Abstract
Background & Aims
Gastrointestinal juvenile polyps may occur in juvenile polyposis syndrome (JPS) or sporadically. JPS is an autosomal-dominant condition caused by a germline defect in SMAD4 or BMPR1A in 50% to 60% of cases, and is characterized by multiple juvenile polyps, predominantly in the colorectum. JPS has an increased risk of gastrointestinal malignancy but sporadic juvenile polyps do not. Cyclooxygenase-2 (COX-2) expression is increased in gastrointestinal tumorigenesis and familial adenomatous polyposis. Inhibition of COX-2 leads to regression of colorectal adenomas in familial adenomatous polyposis patients and inhibits gastrointestinal tumorigenesis. To investigate the role of COX-2 in juvenile polyps, we compared the expression of COX-2 in juvenile polyps from a well-defined group of juvenile polyposis patients and sporadic juvenile polyps.
Methods
COX-2 expression was assessed in 24 genetically well-defined JPS patients and 26 patients with sporadic juvenile polyps using tissue microarray analysis. Two additional markers, Hu-antigen R, a stabilizer of messenger RNA, and CCAAT/enhancer-binding protein β, a transcription factor, both associated with increased COX-2 expression, also were investigated.
Results
Increased COX-2 expression in JPS patients was noted compared with patients with sporadic juvenile polyps (P < .001). Also, JPS patients with a BMPR1A germline defect had higher COX-2 expression than did JPS patients in whom no germline mutation was detected. High COX-2 levels correlated with increased cytoplasmic Hu-antigen R expression in JPS polyps (P = .022), but not in sporadic juvenile polyps.
Conclusions
Juvenile polyposis and sporadic juvenile polyps show distinctive expression profiles of COX-2 that may have clinical implications.
Juvenile polyps occur in about 1% of the pediatric population and most often are sporadic, solitary lesions of the colorectum. 1 These hamartomatous polyps are characterized by distorted and dilated crypts with reactive changes of the epithelium and an abundance of stroma. In contrast, juvenile polyposis syndrome (JPS) is an autosomal-dominant condition characterized by multiple juvenile polyps throughout the gastrointestinal tract.2 In JPS, juvenile polyps often contain relatively less stroma, fewer dilated crypts, and more epithelial proliferative activity than their sporadic counterparts.3 Sporadic juvenile polyps are not associated with an increased risk of gastrointestinal malignancy.4 However, in juvenile polyposis, a recently performed person-year analysis showed a relative risk for colorectal cancer of 34% and a cumulative lifetime risk of 39%.5
Germline mutations in either SMAD4 or BMPR1A are found in 50% to 60% of JPS cases.6–9 The transforming growth factor-β co-receptor endoglin has been suggested as a predisposition gene for JPS, although this is still under debate.9–11 SMAD4, BMPR1A, and endoglin are components of the transforming growth factor-β/bone morphogenetic protein signaling pathway, which is involved in the regulation of cell proliferation and differentiation.12 Patients with a germline SMAD4 mutation may possess a more aggressive gastrointestinal JPS phenotype with higher incidence of neoplastic change compared with those with BMPR1A mutation. 13–15 But much remains unknown about the molecular-genetic phenotype of juvenile polyps. The increased risk of malignancy in JPS patients and the distinctive histologic appearance of JPS polyps suggest differences in molecular biology of JPS versus sporadic juvenile polyps.
Cyclooxygenase-2 (COX-2) is a key enzyme in the conversion of arachidonic acid to prostaglandins and affects several signal transduction pathways modulating inflammation and cell proliferation.16,17 COX-2 may play a crucial role in intestinal tumorigenesis through changes in cellular adhesion, local invasion, and inhibition of apoptosis, and is up-regulated in consecutive stages of the colorectal adenoma-carcinoma sequence in patients with sporadic colorectal cancer and in familial adenomatous polyposis.18–20
Hu-antigen R (HuR) and CCAAT/enhancer-binding protein β (C/EBP-β) interact with COX-2 and may be involved in regulation of COX-2 expression in juvenile polyps. HuR is an messenger RNA (mRNA)-binding protein capable of inhibiting rapid mRNA degradation and is associated with COX-2 expression. 21 Nucleocytoplasmic translocation is necessary for HuR activation.22 C/EBP-β is a transcription factor regulating proliferation and differentiation,23 capable of inducing COX-2 expression. 24 Increased C/EBP-β correlates with invasiveness in human colorectal cancer.25
In this study we compare COX-2 protein expression in polyps of a well-defined group of JPS patients with sporadic juvenile polyps using immunohistochemistry on tissue microarray. HuR and C/EBP-β expression were examined to investigate their relationship to COX-2 expression in JPS and sporadic juvenile polyps.
