Abstract
The JAK2 mutation JAK2V617F is found frequently in patients with myeloproliferative disorders (MPD) and transforms hematopoietic cells to cytokine-independent proliferation when expressed with specific cytokine receptors. The Src homology 2 (SH2) and pleckstrin homology (PH) domain-containing adaptor protein Lnk (SH2B3) is a negative regulator of hematopoietic cytokine signaling. Here, we show that Lnk is a potent inhibitor of JAK2V617F constitutive activity. Lnk down-regulates JAK2V617F-mediated signaling and transformation in hematopoietic Ba/F3-erythropoietin receptor cells. Furthermore, in CFU assays, Lnk-deficient murine bone marrow cells are significantly more sensitive to transformation by JAK2V617F than wild-type (WT) cells. Lnk, through its SH2 and PH domains, interacts with WT and mutant JAK2 and is phosphorylated by constitutively activated JAK2V617F. Finally, we found that Lnk levels are high in CD34+ hematopoietic progenitors from MPD patients and that Lnk expression is induced following JAK2 activation. Our data suggest that JAK2V617F is susceptible to endogenous negative-feedback regulation, providing new insights into the molecular pathogenesis of MPD.
Keywords: cancer, cell lines, signaling cascade, protein kinases/phosphatases
INTRODUCTION
Cytokines regulate proliferation and differentiation of hematopoietic cells by binding to cell surface cytokine receptors. Homodimeric type I cytokine receptors lack intrinsic catalytic activity and mediate ligand-dependent protein phosphorylation through association with tyrosine kinases of the JAK family. The importance of JAK2 to normal hematopoiesis is demonstrated by the severe defects of erythropoiesis in JAK2-deficient mice [1]. Aberrant activation of JAK2 occurs in many human cancers and has a primary role in the pathogenesis of myeloproliferative disorders (MPD), a group of hematopoietic malignancies characterized by expansion of the myeloid lineages [2, 3]. The JAK2 mutation, JAK2V617F, is restricted to myeloid malignancies and is predominant in MPD patients [4,5,6,7,8]; it is present in almost all patients with polycythemia vera and in approximately half of those with essential thrombocytosis and idiopathic myelofibrosis. Expression of JAK2V617F in hematopoietic cells results in transformation to factor-independent growth and cytokine hypersensitivity. Moreover, transfection of hematopoietic stem cells with JAK2V617F causes MPD-like disease in mice [5, 9,10,11,12]. Unlike other activated tyrosine kinases identified in human malignancies, JAK2V617F-mediated cell transformation requires interaction with a homodimeric type I cytokine receptor scaffold {erythropoietin receptor (EpoR), thrombopoietin receptor (Tpo; MPL), or G-CSFR [13,14,15]}. Furthermore, JAK2V617F responds to ligand stimulation, suggesting its activity can be modulated by feedback mechanisms, which normally regulate JAK2.
Adaptor proteins that bind JAK2 and its cognate receptor are important factors in determining the magnitude and duration of JAK2 stimulation [16]. Lnk [also known as Src homology 2 (SH2)B3], an adaptor protein highly expressed in hematopoietic cells, regulates several signaling pathways of cytokine receptors [17]. Lnk-deficient mice exhibit profound abnormalities in hematopoiesis including accumulation of B lymphocytes, enhanced megakaryocytopoiesis and erythropoiesis [18,19,20,21], an increased number of hematopoietic stem cells [22, 23], and enhanced cytokine sensitivity [18,19,20,21,22]. Lnk forms part of an adaptor protein family, together with SH2-B (SH2B1) and APS (SH2B2), whose members share a common domain structure that includes a dimerization domain, a pleckstrin homology (PH) region, and a SH2 domain. The latter binds phosphotyrosines (pTyr) in various signal-transducing proteins and is critical for Lnk inhibition of signaling by c-Kit, MPL, and EpoR [20, 21, 24,25,26]. SH2-B and APS are well-recognized JAK2 regulators in various signaling networks [27,28,29,30]. Increasing evidence suggests that inappropriate function of negative regulators has an important role in oncogenic transformation. The aim of this study was to investigate whether Lnk, an important negative regulator of JAK2, can modulate mutant JAK2V617F-mediated signaling and transformation.
MATERIALS AND METHODS
Patient samples and controls
Peripheral blood was obtained from patients [#1 JAK2V617F-negative agnogenic myeloid metaplasia (AMM); #2 JAK2V617F-negative hypereosinophilic syndrome; #3 JAK2V617F-positive AMM; #4 JAK2V617F-positive AMM] after informed consent, and peripheral blood was obtained from normal volunteers (n=3) to serve as controls. A CD34+ myeloid stem cell population was isolated from low-density mononuclear cells, and purified cell pellets were used to extract RNA.
Mice and cell culture
Lnk-deficient 129/Sv mice were a generous gift from Dr. Anthony J. Pawson (Samuel Lunenfeld Research Institute, Toronto, Canada) [18]. Bone marrow (BM) cells were obtained by flushing femurs and tibias with medium and were cultured for 1 or 2 days in RPMI-1640 medium containing 10% FBS and cytokines, as indicated in specific experiments. The NIH 3T3 and 293T cell lines were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA), and the packaging cell line Plat-E was generously provided by Dr. Toshio Kitamura (University of Tokyo, Japan) [31]. These cell lines were grown in DMEM medium with 10% FCS. The Ba/F3 cell line was obtained from ATCC and grown in RPMI-1640 medium containing 10% FCS and 10% WEHI-3B cell supernatant as a source of IL-3 (WEHI media). Human leukemia cell lines were obtained from ATCC and were cultured in RPMI-1640 medium with 10% FCS. The following leukemia cell lines were used: Hel (erythroleukemia, JAK2V617F-positive), K562 (chronic myelogenous leukemia, BCR-ABL-positive), and NB4 (M3, acute promyelocytic leukemia, PML-RARα-positive).
Expression vectors
The pcDNA3.1/V5 human Lnk and GST Lnk vectors were made as described [26]. The bicistronic retroviral murine stem cell virus (MSCV)-internal ribosomal entry site (IRES)-GFP [monokine induced by IFN-γ (MIG)] murine Lnk [wild-type (WT) and R364E] and JAK2 (WT and V617F) constructs were generously provided by Dr. Wei Tong (Children’s Hospital of Philadelphia, PA, USA) [20] and Dr. Richard A. Van Etten (Tufts New England Medical Center, Boston, MA, USA) [10], respectively. The pcDNA3.1 JAK2 (WT and V617F) constructs, the murine Lnk, and EpoR vectors were kindly provided by Dr. Zhizhuang J. Zhao (University of Oklahoma, Oklahoma City, OK, USA) [8], Dr. Satoshi Takaki (University of Tokyo) [24], and Dr. Alan D. D’Andrea (Harvard Medical School, Boston, MA, USA) [32], respectively.
