Abstract
Hepatic damage occurs in males and ovariectomized (OVX), not in proestrus (PE), females following trauma-hemorrhage (T-H). The mechanism responsible for hepatoprotection remains unknown. We hypothesized protection in PE is a result of enhanced heme oxygenase-1 (HO-1)-derived down-regulation of liver inflammatory responses. PE and OVX rats underwent T-H (midline laparotomy, 60% blood loss). PE rats received vehicle (Veh; saline), HO-1 inhibitor chromium mesoporphyrin IX chloride (CrMP; 2.5 mg/kg), zinc protoporphyrin IX (ZnPP; 25 mg/kg), or Akt/PI-3K inhibitor Wortmannin (Wort; 1 mg/kg) 30 min prior to resuscitation or sham operation i.p. OVX rats received Veh or 17β-estradiol (E2; 1 mg/kg) 30 min before hemorrhage. Rats were killed 2 h thereafter. Following T-H, left ventricular performance was maintained in PE and E2 OVX rats but was depressed in OVX and CrMP-, ZnPP-, and Wort-treated PE rats; liver damage was not evident in PE rats, and CrMP, ZnPP, and Wort abrogated protection; liver HO-1, p38 MAPK, Akt/PI3K, and Bcl-2 expression increased in PE and E2 OVX rats, which was abrogated by CrMP, ZnPP, and Wort, and liver ICAM-1, caspase-3, phospho-IκB-α, and NF-κB expression increased in OVX and CrMP-, ZnPP-, and Wort-PE rats; liver myeloperoxidase, NF-κB DNA-binding activity, TNF-α, IL-6, plasma proinflammatory cytokines, and cytokine-induced neutrophil chemoattractants increased in OVX and CrMP-, ZnPP-, and Wort-PE rats; and plasma estradiol levels and hepatic estrogen receptor-α and -β expression decreased in OVX but were unaltered by CrMP, ZnPP, and Wort. Thus, enhanced HO-1 in PE and E2 OVX females modulates inflammatory responses and protects liver following T-H.
Keywords: estrus cycle, ovariectomy, liver damage, HO-1 inhibitor, PI3K/Akt inhibitor
INTRODUCTION
Studies have shown that cardiac function is depressed in males and females following trauma-hemorrhage (T-H); however, such depression was not observed in proestrus (PE) females [1, 2]. Furthermore, hepatic heme oxygenase (HO) expression and activity are significantly higher in PE rats following T-H [3]. Moreover, the female reproductive cycle is an important variable in the regulation of lung injury following T-H, and the protective effect in PE females is likely mediated via up-regulation of HO-1 [4]. Studies have also indicated that such a gender dimorphism following T-H appears to be hormonally regulated [5,6,7].
Neutrophils are the first line of host defense against invading pathogens. Neutrophils can release superoxide anions and proteolytic enzymes, which diffuse across the endothelium. Neutrophils can also leave the microcirculation and migrate to and adhere to matrix proteins or other cells in the vicinity [8], and activated neutrophils can cause organ damage by releasing proteases and reactive oxygen species [8]. ICAM-1 is known to play a major role in the firm adhesion of neutrophils to the vascular endothelium, which is constitutively present on the surface of endothelial cells and is markedly up-regulated following T-H [9]. In addition to adhesion molecules, chemokines such as cytokine-induced neutrophil chemoattractants (CINC)-1 and -3, members of the CXC chemokine family, are also potent chemotactic factors for neutrophils [10].
Studies have indicated that high estrogen levels in the PE female rats maintain cardiac function following T-H [2, 11]. Furthermore, administration of 17β-estradiol (E2) following T-H in males induces HO-1 mRNA expression, improves cardiac function, and attenuates T-H-induced organ dysfunction and damage [12, 13]. Nonetheless, the precise mechanism by which HO-1 protects organ function remains unknown. We hypothesized that increased HO-1 in PE rats reduces hepatic inflammatory response, including neutrophil infiltration, and thus, protects hepatic function following T-H.
MATERIALS AND METHODS
T-H shock model
T-H was induced in Sprague-Dawley PE and ovariectomized (OVX) adult (200–250 g) female rats (Charles River Laboratories, Wilmington, MA, USA) as described previously [14]. Briefly, rats were fasted overnight but allowed water ad libitum. Following cannulation of the femoral arteries and vein, the rats were allowed to awaken and then bled rapidly through the left femoral artery to a mean arterial pressure (MAP) of 35–40 mmHg within 10 min; MAP was maintained at this level by further blood withdrawal until the animal could no longer maintain that pressure [maximum bleedout (MBO) volume]. MAP was maintained at 40 mmHg by infusing small volumes of Ringer’s lactate for 45 min; the rats were then resuscitated with Ringer’s lactate (4×MBO volume) over 1 h, returned to cages, and allowed food and water ad libitum. In the OVX group, ovariectomy was performed 2 weeks prior to T-H [2]. It should be noted that endogenous estrogen was depleted almost completely after this interval after ovariectomy [2]. The experiments described here were performed in adherence to the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee of the University of Alabama at Birmingham (AL, USA) approved this project.
PE cycle determination and experimental groups
PE cycle was determined in the early morning on the experimental day by vaginal smear as described previously [2]. In groups of PE rats, HO-I inhibitor chromium mesoporphyrin-IX chloride (CrMP; 2.5 mg/kg, Frontier Scientific, Logan, UT, USA) or zinc protoporphyrin IX (ZnPP; 25 mg/kg, Sigma Chemical Co., St. Louis, MO, USA) was administered i.p. at the time of sham operation or 30 min prior to resuscitation. In another group of PE rats, the PI3K/Akt inhibitor Wortmannin (Wort; 1 mg/kg, Sigma Chemical Co.) was administered i.v. 30 min prior to resuscitation. The dose for CrMP and Wort used in this study was the same as in our previous studies [3, 13]; the dose for ZnPP was referenced from Poole et al. [15]. There was a total of 11 groups including PE rats—sham- or T-H-treated with vehicle (Sham-Veh or T-H-Veh), CrMP (Sham-CrMP or T-H-CrMP), ZnPP (Sham-ZnPP or T-H-ZnPP), and Wort (Sham-Wort or T-H-Wort)—or OVX rats—OVX-Sham or OVX-T-H or T-H treated with E2 (OVX-T-H-E2).
OVX female rat preparation and OVX pretreated with E2
Ovariectomy was performed 2 weeks prior to the induction of T-H or sham operation as described previously [2], and plasma estradiol was almost depleted within 2 weeks of OVX. In the E2-pretreated OVX group, water-soluble E2 (1 mg/kg) was administered 30 min prior to the start of blood withdrawal.
Left ventricular (LV) performance
At 2 h after T-H or sham operation, the maximal rate of pressure increase (+dP/dtmax) and decrease (–dP/dtmax) was determined using a heart performance analyzer (Digi-Med, Louisville, KY, USA) [14]. MAP and heart rate (HR) were measured by a blood pressure analyzer (Digi-Med) [14].
Plasma α-GST and estradiol levels
Liver damage was determined by measuring plasma levels of α-GST using a commercially available enzyme immunoassay (EIA) kit according to the manufacturer’s instructions (Biotrin International Ltd., Ireland) [16]. Plasma estradiol levels were measured by an EIA kit (Cayman Chemical Co., Ann Arbor, MI, USA) as described previously [2].
Liver nuclear extraction
Liver tissue (0.5 g) was used for nuclear extraction as described previously [17].