Methods
Tissue Selection
Eighty-two patients, diagnosed between 1985 and 2004 with one or more juvenile polyps, were identified in a retrospective search in the Department of Pathology databases of The Johns Hopkins Hospital in Baltimore, MD, and the Academic Medical Centre in Amsterdam, The Netherlands. The research was performed in accordance with the ethical guidelines of the research review committee of these institutions.
Clinical and family history data were examined and polyps were histologically re-evaluated by an experienced pathologist (G.J.A.O.) to confirm the diagnosis of JPS or sporadic juvenile polyps. Also, all JPS patients underwent thorough genetic analysis through direct sequencing and multiplex ligation-dependent probe amplification analysis.9 JPS was defined as patients with 3 or more juvenile polyps and/or a well-established familial segregation and/or a germline mutation in one of the known JPS genes. Patients with sporadic juvenile polyps had a single sporadic polyp incidentally found and no family history of juvenile polyps. Sporadic juvenile polyps in patients with findings of colorectal mucosal inflammation were excluded.
A total of 50 patients (92 polyps) consisting of 24 JPS patients (median age at diagnosis, 10 y; range, 2–32 y; 65 polyps) and 26 patients with sporadic juvenile polyps (median age at diagnosis, 6 y; range, 1–61 y; 27 polyps) were selected for analysis. Of the 24 selected JPS patients, 7 (29%) had a SMAD4 germline mutation and 6 (25%) carried a BMPR1A germline mutation, 2 of which had a contiguous BMPR1A/PTEN germline deletion.9
Tissue Microarray
Tissue microarrays were constructed from formalin-fixed and paraffin-embedded specimens using a custom-built instrument (Beecher Instruments, Silver Spring, MD). Three core biopsy specimens (0.6-mm cylinders) were taken from the polyp tissue and, if present, also from dysplastic foci within the polyp, in a standardized fashion, and arranged in a new recipient paraffin block. Normal mucosa was included separately when available.
Immunohistochemistry and Scoring
Immunohistochemistry for COX-2 (160112; Cayman Chemical Co., Ann Arbor, MI), HuR (19F1226), and C/EBP-β (sc-7962; Santa Cruz Biotechnology, Santa Cruz, CA) was performed as previously described.27 Immunoreactivity of COX-2,28 HuR,29 and C/EBP-β27 was quantified according to established systems as shown in Table 1. The highest score found determined the overall polyp score. Similarly, patient scores were determined by the highest polyp score found in that particular patient.
Table 1.
Scoring of Immunohistochemistry
| COX-2 | |
| Low | 0: no staining |
| 1: very weak diffuse cytoplasmic staining | |
| High | 2: moderate to strong granular cytoplasmic staining in 10%–50% of cells |
| 3: strong intensity in >50% of cells | |
| HuR | Nuclear and cytoplasmic staining was scored separately as positive (high) or negative (low) in epithelial cells |
| C/EBP-β | Nuclear staining >25% of epithelial cells |
Statistical Analysis
Statistical analysis was performed using the SPSS 15.0 software package (SPSS Inc, Chicago, IL). The chi-square test, or, when appropriate, the Fisher exact test was applied to determine whether the difference in expression between groups (JPS vs sporadic) or correlation between markers within a group were statistically significant (P < .05). Overall patient scores were used when comparing JPS patients with patients with sporadic juvenile polyps for differences in expression of a certain marker. Correlations between markers were determined at the individual polyp level using the overall polyp score.
