-infected mice despite the finding that scrapie neuroinvasion from the tongue was independent of LRS infection (Table 4).
Pathogenicity of the RML scrapie agent in wild-type, LTα null, and muMT null mice following i.n. inoculation.
To investigate scrapie agent neuroinvasion from the nasal cavity, C57BL/6J, LTα null, and muMT mice were inoculated in the nasal cavity with the RML scrapie agent at two different doses and mice were monitored for scrapie agent infection. For the i.n. route of inoculation, only one C57BL/6J mouse developed scrapie while none of the LTα null mice developed disease at the lower dose of scrapie agent (Table 1). In the one scrapie agent-positive C57BL/6J mouse, PrPSc was found in the brain and spleen at clinical disease (Fig. 1A and B, lane 13), but it was not found in the brains of asymptomatic C57BL/6J (Fig. 1A, lane 14) or LTα null (Fig. 1A, lane 16) mice at 365 days postinoculation. At a 10-fold-higher dose of scrapie agent, five out of eight C57BL/6J mice and two out of eight muMT null mice developed scrapie at 236 ± 3.5 days and 226 ± 0 days, respectively, following i.n. inoculation (Table 1). Since these incubation periods were not statistically different (P > 0.05, t test) and partial disease penetrance was observed for both C57BL/6J mice and muMT null mice, these findings suggested that the route(s) of neuroinvasion was similar between the two murine hosts. PrPSc was found in the brain and spleen (Fig. 1B, lane 14; also data not shown) in the second group of scrapie-infected C57BL/6J mice and was found in the brain but not the spleen of scrapie agent-positive muMT null mice following i.n. inoculation (Fig. 1A, lane 15, and B, lanes 15 and 16). PrPSc was detected in a third brain from an asymptomatic muMT null mice after 400 days postinoculation (data not shown). In another transmission study, six out of seven muMT mice developed scrapie at 206 ± 2.2 days following i.n. inoculation of 103.9 LD50 of the RML scrapie agent. These findings suggest that neuroinvasion following i.n. inoculation is independent of LRS infection in a subset of immunodeficient mice (Table 4).
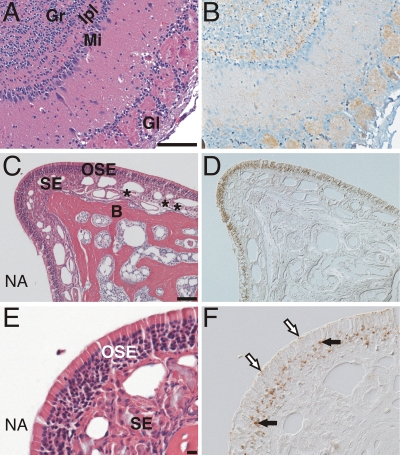
In the nasal cavity, immunohistochemistry detected PrPSc deposits in the nasal-associated lymphoid tissue (NALT) in scrapie agent-positive C57BL/6J mice but not in muMT mice. PrPSc was not observed in any other structures of the nasal cavity, including the olfactory sensory epithelium or nerve fibers, but was prominent in the olfactory bulb, where PrPSc deposits were observed in the granular, internal plexiform, and glomerular layers in both scrapie agent-positive C57BL/6J mice and muMT mice (see Fig. 6A and B). The lack of evidence for scrapie infection in the olfactory neurons in the sensory epithelium and their axons in the subepithelial layer suggests either a low level of infection in neurons and axonal spread to the olfactory bulb or scrapie agent transport along the olfactory nerve to the olfactory bulb in the absence of scrapie agent replication.
FIG. 6.
Distribution of PrPSc in the olfactory system following i.n. inoculation of the prion agent. Serial tissue sections (A and B, C and D, and E and F) were stained with anti-PrP antibody for PrPSc (B, D, and F) or hematoxylin and eosin stain (A, C, and E). PrPSc immunohistochemistry (B) of the olfactory bulb from a muMT mouse with clinical disease following i.n. inoculation of the RML scrapie agent is shown. PrPSc deposition (brown color) was most prominent in the granular layer (Gr), internal plexiform layer (Ipl), and glomerular layer (Gl) in the olfactory bulb (counterstained with hematoxylin). PrPSc deposition was less prominent in the mitral cell layer (Mi) of the olfactory bulb. In a single hamster following i.n. inoculation of the HY TME agent, PrPSc was found in the olfactory sensory epithelium (OSE) (D and F) and olfactory bulb (data not shown) but not in axon bundles (*) that transverse the subepithelial layer (SE) of the nasal mucosa. PrPSc deposition was prominent in the olfactory receptor neuron layer of the OSE (black arrows) and at the edge of the OSE layer in a location consistent with the dendritic knobs of olfactory neurons (white arrows with black outline). Panels D and F were viewed by differential interference contrast microscopy. NA, nasal airway; B, bone. Scale bar is 100 μm.
Pathogenicity of HY and DY strains of the TME agent in hamsters following i.c., i.p., and oral routes of inoculation.