Western blotting
Western blots were performed to measure liver HO-1, p38 MAPK/phospho (p)-p38 MAPK, PI3K (p85)/p-PI3K (p85/55), Akt/p-Akt, ICAM-1, IкB-α/p-IкB-α, NF-кB, caspase-3, Bcl-2, and estrogen receptor (ER) protein levels [17]. Samples were analyzed using electrophoresis on precasted gels (Bio-Rad Laboratories, Hercules, CA, USA). After transfer, the membranes were immunoblotted with anti-HO-1 (Stressgen Bioregions, Ann Arbor, MI, USA); -ICAM-1 (Biosource International, Camarillo, CA, USA); -NF-кB (p65) and -IкB-α/-p-IкB-α (Santa Cruz Biotechnology, Santa Cruz, CA, USA); -p38 MAPK/-p-p38 MAPK, -PI3K (p85)/-p-PI3K (p85/55), -Akt/-p-Akt, -caspase-3, and -Bcl-2 (Cell Signaling Technology, Danvers, MA, USA); and -ER-α and -β (Upstate, Lake Placid, NY, USA) antibodies, respectively, followed by the addition of HRP-conjugated secondary antibody. After the final wash, membranes were probed using ECL dye (Amersham Pharmacia Biotech, Piscataway, NJ, USA) and autoradiographed. Signaling densities were evaluated by Chemilmager 5500 software (Alpha Inotech, San Leandro, CA, USA). Protein levels were quantified by densitometric evaluation and corrected to the corresponding GAPDH (Abcam Inc., Cambridge, MA, USA) or histone H1 (Upstate, Charlottesville, VA, USA).
Liver NF-κB DNA-binding activity
The TransAM™ method was used, and a NF-κB p65 transcription factor assay kit (Active Motif, Carlsbad, CA, USA) was used to detect and quantify liver tissue NF-κB transcription factor DNA-binding activity, according to the manufacturer’s instructions [17, 18].
Liver myeloperoxidase (MPO) activity
Liver MPO activity was measured as described previously [17].
Liver TNF-α, IL-6, and plasma TNF-α, IL-6, and CINCs
Liver TNF-α and IL-6 levels were measured using the supernatant from homogenized liver tissue, and plasma TNF-α, IL-6, and CINC-1 and -3 were measured by rat TNF-α, IL-6, and CINC-1 and -3 ELISA kits (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions as described previously [17].
Statistical analysis
Data are presented as mean ± se. One-way ANOVA and Tukey’s test were used for comparison among different groups. The differences are considered significant at P < 0.05.
RESULTS
LV performance and systemic hemodynamic parameters
As shown in Table 1, no differences were found in the cardiovascular and systemic parameters in various shams. +dP/dtmax and –dP/dtmax were restored in PE females treated with Veh and in OVX females treated with E2 following T-H; however, +dP/dtmax decreased by 39.7%, 35.6%, and 43.9%, and –dP/dtmax decreased by 52.2%, 56.1%, and 63.2% compared with sham in PE females following T-H treated with CrMP, ZnPP, or Wort, respectively (all P<0.05). Moreover, +dP/dtmax and –dP/dt decreased by 52.8% and 72.2% in the OVX group (P<0.05) following T-H compared with shams. In addition, MAP and HR were maintained in the PE- and E2-pretreated OVX groups; however, MAP decreased by 34.7%, 37.2%, and 46.2%, respectively; HR decreased by 19.7%, 23.0%, and 29.5%, respectively, in PE females following T-H treated with CrMP, ZnPP, or Wort (all P<0.05). Similarly, MAP and HR decreased by 41.3%, and HR was decreased by 33.7% in OVX females following T-H (P<0.05). Furthermore, the mortality rate was 14.3% (one of seven), 14.3% (one of seven), 25% (two of eight), and 30.0% (three of nine), respectively, in HO-1 inhibitors CrMP- and ZnPP-treated and Akt/PI3K inhibitor Wort-treated PE and OVX females within 2 h following T-H.
TABLE 1.
Alterations in + dP/dt, –dP/dt, MAP, HR, and Plasma Lactate at 2 h after Sham Operation or T-H
| +dP/dt (mmHg/s) | –dP/dt (mmHg/s) | MAP (mmHg) | HR (beats/min) | Lactate (mg/dL) | |
|---|---|---|---|---|---|
| PE | |||||
| Sham-Veh | 14,065 ± 321 | 10,313 ± 275 | 121 ± 4 | 421 ± 8 | 7 ± 1 |
| Sham-CrMP | 13,928 ± 486 | 10,219 ± 321 | 119 ± 3 | 412 ± 5 | 9 ± 1 |
| Sham-ZnPP | 14,052 ± 412 | 10,195 ± 293 | 118 ± 2 | 413 ± 3 | 10 ± 1 |
| Sham-Wort | 13,978 ± 253 | 10,073 ± 145 | 126 ± 2 | 416 ± 8 | 9 ± 1 |
| T-H-Veh | 13,962 ± 321 | 9052 ± 239 | 105 ± 2 | 388 ± 6a | 10 ± 2 |
| T-H-CrMP | 8579 ± 348a | 4719 ± 367a | 79 ± 4a | 338 ± 8a | 39 ± 4a |
| T-H-ZnPP | 9045 ± 162a | 54527 ± 159a | 76 ± 3a | 324 ± 21a | 41 ± 5a |
| T-H-Wort | 7884 ± 545a | 3794 ± 352a | 65 ± 2a | 297 ± 15a | 55 ± 5a |
| OVX | |||||
| OVX-Veh | 13,997 ± 482 | 10,227 ± 179 | 117 ± 3 | 421 ± 7 | 9 ± 1 |
| OVX-T-H-Veh | 6334 ± 358a | 2685 ± 238a | 71 ± 6a | 279 ± 16a | 45 ± 3a |
| OVX-E2-T-H | 13,836 ± 679 | 8645 ± 775 | 99 ± 3 | 387 ± 9 | 13 ± 2 |
Values are mean ± se (n = 6–7/group) and compared with one-way ANOVA and Tukey’s test;
P < 0.05 versus sham.
Plasma α-GST
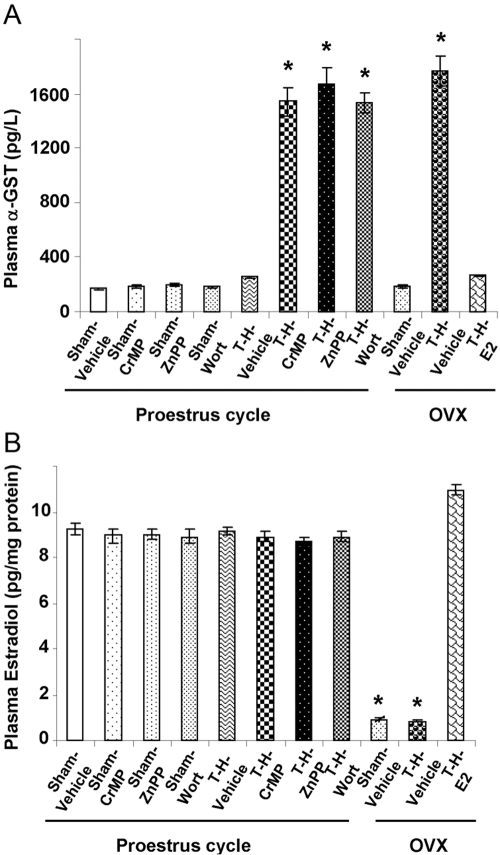
Plasma α-GST levels increased significantly in Veh-treated OVX females (Fig. 1A; P<0.05) after T-H; however, this increase was not evident in PE females or the E2-pretreated OVX group. Moreover, HO-1 inhibitors CrMP and ZnPP and the Akt/PI3K inhibitor Wort abolished the protective effect against liver damage following T-H in PE females (P<0.05).
Fig. 1.