Results
Immunohistochemistry
A total of 50 patients (92 polyps), consisting of 24 JPS patients (65 polyps) and 26 patients with sporadic juvenile polyps (27 polyps), were analyzed. Eighty-one polyps were informative for all 3 markers. Immunohistochemical results for JPS and sporadic polyps are displayed in Figure 1. Epithelial and stromal COX-2 was assessed separately. Stromal COX-2 staining was rare, with the exception of granulation tissue, which formed a positive control. Therefore, only epithelial COX-2 data were included in our analysis. Because nuclear HuR staining was positive in all polyps it was not included in statistical analysis.
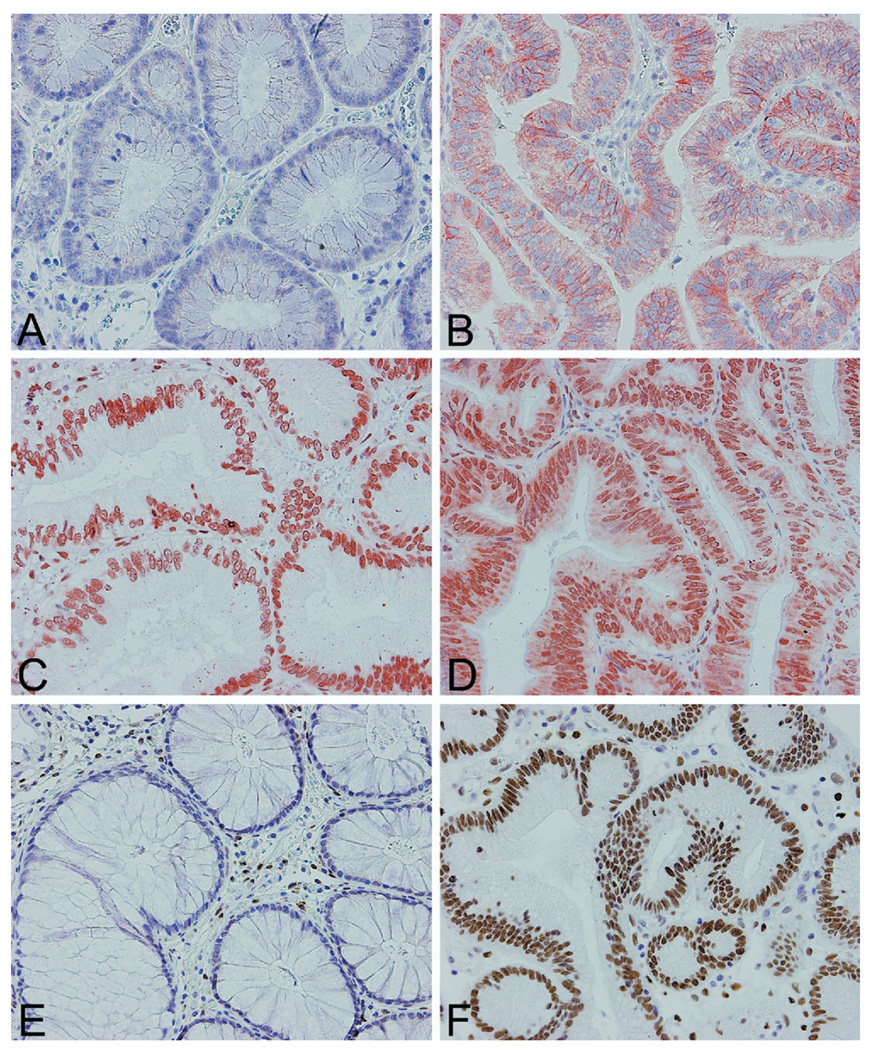
Figure 1. Immunohistochemistry on tissue microarrays for COX-2.
(A) COX-2 low, (B)COX-2high, (C) HuR-negative cytoplasmic staining, (D) HuR-positive cytoplasmic staining, (E) C/EBP-β negative, and (F) C/EBP- β positive. Magnification, 20×, counterstain hematoxylin.
Juvenile Polyposis Versus Sporadic Juvenile Polyps
COX-2 expression was significantly higher in JPS patients compared with patients with sporadic juvenile polyps (P < .001) (Table 2). Of the 65 JPS polyps 14 (22%) contained dysplasia, but no dysplasia was found in sporadic juvenile polyps. To investigate a possible confounding effect of dysplasia, we determined whether dysplasia could be linked to high COX-2 expression. Although high COX-2 expression was relatively more common in dysplastic foci than in nondysplastic polyp tissue, this difference was not significant (P = .257). No statistically significant difference in JPS versus sporadic polyps was found in the expression of HuR (P = .292) and C/EBP-β (P = .234).