A second experimental model was used to investigate the role of the LRS in neuroinvasion from the oral and nasal cavities. Previous studies demonstrated that the HY strain of the TME agent can replicate in both the lymphoreticular and nervous systems of Syrian golden hamsters, while replication of the DY strain of the TME agent is limited to the nervous system (2, 3). The incubation periods in hamsters for HY TME and DY TME were measured following neural (i.e., brain and sciatic nerve) or extraneural (i.e., tongue, peritoneum, oral ingestion, and nasal cavity) routes of inoculation. Following i.c. or intra-sciatic-nerve inoculation, both the HY TME and DY TME agents can induce clinical disease, with incubation periods of 62 ± 2 days or 64 ± 1 days for HY TME and 173 ± 0 days or 211 ± 3.5 days for DY TME (Table 2). Western blot revealed PrPSc was present in the brain and spleen tissue of HY TME-infected hamsters following i.c. inoculation, but it was found only in the brains of DY TME-infected hamsters and not in spleens (Fig. 4A and B, lanes 1 to 4). Therefore, the distribution of PrPSc in hamsters infected with the DY TME agent was similar to that found in LTα and muMT null mice following i.c. inoculation with the RML scrapie agent (Fig. 1). These findings (and those shown in Table 3, described below) indicate that the DY TME agent does not replicate in the spleen or lymph nodes and therefore the DY TME agent can be used to investigate the role of the LRS in neuroinvasion from extraneural sites of inoculation in hamsters.
To investigate neuroinvasion of TME strains from extraneural sites, inoculations were performed by the i.p. route or by oral ingestion. The HY TME agent caused clinical disease at 112 ± 9.7 days or 122 ± 1.1 days following i.p. or oral inoculation, respectively, but disease was not observed after 500 days post-inoculation of the DY TME agent by either of these routes (Table 2). Western blot revealed that PrPSc was present in the brain and spleen tissues of HY TME-infected hamsters following oral exposure but not in those of asymptomatic DY TME hamsters at 600 days postinoculation (Fig. 4A and B, lanes 5 to 8). Tissues from two asymptomatic hamsters at 600 days postinoculation following oral exposure to the DY TME agent were tested in a hamster TME infectivity assay. Homogenates from brain, Peyer's patches, spleen, and SMLN were i.c. inoculated into hamsters, but none of the recipient animals developed clinical symptoms of DY TME by 350 days, and up to 410 days, postinoculation (Table 3). A control inoculum containing 103.1 LD50 of the DY TME agent was also i.c. inoculated into hamsters and resulted in an incubation period of 237 ± 1.7 days. These findings demonstrate that the DY TME agent does not replicate in the LRS following oral ingestion and suggest that the absence of neuroinvasion following oral exposure or i.p. inoculation is due to its inability to replicate in the LRS (Table 4).
Pathogenicity of HY and DY TME agents in hamsters following intratongue inoculation.
To investigate TME neuroinvasion from the oral cavity, the HY TME and DY TME agents were i.t. inoculated into hamsters. Following i.t. inoculation, the HY TME agent produced disease in 82 ± 1 days for three out of three inoculated hamsters while the DY TME agent had an incubation period of 245 ± 5.9 days for five out of eight inoculated hamsters (Table 2). A 10-fold-higher dose of the DY TME agent resulted in an onset of clinical symptoms at 204 ± 0 days for four out of four inoculated hamsters. Western blot for PrPSc revealed deposition in the brains and spleens of symptomatic hamsters with HY TME and in the brains of symptomatic hamsters with DY TME following i.t. inoculation but not in the spleens of DY TME agent-inoculated hamsters (Fig. 4A and B, lanes 9 to 12). These findings suggested that neuroinvasion following i.t. inoculation of the DY TME agent was independent of LRS infection in hamsters.