Alterations in plasma α-GST levels (A) and plasma estradiol levels (B) in PE-sham treated with normal saline (Sham-Vehicle), PE-sham treated with CrMP (Sham-CrMP), PE-sham treated with ZnPP (Sham-ZnPP), PE-sham treated with Wort (Sham-Wort), PE-T-H treated with Veh (T-H-Vehicle), PE-T-H treated with CrMP (T-H-CrMP), PE-T-H treated with ZnPP (T-H-ZnPP), PE-T-H treated with Wort (T-H-Wort), OVX-sham treated with Veh (OVX-Sham-Vehicle), OVX-T-H treated with Veh (OVX-T-H-Vehicle), and OVX-T-H pretreated with E2 (OVX-T-H-E2) groups. There are six rats in each group. Data are expressed as mean ± se and compared by one-way ANOVA and Tukey’s test. *, P < 0.05, versus PE-shams.
Hepatic HO-1 expression
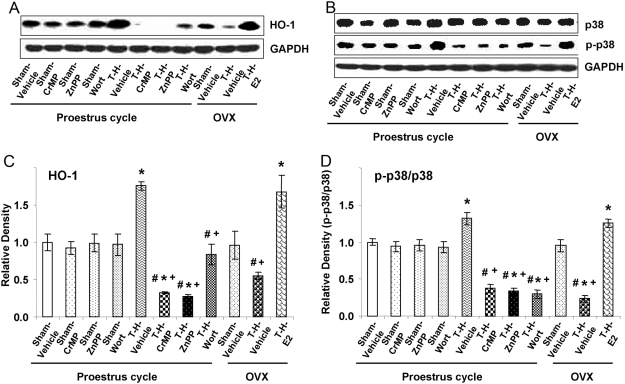
Liver HO-1 protein levels increased significantly in PE (1.6-fold compared with sham; P<0.05) and in E2-pretreated OVX rats (1.4-fold compared with sham; P<0.05) after T-H; however, the increase of HO-1 in PE rats was abrogated by HO-1 inhibitors CrMP and ZnPP. Moreover, hepatic HO-1 was attenuated by the Akt/PI3K inhibitor Wort in females after ovariectomy (Fig. 2, A and B).
Fig. 2.
Alterations in liver HO-1 (A and C) and p38 MAPK (B and D) expression in Sham-Veh, Sham-CrMP, Sham-ZnPP, Sham-Wort, T-H Veh, T-H-CrMP, T-H-ZnPP, T-H-Wort, OVX-Sham-Veh, OVX-T-H-Veh, and OVX-T-H- E2 groups. (A and B) Representative blots are shown. Blots are analyzed densitometrically, and the densitometric values are pooled from four animals in each group and shown in C and D. Data are expressed as mean ± se and compared by one-way ANOVA and Tukey’s test. *, P < 0.05, versus PE-sham; #, P < 0.05, versus PE-T-H; +, P < 0.05, versus OVX-T-H-E2.
Hepatic p38 MAPK expression
Hepatic total p38 MAPK expression was not altered significantly among various groups; however, p-p38 MAPK decreased significantly in HO-1 inhibitor- and Akt/PI3K inhibitor-treated PE females. Moreover, p-P38 MAPK also decreased significantly in OVX females after T-H. In contrast, p-p38 MAPK was enhanced in PE and E2-pretreated OVX females (Fig. 2, C and D).
Hepatic PI3K/Akt expression
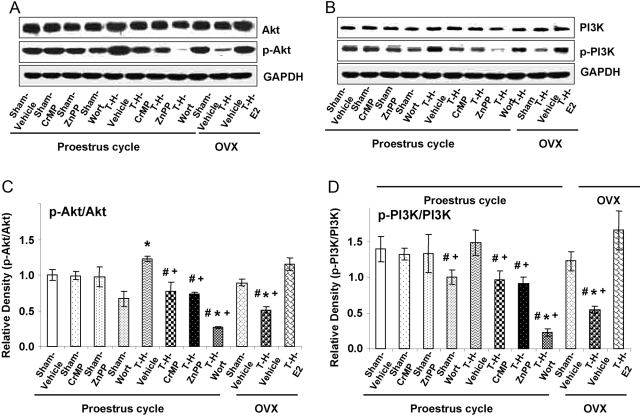
As shown in Figure 3, liver Akt (Fig. 3, A and B) and PI3K (Fig. 3, C and D) protein levels decreased significantly in Akt/PI3K inhibitor Wort-treated PE and OVX females following T-H (P<0.05); however, they were restored in PE and in E2-pretreated OVX females. Moreover, administration of HO-1 inhibitors CrMP and ZnPP in PE females attenuated the decrease in liver Akt/PI3K (P<0.05) after T-H.
Fig. 3.
Alterations in liver Akt (A and C) and PI3K (B and D) expression in Sham-Veh, Sham-CrMP, Sham-ZnPP, Sham-Wort, T-H Veh, T-H-CrMP, T-H-ZnPP, T-H-Wort, OVX-Sham-Veh, OVX-T-H-Veh, OVX-T-H-E2 groups. (A and B) Representative blots are shown. Blots are analyzed densitometrically, and the densitometric values are pooled from four animals in each group and shown in C and D. Data are expressed as mean ± se and compared by one-way ANOVA and Tukey’s test. *, P < 0.05, versus PE-sham; #, P < 0.05, versus PE-T-H; +, P < 0.05, versus OVX-T-H-E2.
Hepatic IкB-α, NF-кB expression, and NF-кB DNA-binding activity
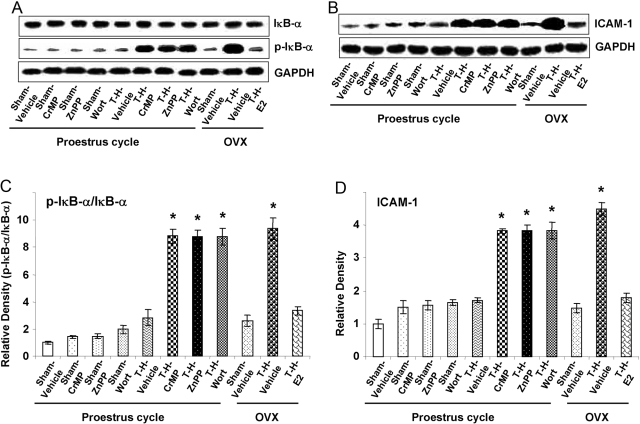
There was no significant difference in total liver IκB-α protein levels among various sham and T-H groups. However, hepatic p-IκB-α (Fig. 4, A and B) and NF-κB (Fig. 5, A and B) expression and NF-κB DNA-binding activity (Fig. 5C) increased significantly in OVX females after T-H (P<0.05). In contrast, the increase in these parameters was prevented in PE and E2-pretreated OVX females following T-H. Moreover, the salutary effect on hepatic p-IκB-α and NF-κB in PE females was abolished by HO-1 inhibitors and the Akt/PI3K inhibitor following T-H.
Fig. 4.
Alterations in liver IκB-α (A and C) ICAM-1 (B and D) expression in Sham-Veh, Sham-CrMP, Sham-ZnPP, Sham-Wort, T-H Veh, T-H-CrMP, T-H-ZnPP, T-H-Wort, OVX-Sham-Veh, OVX-T-H-Veh, and OVX-T-H-E2 groups. (A and B) Representative blots are shown. Blots are analyzed densitometrically, and the densitometric values are pooled from four animals in each group and shown in C and D. Data are expressed as mean ± se and compared by one-way ANOVA and Tukey’s test. *, P < 0.05, versus PE-sham.
Fig. 5.