Transfections
293T and Plat-E cells were transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) and Effectene transfection reagent (Qiagen, Valencia, CA, USA), respectively. To generate Ba/F3 cells stably expressing EpoR and JAK2 (WT or V617F), Ba/F3 cells were electroporated with EpoR and JAK2 vectors using an electroporation apparatus (Electro Square Porator T820). G418 (800 μg/ml) selection was started 2 days later. BaF/EpoR/WT were cultured with Epo (10 U/ml), and BaF/EpoR/V617F were cultured without cytokines.
Retroviral infection
Plat-E cells were transfected with the MIG vectors, and viral supernatants were collected 2 days later. Viral titer was determined by serial dilution of the viral supernatant and infection of NIH 3T3 cells. Supernatants with equal viral titers were used in each experiment. BaF/EpoR/WT, BaF/EpoR/V617F, and BM cells were spin-infected with retroviral supernatants containing 8 μg/ml polybrene (Sigma-Aldrich, St. Louis, MO, USA) at 1900 rpm (Beckman GS-CR) for 90 min at 25°C.
Western blot, immunoprecipitation, and GST pull-down assays
Cells were washed twice with PBS and lysed on ice with lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.5% Nonidet P-40) containing complete protease inhibitor cocktail (Roche, Nutley, NJ, USA) and phosphatase inhibitors (1 mM PMSF, 100 mM NaF, and 10 mM Na3VO4). Proteins were resolved on 4–15% gradient SDS-PAGE and transferred to nitrocellulose membranes (Sigma-Aldrich). Immunoblots were incubated with primary antibodies followed by incubation with appropriate secondary IgG antibody conjugated with HRP (Amersham Pharmacia Biotech, Piscataway, NJ, USA). SuperSignal West Pico and West Dura chemiluminescent substrates (Pierce, Rockford, IL, USA) were used for detection. Band intensities were analyzed by densitometry (Universal Hood.2, Bio-Rad, Hercules, CA, USA). The following antibodies were used for immunoprecipitation and Western blot analysis: Lnk (Serotec, UK; Santa Cruz Biotechnology, Santa Cruz, CA, USA; and Novus Biologicals, Littleton, CO, USA); pJAK2 (Tyr1007/1008), JAK2, and pAKT (Ser473; Cell Signaling Technology, Beverly, MA, USA); pSTAT5A/B (Y694/699; Millipore, Bedford, MA, USA); pERK (Tyr 204), STAT5, AKT, pTyr, and platelet-derived growth factor receptor (PDGFR)β (Santa Cruz Biotechnology); V5 (Invitrogen); and anti-β-actin (Sigma-Aldrich). For immunoprecipitation, lysates (1 mg from cell lines) were incubated in lysis buffer containing protease and phosphatase inhibitors, with specific antibodies and protein A/G agarose beads (Santa Cruz Biotechnology) at 4°C for 16 h. For immunoprecipitation assays using BM cells, cells from Lnk+/+ and Lnk−/− mice (two of each) were cultured 2 days in RPMI 1640 with 10% FBS, 10 ng/ml IL-3, 10 ng/mL IL-6, 10 ng/ml stem cell factor (SCF), 5 U/ml Epo, and 10 ng/ml Tpo. Cells were depleted of cytokines and serum for 4 h and then stimulated with 20 ng/ml SCF, 10 U/ml Epo, and 20 ng/ml Tpo for 30 min. Lysates from 3 × 106 cells were used for immunoprecipitation. For GST pull-down assays, GST or GST-Lnk fusion proteins immobilized on glutathione-sepharose beads (Amersham Pharmacia Biotech) were incubated with lysates from 293T-transfected cells at 4°C for 16 h. Precipitated proteins were washed three times with lysis buffer, eluted with SDS sample buffer, and subjected to Western analysis.
Real-time RT-PCR analysis
Total RNA was extracted using TRIzol reagent (Invitrogen) and processed to cDNA with Superscript III (Invitrogen) according to the manufacturer’s protocols. Lnk and 18S (for endogenous reference) primers and probes were synthesized by Invitrogen and Biosearch Technologies (Novato, CA, USA), respectively. Amplification reactions were done in an iCycler iQ system (Bio-Rad).
Colony assays
BM cells from Lnk+/+ and Lnk−/− mice (three of each) were harvested and cultured for 1 day in RPMI 1640 with 10% FBS, 10 ng/ml IL-3, 10 ng/mL IL-6, and 10 ng/ml SCF. Cells were spin-infected with MIG or MIG-V617F retroviral supernatants and after 2 days, sorted for GFP expression. Positive cells (1.5×104) were plated in triplicates in semisolid methylcellulose (Stem Cell Technologies, Canada) with cytokines (MethoCult GF M3534+3 U/ml Epo) or without (MethoCult M3234). Colony formation was assessed 6 and 12 days later.
RESULTS
Lnk inhibits proliferation of hematopoietic cells expressing JAK2V617F
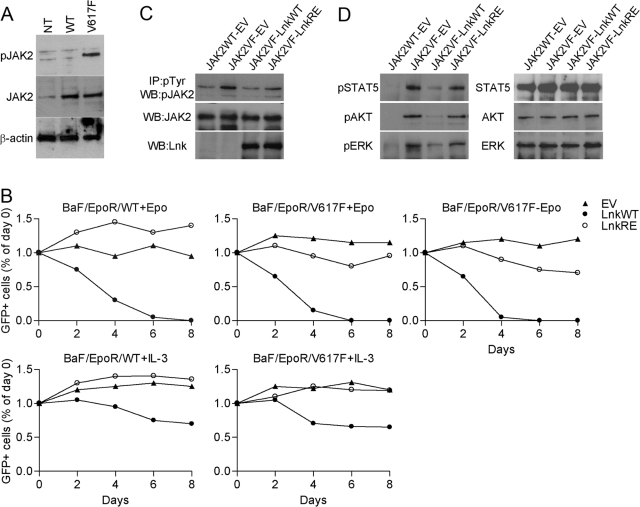
To study the effect of Lnk on JAK2V617F in the murine IL-3-dependent hematopoietic cell line Ba/F3, we generated Ba/F3 cells stably expressing EpoR and WT JAK2 (BaF/EpoR/WT) or JAK2V617F (BaF/EpoR/V617F). Both cell lines expressed higher levels of total JAK2 protein compared with the parental Ba/F3 cells; however, only expression of JAK2V617F resulted in a high level of JAK2 pTyr in the absence of cytokine stimulation (Fig. 1A). Accordingly, although both cell lines responded to Epo treatment, JAK2V617F but not WT JAK2 (JAK2WT) transformed the BaF/EpoR cells to cytokine-independent growth, as has been reported previously [13,14,15]. Subsequently, BaF/EpoR/WT cells were cultured with Epo, and BaF/EpoR/V617F cells were cultured with or without Epo. We used a bicistronic MSCV-IRES-GFP (MIG) vector to introduce WT Lnk (WT), Lnk SH2 domain point mutation (R364E), or vector alone into the Ba/F3 cell lines. The expression of GFP tightly correlates with that of the upstream gene (i.e., Lnk), thus we were able to analyze cell proliferation by measuring GFP levels. Two days after infections, aliquots of cells were analyzed for GFP