Table 2.
Immunohistochemical Results: Juvenile Polyposis Polyps Versus Sporadic Juvenile Polyps
| JPS | Sporadic juvenile polyps | ||||
|---|---|---|---|---|---|
| n | IHC | n | IHC | JPS vs Sporadic | |
| COX-2 | 24 | 54% high | 24 | 4% high | P < .001 |
| HuR | 23 | 57% high | 24 | 50% high | P = .292 |
| C/EBP-β | 22 | 96% positive | 23 | 91% positive | P = .234 |
n, number of patients analyzed; IHC, immunohistochemistry.
JPS patients carrying a BMPR1A germline mutation show a near-significant increase in COX-2 expression compared with JPS patients without germline mutation (P = .086), whereas JPS patients with a SMAD4 germline defect did not (P = .391) (Table 3).
Table 3.
COX-2 Expression in Germline Mutation Carriers Versus Nongermline Mutation Carriers
| No germline mutation | ||||
|---|---|---|---|---|
| n | IHC | |||
| Germline mutation | COX-2 | 11 | 36% high | |
| SMAD4 | n | 7 | P = .391 | |
| IHC | 57% high | |||
| BMPR1A | n | 6 | P = .086 | |
| IHC | 83% high | |||
n, number of patients analyzed; IHC, immunohistochemistry.
Correlation Markers
Thirteen JPS polyps showed high expression of both COX-2 and cytoplasmic HuR. This correlation was statistically significant (P = .022). No such correlation was seen in sporadic juvenile polyps (P = .327). There was no correlation between COX-2 high phenotype and C/EBP-β positivity in either JPS polyps (P = .984), or sporadic polyps (P = .758).
Discussion
COX-2 is up-regulated in consecutive stages of the adenoma–carcinoma sequence in sporadic colorectal cancer and familial adenomatous polyposis.18–20 Chemoprevention using selective (eg, Celecoxib Pfizer Inc, New York, NY) and nonselective (eg, Sulindac Merck & Co, Whitehouse Station, NJ) COX-2 inhibitors reduces the number and size of colorectal adenomas in these patients.30,31 Patients with juvenile polyposis syndrome have a markedly increased relative and absolute risk for colorectal cancer.5 In contrast, sporadic juvenile polyps are not considered to be precursors of colorectal malignancy.
We examined and compared immunostaining of COX-2 and 2 additional molecular markers involved in the regulation of COX-2 expression, C/EBP-β and HuR, in 24 JPS patients and 26 patients with sporadic juvenile polyps. We found a significantly higher COX-2 expression in JPS patients compared with those with sporadic juvenile polyps. Interestingly, although not significant, BMPR1A germline mutation carriers showed an increase in COX-2 expression compared with JPS patients without a detected germline mutation. These findings are in line with Kurland et al,32 who recently described high COX-2 expression in one patient carrying a BMPR1A mutation. JPS patients with a SMAD4 germline mutation on the other hand did not have increased COX-2 expression, even though SMAD4 germline mutation carriers have been described as possessing a more aggressive intestinal phenotype.15 The number of patients in our study group in whom a germline defect was found was limited, therefore these results need be interpreted with caution.
A subset of JPS patients had polyps with dysplastic foci but patients with sporadic juvenile polyps did not. Recently, Brazowski et al33 showed progressively increasing COX-2 expression with increasing degree of dysplasia in JPS. Although a similar trend was seen in our JPS patients we did not find a statistical difference in COX-2 expression between dysplastic foci and nondysplastic polyp tissue. However, to rule out dysplasia as a potential confounding factor we calculated the difference in COX-2 expression in JPS versus sporadic juvenile polyps using polyp scores rather than the overall patient scores and stratified the results by dysplasia. In doing so we excluded polyps containing dysplastic foci from the analysis, that is, nondysplastic JPS polyps versus sporadic juvenile polyps. We found that COX-2 remained significantly higher in JPS compared with sporadic juvenile polyps (data not shown).
