Abstract
Previous studies suggest that prolonged electrical activation of the baroreflex may reduce arterial pressure more than chronic blockade of α1-and β1,2-adrenergic receptors. To determine whether central inhibition of sympathetic outflow has appreciable effects to chronically reduce arterial pressure by actions distinct from well established mechanisms, we hypothesized that chronic baroreflex activation would lower arterial pressure substantially even during complete α1-and β1,2-adrenergic receptor blockade. This hypothesis was tested in 6 dogs during adrenergic blockade (18 days) with and without electrical activation of the carotid baroreflex (7 days). During chronic adrenergic blockade alone, there was a sustained decrease in mean arterial pressure of 21±2 mm Hg (control = 95±4 mm Hg) and nearly a 3-fold increase in plasma norepinephrine concentration (control = 138±6pg/mL), likely due to baroreceptor unloading. In comparison, during adrenergic blockade + prolonged baroreflex activation, plasma norepinephrine concentration decreased to control levels and mean arterial pressure fell an additional 10±1 mm Hg. Because of differences in plasma norepinephrine concentration, we also tested the acute blood pressure lowering effects of MK-467, a peripherally acting α2-antagonist. After administration of MK-467, there was a significantly greater fall in arterial pressure during adrenergic blockade (15±3 mm Hg) than during adrenergic blockade + prolonged baroreflex activation (7±3 mm Hg). These findings suggest that reflex-induced increases in sympathetic activity attenuate reductions in arterial pressure during chronic adrenergic blockade and that inhibition of central sympathetic outflow by prolonged baroreflex activation lowers arterial pressure further by previously undefined mechanisms, possibly by diminishing attendant activation of postjunctional α2-adrenergic receptors.
Keywords: baroreflex, arterial pressure, sympathetic nervous system, α-and β-adrenergic receptors, norepinephrine, renin-angiotensin system
Introduction
The development of technology for chronic electrical stimulation of the afferent limb of the carotid baroreflex has been especially valuable in providing insight into the time dependency and quantitative importance of the mechanisms that account for the long-term blood pressure lowering effects of the baroreflex.1–4 With this methodology for prolonged baroreflex activation (PBA), sustained and controllable reductions in mean arterial pressure (MAP) are associated with distinct reductions in circulating levels of norepinephrine (NE), indicating persistent suppression of central sympathetic outflow. In the present study, we used this methodology to provide a more comprehensive understanding of the role of adrenergic receptors in contributing to the lowering of MAP during chronic suppression of sympathetic outflow by PBA.
In comparing results among different studies, one intriguing observation is that PBA may chronically reduce MAP more than complete blockade of α1-and β1,2- adrenergic receptors.1–2,4–5 Thus, the major goal of this study was to test the hypothesis that central inhibition of sympathetic outflow by PBA has a greater capacity to chronically lower arterial pressure than pharmacological blockade of the postjunctional adrenergic receptors with established roles in mediating the long-term effects of the sympathetic nervous system on arterial pressure. Further, to gain insight into the mechanisms that might account for potential differences in the chronic blood pressure responses to PBA and blockade of α1- and β1,2-adrenergic receptors (AB), we also determined the temporal changes in the sympathetic nervous and renin-angiotensin systems, neurohormonal systems with interconnected and critical roles in long-term control of arterial pressure. Finally, we speculated that greater reductions in arterial pressure during PRA than AB might be due to lower central sympathetic outflow in the former and concomitant diminished activation of vasoconstricting postjunctional α2-adrenergic receptors.6–7 The possibility that this mechanism might contribute to the chronic blood pressure lowering effects of PBA was evaluated acutely during AB and AB+PBA by determining the fall in MAP following bolus administration of MK-467, a peripherally acting α2-antagonist.8
Methods
Animal Preparation
Experiments were conducted in 6 chronically instrumented mongrel dogs weighing 23 to 27 kg). All experimental protocols were performed according to the “Guide for the Care and Use of Laboratory Animals” from the National Institutes of Health and approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee.
Surgical procedures were conducted under isoflurane anesthesia (1.5% to 2.0%) after premedication with acepromazine (0.15 mg/kg. SC) and induction with thiopental (10 mg/kg, SC). The procedures for implantation of vascular catheters in the aorta and vena cava, and implantation of stimulating electrodes around each carotid sinus have been described previously.1–4 Prior to the control period, one of the two arterial catheters was connected to a blood pressure transducer and the lead bodies of the electrodes were attached to a pulse generator. Both the transducer and the pulse generator were worn in the dog jacket. The electrodes and the pulse generator were provided by CVRx, Inc. (Minneapolis, MN).