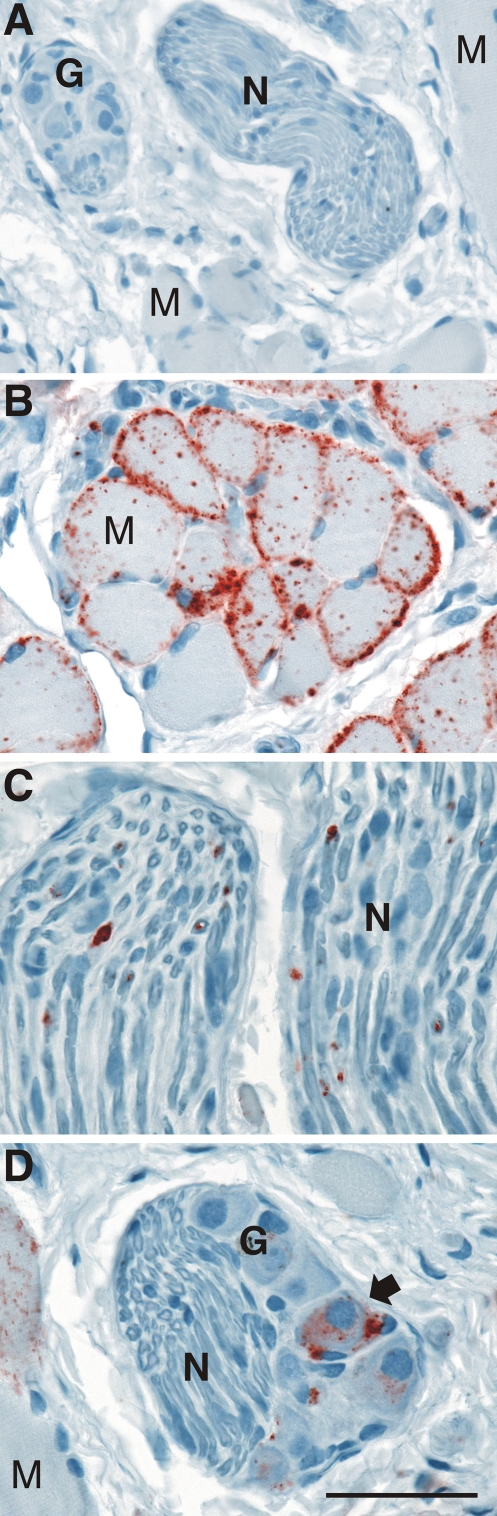
To investigate the route of TME agent neuroinvasion from the oral cavity, the PrPSc distribution was measured by Western blot and immunohistochemistry following i.t. inoculation with the HY TME or DY TME agent. At clinical disease, PrPSc was detected in the tongues of hamsters with HY TME and DY TME by Western blot (Fig. 3B). In HY TME-infected hamsters, the majority of PrPSc was found in skeletal muscle cells, but it was also prevalent in nerve fibers and ganglia of the tongue but to a lesser degree in fungiform papillae in the stratified squamous epithelium (Fig. 5; also data not shown). PrPSc was not found in muscle spindles or serous and mucus glands in the tongues of HY TME-infected hamsters. In clinical DY TME hamsters, PrPSc deposits were found only in nerve bundles and were not observed in skeletal muscle, ganglia, muscle spindles, or taste buds following i.t. inoculation (data not shown). The relative amount of PrPSc deposition in the nerve fibers of the tongue, as measured by monoclonal antibody 3F4 immunohistochemistry, appeared to be significantly less in DY TME infection than in HY TME infection. These findings suggest that following entry of the TME strains into the tongue, they can infect the peripheral nervous system (Table 4).
FIG. 5.
Distribution of PrPSc following i.t. inoculation of the TME agent. Mock-infected (A) or HY TME-infected (B, C, and D) hamsters were examined at clinical disease for the distribution of PrPSc (red chromogen product) in skeletal muscle (A, B, and D), nerve bundles (A, C, and D), and ganglia (A and D) by immunohistochemistry with anti-PrP 3F4 antibody as described in Materials and Methods. PrPSc deposits were preferentially located at the sarcolemma of muscle cells (M) but were also present in a cytoplasmic distribution in striated muscle cells. PrPSc aggregates were sparsely distributed in nerve fibers (N), while in ganglia (G) it was localized to the cell bodies of neurons (arrow). Tissue was counterstained with hematoxylin. Scale bar is 50 μm.
Pathogenicity of HY and DY TME agents in hamsters following i.n. inoculation.
To investigate prion neuroinvasion from the nasal cavity, the HY and DY TME agents were i.n. inoculated into hamsters. An incubation period of 130 ± 5.5 days was observed for the HY TME agent, while clinical symptoms were not observed by 500 days after i.n. inoculation of the DY TME agent (Table 2). In subsequent studies, the incubation period following i.n. inoculation of the HY TME agent was highly variable, ranging from 118 to 229 days with a mean of 158 ± 9.6 (n = 12). PrPSc was detected in the brain and spleen tissue of HY TME-infected hamsters at clinical disease but was not found in these tissues from asymptomatic hamsters following i.n. inoculation of the DY TME agent (Fig. 4A and B, lanes 13 to 16). These findings indicate that neuroinvasion of the TME agent from the hamster nasal cavity was dependent on prior agent replication in the LRS, but the variable incubation period observed for the HY TME agent may suggest that there are multiple routes of prion agent entry following i.n. inoculation.