expression by FACS, and the remaining cells were placed in fresh medium. Cell proliferation was determent by measuring the percentage of GFP-positive cells in cell aliquots every 2 days for 8 days and comparing these levels with that of the percentage of GFP on Day 2 after infection (Day 0, Fig. 1B). In BaF/EpoR/WT and BaF/EpoR/V617F cells, the number of control GFP-expressing cells did not change throughout the experiment (Fig. 1B). On the other hand, the number of GFP-positive BaF/EpoR/WT cells infected with Lnk WT decreased markedly. Similarly, the number of GFP-positive BaF/EpoR/V617F cells cultured in the presence or absence of Epo was reduced dramatically. These results show that in addition to inhibiting Epo-induced growth of cells expressing JAK2WT, Lnk inhibits Epo-mediated and cytokine-independent growth of JAK2V617F-expressing cells. The SH2 domain is essential for Lnk inhibition of Epo-induced JAK2WT activation [21]. As expected, a mutation disrupting the Lnk SH2 domain, R364E, abolished the ability of Lnk to inhibit Epo-dependent growth of BaF/EpoR/WT cells (Fig. 1B, upper panels). In contrast, this mutant retained some inhibitory function in BaF/EpoR/V617F cells, although it was much less profound compared with Lnk WT. Of note, BaF/EpoR/V617F cells appeared to be more sensitive to Lnk WT than BaF/EpoR/WT cells. These results suggest that the mechanism by which Lnk down-regulates JAK2WT and JAK2V617F may differ and may involve several Lnk domains. In parallel experiments, BaF/EpoR/WT and BaF/EpoR/V617F cells, transduced with the MIG vectors, were cultured with IL-3. In these conditions, Lnk-mediated growth inhibition was considerably lower (Fig. 1B, lower panels). These results strongly suggest that Lnk specifically inhibits signaling by EpoR/JAK2WT and EpoR/JAK2V617F.
Fig. 1.
Lnk inhibits proliferation of hematopoietic cells expressing JAK2V617F. (A) Western blot analysis of JAK2 total protein and phosphorylation levels in the Ba/F3-EpoR cells stably expressing JAK2WT (BaF/EpoR/WT) or JAK2V617F (BaF/EpoR/V617F). Cells were depleted of cytokines for 16 h. NT, Nontransfected Ba/F3-EpoR cells. (B) BaF/EpoR/WT and BaF/EpoR/V617F cells were transduced with MSCV-IRES-GFP (MIG) empty vector (EV), MIG WT Lnk (LnkWT), or MIG SH2 mutant Lnk (LnkRE). Two days later (Day 0), the percent of transfected cells was analyzed by measuring GFP (considered as 100%). Subsequently, GFP expression was measured and calculated relative to that of Day 0. Epo (10 U/ml) and IL-3 (10 ng/ml) were added as indicted. Results are representative of three independent experiments. (C and D) BaF/EpoR/WT and BaF/EpoR/V617F cells were transduced with the MIG vectors and cultured for 2 days with (BaF/EpoR/WT) or without Epo (BaF/EpoR/V617F), after which, both cell lines were incubated without Epo for 16 h. FACS-sorted GFP-positive cells were lysed. (C) Levels of pJAK2 were analyzed by immunoprecipitation (IP) with pTyr antibody, followed by Western blot (WB) with pJAK2 antibody. Levels of total JAK2 and Lnk were analyzed by Western blot. (D) Western blot analysis of phosphorylation and total levels of the indicated proteins. Experiments were repeated three times with similar results.
Expression of JAK2V617F results in constitutive pTyr of JAK2, as well as downstream signaling molecules such as STAT5, AKT, and ERK1/2 MAPK. As expected, in the absence of cytokines, these proteins were strongly phosphorylated in BaF/EpoR/V617F cells, whereas only background phosphorylation of JAK2 and no phosphorylation of STAT5, AKT, and ERK1/2 MAPK were detected in cytokine-depleted BaF/EpoR/WT cells. Expression of Lnk WT in BaF/EpoR/V617F cells decreased the phosphorylation of JAK2, STAT5, AKT, and ERK1/2 MAPK, and a mutation in the Lnk SH2 domain greatly impaired Lnk-meditated inhibition (Fig. 1, C and D). Together, our results demonstrate that Lnk is a strong inhibitor of JAK2V617F-mediated signaling and transformation. Furthermore, the Lnk SH2 domain is critical for its inhibitory function, although disruption of this domain may have a greater effect on JAK2WT compared with JAK2V617F.
The SH2 and PH domains of Lnk associate with JAK2WT and JAK2V617F
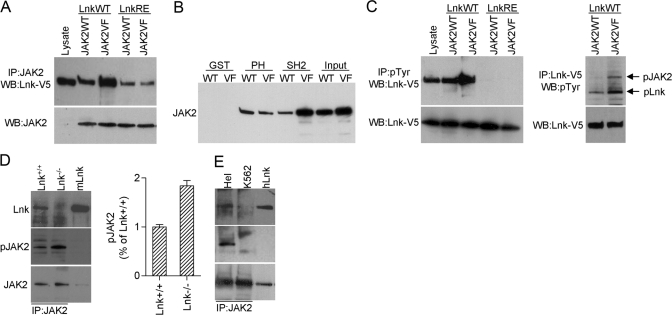
Lnk and its family members SH2-B and APS bind directly to pTyr 813 (Y813) in JAK2 through their SH2 domains [33, 34]. Likewise, Tong and co-workers [35] showed recently that Lnk associates with JAK2V617F, although the protein domains facilitating this interaction were not determined. We examined whether the Lnk SH2 domain is also involved in Lnk-JAK2V617F interaction. V5-tagged Lnk WT or V5-tagged Lnk R392E, which lacks a functional SH2 domain, and JAK2WT or JAK2V617F were expressed in 293T cells, and protein lysates were immunoprecipitated with a JAK2 antibody. Western blot analysis with a V5 antibody showed that Lnk is present in JAK2WT and JAK2V617F immunocomplexes (Fig. 2A). Lnk R392E also associated with JAK2WT and JAK2V617F, although the interaction was much weaker compared with Lnk WT. The interaction between Lnk and JAK2V617F was stronger compared with that of Lnk and JAK2WT. In contrast, Lnk R392E bound to JAK2WT and JAK2V617 with similar affinities, suggesting that this weaker binding is to nonphosphorylated amino acids in JAK2. These results contrast with the Tong et al. study [35], showing that the Lnk SH2 domain mutant does not bind JAK2WT. The reason for this discrepancy is unclear; one possibility is that JAK2 and Lnk expression levels were higher in our system, allowing detection of weaker interactions.
