Abstract
The dystroglycanopathies comprise a clinically and genetically heterogeneous group of muscular dystrophies characterized by deficient glycosylation of α-dystroglycan. Mutations in the fukutin (FKTN) gene have primarily been identified among patients with classic Fukuyama congenital muscular dystrophy (FCMD), a severe form of dystroglycanopathy characterized by CMD, cobblestone lissencephaly and ocular defects. We describe two brothers of Caucasian and Japanese ancestry with normal intelligence and limb-girdle muscular dystrophy (LGMD) due to compound heterozygous FKTN mutations. Muscle biopsy showed a dystrophy with selectively reduced α-dystroglycan glycoepitope immunostaining. Immunoblots revealed hypoglycosylation of α-dystroglycan and loss of laminin binding. FKTN gene sequencing identified two variants: c.340G>A and c.527T>C, predicting missense mutations p.A114T and p.F176S, respectively. Our results provide further evidence for ethnic and allelic heterogeneity and the presence of milder phenotypes in FKTN-dystroglycanopathy despite a substantial degree of α-dystroglycan hypoglycosylation in skeletal muscle.
Keywords: α-Dystroglycan, Dystroglycanopathy, Fukutin (FKTN), Limb-girdle muscular dystrophy (LGMD), Fukuyama congenital muscular dystrophy (FCMD)
1. Introduction
Multiple forms of muscular dystrophy are now known to be caused by defects in the O-linked glycosylation of α-dystroglycan. These dystroglycanopathies span a spectrum of phenotypes from Walker-Warburg syndrome (WWS; OMIM 236670; characterized by severe congenital muscular dystrophy, retinal and anterior chamber eye abnormalities, cobblestone lissencephaly, and hydrocephalus) to mild, adult onset LGMD [1]. Included in this group of disorders is Fukuyama congenital muscular dystrophy (FCMD; OMIM 253800). Patients with typical FCMD display dystrophic changes in skeletal muscle, structural brain malformations, and severe ocular abnormalities. Most patients are never able to walk independently and have moderate to severe cognitive delay. The average life span is less than 20 years [2,3].
The gene implicated in FCMD, FKTN, located on chromosome 9q31, was identified in 1998 by Kobayashi et al. [4]. A 3 kb retro-transposon insertion mutation in the 3′-untranslated region of this gene, descended from a single ancestor, is responsible for the relatively high prevalence of the disorder in Japan [4]. Among the first cases of FKTN-dystroglycanopathy reported outside of Japan are two Turkish patients, both with homozygous null mutations, who suffered from a severe, WWS-like phenotype with early lethality [5,6].
Recently, milder cases of muscular dystrophy associated with FKTN gene mutations have been reported in both Japanese and non-Japanese populations. In 2006, Murakami et al. reported on six Japanese patients, all of whom were compound heterozygotes for the FKTN founder mutation and a point mutation in this gene, with minimal muscle weakness, normal intellect and dilated cardiomyopathy [7]. Other recent papers by Godfrey et al. reported on five non-Japanese children from three families with normal intelligence and a limb-girdle phenotype, caused by heterozygous point mutations in the FKTN gene [8,9]. We report an additional two brothers with a LGMD phenotype, and describe one previously unreported mutation in the FKTN gene.
2. Patients and methods
2.1. Patients
Two brothers with a history of elevated serum creatine kinase (CK) ranging from 1865 to 12,131 IU/L (nl 41–277 IU/L) and mild muscle weakness from a non-consanguineous family were studied. FKTN mutation analysis was performed following abnormal muscle histopathology and immunofluorescence suggestive of an α-dystroglycan glycosylation abnormality.
2.2. Histopathology and immunohistochemistry
Cryosections of skeletal muscle were stained with hematoxylin and eosin (H&E) and underwent standard immunofluorescence staining using a panel of antibodies directed at muscular dystrophy-associated proteins [10]. Fluorescence microscopy was performed for dystrophin (Developmental Studies Hybridoma Bank, rod domain antibody 6A9), β-dystroglycan (Novocastra), and α-dystroglycan (glycoepitope antibody IIH6) [10]. Immunofluorescence was also performed with the core α-dystroglycan antibody, GT20ADG [11].
2.3. Western blots and laminin overlay
Frozen muscle was homogenized and glycoproteins enriched by WGA according to the protocol published in Michele et al. [11]. Immunoblots were performed with the α-dystroglycan glycoepitope antibody IIH6, the α-dystroglycan core antibody GT20ADG, and the β-dystroglycan antibody AP83. Laminin overlays utilized mouse Engelbreth-Holm-Swarm (EHS) laminin and anti-laminin antibody (Sigma) [11].