Alterations in liver NF-κB expression (B) and NF-κB DNA-binding activity (C) in Sham-Veh, Sham-CrMP, Sham-ZnPP, Sham-Wort, T-H Veh, T-H-CrMP, T-H-ZnPP, T-H-Wort, OVX-Sham-Veh, OVX-T-H-Veh, and OVX-T-H-E2 groups. (A) Representative blots are shown. Blots are analyzed densitometrically, and the densitometric values of NF-κB are pooled from four animals in each group and shown in B. NF-κB DNA-binding activity is analyzed from six animals in each group. Data are expressed as mean ± se and compared by one-way ANOVA and Tukey’s test. *, P < 0.05, versus PE-sham.
Hepatic ICAM-1 expression
Liver ICAM-1 protein level increased significantly in the OVX group but not in PE or in E2-pretreated OVX females after T-H (Fig. 4, C and D; P<0.05). The salutary effect on hepatic ICAM-1 expression in PE rats was abrogated by HO-1 inhibitors and the Akt/PI3K inhibitor.
Hepatic Bcl-2 and caspase-3 expression
As indicated in Figure 6, hepatic Bcl-2 protein levels decreased significantly in OVX females after T-H (Fig. 6, A and B; P<0.05); however, Bcl-2 expression was up-regulated/maintained in PE and E2-pretreated OVX females following T-H. In contrast, hepatic-cleaved caspase-3 expression increased significantly in OVX rats after T-H (Fig. 6, C and D; P<0.05); however, the increase of cleaved caspase-3 was not observed in PE or E2-pretreated OVX females. Moreover, HO-1 inhibitors and the Akt/PI3K inhibitor abrogated the salutary effect on hepatic Bcl-2 and cleaved caspase-3 in PE females.
Fig. 6.
Alterations in liver Bcl-2 (A and C) and caspase-3 (B and D) expression in Sham-Veh, Sham-CrMP, Sham-ZnPP, Sham-Wort, T-H Veh, T-H-CrMP, T-H-ZnPP, T-H-Wort, OVX-Sham-Veh, OVX-T-H-Veh, and OVX-T-H-E2 groups. (A and B) Representative blots are shown. Blots are analyzed densitometrically, and the densitometric values are pooled from four animals in each group and shown in C and D. Data are expressed as mean ± se and compared by one-way ANOVA and Tukey’s test. *, P < 0.05, versus PE-sham; #, P < 0.05, versus PE-T-H; +, P < 0.05, versus OVX-T-H-E2.
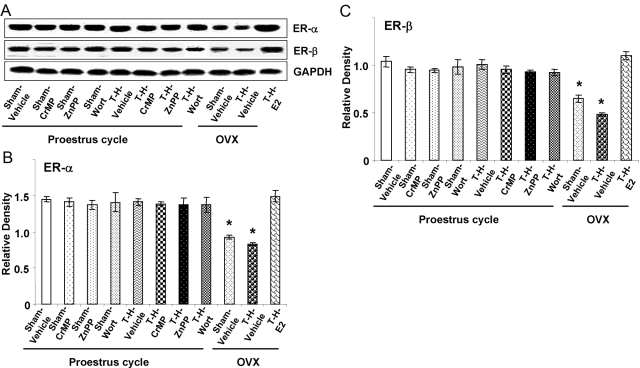
Hepatic ER-α and ER-β expression
Hepatic ER-α (Fig. 7, A and B) and ER-β (Fig. 7, A and C) expression were not affected significantly in PE females after induction of T-H or administration of HO-1 inhibitors or the Akt/PI3K inhibitor; however, both ERs decreased significantly in OVX females in shams and also after T-H (P<0.05). Moreover, administration of E2 before induction of T-H in OVX females restored hepatic ERs.
Fig. 7.
Alterations in liver ER-α (B) and ER-β (C) expression in Sham-Veh, Sham-CrMP, Sham-ZnPP, Sham-Wort, T-H Veh, T-H-CrMP, T-H-ZnPP, T-H-Wort, OVX-Sham-Veh, OVX-T-H-Veh, OVX-T-H-E2 groups. (A) Representative blots are shown. Blots are analyzed densitometrically, and the densitometric values are pooled from four animals in each group and shown in B and C. Data are expressed as mean ± se and compared by one-way ANOVA and Tukey’s test. *, P < 0.05, versus PE-sham.
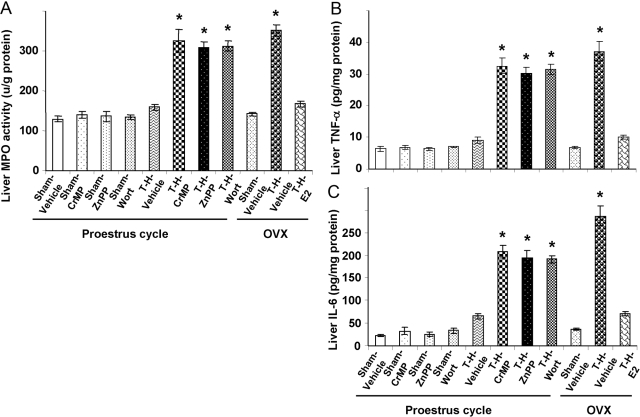
Hepatic MPO activity and proinflammatory cytokines
As shown in Figure 8, hepatic MPO activity (Fig. 8A), hepatic TNF-α (Fig. 8B), and IL-6 (Fig. 8C) increased significantly in OVX females after T-H (P<0.05); however, these increases following T-H were prevented in PE and E2-pretreated OVX females. Moreover, the HO-1 and Akt/PI3K inhibitor abrogated the salutary effect on liver MPO activity and proinflammatory cytokines in PE females.
Fig. 8.
Alterations in liver MPO activity (A), TNF-α (B), and IL-6 (C) in Sham-Veh, Sham-CrMP, Sham-ZnPP, Sham-Wort, T-H Veh, T-H-CrMP, T-H-ZnPP, T-H-Wort, OVX-Sham-Veh, OVX-T-H-Veh, and OVX-T-H-E2 groups. There are six rats in each group. Data are expressed as mean ± se and compared by one-way ANOVA and Tukey’s test. *, P < 0.05, versus PE-sham.
Plasma proinflammatory cytokines and chemokines
As indicated in Figure 9, plasma TNF-α (Fig. 9A), IL-6 (Fig. 9B), CINC-1 (Fig. 9C), and CINC-3 (Fig. 9D) increased significantly after T-H in OVX females (P<0.05) but not in PE or E2-pretreated OVX females. Moreover, administration of the HO-1 and Akt/PI3K inhibitor abrogated the salutary effect on plasma proinflammatory cytokines and chemokines in PE females.
Fig. 9.
Alterations in plasma levels of TNF-α (A), CINC-1, (B), IL-6 (C), and CINC-3 (D) in Sham-Veh, Sham-CrMP, Sham-ZnPP, Sham-Wort, T-H Veh, T-H-CrMP, T-H-ZnPP, T-H-Wort, OVX-Sham-Veh, OVX-T-H-Veh, and OVX-T-H-E2 groups. There are six rats in each group. Data are expressed as mean ± se and compared by one-way ANOVA and Tukey’s test. *, P < 0.05, versus PE-sham.
Plasma estradiol levels
Plasma estradiol levels were significantly higher in PE females compared with OVX groups (Fig. 1B; P<0.05). Administration of the HO-1 and Akt/PI3K inhibitor did not alter the plasma levels of estradiol in PE females following T-H. Moreover, E2 pretreatment in OVX females increased the plasma levels of estradiol significantly.