Fig. 2.
Lnk interacts with JAK2WT and JAK2V617F. (A–C) 293T cells were cotransfected with combinations of JAKWT (JAK2WT), JAK2V617F (JAK2VF), WT Lnk-V5 (LnkWT), or SH2 mutant Lnk-V5 (LnkRE) as indicated. Lysates were immunoprecipitated with JAK2, pTyr, or V5 antibodies and analyzed by Western blot as indicated (A and C, upper panels). JAK2 and Lnk levels in the lysates were analyzed by Western blot with JAK2 (A) and V5 (C) antibodies (lower panels). Lysate from 293T-Lnk-V5-transfected cells was used as control for molecular size (A and C, lysate, first lane). (B) Lysates from 293T cells transfected with JAK2WT or JAK2V617F were incubated with GST, GST-Lnk PH (PH), or GST-Lnk-SH2 (SH2) fusion proteins. GST-protein complexes were analyzed by Western blot with JAK2 antibody. Input represents one-tenth of the lysate used for the pull-downs. (D) BM cells from Lnk+/+ and Lnk−/− mice were starved in RPMI 1640 for 4 h and stimulated with 20 ng/ml SCF, 10 U/ml Epo, and 20 ng/ml Tpo for 30 min. Lysates were immunoprecipitated with JAK2 antibody. Bar graph shows the mean ± sd of pJAK2 from two experiments. Results are expressed as a relative percentage compared with pJAK2 in Lnk+/+ cells. (E) Lysates from Hel and K562 cells were immunoprecipitated with JAK2 antibody. (E and D) Blots were probed with the indicated antibodies. Lysates from 293T Lnk-transfected cells were used to control for molecular size. mLnk, Murine Lnk; hLnk, human Lnk. Shown are representative results from two independent experiments.
Maximal binding of SH2-B and APS to JAK2 requires pJAK2 and the SH2 domain of SH2-B/APS. However, regions outside of the SH2 domains of SH2-B/APS, including the PH domain and the region between the PH and SH2 domains, interact at lower affinity with non-pJAK2 [34, 36]. Our results suggest that similar interactions mediate the binding of Lnk to JAK2. GST pull-down assays were used to study further the interaction of JAKWT and JAKV617F with Lnk PH and SH2 domains. Protein lysates from 293T cells transfected with JAK2WT or JAKV617F were incubated with GST-Lnk PH and GST-Lnk SH2 fusion proteins. A weak interaction was detected between the Lnk PH domain and JAK2WT and JAK2V617F (Fig. 2B). A weak interaction was also detected between the JAK2WT and Lnk SH2 domain, and JAK2V617F bound strongly to the Lnk SH2 domain. As JAK2V617F, but not JAK2WT, is highly phosphorylated in 293T cells (ref. [6] and Fig. 2C), these results suggest that similar to SH2-B and APS, the Lnk SH2 domain binds strongly to pJAK2, and the PH and possibly other Lnk domain(s) facilitate weaker phosphorylation-independent interactions.
Lnk was shown to be phosphorylated by JAK2 in response to Tpo stimulation [35], and we next wished to determine if Lnk is also a substrate for the constitutively active JAK2V617F. Protein lysates from 293T cells cotransfected with Lnk-V5 and JAK2WT or JAK2V617F were immuoprecipitated with an anti-pTyr antibody, followed by Western blot with V5 antibody. Results showed more pLnk protein in pTyr precipitates from cells expressing JAK2V617F compared with cells expressing JAK2WT (Fig. 2C, left panel). Hence, more pLnk binds to pTyr proteins (including JAK2) in JAK2V617F-expressing cells compared with JAK2WT-expressing cells. pLnk was abolished in the Lnk R392E mutant, showing that the SH2 domain is required for its phosphorylation by JAK2WT and JAK2V617F. Reciprocal immuoprecipitations with V5 antibody (to precipitate Lnk) followed by Western blot with pTyr antibody showed that the phosphorylation levels of JAK2 and Lnk are higher in 293T cells expressing JAK2V617F compared with cells expressing JAK2WT (Fig. 2C, right panel). These results strongly suggest that Lnk is a JAK2V617F substrate.
To examine whether the Lnk–JAK2 interaction is physiologically relevant, we preformed immunoprecipitation using primary cells. Murine BM cells from WT (Lnk+/+) and Lnk nullizygous (Lnk−/−) mice cultured in vitro with cytokines for 2 days were starved for 4 h and stimulated with cytokines (SCF, Epo, and Tpo) for 30 min. Protein lysates were immunoprecipitated with a JAK2 antibody followed by Western blotting with Lnk antibody. Lnk was present in JAK2 immunocomplexes in WT cells, and a band corresponding to Lnk was not detected in Lnk-deficient cells (Fig. 2D). Furthermore, following cytokine stimulation, JAK2 phosphorylation levels were approximately twofold higher (average results of two independent experiments) in Lnk−/− cells. Thus, endogenously expressed Lnk interacts with JAK2, and the absence of Lnk results in enhanced activation of JAK2 in BM cells in response to cytokine stimulation. To determine if endogenously expressed Lnk and JAK2V617F interact, we used JAK2V617F-positive Hel cells. Immuoprecipitations with JAK2 antibody demonstrated that Lnk is present in JAK2V617F immunocomplexes (Fig. 2E). On the other hand, in BCR-ABL-positive K562 cells, we could not detect Lnk in JAK2 immunocomplexes.
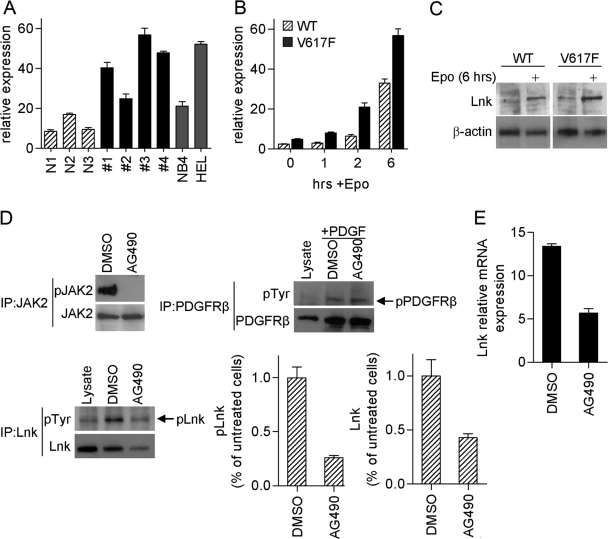
Lnk levels are elevated in MPD patients, and Lnk expression is induced by activated JAK2
To determine whether Lnk is associated with MPD, we measured Lnk expression in CD34+ hematopoietic progenitor BM cells from four MPD patients (two JAK2V617F-negative; two JAK2V617F-positive) and from three healthy donors. Real-time PCR analysis showed that mRNA levels of Lnk are higher in all MPD patient samples compared with control (Fig. 3A). Clearly, these results need to be extended; however, they suggest that Lnk may be highly expressed in some MPD. We speculated that high levels of Lnk in MPD patients may be a result of activated cytokine signaling, a predominant feature of this disease. To explore this possibility further, we measured Lnk expression in the BaF/EpoR/WT and BaF/EpoR/V617F cells, cultured without cytokines for 16 h. Real-time PCR analysis demonstrated that Lnk mRNA levels are indeed higher (approximately twofold, at 0 h, Fig. 3B) in JAK2V617F-expressing cells. Furthermore, Epo stimulation induced Lnk mRNA rapidly in both cell lines, demonstrating that EpoR-JAK2 signaling induces Lnk expression (Fig. 3B). Western blot analysis showed a parallel increase in Lnk protein levels (Fig. 3C).