2.4. Mutation analysis
Genomic DNA was extracted from whole blood using Puregene reagents (Gentra Systems, Inc., Minneapolis, MN). Amplification of genomic DNA for FKTN sequence analysis was performed in 50 µL reaction volumes containing 1× PCR buffer (Qiagen), 1.5 mM MgCl2, 200 µM dNTPs, 1.0 µM each of FKTN forward and reverse primers, 1 U of taq polymerase (Qiagen) and 150 ng of patient DNA. Cycling parameters were as follows: 5 min at 95 °C; followed by 10 cycles of 30 s at 95 °C, 30 s at 65 °C with decreases of 0.5 °C/cycle, 60 s at 72 °C; followed by 25 cycles of 30 s at 95 °C, 30 s at 60 °C, 60 s at 72 °C; followed by 7 min at 72 °C.
Sequence analysis of FKTN exons 2–10 was performed by cycle sequencing 5 ng of template in 10 µL reactions with Big Dye Terminator 3.1 (Applied Biosystems Inc., Foster City, CA) as recommended by the manufacturer. An ABI3130 instrument and Seqscape software (ABI) was used for analysis. Patient results were compared to reference sequence NM_006731.2 (UCSC Genome Browser, NCBI Build 36.1) to identify sequence variations.
Additionally, each of the five genes known to be associated with α-dystroglycanopathy, FKRP, POMT1, POMT2, POMGnT1, and LARGE, was studied. The coding exons and 50–100 bp of flanking noncoding sequence were amplified and bi-directionally sequenced using standard procedures. The patient’s sequence was then compared to the following reference sequences: NM_024301 (FKRP), NM_007171.2 (POMT1), NM_013382 (POMT2), NM_017739 (POMGnT1), NC_000022.9 (LARGE) (UCSC Genome Browser, NCBI Build 36.1).
3. Results
3.1. Clinical findings
Patient 1 is a boy who was born at full term after an uneventful pregnancy to a healthy mother of Japanese ancestry and healthy father of Caucasian ancestry. His early development was normal. Around 9 months of age, the child’s growth, which had been slightly below the 5th percentile since birth, began to plateau. At 13 months of age, he was seen in the metabolic clinic for failure to thrive and elevated transaminases. His height and weight were both below the 3rd percentile. Head circumference was between the 25th and 50th percentiles. Physical exam was normal, including muscle tone, strength and deep tendon reflexes. Initial workup was remarkable for an elevated CK of 3110 IU/L. ALT and AST were slightly increased at 96 U/L (nl 9–80 U/L) and 77 U/L (nl 1–55 U/L), respectively. An acylcarnitine profile, carnitine levels and urine organic acids were normal. At 17 months of age, the patient’s CK remained elevated (1865 IU/L). Both parents had normal CK levels. Muscle weakness was first noted at 21 months of age and by 24 months, physical exam was remarkable for incipient pectus excavatum, modified Gowers’ maneuver and decreased deep tendon reflexes. There was no evidence of calf hypertrophy and cognitive and motor development continued to be normal. The patient was referred for muscle biopsy. Cardiac and ophthalmology evaluations were normal. Calf hypertrophy was evident by 2 years, 9 months of age. At 5 years of age, the patient’s physical exam was unchanged and his cognitive and social development remained normal. He has been hospitalized once for pneumonia. CK levels have ranged between 1865 and 12,131 IU/L.
Patient 2 is the older brother of patient 1. He was referred at 4 years of age, after his brother’s diagnosis was made. He rolled over at 6 months, sat up at 8 months and walked at 12 months. His initial physical exam revealed subtle abnormalities, including mild hypotonia, decreased muscle strength, calf hypertrophy, partial Gowers’ maneuver and decreased deep tendon reflexes. Head circumference was between the 25th and 50th percentiles. Cardiac and ophthalmology exams were normal. At 4 years, 5 months of age, incipient lumbar lordosis and pectus excavatum were noted. Follow-up, 6 months later, revealed waddling gait. He was last seen at 6 years, 11 months of age and was stable. His gait had improved. CK levels have ranged between 4068 and 9955 U/L. Both brothers receive physical therapy.