DISCUSSION
Our results indicate that hepatic damage was not evident in PE females but was markedly evident in OVX rats following T-H. This is closely correlated with various findings including normal LV performance, increased hepatic HO-1, p-p38 MAPK, p-Akt, p-PI3K, and Bcl-2 expression, and maintained hepatic ICAM-1, p-IκB-α, NF-κB, and caspase-3 expression in PE females following T-H. Moreover, liver NF-κB DNA-binding activity, MPO activity, TNF-α and IL-6, and plasma TNF-α, IL-6, and CINC-1 and -3 were maintained at the levels similar to shams in the PE but not in the OVX rats following T-H. In addition, hepatic ER-α and ER-β expression was normal following T-H in PE females. However, the HO-1 inhibitor (CrMP or ZnPP) and Akt/PI3K inhibitor Wort abrogated the protective effect on the liver in PE rats, and all of the above parameters were similar to the OVX group following T-H. Interestingly, E2 pretreatment prior to induction of T-H in OVX females provided almost similar results as in PE females following T-H.
Our previous studies have shown that cardiac function was restored in PE rats, and the maintenance of cardiac function following T-H in PE females was a result of high plasma estradiol levels. In contrast, females in all other stages of the estrus cycle have significantly lower estradiol, and OVX females have the lowest plasma estradiol levels [2]. Support for the notion that estradiol plays a major role in cardioprotection came from studies that showed that OVX females respond to T-H in a manner even worse than males [2]. Furthermore, administration of E2 in OVX females or in males normalizes the depressed cardiovascular and immunological responses following T-H [11, 14, 19]. Thus, it appears that maintenance or depression of cardiac function following T-H is dependent on the prevailing estrogen levels at the time of injury.
Gender-specific differences in hepatic HO expression and activity have been shown in previous studies [3]. HO-1 activity was found to be significantly higher in PE females compared with males following T-H, and the elevated HO-1 protein expression was hepatoprotective, as it maintained microcirculation via increased production of carbon monoxide (CO) [3]. The results from this study indicate that administration of HO-1 inhibitors CrMP or ZnPP after T-H in PE rats did not alter plasma levels of estradiol or hepatic ER expressions; however, hepatic HO-1 and almost all observed HO-1 upstream signaling pathways including p38 MAPK, Akt, PI3K, ICAM-1, p-IκB-α, NF-κB, caspase-3, and Bcl-2 were altered significantly following T-H under those conditions. Moreover, liver MPO activity, NF-κB DNA-binding activity, TNF-α and IL-6, and plasma TNF-α, IL-6, and CINC-1 and -3 levels increased significantly after administration of the HO-1 inhibitor.
Although three HO isoforms have been identified, HO-1 is the only inducible form [20, 21] and is also known as Hsp32 [21]. The products of HO-catalyzed reactions, particularly CO and biliverdin/bilirubin, exert protective effects against oxidative and other noxious stimuli in several organs [21, 22]. Studies have suggested that CO generated via HO may act as a vasodilator in the liver following T-H [23, 24]. Although T-H induced the expression of HO-I in the liver of male rats [24], the expression of HO-2 remained unchanged under those conditions [25]. There is increasing evidence supporting the notion that HO-1 is protective in several disparate models of hepatic stress and the metabolic products of HO-catalyzed heme breakdown have been shown to play a pivotal role in cellular defense including hepatoprotection [21]. Studies have shown that E2 induces an increase in HO-1 in the liver [3, 12], intestine [4], lung [26], and heart [12]. Thus, it is clear that the significant increase in hepatic HO-1 in PE females following T-H is likely a result of higher plasma levels of estradiol.
The present results indicate that the increase of hepatic HO-1 was mediated via up-regulation of hepatic p38 MAPK and Akt/PI3K pathways following T-H, and this is in agreement with our previous results [13, 27]. In this regard, our previous studies have shown that the E2-mediated cardioprotection after T-H was mediated via activation of the p38 MAPK pathway [27, 28], and we have further evidence that activation of cardiac p38 MAPK is derived subsequent to heat shock protein-27 and αB-crystallin activation [27]. Moreover, coadministration of E2 and p38 MAPK inhibitor SB203580 blocked cardiac p38 MAPK activation and partially inhibited the cardioprotection derived from E2 [27, 28]. We have also reported that hepatic HO-1 is Akt-dependent in E2-treated male rats following T-H, and coadministration of E2 and the Akt inhibitor Wort abolished the increase in hepatic HO-1 and the protective effect of E2 on the liver [13]. The present study demonstrates that the increase in hepatic HO-1 is Akt/PI3K-dependent, not only in males but also in females following T-H. Ovariectomy depletes endogenous estradiol, and as indicated in this study, hepatic HO-1, p38 MAPK, and Akt/PI3K decreased significantly following T-H in OVX females; however, those signaling pathways were restored/enhanced in E2-pretreated OVX females, in which plasma levels of estradiol and hepatic ERs recovered to similar levels as seen in PE females. The results demonstrate further that the increase in p38 MAPK and Akt/PI3K following T-H was a result of high estradiol levels and restored hepatic ERs, and the increased HO-1 after T-H was p38 MAPK- and Akt/PI3K-dependent in PE females. Thus, the PE females are more tolerant to T-H, as they restore cell signaling pathways following adverse circulatory conditions. The results also suggest that administration of E2 in OVX animals or even males following T-H produces salutary effects on cell signaling pathways under those conditions.
Neutrophils are one of the primary defense mechanisms against invading microorganisms. They are also involved in the removal of necrotic cells. However, under certain conditions, recruitment of neutrophils can aggravate existing injury. Neutrophil infiltration plays an important role in reperfusion tissue injury, which is mediated by adhesion molecules such as selectins, β2-integrins, and ICAM-1 [29, 30]. Studies have shown that neutrophils are activated in the early phase of hemorrhagic shock [31], and hepatic damage is associated with an increase in neutrophil accumulation in the liver following T-H [32, 33]. The activated neutrophils infiltrate the injured liver in parallel with increased expression of adhesion molecules on endothelial cells and elevated local chemokine/cytokine levels following T-H. It has also been suggested that T-H increases endothelial cell P-selectin and ICAM-1 in the liver [34]. The up-regulation of ICAM-1 expression appears to be a crucial step leading to cellular recruitment and diapedesis [35]. Moreover, it has been suggested that inhibition of the adhesion molecules should prevent neutrophil infiltration, thus providing protection against organ injury [29].
Advances in unveiling the signal transduction network indicate that a battery of redox-sensitive transcription factors, such as AP-1, NF-κB, and NF-E2-related factor 2 and their upstream kinases including MAPKs, plays an important regulatory role in HO-1 gene induction [21]. It has been reported that the NF-κB family, one of the transcriptional factors, is activated in the liver, lung, and heart following adverse circulatory conditions [36, 37]. T-H activates NF-κB and produces liver injury [38, 39]. It has been reported that NF-кB and IкB-α interact in an autoregulatory mechanism [40]. Our studies have indicated that the normalized cardiac function following T-H in PE females may be a result of inhibition of the NF-кB/IкB system [2]. Studies have also shown that the NF-κB signaling cascade is important in ICAM-1 activation [41]. Nonetheless, further studies are needed to clarify the precise mechanism, i.e., the upstream pathways of HO-1, by which estradiol increases hepatic HO-1.
Studies have also demonstrated that CINC-1 and -3 gene expression appears to be related to the activation of NF-κB, and a rapid increase in NF-κB DNA-binding activity after ischemia/reperfusion injury in the liver precedes the increase in the expression of CINC-1 [42]. Furthermore, it has been reported that the reduction in hepatic neutrophil recruitment is associated with decreased activation of NF-κB and expression of CINC-3 [43]. The present study shows clearly that hepatic NF-κB, ICAM-1, and CINCs, along with MPO (an index of neutrophil infiltration) increased significantly following T-H in OVX rats; however, such increases were not evident in PE or E2-pretreated OVX females under those conditions. Moreover, HO-1 inhibitors CrMP and ZnPP and the Akt/PI3K inhibitor Wort abrogated the salutary effects on those signal pathways in PE females following T-H.