Immunohistochemistry of clinical HY TME hamsters revealed PrPSc deposits in the NALT and a mild level of staining in the olfactory bulb following i.n. inoculation in the initial study (n = 2) (data not shown). There was no evidence of PrPSc in the olfactory sensory epithelium (OSE) or the nerve fibers in the subepithelial layer in the nasal cavity of this group. In a second study, following i.n. inoculation of the HY TME agent, the olfactory bulbs (15 to 40 mg) from hamsters at 70 and 91 days postinoculation were enriched for PrPSc and analyzed by Western blot, but PrPSc was not found (n = 4) (data not shown). This suggests that initial entry of the HY TME agent was not via the olfactory nerve with subsequent transport to the olfactory bulb. However, in this trial, immunohistochemistry analysis of hamsters with clinical TME revealed PrPSc in the OSE and olfactory bulb. PrPSc deposition in the OSE was observed in only one of five hamsters, and it was widely distributed in the olfactory sensory epithelium but not in the nerve fibers of the nasal cavity (Fig. 6C to D). At high magnification, PrPSc was found in the sensory epithelium in areas consistent with deposition in olfactory receptor neurons and their dendrites and dendritic knobs (Fig. 6E and F). There was also intense PrPSc deposition in the olfactory bulb in this hamster, including in the glomerular layer (data not shown). The glomeruli are synapse-rich structures containing the distal nerve terminals of the olfactory receptor neurons whose cell bodies lie in the OSE. Therefore, the olfactory tract between the nasal mucosa and olfactory bulb had evidence of HY TME infection in a subset of hamsters, which raises the possibility that neuroinvasion occurred via the olfactory nerve in these cases. Alternatively, infection of the OSE may be due to centrifugal spread from the olfactory bulb. Given the large surface area of the nasal turbinates, limited sampling could account for our failure to detect PrPSc in the remainder of the HY TME-infected hamsters by immunohistochemistry.
DISCUSSION
In this work, we examined the role of the LRS in prion neuroinvasion from the oral and nasal mucosa since these are potential sites of prion agent entry into a host, most notably in ruminants. Since these mucosal tissues are innervated by cranial nerves that originate in or project to the brain, we propose that if the prion agent can directly enter these nerve fibers, then agent replication in the LRS would not be necessary for spread of the prion agent to the central nervous system and for disease induction. Using mice that develop scrapie following i.c. inoculation, but that are not susceptible to disease by the i.p. route due to their resistance to RML scrapie agent replication in the LRS, we provide evidence for prion neuroinvasion from the oral and nasal mucosa in the absence of LRS replication. Inoculation of LTα null and muMT mice in the tongue or nasal cavity with the RML scrapie agent resulted in a scrapie incubation period that was similar to that for wild-type mice and with scrapie agent infectivity and PrPSc deposition in the brains of immunodeficient mice but not in the spleens. These findings are consistent with findings of previous studies that demonstrate an absence of scrapie agent replication in the spleen and lymph nodes of muMT and LTα null mice and in those of SCID mice (23, 24, 30, 34) and, therefore, argue against the requirement for scrapie agent replication in the LRS prior to prion neuroinvasion following i.t. and i.n. inoculation.
LRS-independent scrapie agent neuroinvasion from the oral and nasal mucosa of mice was observed even though there was no direct evidence for infection of the peripheral nervous system at either site of inoculation. PrPSc was not detected in the tongue and nasal cavity of LTα null and muMT mice despite the presence of nerve fibers that prominently transverse these tissues. One possible explanation for scrapie agent neuroinvasion from these sites is that there was a low level of scrapie agent replication that resulted in spread into peripheral nerves, but the levels were below the sensitivity of detection for PrPSc immmunohistochemistry or Western blot. Alternatively, the scrapie agent could have entered nerve fibers in the absence of scrapie agent replication and was directly transported along cranial nerves to nerve cell bodies distal to the site of inoculation. From the tongue, either retrograde or transganglionic spread along cranial nerves (there are one motor and three sensory cranial nerves in the tongue) and/or autonomic nerve fibers could account for LRS-independent prion neuroinvasion. However, given the absence of scrapie in LTα null and muMT mice following i.p. inoculation and the short incubation period following i.t. inoculation compared to that for i.p. inoculation in C57BL/6J mice, scrapie agent neuroinvasion was likely via one or more of the cranial nerves in the tongue. A prior study provides evidence for prion neuroinvasion via the hypoglossal nerve, which innervates the skeletal muscles in the tongue, following i.t. inoculation of the HY TME agent (4). From the murine nasal cavity, LRS-independent scrapie agent neuroinvasion could also occur via cranial nerves (olfactory, vomeronasal, and/or trigeminal cranial nerves) or through the autonomic nervous system in LTα null and muMT mice. The precise pathway(s) of scrapie agent neuroinvasion from the oral and nasal mucosa in these murine models will require a temporal and spatial analysis to trace the progression of scrapie agent infection of the nervous system. In a previous study, aged muMT mice that were i.p. inoculated with the RML scrapie agent did not develop clinical symptoms and PrPSc was not found in the spleen, but in asymptomatic mice that were more than 285 days old, PrPSc and scrapie agent infectivity were found in the brain (14). Although we did not detect PrPSc in the brains of aged, asymptomatic muMT or LTα null mice inoculated by the i.p. route, in this prior study and the current one, the findings suggest that in the absence of scrapie agent replication in the LRS, the prion agent can enter the nervous system at peripheral sites and spread to the brain. The different outcomes between these two studies following peripheral scrapie agent inoculation—clinical disease versus asymptomatic scrapie agent infection—could be due to infection of cranial nerves following inoculation by the i.t. and i.n. routes versus infection of spinal or autonomic nerves, as in the case of an i.p. inoculation. The greater the distance from the brain, the longer it could take to establish infection in the central nervous system in the absence of an LRS scrapie agent amplification phase. In this scenario, there would be support for our hypothesis that the cranial nerves that innervate the oral and nasal mucosa play a role in prion neuroinvasion. Based on the lack of evidence for PrPSc deposition in murine tongue and nasal cavity of muMT mice, we conclude that either the level of scrapie agent infection in axons in these tissues is below the limit of detection for PrPSc and/or the prion agent can enter nerve fibers and is transported along axons to distal nerve cell bodies in the brain or ganglia in the absence of agent replication.