Fig. 3.
High levels of Lnk are associated with MPD. (A) Lnk expression was measured by real-time PCR in CD34+ hematopoietic progenitor cells from MPD patients (JAK2V617F-negative #1 and #2; JAK2V617F-positive #3 and #4), as well as normal donors (N1–N3) and cell lines NB4 (JAK2WT) and Hel (JAK2V617F). Relative Lnk mRNA levels are expressed in arbitrary units as a ratio of Lnk transcripts:18S transcripts. Data represent the mean ± sd of triplicate samples. (B and C) Real-time PCR (B) and Western blot (C) analysis of Lnk expression in Ba/F3 cells stably expressing EpoR and JAK2WT (WT) or JAK2V617F (V617F). Cells were depleted of cytokines for 16 h and then stimulated with Epo (10 U/ml) for the indicated times. Experiments were repeated three times. (D and E) Hel cells were treated for 4 h with JAK2 inhibitor (AG490, 50 μM) or vehicle alone (DMSO). (D) pJAK2 was analyzed by immunoprecipitation with JAK2 antibody followed by Western blot with pJAK2 antibody; JAK2 protein level was analyzed by Western blot. pLnk was analyzed by immunoprecipitation with pTyr antibody followed by Western blot with Lnk antibody; total Lnk levels were analyzed by Western blot. Bar graphs show the mean ± sd of pLnk and Lnk from three experiments. Results are expressed as a relative percentage compared with untreated cells. As a negative control, pPDGFRβ was analyzed in cells stimulated with PDGF-BB (20 ng/ml) for the last 5 min of AG490/DMSO treatment. Lysates from 293T Lnk- and PDGFRβ-transfected cells were used to control for molecular size. (E) Lnk mRNA levels were measured by real-time PCR. Relative Lnk mRNA levels are expressed in arbitrary units as a ratio of Lnk transcripts:18S transcripts. Data represent the mean ± sd of triplicate samples. Shown are representative results from three experiments.
To examine further the effect of JAK2V617F on Lnk, we used a JAK2 inhibitor AG490 to inhibit JAK2V617F in Hel cells (Fig. 3, D and E). Treatment with the JAK2 inhibitor abolished pJAK2 completely (Fig. 3D, upper left panel). pLnk, as well as total protein and mRNA levels, sharply decreased, although some pLnk was still detected (Fig. 3D, lower panels). These results suggest that inhibition of JAK2V617F attenuates Lnk expression and pLnk; however, other signaling pathways likely also contribute to Lnk regulation. PDGFRβ served as a negative control, and as expected, its phosphorylation levels did not change in AG490-treated cells (Fig. 3D, upper right panel). Together, our results demonstrate that endogenous Lnk is activated by cytokine-stimulated EpoR-JAK2 and by constitutively active JAK2V617F.
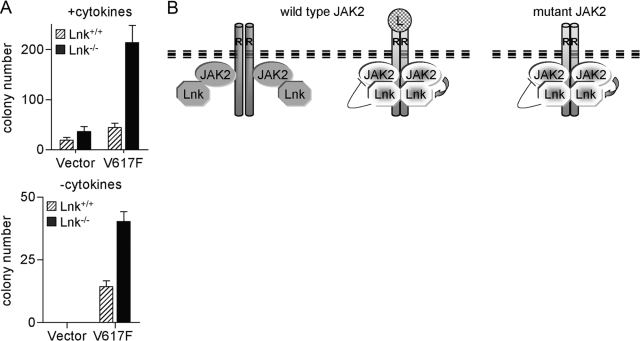
Lnk inhibits transformation by JAK2V617F in primary murine myeloid cells
The above findings suggest that the constitutive JAK2 signaling in MPD induces Lnk expression, which in turn, feeds back to attenuate the signaling itself. To determine whether Lnk can inhibit the transforming potential of mutant JAK2, we compared the ability of JAK2V617F to transform primary myeloid cells from BM of WT and Lnk nullizygous mice. Isolated BM cells were infected with MIG or MIG-JAK2V617F vectors and plated in methylcellulose. Previous reports demonstrated that expression of JAK2V617F in BM cells stimulated clonogenic growth and cytokine hypersensitivity [9,10,11,12]. Aligned with these data, in the presence of cytokines, JAK2V617F leads to an increase in total myeloerythroid colonies [CFU-erythroid (E), burst-forming unit (BFU)-E, CFU-M, CFU-GM, and CFU-G) in progenitors derived from Lnk+/+ and Lnk−/− mice (approximately two- and sixfold, respectively, Fig. 4). However, the increase was significantly higher in Lnk−/− cells (P<0.01). Similarly, although BM cells from either genotype were transformed by JAK2V617F, leading to cytokine-independent growth (mainly CFU-E), the Lnk−/− cells produced a higher (approximately threefold) number of colonies (P<0.01). Consistent with previous work [18,19,20,21, 23], the Lnk−/− BM cells formed more hematopoietic colonies than the WT cells. These data demonstrate that the absence of Lnk leads to a marked increased sensitivity of BM progenitors to JAK2V617F, indicating that mutant JAK2 signaling is under negative regulation by endogenously expressed Lnk.
Fig. 4.
Lnk inhibits JAK2V617F-induced transformation in primary murine cells. (A) BM cells from Lnk+/+ and Lnk−/− mice (three of each) were transduced with MIG vector alone or MIG-JAK2V617F and sorted for GFP expression. Methylcellulose CFU assays were performed in the absence or presence of cytokines. Total colony numbers (including CFU-E, BFU-E, CFU-M, CFU-GM, and CFU-G) were counted on Day 6 (with cytokines) and Day 12 (without cytokines). Data represent the mean ± sd of triplicate samples and are representative of two experiments. (B) A model showing Lnk interactions with WT and mutant JAK2. In the absence of ligand (L), Lnk interacts weakly with non-pWT JAK2. Following ligand stimulation, activated JAK2 induces signaling by phosphorylating downstream positive and negative regulators, including Lnk. Consequently, Lnk strongly binds to pTyr in JAK2 and the receptor (R), thereby attenuating the activated receptor/JAK2 complex. In cells expressing mutant JAK2, downstream positive and negative regulators are constitutively activated, leading to an overall enhanced signaling. Dark gray shapes, Nonphosphorylated proteins; bright shapes, phosphorylated proteins.