With other studies showing intestinal polyp regression through COX-2 inhibition, our results may have clinical implications for JPS patients. Future in vivo testing should be performed to determine the effect of COX-2 inhibition on gastrointestinal polyp formation in JPS animal models.34–37 Although COX-2 inhibition has proven effective in colorectal adenoma prevention, the use of COX-2 inhibitors increases the risk of cardiovascular events and thus may not be suitable for routine prevention purposes.38,39 However, the patients in these studies were above middle age (median age of patients > 50) and the findings therefore may not be applicable to children and adolescents suffering from juvenile polyposis.
HuR is an mRNA-binding protein capable of inhibiting rapid mRNA degradation by selectively binding AU-rich elements in the 3′ untranslated regions of mRNAs.40 COX-2 mRNA contains HuR-binding AU-rich elements and cytoplasmic expression of HuR is associated with high COX-2 expression and poor prognosis in several human malignancies, including colorectal cancer.29,41,42 Our data showed a correlation between high COX-2 expression and high cytoplasmic HuR expression in JPS, but not in sporadic juvenile polyps. However, no difference was found in cytoplasmic HuR expression in JPS versus sporadic juvenile polyps. Therefore, the difference found in correlation between COX-2 and HuR expression in JPS and sporadic juvenile polyps may be explained mainly by the difference in COX-2 expression in both groups. Also, correlation between COX-2 and HuR was found in SMAD4, but not in BMPR1A mutation carriers, whereas increased COX-2 expression was noted only in BMPR1A mutation carriers. HuR expression was similar in patients with a SMAD4 or BMPR1A germline mutation. Based on these results it remains unclear whether HuR is involved in up-regulation of COX-2 in JPS. It is feasible that regulation of COX-2 expression is governed by different mechanisms in SMAD4 versus BMPR1A mutation carriers.
C/EBP-β is a transcription factor regulating proliferation and differentiation23 capable of inducing COX-2 expression and present in normal colorectal epithelial cells within the proliferative zone.25 Generally, an increase in proliferative activity is seen in JPS compared with sporadic juvenile polyps. We found a C/EBP-β–positive phenotype in more than 90% of both JPS and sporadic juvenile polyps. No correlation between C/EBP-β and COX-2 expression was observed.
In summary, evaluation of COX-2 status, and COX-2–regulating molecules HuR and C/EBP-β, showed a significantly higher COX-2 expression in JPS patients compared with patients with sporadic juvenile polyps. Also, our results suggest JPS patients carrying a BMPR1A germline defect may have higher COX-2 expression than those in whom no germline defect was found. In this light, investigation of the effect of COX-2 inhibitors on polyp size and disease progression in JPS patients may be worthwhile. Additional research on the mechanisms of COX-2 regulation in juvenile polyps may be of interest.
Acknowledgments
The authors disclose the following: Supported by The Netherlands Digestive Disease Foundation (MLDS WS 04-06), The John G. Rangos, Sr. Charitable Foundation, The Clayton Fund, and National Institutes of Health grants CA 53801, 63721, 51085, and P50 CA 93-16. The study sponsors were not involved in the study design, collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
The authors are indebted to Mr. Folkert H. Morsink for technical support.
Abbreviations used in this paper
- C/EBP-β
CCAAT/enhancer-binding protein β
- COX-2
cyclooxygenase-2
- HuR
Hu-antigen R
- JPS
juvenile polyposis syndrome
- mRNA
messenger RNA.