LRS-independent prion neuroinvasion from the tongue or nasal cavity of LTα null and muMT mice that lack evidence for PrPSc at these sites suggests that the portal of prion agent entry may not always reveal evidence of infection. Therefore, it may not be possible to readily assess the prevalence of prion neuroinvasion from the oral and nasal mucosa in natural prion diseases in the absence of PrPSc deposition at these mucosa. More specifically, there would have to be evidence of prion infection at the early stages of neuroinvasion at these sites and subsequent centripetal spread to the brain. This pattern should be present prior to infection of the brain in order to rule out the possibility of centrifugal prion spread to these mucosa following establishment of infection in the brain. Possibly, it is the latter pathway that results in a measurable prion infection in the tongue and nasal mucosa of sheep and/or goats with natural or experimental scrapie and deer experimentally inoculated with chronic wasting disease (15, 16; R. A. Bessen, unpublished). Prion replication in neurons and spread along nerve fibers to the oral and nasal mucosa could result in PrPSc deposition in these mucosal tissues in larger amounts than would be expected by prion replication that is restricted to the site of prion agent entry into the nervous system (i.e., nerve terminals and axons). It is unknown whether natural prion infections are acquired via infection of cranial nerves in the oral or nasal mucosa; this may be difficult to determine if initial agent replication at these sites is very low or nonexistent and neuroinvasion involves uptake and transport in nerve fibers to nerve cell bodies distal to the site of prion exposure. Interestingly, experimental inoculation of the scrapie agent into the tongue of sheep results in clinical disease within 1 month of the onset of symptoms following i.c. inoculation of sheep (i.e., 18.3 months versus 17.6 months) (17). This suggests that the i.t. route is very efficient, since i.c. inoculation typically results in the shortest incubation period among the experimental routes of inoculation. The high efficiency of the i.t. route was previously described for hamsters infected with the HY TME agent, in which the i.t. route was 10- to 100-fold more efficient than oral ingestion (4). It is also noteworthy that superficial lesions on the surface of the tongue also resulted in a shorter incubation period in hamsters compared to the absence of lesions following oral exposure to the HY TME agent, but it has not been established whether this can occur in an LRS-independent manner (4). Sheep have also been i.n. inoculated with the scrapie agent and demonstrated evidence of PrPSc in the palatine tonsil by 12 months postinfection, but only one sheep survived to the onset of clinical disease at 46 months postinoculation (18).
Using the HY and DY TME model with hamsters to investigate the role of the LRS in prion neuroinvasion, there were similarities and differences in susceptibility following neural and extraneural routes of inoculation compared to RML scrapie infection using murine models. Hamsters inoculated with the HY TME agent by each route developed disease and had evidence of PrPSc in the brain and LRS, which was analogous to infection of C57BL/6J mice with the RML scrapie agent. Inoculation with the DY TME strain was used to determine whether the i.t. and i.n. routes were dependent on LRS infection for neuroinvasion. For the DY TME agent, (i) hamsters were susceptible to TME following i.c., sciatic nerve, and i.t. inoculation but not following i.n. and i.p. inoculation or oral ingestion, (ii) PrPSc deposits were present in the brain but not the spleen following i.c. and i.t. inoculation, and (iii) PrPSc was located in nerve fibers of the tongue following i.t. inoculation. Based on these findings, we conclude that neuroinvasion from the tongue in hamsters is independent of the LRS and likely occurs via retrograde transport in the nerve fibers in the tongue. Prior studies support a role for the hypoglossal nerve in TME neuroinvasion from the tongue (4). As determined by immunohistochemistry, DY TME agent infection was restricted to nerve fibers, which is consistent with neuroinvasion via the hypoglossal nerve but does not exclude invasion via other types of nerve fibers.