Experimental Protocol
Following recovery from surgery the dogs were placed in metabolic cages in a temperature and humidity-controlled room with a 12-hour light/dark cycle. During a 3 week postoperative period and throughout the study, the dogs were given free access to water and maintained on a fixed daily diet of two 15.5 oz. cans of prescription heart diet (H/D; Hill’s Pet Products) supplemented with 5 ml of vitamin syrup. Two cans of H/D provide ~5 mmol of sodium and ~55 mmol of potassium. Additionally, the dogs received a continuous intravenous infusion of isotonic saline at a rate of 350 mL/day, thus providing a total daily sodium intake of ~60 mmol. Water consumption was monitored daily, and 24-h urine samples were collected at 11 am each day at the time of feeding.
The dogs were given ~3 weeks to acclimate to the laboratory environment and to establish electrolyte and fluid balance. During this time they were trained to lie quietly in their cages for several hours each morning to permit blood sampling under these controlled conditions. Arterial pressure and heart rate were monitored continuously, 24hours/day, throughout the study.
After steady state conditions were achieved at the end of the third postoperative week, control measurements were made. The control period was followed by an 18 day experimental period during which time the dogs were administered prazosin and propranolol to block α1- and β1,2-adrenergic receptors, respectively. Prazosin (5mg/kg/day) was administered orally every 8 hours, whereas propranolol was continuously infused (10mg/kg/day) by adding the drug to the 24-hour intravenous saline infusion. After 7 days of AB, the carotid baroreflex was electrically activated for 7 days (days 8–14) while continuing AB. The stimulation parameters for bilateral activation of the carotid baroreflex have been described previously.1–4 Subsequently, activation of the carotid baroreflex was discontinued during the last 4 days (days 15–18) of AB, initiating a 4-day recovery period from baroreflex activation. Finally, after 18 days of AB, a final recovery period was initiated with the dogs monitored for an additional 7 days.
On intermittent days throughout the control, experimental, and recovery periods, arterial blood samples (~10 ml) were taken while the dogs were recumbent and in a resting state. Blood samples were analyzed for hematocrit, plasma renin activity (PRA), and the plasma concentrations of sodium, potassium, protein, aldosterone, cortisol, and NE. Completeness of adrenergic blockade was tested on days in which blood samples were not taken. This was assessed by comparing arterial pressure and heart rate responses to bolus IV injections of phenylephrine (α1-agonist,100 µg) and isoproterenol (β1,2-agonist, 2µg) during AB and during the control period. The contribution of α2-adrenergic receptors to the maintenance of arterial pressure was assessed acutely by determining the magnitude of the fall in arterial pressure after bolus IV injection of the peripherally acting α2-adrenergic receptor antagonist MK-467 (L-659,066, 400 µg/kg).8 This evaluation was made on day 14 of AB+PBA and again on day18 of AB alone. Changes in arterial pressure in response to MK-467 injection were also determined during the control period when the adrenergic receptors were unblocked. As MK-467 does not readily penetrate the blood brain barrier, changes in arterial pressure after drug administration reflect effects of the α2-adrenergic receptor antagonist on adrenergic receptors in the peripheral circulation.8
Analytical Methods
The plasma levels of hormones were measured by radioimmunoassay.1–4 Plasma concentrations of NE were determined by high-performance liquid chromatography (HPLC) with electrochemical detection (Agilent 1100), as previously described.2–4 Hematocrit and the plasma concentrations of sodium, potassium, and protein were measured by standard techniques.1–4
The daily hemodynamic values presented for MAP and heart rate were averaged from the 20-hour period extending from 11:30–7:30 am. The hours excluded from the 24-hour recordings included the time required for flushing catheters, calibrating pressure transducers, feeding, and cleaning cages.
Statistical Analysis
Results are expressed as mean±SE. A 1-way repeated measures ANOVA was used to compare daily values to either control or day 7 of adrenergic blockade. Significant differences were established using Dunnett’s t test for multiple comparisons. Maximal arterial pressure responses to MK-467 were determined within 5 minutes of drug injection. Changes in arterial pressure and heart rate in response to MK-467 during AB and AB+PBA were compared by the Student t-test for paired observations. Evaluation of the efficacy of adrenergic blockade was based upon maximal arterial pressure and heart rate responses to bolus injections of adrenergic receptor agonists (~ 30 seconds after drug administration). Arterial pressure and heart rate responses to agonist injections were compared by the Student t-test for paired observations. Statistical significance was considered to be P<0.05.