3.2. Histopathology
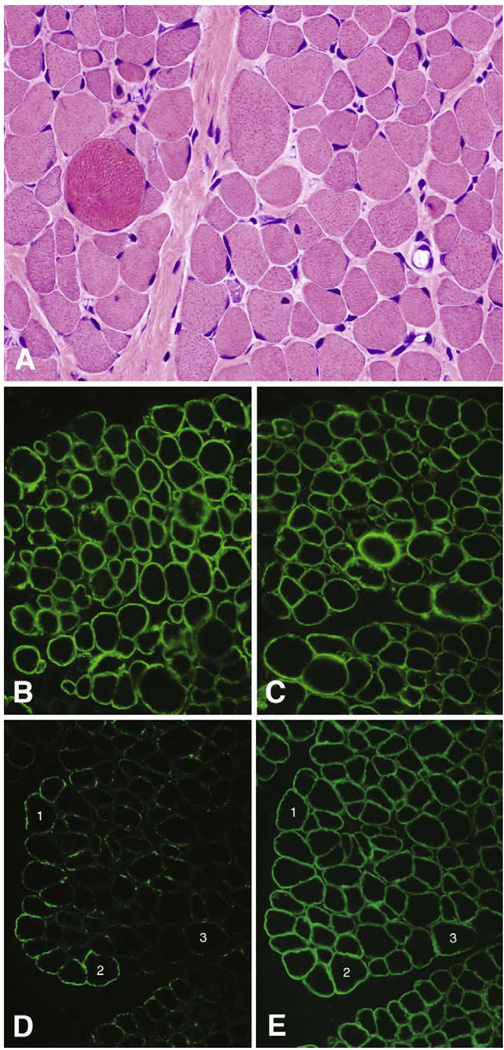
Small numbers of necrotic and regenerating myofibers, sometimes clustered, were identified in the biopsy from patient 1 performed at age 2. There was a marked degree of variation in fiber size due to both atrophic and hypertrophic fibers in an intermixed pattern in association with mild endomysial fibrosis (Fig. 1A). No lymphocytic inflammation was noted.
Fig. 1.
Histology and immunofluorescence. Mildly dystrophic histopathology is evident in the H&E photomicrograph (A); shown here are atrophy, hypertrophy, muscle fiber regeneration, and mild endomysial fibrosis. α-Dystroglycan ranges from negative to nearly normal with the glycoepitope antibody IIH6 (D), while dystrophin (B), α-dystroglycan using the core αDG antibody GT20ADG (C), and β-dystroglycan (E) stain normally. All images photographed with a 40× objective. Numbers in the immunofluorescence panels indicate the same muscle fibers in adjacent cryosections.
3.3. Immunostaining
Immunofluorescence staining showed normal expression of dystrophin (Fig. 1B), utrophin, spectrin, sarcoglycans, merosin, and collagen VI (data not shown). β-Dystroglycan also stained normally (Fig. 1E), while α-dystroglycan staining with glycosylation-dependent antibodies varied in a mosaic pattern from absent to nearly normal (IIH6 is shown in Fig. 1D). Immunofluorescence with the α-dystroglycan core antibody, GT20ADG [11], shows that α-dystroglycan is present at the sarcolemma of all muscle fibers (Fig. 1C).
3.4. Western blots and laminin overlay
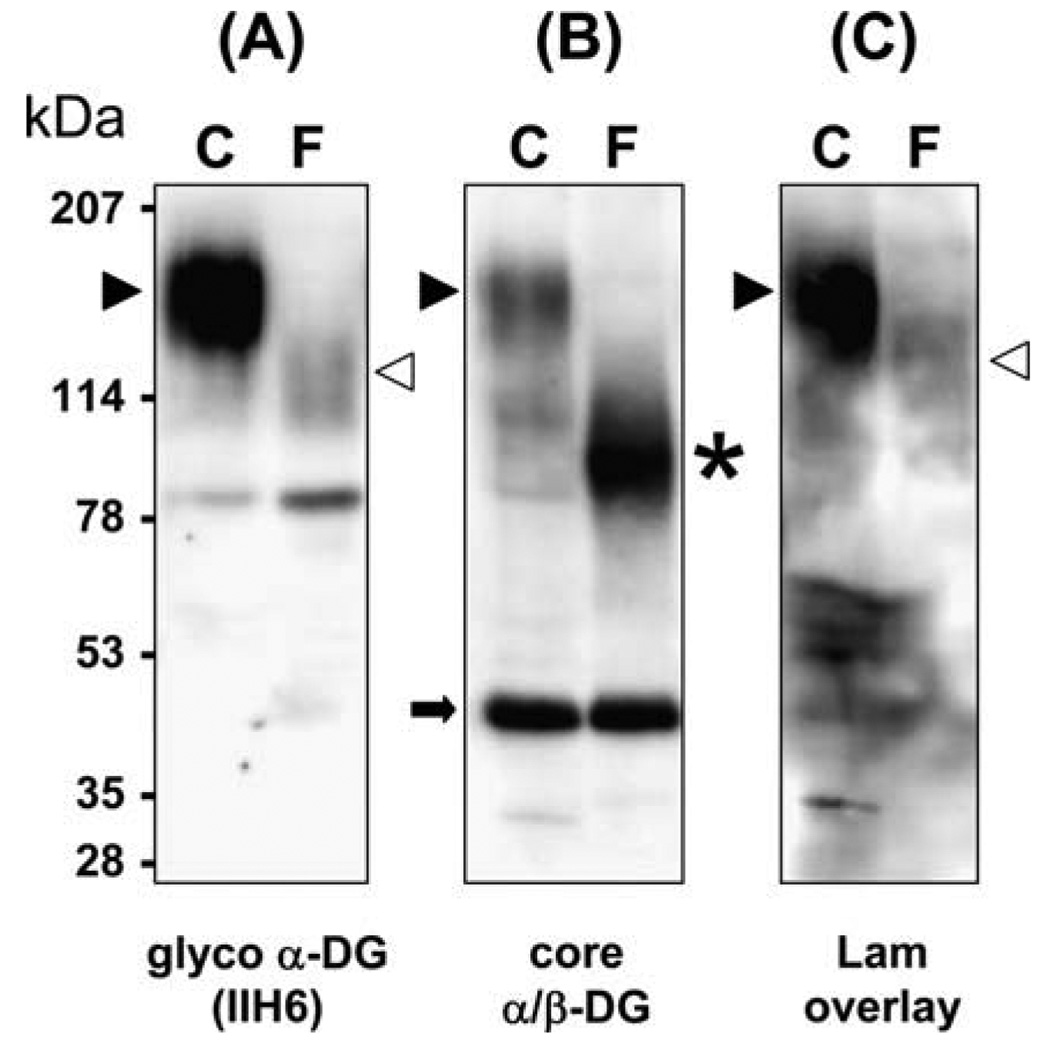
Immunoblots show less fully glycosylated α-dystroglycan in the patient compared to control (Fig. 2A); the small amount of functionally glycosylated α-dystroglycan detected in the patient has a reduced molecular weight compared to control. On the other hand, β-dystroglycan appears normal (Fig. 2B). Lower molecular weight, hypoglycosylated α-dystroglycan is revealed in the patient by the core α-dystroglycan antibody (Fig. 2B). The molecular weight shift to ~90 kDa observed in our patient is similar to that previously reported in MEB, FCMD, and WWS patients [11,12]. The residual laminin binding observed in the overlay assay (Fig. 2C) corresponds to the low binding affinity of α-dystroglycan to IIH6 (Fig. 2A).
Fig. 2.