Conversely, the absence of disease following i.n. inoculation with the DY TME agent suggests that TME neuroinvasion from the nasal cavity is dependent on LRS infection. The presence of PrPSc in the NALT of HY TME agent-infected hamsters was consistent with findings of prior studies (12, 21) and suggests that either early infection at this site leads to prion neuroinvasion via the nerve fibers that innervate the LRS or PrPSc deposition in the NALT is not related to initial agent entry but is a result of systematic spread of prion infection in the LRS during infection. HY TME infection was also found in the olfactory bulb and sometimes in the OSE at clinical disease but not in nerve fibers in the nasal cavity. These studies do not determine whether HY TME infection of the OSE was the initial site of infection or was a target following centrifugal spread, possibly via the olfactory nerve, after infection was established in the olfactory bulb. The absence of PrPSc in the olfactory bulb midway through the incubation period suggests the latter pathway is more probable. Additional analysis is needed to map the route(s) of HY TME spread in the LRS and nervous systems in order to determine the pathway of neuroinvasion following i.n. inoculation. Given the variable length of the incubation period following i.n. inoculation of the HY TME agent, it cannot be excluded that multiple pathways of neuroinvasion, possibly LRS dependent and LRS independent, are involved, including direct infection of nerve fibers at this site (e.g., olfactory, vomeronasal, trigeminal, or autonomic fibers). These routes of entry are not necessarily inconsistent with the inability of the DY TME agent to cause disease following i.n. inoculation, since the number of hosts challenged was low and there was a variable incubation period following i.n. inoculation of hamsters with the HY TME agent. This could be more thoroughly tested by increasing the number of animals i.n. inoculated with the DY TME agent in order to determine whether a small percentage of hamsters are susceptible to disease, which would suggest that direct infection of nerve fibers can occur in a subset of infections. The findings with the TME hamster model are distinct from those for RML scrapie infection in mice, in which both wild-type and muMT mice developed scrapie following i.n. inoculation, suggesting neuroinvasion does not require replication in the LRS, despite the lack of evidence for scrapie infection in the olfactory sensory neurons.
Using murine and hamster models of prion disease, we demonstrated that neuroinvasion following inoculation of the tongue was independent of prion replication in the LRS in hamsters and mice despite the absence of PrPSc in the tongues of mice. Direct application of the prion agent to the nasal mucosa resulted in prion neuroinvasion in mice but not in DY TME agent-inoculated hamsters, suggesting that the role of the LRS in prion neuroinvasion from mucosal sites is partially dependent on the agent strain-host species combination. Our findings also suggest that an absence of PrPSc deposition in the tongue and nasal mucosa in cases of LRS-independent neuroinvasion would result in an underestimation of the role of these mucosal sites as a portal of entry in natural prion diseases.
When the manuscript for this article was in revision, a study by Sbriccoli et al. was published that investigated the temporal and spatial pattern of PrPSc following intransal inoculation of hamsters with the 263K strain of the scrapie agent. Their findings demonstrate that prion neuroinvasion is not likely via the olfactory nerve but may involve agent entry via the trigeminal nerve or autonomic nervous system (35).
Acknowledgments
This work was supported by Public Health Service grant R01 AI055043 from the National Institute of Allergy and Infectious Diseases, by the National Research Initiative of the USDA Cooperative State Research, Education, and Extension Service, grant number 2006-35201-16626, by U.S. Fish and Wildlife Service grant 1448-60181-03-G739, and by NIH grant P20 RR020185-01 from the National Center for Research Resources and the Murphy Foundation.
Special thanks go to Hal Shearin, John Bailey, and Katie Nicoll for technical assistance and to Renee Arens for animal care.
Footnotes
Published ahead of print on 15 April 2009.
REFERENCES
- 1.Banks, T. A., B. T. Rouse, M. K. Kerley, P. J. Blair, V. L. Godfrey, N. A. Kuklin, D. M. Bouley, J. Thomas, S. Kanangat, and M. L. Mucenski. 1995. Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J. Immunol. 1551685-1693. [PubMed] [Google Scholar]
- 2.Bartz, J. C., J. M. Aiken, and R. A. Bessen. 2004. Delay in onset of prion disease for the HY strain of transmissible mink encephalopathy as a result of prior peripheral inoculation with the replication-deficient DY strain. J. Gen. Virol. 85265-273. [DOI] [PubMed] [Google Scholar]