Results
Arterial Pressure and Heart Rate
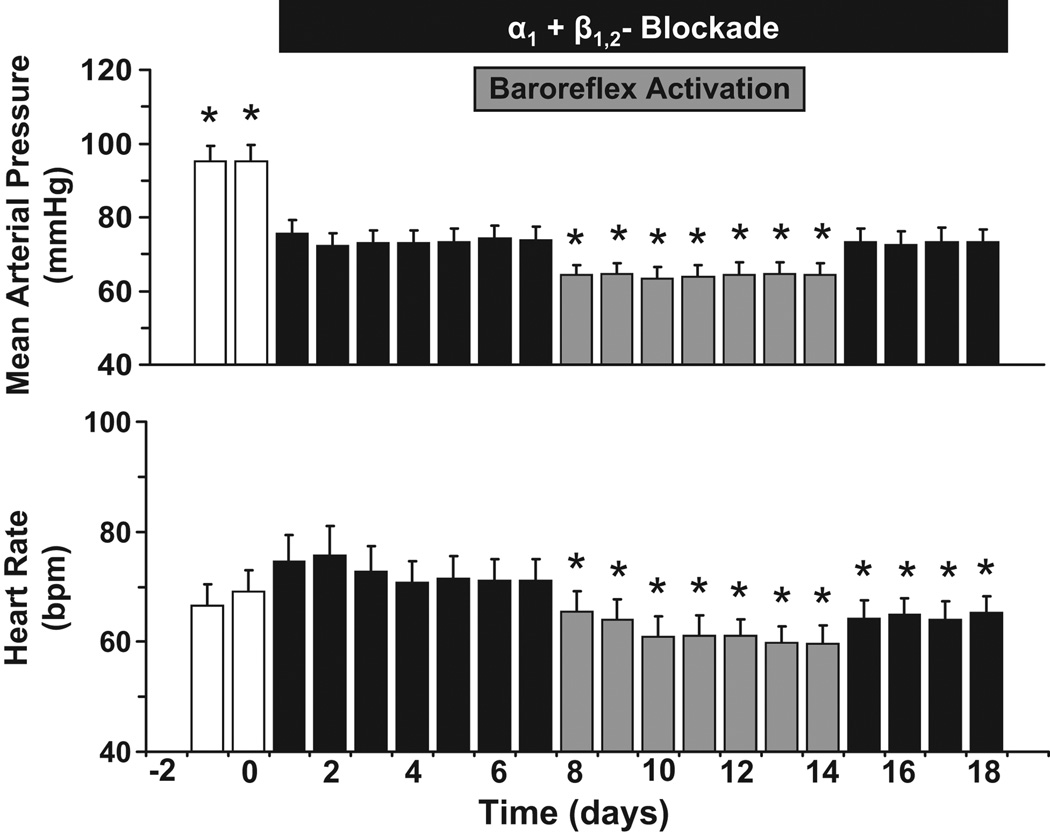
Figure 1 illustrates the changes in MAP and heart rate in response to AB and AB+PBA. During the control period, basal values for MAP and heart rate were 95±4 mm Hg and 65±3 bpm, respectively. Within 24 hours, there was a substantial fall in MAP during AB that was sustained on the subsequent days before PBA. After 7 days of AB, MAP was reduced 21±2 mm Hg, but there were no significant change in heart rate. Moreover, during AB+PBA, there was a further appreciable reduction in MAP, which was usually apparent immediately after initiating baroreflex activation. Within the first 24 hours of PBA, MAP decreased an additional 10±1 mm Hg and remained at this reduced level for the duration of the 7 day period of baroreflex activation (day 14 = 64±3 mmHg). Thus, during AB+PBA, MAP was ~ 30mmHg lower than control levels. Throughout the entire period of PBA, heart rate was decreased, and on the last day of baroreflex activation heart rate was 11±2 bpm lower than on day 7 of AB. During the 4 days after AB+PBA when AB was maintained, MAP returned to the levels observed on day 7 of AB; however, recovery of heart rate was incomplete. During the 7 day recovery period following AB (not shown), both MAP and heart rate returned to control levels, and on the last day of the recovery period MAP and heart rate were 93±5 mm Hg and 63±3 bpm, respectively.