Western blot (A and B) and laminin overlay (C). Control lanes in each blot are labeled C. Patient lanes are labeled F. Fully glycosylated α-dystroglycan is marked by black arrowheads. The position of partially glycosylated α-dystroglycan is marked by white arrowheads. The asterisk is hypoglycosylated α-dystroglycan detected in the patient by the core antibody. β-Dystroglycan is marked by the black arrow. Compared to the control, the patient’s muscle shows a reduced amount of fully glycosylated α-dystroglycan (A), but normal β-dystroglycan (B). However, the core α-dystroglycan antibody reveals that lower molecular weight α-dystroglycan is present (B). Laminin binding is nearly absent in the laminin (Lam) overlay of the patient’s muscle (C). The molecular weight of the patient’s laminin-bound band in blot C correlates with the α-dystroglycan detected by IIH6 in blot A.
3.5. Mutation analysis
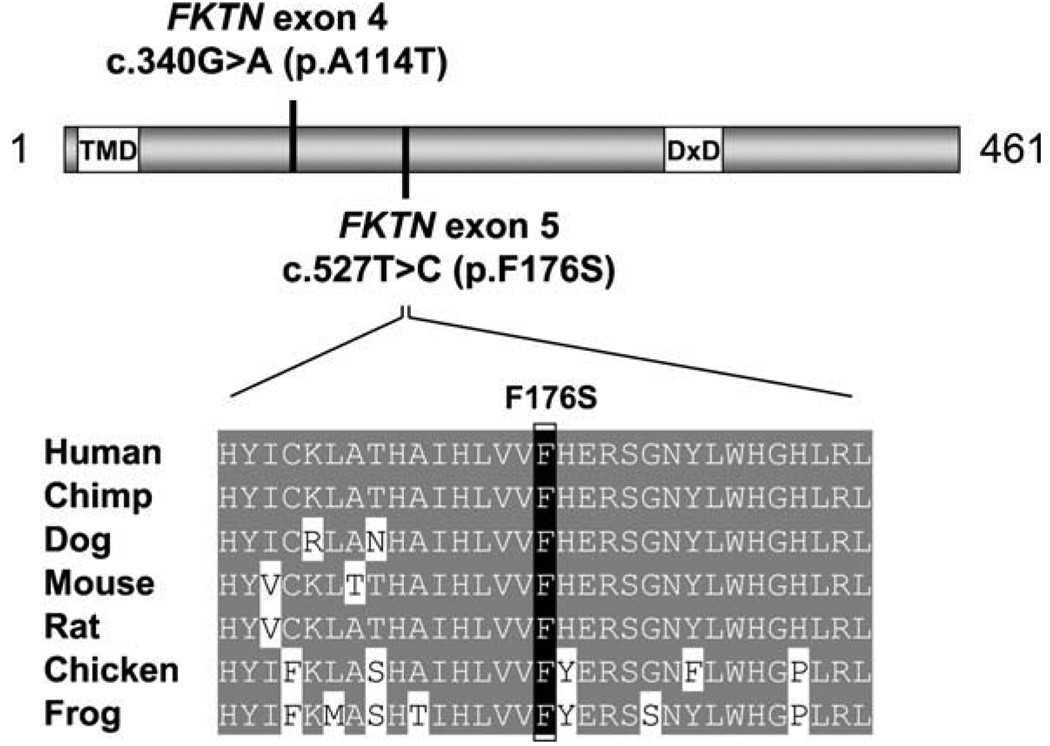
All six genes known to be associated with deficient glycosylation of α-dystroglycan were studied. Due to the relatively frequent occurrence of FKRP mutations among individuals with a LGMD phenotype, sequencing of this gene was performed first in patient 1 with normal results. Given the patient’s Japanese ancestry, mutation analysis for the 3 kb retrotransposon insertion mutation in the FKTN gene was subsequently performed. Despite the fact that this yielded negative results, it was followed by analysis of the coding sequence and intron-exon junctions of the FKTN gene, revealing two heterozygous missense mutations. The c.340G>A mutation, found in exon 4, predicts an alanine (A) to threonine (T) amino acid substitution at position 114 and the exon 5 c.527T>C variant predicts a phenylalanine (F) to serine (S) substitution at residue 176. The c.340G>A mutation has been recently described in a pair of siblings with LGMD and no mental retardation [9], The c.527T>C variant is a novel mutation. This change is predicted to be deleterious based on the fact that it disrupts a highly conserved residue (Fig. 3). Furthermore, a phenylalanine to serine change is not chemically conservative and is usually pathogenic [13]. Mutation analysis of 90 unrelated individuals failed to identify the same variant. It is also not present among any of the 295 patients with FKTN sequence variants described in the Leiden database. Parental studies revealed that the c.340G>A mutation was inherited from the patients’ father and the c.527T>C mutation from their mother. No mutations were detected in POMT1, POMT2, POMGnT1 or LARGE.
Fig. 3.