DISCUSSION
In the present study, we show that key transforming signals emanating from mutant JAK2V617F are under negative regulation by Lnk, a protein known to be required for normal hematopoiesis. Although Lnk has been shown to down-regulate JAK2 activity, Lnk family members, SH2-B and APS, have been described as activators and inhibitors of JAK2 in a variety of signaling pathways in different tissues [27,28,29,30]. Structure-function studies suggested one model, in which different regions within SH2-B/APS facilitate these conflicting functions; their SH2 domain stimulates, although their PH domain and other regions inhibit, JAK2 [34, 36]. Other studies proposed that the mechanism of JAK2 regulation by SH2-B/APS involves formation of complexes containing dimerized SH2-B/APS and JAK2 proteins, leading to JAK2 transphosphorylation [37, 38]. Our results suggest that similar to SH2-B/APS, Lnk strongly binds through its SH2 domain to pJAK2WT and pJAK2V617F, whereas other Lnk regions facilitate weaker interactions with non-pJAK2. Recently, Bersenev et al. [35] identified pY813 and pY613 in JAK2 as the major and minor binding sites for the Lnk SH2 domain, respectively. In that study, no other Lnk regions were shown to bind JAK2. Additional studies will aim to identify the site(s) within JAK2 that interact with additional regions of Lnk. Further in vitro experiments will also examine the effect of different Lnk regions on JAK2 activity. Whether binding of the recombinant Lnk SH2 domain can stimulate (such as SH2-B and APS SH2 domains, which share, respectively, 68% and 65% identity with the Lnk SH2 domain) or inhibit pJAK2 is another question that will be addressed in future in vitro studies. Regardless of the mechanism, inhibition of JAK2WT and JAK2V617F by Lnk appears to be one of its physiologically relevant functions, at least in hematopoietic cells.
It has been suggested that the conformation of activated JAK2V617F is different than that of JAK2WT [15, 39]. We found that although the SH2 domain is essential for Lnk regulation of JAK2WT and JAK2V617F, specific domains of Lnk may have different effects on the mutant verses WT proteins. A recent study showed that a point mutation at Y613 (Y613E) of JAK2, a minor site for Lnk SH2 domain–JAK2 interaction, led to constitutive activation of JAK2 when EpoR was present [40]. Given the close proximity of Y613 and V617, we speculate that the differential Lnk regulation of JAK2WT and V617F is mediated through binding of Lnk to Y613. Addressing this issue may have therapeutic value, as one challenge facing development of JAK2 inhibitors is obtaining an inhibitor with preferential activity against mutant rather than WT JAK2.
The molecular basis of how Lnk modulates JAK2 is currently unknown. A conserved tyrosine in the C terminus of Lnk, SH2-B, and APS has been identified as a c-Cbl recruitment site for APS [41]. Indeed, APS regulates a number of signaling pathways, including those of PDGF [42], insulin [43, 44], c-Kit [45], and JAK-STAT [28], in collaboration with c-Cbl. However, this site is important, but not required, for the negative regulation by Lnk of Epo-dependent erythroblast survival [21] and is actually dispensable for the Lnk inhibition of lymphocyte development [45] and MPL signaling in megakaryocytes [20]. Lnk also does not appear to inhibit expression or alter cellular localization of its targets [20, 21, 23, 35]. Several possible mechanisms by which Lnk may attenuate JAK2 activity include competition with JAK2-positive regulators, including SH2-B, which like Lnk, strongly binds to JAK2 Y813; Lnk may recruit other JAK2-negative regulators such as suppressor of cytokine signaling (SOCS) proteins; and binding of Lnk to activated JAK2 may promote a conformational change within JAK2, resulting in inactivation of its kinase activity.
JAK2V617F is a weak gain-of-function mutation, only changing the basal activation but not other biological properties of JAK2 [13,14,15,16, 39]. The mutation is located in the JH2 pseudo-kinase autoinhibitory domain of JAK2 and is thought to disrupt JH2-mediated inhibition of the kinase domain JH1, resulting in constitutive enzymatic activity. In addition, a recent study demonstrated that JAK2V617F escapes negative regulation by SOCS3, a known inhibitor of JAK2 [46]. Thus, although some normal inhibitory mechanisms, e.g., JH2 and SOCS3, are unable to attenuate JAK2V617F, we show that other negative regulators such as Lnk retain the capacity to restrain its activity.
An additional, intriguing finding from our study is that JAK2V617F positively regulates Lnk expression and that Lnk expression may be high in some MPD patients. Likewise, we found that Lnk levels are high in acute myeloid leukemia and myelodysplastic syndrome patient samples (unpublished data). Although a role for Lnk in MPD pathogenesis is intuitive [18], a correlation between high levels of Lnk and MPD appears paradoxical; as we have shown, the proliferation of leukemic cells transformed by JAK2V617F is suppressed by Lnk, and Lnk−/− progenitors are more sensitive to transformation by mutant JAK2. The ultimate result of activating tyrosine kinase mutations is malignant transformation. However, studies have strongly suggested that deregulated oncogenes induce a multifaceted negative-feedback response that attenuates the oncogenic signal itself [47, 48]. Our results suggest that Lnk may function in such a negative-feedback loop: Lnk expression is induced by constitutively active JAK2; subsequently, Lnk inhibits the oncogenic JAK2 signaling (Fig. 4B). These data support the hypothesis wherein malignant transformation is not merely the consequence of hyperactivation of growth-promoting genes but actually reflects a shift in the balance between proproliferative and antiproliferative genes, and the net result is an increase in the risk for initiation and progression of cancer. Furthermore, “oncogene addiction” may in part be explained by these observations; therapeutically suppressing mutant JAK2 results in unopposed growth inhibition by the elevated levels of Lnk.
By targeting some of the major cytokine receptor signaling pathways (i.e., c-Kit MPL and EpoR), Lnk plays critical, nonredundant roles in hematopoietic cells. Lnk has not been associated previously with hematopoietic malignancies. However, Lnk−/− mice show profound perturbation in hematopoiesis and cytokine signaling, hinting that Lnk might be implicated in hematopoietic-proliferative disorders. Our work suggests that Lnk functions as a physiological relevant inhibitor of JAK2V617F.
Acknowledgments
This work was supported by National Institutes of Health grants, University of California Los Angeles (UCLA) Cancer Gene Medicine Training Grant, and also, in part, the Parker Hughes Trust, the Inger Foundation, the Mary Barry Foundation, and the Deutsche Krebshilfe Foundation. H. P. K. is a member of the UCLA Jonsson Comprehensive Cancer Center and holds the endowed Mark Goodson Chair of Oncology Research at Cedars-Sinai Medical Center/UCLA School of Medicine. S. Gueller holds the Tower Cancer Research Foundation Fellowship.