- 3.Bartz, J. C., C. Dejoia, T. Tucker, A. E. Kincaid, and R. A. Bessen. 2005. Extraneural prion neuroinvasion without lymphoreticular system infection. J. Virol. 7911858-11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartz, J. C., A. E. Kincaid, and R. A. Bessen. 2003. Rapid prion neuroinvasion following tongue infection. J. Virol. 77583-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benestad, S. L., P. Sarradin, B. Thu, J. Schonheit, M. A. Tranulis, and B. Bratberg. 2003. Cases of scrapie with unusual features in Norway and designation of a new type, Nor98. Vet. Rec. 153202-208. [DOI] [PubMed] [Google Scholar]
- 6.Bessen, R. A., and R. F. Marsh. 1994. Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J. Virol. 687859-7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bessen, R. A., and R. F. Marsh. 1992. Identification of two biologically distinct strains of transmissible mink encephalopathy in hamsters. J. Gen. Virol. 73329-334. [DOI] [PubMed] [Google Scholar]
- 8.Buschmann, A., and M. H. Groschup. 2005. Highly bovine spongiform encephalopathy-sensitive transgenic mice confirm the essential restriction of infectivity to the nervous system in clinically diseased cattle. J. Infect. Dis. 192934-942. [DOI] [PubMed] [Google Scholar]
- 9.Buyukmihci, N., F. Goehring-Harmon, and R. F. Marsh. 1983. Neural pathogenesis of experimental scrapie after intraocular inoculation of hamsters. Exp. Neurol. 81396-406. [DOI] [PubMed] [Google Scholar]
- 10.Buyukmihci, N., M. Rorvik, and R. F. Marsh. 1980. Replication of the scrapie agent in ocular neural tissues. Proc. Natl. Acad. Sci. USA 771169-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casalone, C., C. Corona, M. I. Crescio, F. Martucci, M. Mazza, G. Ru, E. Bozzetta, P. L. Acutis, and M. Caramelli. 2005. Pathological prion protein in the tongues of sheep infected with naturally occurring scrapie. J. Virol. 795847-5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeJoia, C., B. Moreaux, K. O'Connell, and R. A. Bessen. 2006. Prion infection of oral and nasal mucosa. J. Virol. 804546-4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espinosa, J. C., M. Morales, J. Castilla, M. Rogers, and J. M. Torres. 2007. Progression of prion infectivity in asymptomatic cattle after oral bovine spongiform encephalopathy challenge. J. Gen. Virol. 881379-1383. [DOI] [PubMed] [Google Scholar]
- 14.Frigg, R., M. A. Klein, I. Hegyi, R. M. Zinkernagel, and A. Aguzzi. 1999. Scrapie pathogenesis in subclinically infected B-cell-deficient mice. J. Virol. 739584-9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadlow, W. J., C. M. Eklund, R. C. Kennedy, T. A. Jackson, H. W. Whitford, and C. C. Boyle. 1974. Course of experimental scrapie virus infection in the goat. J. Infect. Dis. 129559-567. [DOI] [PubMed] [Google Scholar]
- 16.Hadlow, W. J., R. C. Kennedy, and R. E. Race. 1982. Natural infection of Suffolk sheep with scrapie virus. J. Infect. Dis. 146657-664. [DOI] [PubMed] [Google Scholar]
- 17.Hamir, A. N., R. A. Kunkle, M. S. Bulgin, R. G. Rohwer, L. Gregori, and J. A. Richt. 2008. Experimental transmission of scrapie agent to susceptible sheep by intralingual or intracerebral inoculation. Can. J. Vet. Res. 7263-67. [PMC free article] [PubMed] [Google Scholar]
- 18.Hamir, A. N., R. A. Kunkle, J. A. Richt, J. M. Miller, and J. J. Greenlee. 2008. Experimental transmission of US scrapie agent by nasal, peritoneal, and conjunctival routes to genetically susceptible sheep. Vet. Pathol. 457-11. [DOI] [PubMed] [Google Scholar]
- 19.Heggebo, R., C. M. Press, G. Gunnes, L. Gonzalez, and M. Jeffrey. 2002. Distribution and accumulation of PrP in gut-associated and peripheral lymphoid tissue of scrapie-affected Suffolk sheep. J. Gen. Virol. 83479-489. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann, C., U. Ziegler, A. Buschmann, A. Weber, L. Kupfer, A. Oelschlegel, B. Hammerschmidt, and M. H. Groschup. 2007. Prions spread via the autonomic nervous system from the gut to the central nervous system in cattle incubating bovine spongiform encephalopathy. J. Gen. Virol. 881048-1055. [DOI] [PubMed] [Google Scholar]
- 21.Kincaid, A. E., and J. C. Bartz. 2007. The nasal cavity is a route for prion infection in hamsters. J. Virol. 814482-4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350423-426. [DOI] [PubMed] [Google Scholar]
- 23.Klein, M. A., R. Frigg, E. Flechsig, A. J. Raeber, U. Kalinke, H. Bluethmann, F. Bootz, M. Suter, R. M. Zinkernagel, and A. Aguzzi. 1997. A crucial role for B cells in neuroinvasive scrapie. Nature 390687-690. [DOI] [PubMed] [Google Scholar]
- 24.Lasmezas, C. I., J. Y. Cesbron, J. P. Deslys, R. Demaimay, K. T. Adjou, R. Rioux, C. Lemaire, C. Locht, and D. Dormont. 1996. Immune system-dependent and -independent replication of the scrapie agent. J. Virol. 701292-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mabbott, N. A., F. Mackay, F. Minns, and M. E. Bruce. 2000. Temporary inactivation of follicular dendritic cells delays neuroinvasion of scrapie. Nat. Med. 6719-720. [DOI] [PubMed] [Google Scholar]