Novel FKTN mutation. The relative locations of the exon 4 and exon 5 mutations are mapped on a diagram of Fukutin. Additionally, the location of the transmembrane domain (TMD) and the conserved DxD motif are indicated. Alignment of Fukutin amino acid sequences surrounding the novel exon 5 mutation demonstrates conservation of the F176 residue in human, chimpanzee, dog, mouse, rat, chicken, and frog.
4. Discussion
Fukuyama congenital muscular dystrophy (FCMD) is a severe congenital muscular dystrophy occurring predominantly in Japan. Affected children present with generalized hypotonia in infancy. Ambulation is rare and individuals with the disorder are often bed-ridden before they reach adolescence [2]. Severe mental retardation and brain malformations, most frequently cobblestone lissencephaly and cerebellar cysts, are common [2,14,15]. Ophthalmic abnormalities, typically involving the retina, are also frequent [16].
Classic FCMD patients have mutations in the FKTN gene, located on chromosome 9q31 [17]. A 3-kb retrotransposon insertion mutation in the 3′UTR of this gene has been identified in greater than 80% of FCMD chromosomes in the Japanese population [4,18,19]. Due to this founder mutation, FCMD is the second most common muscular dystrophy in Japan after Duchenne Muscular Dystrophy [2]. The 10-exon gene spans over 100 kb of genomic DNA and encodes a novel protein composed of 461 amino acids [20]. Identification of the FKTN gene has allowed for molecular confirmation in patients clinically suspected of having FCMD.
In this report, we describe two brothers of Japanese and Caucasian ancestry with elevated CK, mild muscle weakness and normal cognition who were found to carry two missense mutations in the FKTN gene. The clinical presentation of these patients is significant due to their onset in the toddler period and mild symptoms. Both boys had normal early physical exams followed by slowly progressive muscle involvement beginning between one and a half and 2 years of age. At ages four and six, they remain ambulant. Furthermore, the two boys have normal neurodevelopment and demonstrate age-appropriate cognition. Additionally, they lack any cardiac or ocular abnormalities. It is possible that cardiac involvement may occur over time and therefore, the patients should continue with annual cardiac evaluations including echocardiogram or cardiac MRI. The latter modality of heart imaging may be more sensitive for early detection of cardiac involvement in dystroglycanopathy patients based on the findings of two recent studies involving 22 LGMD2I patients [21,22].