Apoptosis is a morphologically distinct form of cell death that has been shown to contribute to hepatocellular injury [44]. Induction of necrotic or apoptotic pathways in hepatocellular injury depends on the degree of ischemic injury and the adequacy of restoration of oxygen supply to the cells, as oxidative metabolism is of paramount importance in the regulation of programmed cell death [45]. Moreover, those agents that induce apoptosis are oxidants or stimulators of oxidative metabolism [46, 47], suggesting that the aggravation of hepatocellular injury that occurs after resuscitation following hemorrhage may depend on the generation of free oxygen radicals [48] and subsequent induction of apoptotic pathways [49]. Our results indicate that increased HO-1 following T-H in PE females attenuated hepatocellular apoptosis as shown by increased hepatic Bcl-2 and decreased, cleaved caspase-3; however, HO-1 inhibitors and the Akt/PI3K inhibitor abrogated the salutary effect seen in PE females. It has been demonstrated that Bcl-2 has an antiapoptotic effect [50,51,52,53], and the mitochondrial release of cytochrome c is a major target for the antiapoptotic effects of Bcl-2 [52]. A decrease in Bcl-2 levels or the inhibition of Bcl-2 activity might provoke apoptosis or at least sensitize cells to apoptotic death [53]. It has also been reported that Bcl-2 can protect from in vivo Fas-mediated cytotoxity and is therefore able to prevent fulminant hepatic failure [51]. In the final or execution phase of apoptosis, caspases, a new family of proteases, dismantle the cell by sequential activation and cleavage of key proteins [54]. Caspases are present in the cytosol of most cells as zymogens and need to be activated to fully functional proteases by cleavage of the proenzyme by proteolytic steps [55]. So far, 14 mammalian caspases have been identified [56]. Functionally, these proteases divide into two major subfamilies: Caspases-1, -4, and -5 are related to IL-1β-converting enzyme, and other caspases perform an elaborate Caenorhabditis elegans cell-death protein (CED-3) function to mediate apoptosis [55]. It has been reported that caspases-3 and -7 perform the proteolytic cleavage events [55]. Certain caspases (-3, -6, -7) are called effectors or downstream caspases, as they cleave key substrates and eventually lead to apoptotic cell death [55]. Interestingly, ovariectomy in female rats decreased hepatic Bcl-2 and increased cleaved caspase-3; however, E2 supplementary treatment prior to the induction of T-H in OVX females restored hepatic Bcl-2 and prevented the increase in hepatic cleaved caspase-3.
ER-α has been demonstrated to be present predominantly in the liver [57, 58]. Our recent study has also shown that the ER-α but not ER-β agonist attenuated hepatic injury after T-H [59]. The present study showed that hepatic ERs were not significantly different in PE females following T-H. A significant decrease in hepatic ER-α and ER-β expression was, however, seen in OVX females. Interestingly, E2 pretreatment in OVX females restored hepatic ER-α and ER-β following T-H. Administration of HO-1 inhibitors and the Akt/PI3K inhibitor after T-H did not significantly alter hepatic ERs and the plasma estradiol levels in PE females. These findings imply that the loss of a hepatic-protective effect seen in PE females by HO-1 inhibitors is neither a result of a decrease in plasma estradiol levels nor a decrease in hepatic ERs.
The current study examined only a single time-point, i.e., 2 h after T-H and resuscitation, and thus, it remains unclear whether the hepatoprotective effects seen in PE rats are sustained for a longer time after T-H. Our previous studies have shown that if the improvement in organ function by any pharmacological agent were evident early after treatment, then those salutary effects are sustained for prolonged intervals, and they improved the survival of animals [5, 6, 11]. Thus, although a time-point other than 2 h was not examined in this study, based on our previous studies, it would appear that the hepatoprotective effects of higher estradiol levels in PE females would be evident, even if those effects were measured at another time-point following T-H or resuscitation.
In summary, our results indicate that cardiac function was depressed significantly, and liver damage occurred following T-H in OVX females; however, these parameters were maintained in PE females under those conditions. In addition to the restoration of cardiac and hepatic function in PE females following T-H, liver HO-1 increased significantly via up-regulation of hepatic p38 MAPK and Akt/PI3K. The up-regulation of hepatic p38 MAPK and Akt/PI3K prevented neutrophil infiltration into the liver, as evidenced by normalization of hepatic ICAM-1 expression, liver MPO activity, and plasma CINCs and proinflammatory cytokines. Although administration of HO-1 inhibitors and the Akt/PI3K inhibitor following T-H did not alter plasma estradiol levels or hepatic ERs in PE females, it abrogated the increase in hepatic HO-1 expression and related upstream signaling pathways. Furthermore, the increase in liver HO-1 expression and the normalization of liver ICAM-1 expression after T-H in PE females were abolished by HO-1 inhibitors and Akt/PI3K inhibitor administration. Moreover, the liver NF-кB/IкB-α system and apoptotic pathways were activated in OVX females and in PE females treated with HO-1 inhibitors and the Akt/PI3K inhibitor; however, administration of E2 in OVX females before the induction of T-H restored the hepatic NF-кB/IкB-α system and apoptotic pathways. In addition, the increase in liver and plasma proinflammatory cytokines and plasma chemokines in OVX females was prevented in PE and in E2-pretreated OVX females following T-H. These data collectively suggest that up-regulation of hepatic HO-1 plays an important role in hepatoprotection following T-H in PE females. This is likely through up-regulation of p38 MAPK and Akt/PI3K and down-regulation of hepatic neutrophil infiltration, inflammatory response, and hepatocyte apoptosis. Thus, up-regulation of hepatic HO-1 in PE females appears to be one of the mechanisms responsible for hepatoprotection following T-H.
Acknowledgments
This work supported by NIH grant R37 GM39519 (I. H. C.). The authors thank Ms. Bobbi Smith for her assistance with manuscript preparation.