Figure 1.
Changes in mean arterial pressure (MAP) and heart rate during adrenergic blockade (AB) and adrenergic blockade + prolonged baroreflex activation (AB+PBA). Values are mean±SEM (n=6). *P<0.05 versus day 7 of AB. bpm, beats/min.
Urinary Electrolyte Excretion
During the control period the excretion rates of sodium and potassium were 59±2 and 42±4 mmol/d, respectively, reflecting the intake of these electrolytes. During the first 24-hours of AB and coinciding with the initial drop in MAP, there was modest sodium retention before daily sodium balance was reestablished on subsequent days. A similar pattern in sodium excretion occurred with initiation of PBA. There were no significant changes in potassium excretion during AB or AB+PBA.
Neurohormonal Profile
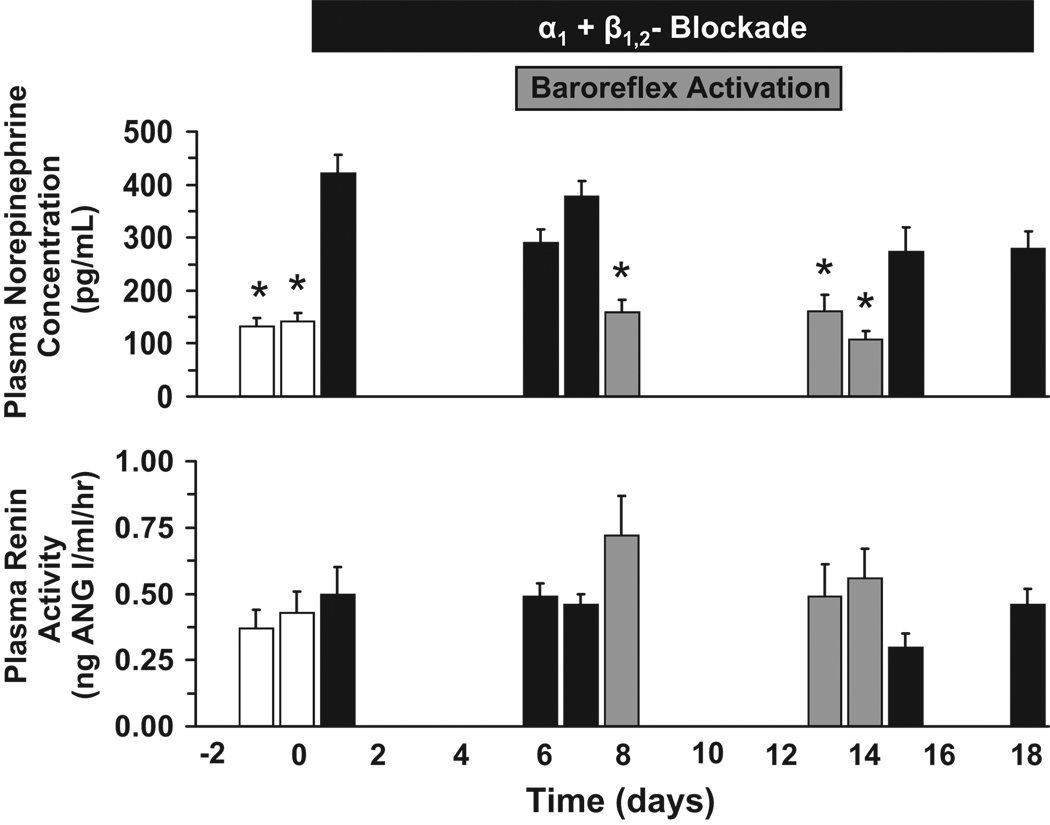
Changes in PRA and in the plasma concentrations of NE during AB and AB+PBA are illustrated in Figure 2. During the initial 7 days of AB, there was an approximately 3-fold increase in plasma NE concentration above control levels (control = 138±14pg/mL). However, central sympathoinhibition by sustained activation of the baroreflex reduced these high plasma levels of NE back to control levels in parallel with the additional fall in MAP (Figure 1). During the 4 days following AB+PBA when AB was continued, plasma NE concentration returned to the elevated levels observed on day 7 before PBA. An important observation was the absence of an increase in PRA (control = 0.55±0.05 ng ANG I/mL/hr) during AB and AB+PBA, despite substantial reductions in MAP that reached ~30 mmHg below control levels during AB+PBA. Finally, by the end of the 7 day recovery period following AB, values for both plasma NE concentration (122±13pg/mL) and PRA (control = 0.48±0.12 ng ANG I/mL/hr) were similar to control.