References
- 1.Nugent KP, Talbot IC, Hodgson SV, et al. Solitary juvenile polyps: not a marker for subsequent malignancy. Gastroenterology. 1993;105:698–700. doi: 10.1016/0016-5085(93)90885-g. [DOI] [PubMed] [Google Scholar]
- 2.Jass JR, Williams CB, Bussey HJ, et al. Juvenile polyposis—a precancerous condition. Histopathology. 1988;13:619–630. doi: 10.1111/j.1365-2559.1988.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 3.Aaltonen LA, Jass JR, Howe JR. Juvenile polyposis. Pathology and genetics of tumours of the digestive system. 2000:130–132. [Google Scholar]
- 4.Giardiello FM, Hamilton SR, Kern SE, et al. Colorectal neoplasia in juvenile polyposis or juvenile polyps. Arch Dis Child. 1991;66:971–975. doi: 10.1136/adc.66.8.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brosens LA, van Hattem A, Hylind LM, et al. Risk of colorectal cancer in juvenile polyposis. Gut. 2007;56:965–967. doi: 10.1136/gut.2006.116913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howe JR, Roth S, Ringold JC, et al. Mutations in the SMAD4/DPC4 gene in juvenile polyposis. Science. 1998;280:1086–1088. doi: 10.1126/science.280.5366.1086. [DOI] [PubMed] [Google Scholar]
- 7.Howe JR, Bair JL, Sayed MG, et al. Germline mutations of the gene encoding bone morphogenetic protein receptor 1A in juvenile polyposis. Nat Genet. 2001;28:184–187. doi: 10.1038/88919. [DOI] [PubMed] [Google Scholar]
- 8.Aretz S, Stienen D, Uhlhaas S, et al. High proportion of large genomic deletions and a genotype phenotype update in 80 unrelated families with juvenile polyposis syndrome. J Med Genet. 2007;44:702–709. doi: 10.1136/jmg.2007.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Hattem WA, Brosens LAA, de Leng WW, et al. Large genomic deletions of SMAD4, BMPR1A or PTEN in juvenile polyposis. Gut. 2007;57:623–627. doi: 10.1136/gut.2007.142927. [DOI] [PubMed] [Google Scholar]
- 10.Howe J, Haidle J, Lal G, et al. ENG mutations in MADH4/BMPR1A mutation negative patients with juvenile polyposis. Clin Genet. 2007;71:91–92. doi: 10.1111/j.1399-0004.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- 11.Sweet K, Willis J, Zhou XP, et al. Molecular classification of patients with unexplained hamartomatous and hyperplastic polyposis. JAMA. 2005;294:2465–2473. doi: 10.1001/jama.294.19.2465. [DOI] [PubMed] [Google Scholar]
- 12.Massague J. TGFbeta signaling: receptors, transducers, and Mad proteins. Cell. 1996;85:947–950. doi: 10.1016/s0092-8674(00)81296-9. [DOI] [PubMed] [Google Scholar]
- 13.Sayed MG, Ahmed AF, Ringold JR, et al. Germline SMAD4 or BMPR1A mutations and phenotype of juvenile polyposis. Ann Surg Oncol. 2002;9:901–906. doi: 10.1007/BF02557528. [DOI] [PubMed] [Google Scholar]
- 14.Friedl W, Uhlhaas S, Schulmann K, et al. Juvenile polyposis: massive gastric polyposis is more common in MADH4 mutation carriers than in BMPR1A mutation carriers. Hum Genet. 2002;111:108–111. doi: 10.1007/s00439-002-0748-9. [DOI] [PubMed] [Google Scholar]
- 15.Handra-Luca A, Condroyer C, de Moncuit C, et al. Vessels’ morphology in SMAD4 and BMPR1A-related juvenile polyposis. Am J Med Genet A. 2005;138:113–117. doi: 10.1002/ajmg.a.30897. [DOI] [PubMed] [Google Scholar]
- 16.Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci U S A. 1992;89:7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tazawa R, Xu XM, Wu KK, et al. Characterization of the genomic structure, chromosomal location and promoter of human prostaglandin H synthase-2 gene. Biochem Biophys Res Commun. 1994;203:190–199. doi: 10.1006/bbrc.1994.2167. [DOI] [PubMed] [Google Scholar]
- 18.Eberhart CE, Coffey RJ, Radhika A, et al. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 19.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 20.Brosens LA, Iacobuzio-Donahue CA, Keller JJ, et al. Increased cyclooxygenase-2 expression in duodenal compared with colonic tissues in familial adenomatous polyposis and relationship to the −765G -> C COX-2 polymorphism. Clin Cancer Res. 2005;11:4090–4096. doi: 10.1158/1078-0432.CCR-04-2379. [DOI] [PubMed] [Google Scholar]