References
- Holbrook T L, Hoyt D B, Stein M B, Sieber W J. Gender differences in long-term posttraumatic stress disorder outcomes after major trauma: women are at higher risk of adverse outcomes than men. J Trauma. 2002;53:882–888. doi: 10.1097/00005373-200211000-00012. [DOI] [PubMed] [Google Scholar]
- Yang S, Choudhry M A, Hsieh Y C, Hu S, Rue L W, III, Bland K I, Chaudry I H. Estrus cycle: influence on cardiac function following trauma-hemorrhage. Am J Physiol Heart Circ Physiol. 2006;291:H2807–H2815. doi: 10.1152/ajpheart.00195.2006. [DOI] [PubMed] [Google Scholar]
- Toth B, Yokoyama Y, Kuebler J F, Schwacha M G, Rue L W, III, Bland K I, Chaudry I H. Sex differences in hepatic heme oxygenase expression and activity following trauma and hemorrhagic shock. Arch Surg. 2003;138:1375–1382. doi: 10.1001/archsurg.138.12.1375. [DOI] [PubMed] [Google Scholar]
- Yu H P, Choudhry M A, Shimizu T, Hsieh Y C, Schwacha M G, Yang S, Chaudry I H. Mechanism of the salutary effects of flutamide on intestinal myeloperoxidase activity following trauma-hemorrhage: up-regulation of estrogen receptor-{β}-dependent HO-1. J Leukoc Biol. 2006;79:277–284. doi: 10.1189/jlb.0705363. [DOI] [PubMed] [Google Scholar]
- Ray P, Ghosh S K, Zhang D H, Ray A. Repression of interleukin-6 gene expression by 17 β-estradiol: inhibition of the DNA-binding activity of the transcription factors NF-IL6 and NF-κ B by the estrogen receptor. FEBS Lett. 1997;409:79–85. doi: 10.1016/s0014-5793(97)00487-0. [DOI] [PubMed] [Google Scholar]
- Remmers D E, Cioffi W G, Bland K I, Wang P, Angele M K, Chaudry I H. Testosterone: the crucial hormone responsible for depressing myocardial function in males after trauma-hemorrhage. Ann Surg. 1998;227:790–799. doi: 10.1097/00000658-199806000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry M A, Schwacha M G, Hubbard W J, Kerby J D, Rue L W, Bland K I, Chaudry I H. Gender differences in acute response to trauma-hemorrhage. Shock. 2005;24:101–106. doi: 10.1097/01.shk.0000191341.31530.5e. [DOI] [PubMed] [Google Scholar]
- Olanders K, Sun Z, Borjesson A, Dib M, Andersson E, Lasson A, Ohlsson T, Andersson R. The effect of intestinal ischemia and reperfusion injury on ICAM-1 expression, endothelial barrier function, neutrophil tissue influx, and protease inhibitor levels in rats. Shock. 2002;18:86–92. doi: 10.1097/00024382-200207000-00016. [DOI] [PubMed] [Google Scholar]
- Dayal S D, Hasko G, Lu Q, Xu D Z, Caruso J M, Sambol J T, Deitch E A. Trauma/hemorrhagic shock mesenteric lymph upregulates adhesion molecule expression and IL-6 production in human umbilical vein endothelial cells. Shock. 2002;17:491–495. doi: 10.1097/00024382-200206000-00009. [DOI] [PubMed] [Google Scholar]
- Meldrum D R, McIntyre R C, Sheridan B C, Cleveland J C, Jr, Fullerton D A, Harken A H. L-arginine decreases alveolar macrophage proinflammatory monokine production during acute lung injury by a nitric oxide synthase-dependent mechanism. J Trauma. 1997;43:888–893. doi: 10.1097/00005373-199712000-00003. [DOI] [PubMed] [Google Scholar]
- Jarrar D, Wang P, Cioffi W G, Bland K I, Chaudry I H. The female reproductive cycle is an important variable in the response to trauma-hemorrhage. Am J Physiol Heart Circ Physiol. 2000;279:H1015–H1021. doi: 10.1152/ajpheart.2000.279.3.H1015. [DOI] [PubMed] [Google Scholar]
- Szalay L, Shimizu T, Schwacha M G, Choudhry M A, Rue L W, III, Bland K I, Chaudry I H. Mechanism of salutary effects of estradiol on organ function after trauma-hemorrhage: upregulation of heme oxygenase. Am J Physiol Heart Circ Physiol. 2005;289:H92–H98. doi: 10.1152/ajpheart.01247.2004. [DOI] [PubMed] [Google Scholar]
- Hsu J T, Kan W H, Hsieh C H, Choudhry M A, Schwacha M G, Bland K I, Chaudry I H. Mechanism of estrogen-mediated attenuation of hepatic injury following trauma-hemorrhage: Akt-dependent HO-1 up-regulation. J Leukoc Biol. 2007;82:1019–1026. doi: 10.1189/jlb.0607355. [DOI] [PubMed] [Google Scholar]
- Yang S, Zheng R, Hu S, Ma Y, Choudhry M A, Messina J L, Rue L W, III, Bland K I, Chaudry I H. Mechanism of cardiac depression after trauma-hemorrhage: increased cardiomyocyte IL-6 and effect of sex steroids on IL-6 regulation and cardiac function. Am J Physiol Heart Circ Physiol. 2004;287:H2183–H2191. doi: 10.1152/ajpheart.00624.2003. [DOI] [PubMed] [Google Scholar]
- Poole B, Wang W, Chen Y C, Zolty E, Falk S, Mitra A, Schrier R. Role of heme oxygenase-1 in endotoxemic acute renal failure. Am J Physiol Renal Physiol. 2005;289:F1382–F1385. doi: 10.1152/ajprenal.00402.2004. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Yu H P, Hsieh Y C, Choudhry M A, Suzuki T, Bland K I, Chaudry I H. Flutamide attenuates pro-inflammatory cytokine production and hepatic injury following trauma-hemorrhage via estrogen receptor-related pathway. Ann Surg. 2007;245:297–304. doi: 10.1097/01.sla.0000232523.88621.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Hu S, Choudhry M A, Rue L W, III, Bland K I, Chaudry I H. Anti-rat soluble IL-6 receptor antibody down-regulates cardiac IL-6 and improves cardiac function following trauma-hemorrhage. J Mol Cell Cardiol. 2007;42:620–630. doi: 10.1016/j.yjmcc.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Renard P, Ernest I, Houbion A, Art M, Le Calvez H, Raes M, Remacle J. Development of a sensitive multi-well colorimetric assay for active NFκB. Nucleic Acids Res. 2001;29:E21. doi: 10.1093/nar/29.4.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima Y, Wang P, Jarrar D, Cioffi W G, Bland K I, Chaudry I H. Preinduction of heat shock proteins protects cardiac and hepatic functions following trauma and hemorrhage. Am J Physiol Regul Integr Comp Physiol. 2000;278:R352–R359. doi: 10.1152/ajpregu.2000.278.2.R352. [DOI] [PubMed] [Google Scholar]
- Maines M D. The heme oxygenase system: update 2005. Antioxid Redox Signal. 2005;7:1761–1766. doi: 10.1089/ars.2005.7.1761. [DOI] [PubMed] [Google Scholar]
- Farombi E O, Surh Y J. Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. J Biochem Mol Biol. 2006;39:479–491. doi: 10.5483/bmbrep.2006.39.5.479. [DOI] [PubMed] [Google Scholar]
- Zuckerbraun B S, Billiar T R. Heme oxygenase-1: a cellular Hercules. Hepatology. 2003;37:742–744. doi: 10.1053/jhep.2003.50139. [DOI] [PubMed] [Google Scholar]
- Bauer M, Pannen B H, Bauer I, Herzog C, Wanner G A, Hanselmann R, Zhang J X, Clemens M G, Larsen R. Evidence for a functional link between stress response and vascular control in hepatic portal circulation. Am J Physiol. 1996;271:G929–G935. doi: 10.1152/ajpgi.1996.271.5.G929. [DOI] [PubMed] [Google Scholar]
- Pannen B H, Kohler N, Hole B, Bauer M, Clemens M G, Geiger K K. Protective role of endogenous carbon monoxide in hepatic microcirculatory dysfunction after hemorrhagic shock in rats. J Clin Invest. 1998;102:1220–1228. doi: 10.1172/JCI3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer I, Wanner G A, Rensing H, Alte C, Miescher E A, Wolf B, Pannen B H, Clemens M G, Bauer M. Expression pattern of heme oxygenase isoenzymes 1 and 2 in normal and stress-exposed rat liver. Hepatology. 1998;27:829–838. doi: 10.1002/hep.510270327. [DOI] [PubMed] [Google Scholar]
- Yu H P, Yang S, Hsieh Y C, Choudhry M A, Bland K I, Chaudry I H. Maintenance of lung myeloperoxidase activity in PE females after trauma-hemorrhage: upregulation of heme oxygenase-1. Am J Physiol Lung Cell Mol Physiol. 2006;291:L400–L406. doi: 10.1152/ajplung.00537.2005. [DOI] [PubMed] [Google Scholar]