- 26.Mabbott, N. A., A. Williams, C. F. Farquhar, M. Pasparakis, G. Kollias, and M. E. Bruce. 2000. Tumor necrosis factor alpha-deficient, but not interleukin-6-deficient, mice resist peripheral infection with scrapie. J. Virol. 743338-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manuelidis, L., I. Zaitsev, P. Koni, Z. Y. Lu, R. A. Flavell, and W. Fritch. 2000. Follicular dendritic cells and dissemination of Creutzfeldt-Jakob disease. J. Virol. 748614-8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McBride, P. A., P. Eikelenboom, G. Kraal, H. Fraser, and M. E. Bruce. 1992. PrP protein is associated with follicular dendritic cells of spleens and lymph nodes in uninfected and scrapie-infected mice. J. Pathol. 168413-418. [DOI] [PubMed] [Google Scholar]
- 29.McBride, P. A., W. J. Schulz-Schaeffer, M. Donaldson, M. Bruce, H. Diringer, H. A. Kretzschmar, and M. Beekes. 2001. Early spread of scrapie from the gastrointestinal tract to the central nervous system involves autonomic fibers of the splanchnic and vagus nerves. J. Virol. 759320-9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montrasio, F., R. Frigg, M. Glatzel, M. A. Klein, F. Mackay, A. Aguzzi, and C. Weissmann. 2000. Impaired prion replication in spleens of mice lacking functional follicular dendritic cells. Science 2881257-1259. [DOI] [PubMed] [Google Scholar]
- 31.Mulcahy, E. R., J. C. Bartz, A. E. Kincaid, and R. A. Bessen. 2004. Prion infection of skeletal muscle cells and papillae in the tongue. J. Virol. 786792-6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muramoto, T., T. Kitamoto, J. Tateishi, and I. Goto. 1992. The sequential development of abnormal prion protein accumulation in mice with Creutzfeldt-Jakob disease. Am. J. Pathol. 1401411-1420. [PMC free article] [PubMed] [Google Scholar]
- 33.Orge, L., A. Galo, C. Machado, C. Lima, C. Ochoa, J. Silva, M. Ramos, and J. P. Simas. 2004. Identification of putative atypical scrapie in sheep in Portugal. J. Gen. Virol. 853487-3491. [DOI] [PubMed] [Google Scholar]
- 34.Prinz, M., F. Montrasio, M. A. Klein, P. Schwarz, J. Priller, B. Odermatt, K. Pfeffer, and A. Aguzzi. 2002. Lymph nodal prion replication and neuroinvasion in mice devoid of follicular dendritic cells. Proc. Natl. Acad. Sci. USA 1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sbriccoli, M., F. Cardone, A. Valanzano, M. Lu, S. Graziano, A. De Pascalis, L. Ingrosso, G. Zanusso, S. Monaco, M. Bentivoglio, and M. Pocchiari. 2009. Neuroinvasion of the 263K scrapie strain after intranasal administration occurs through olfactory-unrelated pathways. Acta Neuropathol. 117175-184. [DOI] [PubMed] [Google Scholar]
- 36.Shlomchik, M. J., K. Radebold, N. Duclos, and L. Manuelidis. 2001. Neuroinvasion by a Creutzfeldt-Jakob disease agent in the absence of B cells and follicular dendritic cells. Proc. Natl. Acad. Sci. USA 989289-9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sigurdson, C. J., T. R. Spraker, M. W. Miller, B. Oesch, and E. A. Hoover. 2001. PrP(CWD) in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. J. Gen. Virol. 822327-2334. [DOI] [PubMed] [Google Scholar]
- 38.Sigurdson, C. J., E. S. Williams, M. W. Miller, T. R. Spraker, K. I. O'Rourke, and E. A. Hoover. 1999. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus). J. Gen. Virol. 802757-2764. [DOI] [PubMed] [Google Scholar]
- 39.Terry, L. A., S. Marsh, S. J. Ryder, S. A. Hawkins, G. A. Wells, and Y. I. Spencer. 2003. Detection of disease-specific PrP in the distal ileum of cattle exposed orally to the agent of bovine spongiform encephalopathy. Vet. Rec. 152387-392. [DOI] [PubMed] [Google Scholar]
- 40.Valdez, R. A., M. J. Rock, A. K. Anderson, and K. I. O'Rourke. 2003. Immunohistochemical detection and distribution of prion protein in a goat with natural scrapie. J. Vet. Diagn. Investig. 15157-162. [DOI] [PubMed] [Google Scholar]
- 41.van Keulen, L. J., B. E. Schreuder, M. E. Vromans, J. P. Langeveld, and M. A. Smits. 1999. Scrapie-associated prion protein in the gastrointestinal tract of sheep with natural scrapie. J. Comp. Pathol. 12155-63. [DOI] [PubMed] [Google Scholar]
- 42.van Keulen, L. J., M. E. Vromans, and F. G. van Zijderveld. 2002. Early and late pathogenesis of natural scrapie infection in sheep. APMIS 11023-32. [DOI] [PubMed] [Google Scholar]
- 43.Zanusso, G., S. Ferrari, F. Cardone, P. Zampieri, M. Gelati, M. Fiorini, A. Farinazzo, M. Gardiman, T. Cavallaro, M. Bentivoglio, P. G. Righetti, M. Pocchiari, N. Rizzuto, and S. Monaco. 2003. Detection of pathologic prion protein in the olfactory epithelium in sporadic Creutzfeldt-Jakob disease. N. Engl. J. Med. 348711-719. [DOI] [PubMed] [Google Scholar]