In addition to their mild clinical presentation, our patients are also unique from an ethnic and molecular standpoint. Their father is of European descent and their mother, Japanese. The children, however, do not possess the common Japanese founder mutation. Rather, the brothers have two FKTN missense mutations, one of which, c.527T>C, has not been previously reported. This is significant, as the vast majority of patients reported have been either homozygous or heterozygous for the common retrotransposon insertion.
Due to the wide spectrum of clinical presentations and the lack of consistent genotype-phenotype correlations, a muscle biopsy is frequently required for the diagnosis of patients with elevated CK and muscle weakness, as was performed in this case. The constellation of myopathic features in the muscle of our patient was typical of a muscular dystrophy and the immunophenotype was consistent with an α-dystroglycan glycosylation abnormality.
FCMD is one of a clinically and genetically heterogeneous group of disorders, termed dystroglycanopathies, which are caused by deficient glycosylation of α-dystroglycan. Other disorders in this group include WWS (OMIM 236670), muscle-eye-brain disease (MEB, OMIM 253280), congenital muscular dystrophy type IC (MDCIC, OMIM 606612) and congenital muscular dystrophy type ID (MDCID, OMIM 608840) [23]. α-Dystroglycan is a central component of the dystrophin-glycoprotein complex (DGC), which serves as a high affinity cell surface receptor for laminin. The DGC links the actin cytoskeleton to the laminins and other protein components of the extracellular matrix. It is known to be expressed in skeletal, cardiac and smooth muscle, as well as brain and peripheral nerve [24–26]. Mutations in several genes other than FKTN have been identified among patients with dystroglycanopathies. Specifically, MEB is most often caused by mutations in POMGnT1, WWS is commonly caused by mutations in POMT1 or POMT2, and MDCIC and 1D are defined by mutations in FKRP and LARGE, respectively [24]. As more patients are genetically characterized, it is becoming evident that mutations in any of these six genes may cause any of the clinical phenotypes [9].
A small number of atypically affected patients with FKTN mutations have been reported within the Japanese population. In 2006, Murakami et al. described six patients from four families, all of whom were compound heterozygotes for the common retrotransposon insertion mutation and a missense mutation, presenting with dilated cardiomyopathy [7].
Confirmed cases of FKTN-dystroglycanopathy outside of Japan are rare. In 2003, de Bernabé et al. described a Turkish boy of consanguineous descent who was homozygous for a novel nonsense mutation in the FKTN gene: c.345_346GC → >CT [6]. He presented with a WWS-like phenotype characterized by congenital hypotonia, ocular abnormalities, and hydrocephalus in association with gyral abnormalities and absence of the corpus callosum and died at 4.5 months of age. Another Turkish patient, also with severe brain and eye abnormalities, homozygous for a novel 1 bp insertion mutation in exon 4 of the FKTN gene was reported in 2003 by Silan et al. [5]. A study of fetuses with cobblestone lissencephaly from 41 families in France failed to identify any FKTN mutations [27], while five families from a cohort of 40 with WWS (14 families from the Middle East and 26 families from the USA or Europe) did have mutant FKTN [28]. An additional four USA Ashkenazi Jewish families with WWS were found to have a founder mutation in FKTN [29].
In 2006, a milder phenotype with a variant form of LGMD (LGMD2L) and no brain involvement was described by Godfrey et al. in three patients, one of Israeli origin and the other two, siblings of Jewish and Indian descent, with heterozygous FKTN mutations [8]. In a subsequent paper, Godfrey et al. reported two additional non-Japanese siblings with a similar mild phenotype and a third patient who presented with CMD but no neurological involvement [9]. Despite the milder skeletal muscle phenotype of these patients [8,9] and those reported by Murakami et al. [7], muscle biopsies show a reduction in fully glycosylated α-dystroglycan similar to the patient presented here (Fig. 2) and severe forms of CMD, such as FCMD and MEB [11]. This emphasizes that immunophenotype may correlate poorly with clinical severity as suggested by Jimenez-Mallebrera et al. in an immunohistochemistry study of dystroglycanopathy patients ranging from WWS to LGMD without mental retardation [30].
One of the mutations identified in our family, c.340G>A, was also detected in one of the sibling pairs described by Godfrey et al. [9]. In these siblings, the mutation was inherited heterozygously with a previously unreported frameshift mutation, c.859delA, in exon 7 [9]. These children had a similar age of onset to the siblings described in this paper, reportedly at 4 years of age and 9 months of age. CK levels for the two sibling pairs are also similar. The siblings reported by Godfrey et al. have had levels of 5700 and 9000 IU/L. Our patients’ levels have ranged between 1865 and 12,131 IU/L. The two sets of siblings have comparable motor skills, characterized by the ability to ambulate independently and even run. Calf hypertrophy, which has been documented in our patients, was also been described in one of the siblings reported by Godfrey et al. Mild lumbar lordosis has been documented in the elder of our two patients, but not in the siblings described by Godfrey et al. One of these children, however, was reportedly born with congenital dislocation of the hip. No information is available on the ophthalmologic or cardiac status of the patients described by Godfrey et al. Both sets of siblings have normal intelligence.