Figure 2.
Neurohormonal responses to AB and AB+PBA. Values are mean±SEM (n=6). *P<0.05 versus day 7 of AB.
Control values for plasma aldosterone and cortisol concentration were 2.2±0.3ng/dL and 1.4±0.0.2µg/dL, respectively. In parallel with the small increase in plasma potassium concentration (see below), plasma aldosterone concentration tended to increase slightly during AB and AB+PBA, but this was not statistically significant. There were no statistically significant changes in plasma cortisol concentration throughout this study.
Acute Arterial Pressure and Heart Rate Responses to MK-467
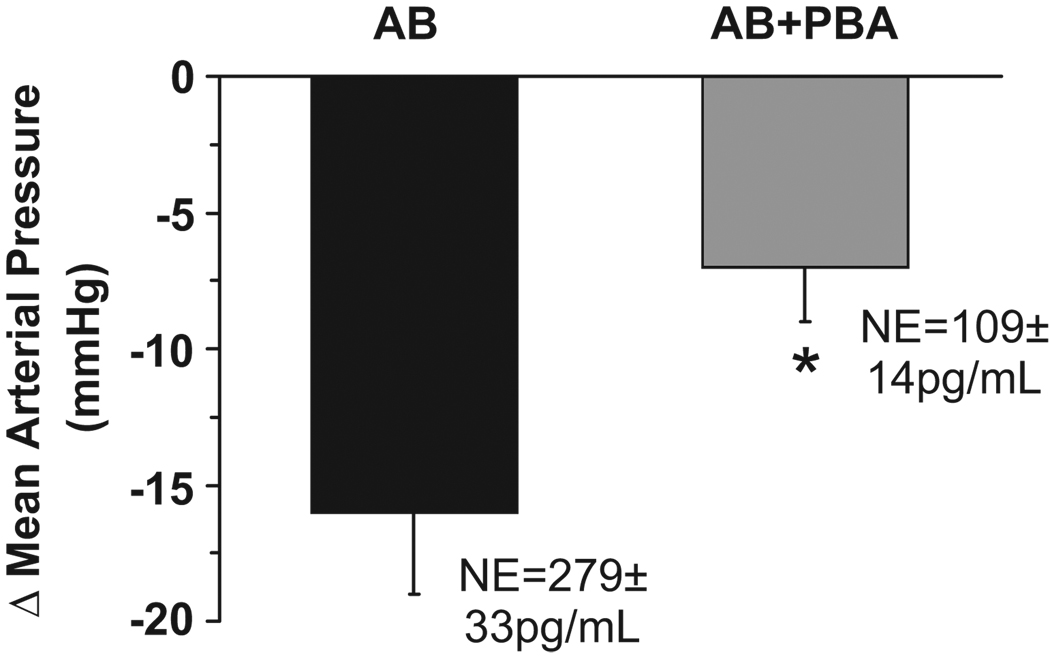
The acute changes in MAP in response to MK-467 during AB and AB+PBA are illustrated in Figure 3. In the presence of increased sympathetic activity during AB, as reflected by high circulating levels of NE, MAP decreased 15±3 mmHg (pre-injection MAP = 76±3 mmHg) and heart rate increased modestly from 67±5 to 80±5 bpm after acute administration of the α2-antagonist. In contrast, when plasma NE was reduced to control levels during AB+PBA, MAP decreased only 7±3 mmHg (pre-injection MAP = 63±2 mmHg) after MK-467. Heart rate (control = 56±3 bpm) tended to increase after injection of MK-467, but this response was not statistically significant. Additionally, MAP and heart rate responses to MK-467 were also determined under control conditions when α1-and β1,2-adrenergic receptors were unblocked. As reported by others,9 under control conditions when adrenergic receptors were unblocked, both MAP (control = 113±6 mmHg) and heart rate (control = 71±3 bpm) increased after MK-467 by 13±3 mmHg and 37±6 bpm, respectively.
Figure 3.
Acute reductions in MAP in response to bolus IV injection of the pereipherally acting α2-antagonist MK-467 during AB and AB+PBA. Values are mean±SEM (n=6). *P<0.05 versus AB.