- 21.Sengupta S, Jang BC, Wu MT, et al. The RNA-binding protein HuR regulates the expression of cyclooxygenase-2. J Biol Chem. 2003;278:25227–25233. doi: 10.1074/jbc.M301813200. [DOI] [PubMed] [Google Scholar]
- 22.Fan XC, Steitz JA. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 1998;17:3448–3460. doi: 10.1093/emboj/17.12.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umek RM, Friedman AD, McKnight SL. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991;251:288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- 24.Eisinger AL, Nadauld LD, Shelton DN, et al. The APC tumor suppressor gene regulates expression of cyclooxygenase-2 by a mechanism that involves retinoic acid. J Biol Chem. 2006;281:20474–20482. doi: 10.1074/jbc.M602859200. [DOI] [PubMed] [Google Scholar]
- 25.Rask K, Thorn M, Ponten F, et al. Increased expression of the transcription factors CCAAT-enhancer binding protein-beta (C/EBBeta) and C/EBzeta (CHOP) correlate with invasiveness of human colorectal cancer. Int J Cancer. 2000;86:337–343. doi: 10.1002/(sici)1097-0215(20000501)86:3<337::aid-ijc6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Furneaux H, Cheng H, et al. HuR regulates p21 Mrna stabilization by UV light. Mol Cell Biol. 2000;20:760–769. doi: 10.1128/mcb.20.3.760-769.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milne AN, Carvalho R, Morsink FM, et al. Early-onset gastric cancers have a different molecular expression profile than conventional gastric cancers. Mod Pathol. 2006;19:564–572. doi: 10.1038/modpathol.3800563. [DOI] [PubMed] [Google Scholar]
- 28.Buskens CJ, Van Rees BP, Sivula A, et al. Prognostic significance of elevated cyclooxygenase 2 expression in patients with adenocarcinoma of the esophagus. Gastroenterology. 2002;122:1800–1807. doi: 10.1053/gast.2002.33580. [DOI] [PubMed] [Google Scholar]
- 29.Erkinheimo TL, Lassus H, Sivula A, et al. Cytoplasmic HuR expression correlates with poor outcome and with cyclooxygenase 2 expression in serous ovarian carcinoma. Cancer Res. 2003;63:7591–7594. [PubMed] [Google Scholar]
- 30.Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 31.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 32.Kurland JE, Beck SE, Solomon CJ, et al. Cyclooxygenase-2 expression in polyps from a patient with juvenile polyposis syndrome with mutant BMPR1A. J Pediatr Gastroenterol Nutr. 2007;44:318–325. doi: 10.1097/MPG.0b013e31802e98e5. [DOI] [PubMed] [Google Scholar]
- 33.Brazowski E, Rozen P, Misonzhnick-Bedny F, et al. Characteristics of familial juvenile polyps expressing cyclooxygenase-2. Am J Gastroenterol. 2005;100:130–138. doi: 10.1111/j.1572-0241.2005.40775.x. [DOI] [PubMed] [Google Scholar]
- 34.Haramis AP, Begthel H, van den Born M, et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 35.Takaku K, Miyoshi H, Matsunaga A, et al. Gastric and duodenal polyps in Smad4 (Dpc4) knockout mice. Cancer Res. 1999;59:6113–6117. [PubMed] [Google Scholar]
- 36.Hohenstein P, Molenaar L, Elsinga J, et al. Serrated adenomas and mixed polyposis caused by a splice acceptor deletion in the mouse Smad4 gene. Genes Chromosomes Cancer. 2003;36:273–282. doi: 10.1002/gcc.10169. [DOI] [PubMed] [Google Scholar]
- 37.He XC, Zhang J, Tong WG, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 38.Baron JA, Sandler RS, Bresalier RS, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131:1674–1682. doi: 10.1053/j.gastro.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 39.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 40.Brennan CM, Steitz JA. HuR and mRNA stability. Cell Mol Life Sci. 2001;58:266–277. doi: 10.1007/PL00000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez de Silanes I, Fan J, Yang X, et al. Role of the RNA-binding protein HuR in colon carcinogenesis. Oncogene. 2003;22:7146–7154. doi: 10.1038/sj.onc.1206862. [DOI] [PubMed] [Google Scholar]
- 42.Denkert C, Koch I, von Keyserlingk N, et al. Expression of the ELAV-like protein HuR in human colon cancer: association with tumor stage and cyclooxygenase-2. Mod Pathol. 2006;19:1261–1269. doi: 10.1038/modpathol.3800645. [DOI] [PubMed] [Google Scholar]