- Hsu J T, Hsieh Y C, Kan W H, Chen J G, Choudhry M A, Schwacha M G, Bland K I, Chaudry I H. Role of p38 mitogen-activated protein kinase pathway in estrogen-mediated cardioprotection following trauma-hemorrhage. Am J Physiol Heart Circ Physiol. 2007;292:H2982–H2987. doi: 10.1152/ajpheart.01303.2006. [DOI] [PubMed] [Google Scholar]
- Kan W H, Hsu J T, Ba Z F, Schwacha M G, Chen J, Choudhry M A, Bland K I, Chaudry I H. p38 MAPK-dependent eNOS upregulation is critical for 17β-estradiol-mediated cardioprotection following trauma-hemorrhage. Am J Physiol Heart Circ Physiol. 2008;294:H2627–H2636. doi: 10.1152/ajpheart.91444.2007. [DOI] [PubMed] [Google Scholar]
- Monson K M, Dowlatshahi S, Crockett E T. CXC-chemokine regulation and neutrophil trafficking in hepatic ischemia-reperfusion injury in P-selectin/ICAM-1 deficient mice. J Inflamm (Lond) 2007;4:11. doi: 10.1186/1476-9255-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deree J, Lall R, Melbostad H, Grant M, Hoyt D B, Coimbra R. Neutrophil degranulation and the effects of phosphodiesterase inhibition. J Surg Res. 2006;133:22–28. doi: 10.1016/j.jss.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Tani T, Endo Y, Hanasawa K, Tsuchiya M, Kodama M. Elevation of plasma peptidoglycan and peripheral blood neutrophil activation during hemorrhagic shock: plasma peptidoglycan reflects bacterial translocation and may affect neutrophil activation. Crit Care Med. 2002;30:77–82. doi: 10.1097/00003246-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Lehnert M, Arteel G E, Smutney O M, Conzelmann L O, Zhong Z, Thurman R G, Lemasters J J. Dependence of liver injury after hemorrhage/resuscitation in mice on NADPH oxidase-derived superoxide. Shock. 2003;19:345–351. doi: 10.1097/00024382-200304000-00009. [DOI] [PubMed] [Google Scholar]
- Kuebler J F, Yokoyama Y, Jarrar D, Toth B, Rue L W, III, Bland K I, Wang P, Chaudry I H. Administration of progesterone after trauma and hemorrhagic shock prevents hepatocellular injury. Arch Surg. 2003;138:727–734. doi: 10.1001/archsurg.138.7.727. [DOI] [PubMed] [Google Scholar]
- Xu D Z, Lu Q, Adams C A, Issekutz A C, Deitch E A. Trauma-hemorrhagic shock-induced up-regulation of endothelial cell adhesion molecules is blunted by mesenteric lymph duct ligation. Crit Care Med. 2004;32:760–765. doi: 10.1097/01.ccm.0000114815.88622.9d. [DOI] [PubMed] [Google Scholar]
- Entman M L, Youker K, Shappell S B, Siegel C, Rothlein R, Dreyer W J, Schmalstieg F C, Smith C W. Neutrophil adherence to isolated adult canine myocytes. Evidence for a CD18-dependent mechanism. J Clin Invest. 1990;85:1497–1506. doi: 10.1172/JCI114596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer C, Kalff J C, Omert L, Tsukada K, Loeffert J E, Watkins S C, Billiar T R, Tweardy D J. Interleukin-6 production in hemorrhagic shock is accompanied by neutrophil recruitment and lung injury. Am J Physiol. 1998;275:L611–L621. doi: 10.1152/ajplung.1998.275.3.L611. [DOI] [PubMed] [Google Scholar]
- Meldrum D R, Shenkar R, Sheridan B C, Cain B S, Abraham E, Harken A H. Hemorrhage activates myocardial NFκB and increases TNF-α in the heart. J Mol Cell Cardiol. 1997;29:2849–2854. doi: 10.1006/jmcc.1997.0506. [DOI] [PubMed] [Google Scholar]
- Hierholzer C, Harbrecht B, Menezes J M, Kane J, MacMicking J, Nathan C F, Peitzman A B, Billiar T R, Tweardy D J. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med. 1998;187:917–928. doi: 10.1084/jem.187.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddipati J P, Sundar S V, Calemine J, Seth P, Sidhu G S, Maheshwari R K. Differential regulation of cytokines and transcription factors in liver by curcumin following hemorrhage/resuscitation. Shock. 2003;19:150–156. doi: 10.1097/00024382-200302000-00011. [DOI] [PubMed] [Google Scholar]
- Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, Akira S. Transcription factors NF-IL6 and NF-κ B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci USA. 1993;90:10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebuck K A, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999;66:876–888. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- Yamada S, Iida T, Tabata T, Nomoto M, Kishikawa H, Kohno K, Eto S. Alcoholic fatty liver differentially induces a neutrophil-chemokine and hepatic necrosis after ischemia-reperfusion in rat. Hepatology. 2000;32:278–288. doi: 10.1053/jhep.2000.9604. [DOI] [PubMed] [Google Scholar]
- Kato A, Gabay C, Okaya T, Lentsch A B. Specific role of interleukin-1 in hepatic neutrophil recruitment after ischemia/reperfusion. Am J Pathol. 2002;161:1797–1803. doi: 10.1016/S0002-9440(10)64456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler J M, Cohen G M, MacFarlane M. Different subcellular distribution of caspase-3 and caspase-7 following Fas-induced apoptosis in mouse liver. J Biol Chem. 1998;273:10815–10818. doi: 10.1074/jbc.273.18.10815. [DOI] [PubMed] [Google Scholar]
- Leist M, Single B, Castoldi A F, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A M, Xu F H, Fady C, Jacoby F J, Duffey D C, Tu Y, Lichtenstein A. Apoptotic vs. nonapoptotic cytotoxicity induced by hydrogen peroxide. Free Radic Biol Med. 1997;22:73–83. doi: 10.1016/s0891-5849(96)00235-3. [DOI] [PubMed] [Google Scholar]
- Cai J, Jones D P. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J Biol Chem. 1998;273:11401–11404. doi: 10.1074/jbc.273.19.11401. [DOI] [PubMed] [Google Scholar]
- Mota-Filipe H, McDonald M C, Cuzzocrea S, Thiemermann C. A membrane-permeable radical scavenger reduces the organ injury in hemorrhagic shock. Shock. 1999;12:255–261. doi: 10.1097/00024382-199910000-00002. [DOI] [PubMed] [Google Scholar]
- Lemasters J J, Nieminen A L, Qian T, Trost L C, Elmore S P, Nishimura Y, Crowe R A, Cascio W E, Bradham C A, Brenner D A, Herman B. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998;1366:177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- Oltvai Z N, Milliman C L, Korsmeyer S J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Lacronique V, Mignon A, Fabre M, Viollet B, Rouquet N, Molina T, Porteu A, Henrion A, Bouscary D, Varlet P, Joulin V, Kahn A. Bcl-2 protects from lethal hepatic apoptosis induced by an anti-Fas antibody in mice. Nat Med. 1996;2:80–86. doi: 10.1038/nm0196-80. [DOI] [PubMed] [Google Scholar]
- Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Nicholson D W. From bench to clinic with apoptosis-based therapeutic agents. Nature. 2000;407:810–816. doi: 10.1038/35037747. [DOI] [PubMed] [Google Scholar]
- Cohen G M. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust C, Gores G J. Apoptosis and liver disease. Am J Med. 2000;108:567–574. doi: 10.1016/s0002-9343(00)00370-3. [DOI] [PubMed] [Google Scholar]
- Earnshaw W C, Martins L M, Kaufmann S H. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- Kuiper G G, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson J A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Moore R, Hess H A, Guo G L, Gonzalez F J, Korach K S, Maronpot R R, Negishi M. Estrogen receptor α mediates 17α-ethynylestradiol causing hepatotoxicity. J Biol Chem. 2006;281:16625–16631. doi: 10.1074/jbc.M602723200. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Yu H P, Suzuki T, Szalay L, Hsieh Y C, Choudhry M A, Bland K I, Chaudry I H. The role of estrogen receptor subtypes in ameliorating hepatic injury following trauma-hemorrhage. J Hepatol. 2007;46:1047–1054. doi: 10.1016/j.jhep.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]