References
- Parganas E, Wang D, Stravopodis D, Topham D J, Marine J C, Teglund S, Vanin E F, Bodner S, Colamonici O R, van Deursen J M, Grosveld G, Ihle J N. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- Campbell P J, Green A R. The myeloproliferative disorders. N Engl J Med. 2006;355:2452–2466. doi: 10.1056/NEJMra063728. [DOI] [PubMed] [Google Scholar]
- Levine R L, Pardanani A, Tefferi A, Gilliland D G. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer. 2007;7:673–683. doi: 10.1038/nrc2210. [DOI] [PubMed] [Google Scholar]
- Baxter E J, Scott L M, Campbell P J, East C, Fourouclas N, Swanton S, Vassiliou G S, Bench A J, Boyd E M, Curtin N, Scott M A, Erber W N, Green A R. Cancer Genome Project Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- James C, Ugo V, Le Couedic J P, Staerk J, Delhommeau F, Lacout C, Garcon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval J L, Constantinescu S N, Casadevall N, Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signaling causes polycythemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- Levine R L, Wadleigh M, Cools J, Ebert B L, Wernig G, Huntly B J, Boggon T J, Wlodarska I, Clark J J, Moore S, Adelsperger J, Koo S, Lee J C, Gabriel S, Mercher T, D'Andrea A, Fröhling S, Döhner K, Marynen P, Vandenberghe P, Mesa R A, Tefferi A, Griffin J D, Eck M J, Sellers W R, Meyerson M, Golub T R, Lee S J, Gilliland D G. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Kralovics R, Passamonti F, Buser A S, Teo S S, Tiedt R, Passweg J R, Tichelli A, Cazzola M, Skoda R C. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- Zhao R, Xing S, Li Z, Fu X, Li Q, Krantz S B, Zhao Z J. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280:22788–22792. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacout C, Pisani D F, Tulliez M, Gachelin F M, Vainchenker W, Villeval J L. JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood. 2006;108:1652–1660. doi: 10.1182/blood-2006-02-002030. [DOI] [PubMed] [Google Scholar]
- Zaleskas V M, Krause D S, Lazarides K, Patel N, Hu Y, Li S, Van Etten R A. Molecular pathogenesis and therapy of polycythemia induced in mice by JAK2 V617F. PLoS ONE. 2006;1:e18. doi: 10.1371/journal.pone.0000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig G, Mercher T, Okabe R, Levine R L, Lee B H, Gilliland D G. Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood. 2006;107:4274–4281. doi: 10.1182/blood-2005-12-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedt R, Hao-Shen H, Sobas M A, Looser R, Dirnhofer S, Schwaller J, Skoda R C. Ratio of mutant JAK2–V617F to wild type JAK2 determines the MPD phenotypes in transgenic mice. Blood. 2008;111:3931–3940. doi: 10.1182/blood-2007-08-107748. [DOI] [PubMed] [Google Scholar]
- Lu X, Levine R, Tong W, Wernig G, Pikman Y, Zarnegar S, Gilliland D G, Lodish H. Expression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation. Proc Natl Acad Sci USA. 2005;102:18962–18967. doi: 10.1073/pnas.0509714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig G, Gonneville J R, Crowley B J, Rodrigues M S, Reddy M M, Hudon H E, Walz C, Reiter A, Podar K, Royer Y, Constantinescu S N, Tomasson M H, Griffin J D, Gilliland D G, Sattler M. The Jak2V617F oncogene associated with myeloproliferative diseases requires a functional FERM domain for transformation and for expression of the Myc and Pim proto-oncogenes. Blood. 2008;111:3751–3759. doi: 10.1182/blood-2007-07-102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Huang L J, Lodish H F. Dimerization by a cytokine receptor is necessary for constitutive activation of JAK2V617F. J Biol Chem. 2008;283:5258–5266. doi: 10.1074/jbc.M707125200. [DOI] [PubMed] [Google Scholar]
- Ihle J N, Gilliland D G. Jak2: normal function and role in hematopoietic disorders. Curr Opin Genet Dev. 2007;17:8–14. doi: 10.1016/j.gde.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Rudd C E. Lnk adaptor: novel negative regulator of B cell lymphopoiesis. Sci STKE. 2001;2001:PE1. doi: 10.1126/stke.2001.85.pe1. [DOI] [PubMed] [Google Scholar]
- Velazquez L, Cheng A M, Fleming H E, Furlonger C, Vesely S, Bernstein A, Paige C J, Pawson T. Cytokine signaling and hematopoietic homeostasis are disrupted in Lnk-deficient mice. J Exp Med. 2002;195:1599–1611. doi: 10.1084/jem.20011883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki S, Morita H, Tezuka Y, Takatsu K. Enhanced hematopoiesis by hematopoietic progenitor cells lacking intracellular adaptor protein, Lnk. J Exp Med. 2002;195:151–160. doi: 10.1084/jem.20011170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W, Lodish H F. Lnk inhibits Tpo-mpl signaling and Tpo-mediated megakaryocytopoiesis. J Exp Med. 2004;200:569–580. doi: 10.1084/jem.20040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W, Zhang J, Lodish H F. Lnk inhibits erythropoiesis and Epo-dependent JAK2 activation and downstream signaling pathways. Blood. 2005;105:4604–4612. doi: 10.1182/blood-2004-10-4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buza-Vidas N, Antonchuk J, Qian H, Mansson R, Luc S, Zandi S, Anderson K, Takaki S, Nygren J M, Jensen C T, Jacobsen S E. Cytokines regulate postnatal hematopoietic stem cell expansion: opposing roles of thrombopoietin and LNK. Genes Dev. 2006;20:2018–2023. doi: 10.1101/gad.385606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa H, Kubo-Akashi C, Nobuhisa I, Kwon S M, Iseki M, Taga T, Takatsu K, Takaki S. Enhanced engraftment of hematopoietic stem/progenitor cells by the transient inhibition of an adaptor protein, Lnk. Blood. 2006;107:2968–2975. doi: 10.1182/blood-2005-05-2138. [DOI] [PubMed] [Google Scholar]
- Nobuhisa I, Takizawa M, Takaki S, Inoue H, Okita K, Ueno M, Takatsu K, Taga T. Regulation of hematopoietic development in the aorta-gonad-mesonephros region mediated by Lnk adaptor protein. Mol Cell Biol. 2003;23:8486–8494. doi: 10.1128/MCB.23.23.8486-8494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Dondi E, Chaix A, de Sepulveda P, Kubiseski T J, Varin-Blank N, Velazquez L. Lnk adaptor protein down-regulates specific Kit-induced signaling pathways in primary mast cells. Blood. 2008;112:4039–4047. doi: 10.1182/blood-2008-05-154849. [DOI] [PubMed] [Google Scholar]