This report further confirms FKTN mutations as a cause of LGMD without mental retardation. It is likely that a wide variety of over-lapping phenotypes may be caused by mutations in the FKTN gene, and each of the other genes associated with aberrant glycosylation of α-dystroglycan. Therefore, the diagnosis of a dystroglycanopathy should be considered for all patients with clinically suspected muscular dystrophy, regardless of the presence or absence of additional neurological and/or ocular features.
Acknowledgements
The authors thank Patricia Nienaber for technical assistance in the preparation of anti-α-dystroglycan antibodies and Terese Nelson for technical assistance in performing the immunofluorescence staining of muscle cryosections. This work was funded in part by the Wellstone Muscular Dystrophy Cooperative Research Center Grant NS053672 (T.W., K.P.C., and S.A.M.). The clinical work was supported by the Commission for Families and Children of Orange County.
References
- 1.Martin PT. The dystrogly canopathies: the new disorders of O-linked glycosylation. Semin Pediatr Neurol. 2005;12:152–158. doi: 10.1016/j.spen.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukuyama Y, Osawa M, Suzuki H. Congenital progressive muscular dystrophy of the Fukuyama type – clinical, genetic and pathological considerations. Brain Dev. 1981;3:1–29. doi: 10.1016/s0387-7604(81)80002-2. [DOI] [PubMed] [Google Scholar]
- 3.Saito K, Osawa M, Wang ZP, et al. Haplotype-phenotype correlation in Fukuyama congenital muscular dystrophy. Am J Med Genet. 2000;92:184–190. doi: 10.1002/(sici)1096-8628(20000529)92:3<184::aid-ajmg5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi K, Nakahori Y, Miyake M, et al. An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature. 1998;394:388–392. doi: 10.1038/28653. [DOI] [PubMed] [Google Scholar]
- 5.Silan F, Yoshioka M, Kobayashi K, et al. A new mutation of the fukutin gene in a non-Japanese patient. Ann Neurol. 2003;53:392–396. doi: 10.1002/ana.10491. [DOI] [PubMed] [Google Scholar]
- 6.de Bernabe DB, van Bokhoven H, van Beusekom E, et al. A homozygous nonsense mutation in the fukutin gene causes a Walker-Warburg syndrome phenotype. J Med Genet. 2003;40:845–848. doi: 10.1136/jmg.40.11.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murakami T, Hayashi YK, Noguchi S, et al. Fukutin gene mutations cause dilated cardiomyopathy with minimal muscle weakness. Ann Neurol. 2006;60:597–602. doi: 10.1002/ana.20973. [DOI] [PubMed] [Google Scholar]
- 8.Godfrey C, Escolar D, Brockington M, et al. Fukutin gene mutations in steroid-responsive limb girdle muscular dystrophy. Ann Neurol. 2006;60:603–610. doi: 10.1002/ana.21006. [DOI] [PubMed] [Google Scholar]
- 9.Godfrey C, Clement E, Mein R, et al. Refining genotype phenotype correlations in muscular dystrophies with defective glycosylation of dystroglycan. Brain. 2007;130:2725–2735. doi: 10.1093/brain/awm212. [DOI] [PubMed] [Google Scholar]
- 10.Moore SA, Shilling CJ, Westra S, et al. Limb-girdle muscular dystrophy in the United States. J Neuropathol Exp Neurol. 2006;65:995–1003. doi: 10.1097/01.jnen.0000235854.77716.6c. [DOI] [PubMed] [Google Scholar]
- 11.Michele DE, Barresi R, Kanagawa M, et al. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- 12.Kim DS, Hayashi YK, Matsumoto H, et al. POMT1 mutation results in defective glycosylation and loss of laminin-binding activity in α-DG. Neurology. 2004;62:1009–1011. doi: 10.1212/01.wnl.0000115386.28769.65. [DOI] [PubMed] [Google Scholar]
- 13.Miller MP, Kumar S. Understanding human disease mutations through the use of interspecific genetic variation. Hum Mol Genet. 2001;10:2319–2328. doi: 10.1093/hmg/10.21.2319. [DOI] [PubMed] [Google Scholar]
- 14.Aida N, Yagishita A, Takada K, Katsumata Y. Cerebellar MR in Fukuyama congenital muscular dystrophy: polymicrogyria with cystic lesions. AJNR Am J Neuroradiol. 1994;15:1755–1759. [PMC free article] [PubMed] [Google Scholar]
- 15.Barkovich AJ. Neuroimaging manifestations and classification of congenital muscular dystrophies. AJNR Am J Neuroradiol. 1998;19:1389–1396. [PMC free article] [PubMed] [Google Scholar]
- 16.Hino N, Kobayashi M, Shibata N, Yamamoto T, Saito K, Osawa M. Clinicopathological study on eyes from cases of Fukuyama type congenital muscular dystrophy. Brain Dev. 2001;23:97–107. doi: 10.1016/s0387-7604(01)00189-9. [DOI] [PubMed] [Google Scholar]
- 17.Toda T, Segawa M, Nomura Y, et al. Localization of a gene for Fukuyama type congenital muscular dystrophy to chromosome 9q31–33. Nat Genet. 1993;5:283–286. doi: 10.1038/ng1193-283. [DOI] [PubMed] [Google Scholar]
- 18.Toda T, Ikegawa S, Okui K, et al. Refined mapping of a gene responsible for Fukuyama-type congenital muscular dystrophy: evidence for strong linkage disequilibrium. Am J Hum Genet. 1994;55:946–950. [PMC free article] [PubMed] [Google Scholar]
- 19.Toda T, Miyake M, Kobayashi K, et al. Linkage-disequilibrium mapping narrows the Fukuyama-type congenital muscular dystrophy (FCMD) candidate region to < 100 kb. Am J Hum Genet. 1996;59:1313–1320. [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi K, Sasaki J, Kondo-Iida E, et al. Structural organization, complete genomic sequences and mutational analyses of the Fukuyama-type congenital muscular dystrophy gene, fukutin. FEBS Lett. 2001;489:192–196. doi: 10.1016/s0014-5793(01)02088-9. [DOI] [PubMed] [Google Scholar]
- 21.Gaul C, Deschauer M, Tempelmann C, et al. Cardiac involvement in limb-girdle muscular dystrophy 2I: conventional cardiac diagnostic and cardiovascular magnetic resonance. J Neurol. 2006;253:1317–1322. doi: 10.1007/s00415-006-0213-0. [DOI] [PubMed] [Google Scholar]
- 22.Wahbi K, Meune C, Hamouda EH, et al. Cardiac assessment of limb-girdle muscular dystrophy 2I patients: an echocardiography, Holter ECG and magnetic resonance imaging study. Neuromuscul Dis. 2008;18:650–655. doi: 10.1016/j.nmd.2008.06.365. [DOI] [PubMed] [Google Scholar]
- 23.Longman C, Brockington M, Torelli S, et al. Mutations in the human LARGE gene cause MDC1D, a novel form of congenital muscular dystrophy with severe mental retardation and abnormal glycosylation of alpha-dystroglycan. Hum Mol Genet. 2003;12:2853–2861. doi: 10.1093/hmg/ddg307. [DOI] [PubMed] [Google Scholar]
- 24.Kanagawa M, Toda T. The genetic and molecular basis of muscular dystrophy: roles of cell-matrix linkage in the pathogenesis. J Hum Genet. 2006;51:915–926. doi: 10.1007/s10038-006-0056-7. [DOI] [PubMed] [Google Scholar]
- 25.Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119:199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- 26.Michele DE, Campbell KP. Dystrophin-glycoprotein complex: post-translational processing and dystroglycan function. J Biol Chem. 2003;278:15457–15460. doi: 10.1074/jbc.R200031200. [DOI] [PubMed] [Google Scholar]
- 27.Bouchet C, Gonzales M, Villaumier-Barrot S, et al. Molecular heterogeneity in fetal forms of type II lissencephaly. Hum Mutat. 2007;28:1020–1027. doi: 10.1002/humu.20561. [DOI] [PubMed] [Google Scholar]
- 28.Manzini MC, Gleason D, Chang BS, et al. Ethnically diverse causes of Walker-Warburg syndrome (WWS): FCMD mutations are a more common cause of WWS outside of the Middle East. Hum Mutation. 2008;29:E231–E234. doi: 10.1002/humu.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang W, Winder TL, LeDuc C, Simpson L, Millar WS, Dungan J, Ginsberg N, Plaga P, Moore SA, Chung W. Founder Fukutin mutation causes Walker-Warburg syndrome in four Ashkenazi Jewish families. Prenat Diag. doi: 10.1002/pd.2238. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jimenez-Mallebrera C, Torelli S, Feng L, et al. A comparative study of α-dystroglycan glycosylation in dystroglycanopathies suggests that the hypoglycosylation of α-dystroglycan does not consistently correlate with clinical severity. Brain Pathol. 2008 doi: 10.1111/j.1750-3639.2008.00198.x. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]