- Gery S, Gueller S, Chumakova K, Kawamata N, Liu L, Koeffler H P. Adaptor protein Lnk negatively regulates the mutant MPL, MPLW515L associated with myeloproliferative disorders. Blood. 2007;110:3360–3364. doi: 10.1182/blood-2007-05-089326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui L, Mathews L S, Hotta K, Gustafson T A, Carter-Su C. Identification of SH2-Bβ as a substrate of the tyrosine kinase JAK2 involved in growth hormone signaling. Mol Cell Biol. 1997;17:6633–6644. doi: 10.1128/mcb.17.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakioka T, Sasaki A, Mitsui K, Yokouchi M, Inoue A, Komiya S, Yoshimura A. APS, an adaptor protein containing pleckstrin homology (PH) and Src homology-2 (SH2) domains inhibits the JAK-STAT pathway in collaboration with c-Cbl. Leukemia. 1999;13:760–767. doi: 10.1038/sj.leu.2401397. [DOI] [PubMed] [Google Scholar]
- Ren D, Zhou Y, Morris D, Li M, Li Z, Rui L. Neuronal SH2B1 is essential for controlling energy and glucose homeostasis. J Clin Invest. 2007;117:397–406. doi: 10.1172/JCI29417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maures T J, Kurzer J H, Carter-Su C. SH2B1 (SH2-B) and JAK2: a multifunctional adaptor protein and kinase made for each other. Trends Endocrinol Metab. 2007;18:38–45. doi: 10.1016/j.tem.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- D'Andrea A D, Lodish H F, Wong G G. Expression cloning of the murine erythropoietin receptor. Cell. 1989;57:277–285. doi: 10.1016/0092-8674(89)90965-3. [DOI] [PubMed] [Google Scholar]
- Kurzer J H, Argetsinger L S, Zhou Y J, Kouadio J L, O'Shea J J, Carter-Su C. Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-B β. Mol Cell Biol. 2004;24:4557–4570. doi: 10.1128/MCB.24.10.4557-4570.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzer J H, Saharinen P, Silvennoinen O, Carter-Su C. Binding of SH2-B family members within a potential negative regulatory region maintains JAK2 in an active state. Mol Cell Biol. 2006;26:6381–6394. doi: 10.1128/MCB.00570-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersenev A, Wu C, Balcerek J, Tong W. Lnk controls mouse hematopoietic stem cell self-renewal and quiescence through direct interactions with JAK2. J Clin Invest. 2008;118:2832–2844. doi: 10.1172/JCI35808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui L, Gunter D R, Herrington J, Carter-Su C. Differential binding to and regulation of JAK2 by the SH2 domain and N-terminal region of SH2-Bβ. Mol Cell Biol. 2000;20:3168–3177. doi: 10.1128/mcb.20.9.3168-3177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhe-Paganon S, Werner E D, Nishi M, Hansen L, Chi Y I, Shoelson S E. A phenylalanine zipper mediates APS dimerization. Nat Struct Mol Biol. 2004;11:968–974. doi: 10.1038/nsmb829. [DOI] [PubMed] [Google Scholar]
- Nishi M, Werner E D, Oh B C, Frantz J D, Dhe-Paganon S, Hansen L, Lee J, Shoelson S E. Kinase activation through dimerization by human SH2-B. Mol Cell Biol. 2005;25:2607–2621. doi: 10.1128/MCB.25.7.2607-2621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staerk J, Kallin A, Royer Y, Diaconu C C, Dusa A, Demoulin J B, Vainchenker W, Constantinescu S N. JAK2, the JAK2 V617F mutant and cytokine receptors. Pathol Biol (Paris) 2007;55:88–91. doi: 10.1016/j.patbio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Funakoshi-Tago M, Pelletier S, Moritake H, Parganas E, Ihle J N. Jak2 FERM domain interaction with the erythropoietin receptor regulates Jak2 kinase activity. Mol Cell Biol. 2008;28:1792–1801. doi: 10.1128/MCB.01447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Hubbard S R. Structural characterization of a novel Cbl phosphotyrosine recognition motif in the APS family of adapter proteins. J Biol Chem. 2005;280:18943–18949. doi: 10.1074/jbc.M414157200. [DOI] [PubMed] [Google Scholar]
- Yokouchi M, Wakioka T, Sakamoto H, Yasukawa H, Ohtsuka S, Sasaki A, Ohtsubo M, Valius M, Inoue A, Komiya S, Yoshimura A. APS, an adaptor protein containing PH and SH2 domains, is associated with the PDGF receptor and c-Cbl and inhibits PDGF-induced mitogenesis. Oncogene. 1999;18:759–767. doi: 10.1038/sj.onc.1202326. [DOI] [PubMed] [Google Scholar]
- Hu J, Liu J, Ghirlando R, Saltiel A R, Hubbard S R. Structural basis for recruitment of the adaptor protein APS to the activated insulin receptor. Mol Cell. 2003;12:1379–1389. doi: 10.1016/s1097-2765(03)00487-8. [DOI] [PubMed] [Google Scholar]
- Ahmed Z, Smith B J, Pillay T S. The APS adapter protein couples the insulin receptor to the phosphorylation of c-Cbl and facilitates ligand-stimulated ubiquitination of the insulin receptor. FEBS Lett. 2000;475:31–34. doi: 10.1016/s0014-5793(00)01621-5. [DOI] [PubMed] [Google Scholar]
- Wollberg P, Lennartsson J, Gottfridsson E, Yoshimura A, Rönnstrand L. The adapter protein APS associates with the multifunctional docking sites Tyr-568 and Tyr-936 in c-Kit. Biochem J. 2003;370:1033–1038. doi: 10.1042/BJ20020716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hookham M B, Elliott J, Suessmuth Y, Staerk J, Ward A C, Vainchenker W, Percy M J, McMullin M F, Constantinescu S N, Johnston J A. The myeloproliferative disorder-associated JAK2 V617F mutant escapes negative regulation by suppressor of cytokine signaling 3. Blood. 2007;109:4924–4929. doi: 10.1182/blood-2006-08-039735. [DOI] [PubMed] [Google Scholar]
- Courtois-Cox S, Genther Williams S M, Reczek E E, Johnson B W, McGillicuddy L T, Johannessen C M, Hollstein P E, MacCollin M, Cichowski K. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell. 2006;10:459–472. doi: 10.1016/j.ccr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S V, Gajowniczek P, Way I P, Lee D Y, Jiang J, Yuza Y, Classon M, Haber D A, Settleman J. A common signaling cascade may underlie “addiction” to the Src, BCR-ABL, and EGF receptor oncogenes. Cancer Cell. 2006;10:425–435. doi: 10.1016/j.ccr.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]