Hematocrit and Plasma Concentrations of Electrolytes and Protein
In association with the modest retention of sodium on day 1, there were small (5–10 %), but nevertheless significant, reductions in both hematocrit (control = 0.38±0.01) and plasma protein (control = 6.5±0.2 g/dL) concentration during AB that were sustained during AB+PBA. Reductions in hematocrit and plasma protein concentration tended to wane by day 18 of AB and returned fully to control levels by the end of the 7 day recovery period. Plasma potassium concentration (control = 4.6±0.2 mmol/L) increased significantly to 5.1±0.3mmol/L during AB and remained at this level throughout AB+PBA and the subsequent 4 days of AB before recovering to control levels by the end of the 7 day recovery period. There were no significant changes in plasma sodium concentration (control = 150±1 mmol/dL) during the study.
Evaluation of Adrenergic Blockade
MAP and heart rate responses to α1- and β1,2- agonists indicated that complete blockade of adrenergic receptors was achieved during AB (Table). Increases in MAP and baroreflex-mediated reductions in heart rate were 37 ±3 mmHg and 24±5 bpm, respectively, in response to bolus injection of the α1-agonist phenylephrine in the unblocked state. Following administration of the β1,2-agonist isoproterenol, MAP decreased 21±2 mmHg and heart rate increased 37±4 bpm. These responses to agonist administration were completely blocked during chronic AB.
Table.
Responses to Adrenergic Agonists
| Control | Adrenergic Blockade | |||
|---|---|---|---|---|
| Agonist | MAP, mmHg | HR, bpm | MAP, mmHg | HR, bpm |
| Phenylephrine | 37 ± 3 | −24 ± 5 | 1 ± 1* | −3 ± 2* |
| Isoproterenol | −21 ± 2 | 37 ± 4 | −1 ± 1* | 1 ± 1* |
Values are means ± SE; n=6. MAP indicates mean arterial pressure; HR, heart rate.
p < 0.05 vs control
Discussion
The most significant and novel finding in this study is that central suppression of sympathetic outflow by PBA has substantial chronic effects to lower arterial pressure by a mechanism(s) independent of decreasing activation of α1-and β-adrenergic receptors. The importance of this additional mechanism(s) to the chronic regulation of arterial pressure is reflected by the relatively large fall in arterial pressure that occurred in response to baroreflex activation during AB. While the fall in MAP during AB alone was pronounced, it was substantially greater during AB+PBA. Thus, to the best of our knowledge, this is the first study to clearly demonstrate a quantitatively significant sustained reduction in arterial pressure during inhibition of central sympathetic outflow that is not mediated by diminished activation of peripheral α1-and β-adrenergic receptors. As the kidneys play a critical role in long-term regulation of arterial pressure,10–12 an important implication of this study is that PBA chronically enhances sodium excretion by mechanisms that previously have not been established.
An interesting and provocative finding in this study, with potential clinical relevance to antihypertensive therapy, was the sustained increase in plasma NE concentration during chronic AB. Presumably, the sustained activation of the sympathetic nervous system during AB was a response to the persistent reduction in MAP and attendant unloading of arterial baroreceptors. However, this interpretation is discordant with the notion that baroreflexes completely reset in the direction of change in ambient pressure and, consequently, do not play a role in long-term control of arterial pressure.11–13 On the other hand, this notion is inconsistent with recent observations in hypertensive animals.11–13 Of more direct relevance to the present study, however, is the paucity of information relating to baroreflex regulation of sympathetic activity during chronic reductions in arterial pressure, including during antihypertensive therapy. Despite technical limitations precluding an unambiguous quantitative evaluation of baroreflex resetting during long-term reductions in arterial pressure, recent experimental studies by Thrasher have demonstrated that resetting of the baroreflex is incomplete during sustained unloading (over 1 month) of carotid baroreceptors.14 In addition, two recent clinical studies have reported sustained increases in postganglionic sympathetic nerve activity to skeletal muscle for up to 3 months of antihypertensive therapy with either a thiazide diuretic or a thiazide-angiotensin receptor blocker combination.15–16 Thus, the current finding of a sustained increase in plasma NE concentration during AB is in accord with these recent experimental and clinical investigations and contributes to the growing body of evidence that resetting of the baroreflex is incomplete during sustained alterations in arterial pressure.11–13 Further, the present study, although conducted in normotensive animals only, suggests one potential adverse effect of increased sympathetic activation during AB and possibly diuretic therapy: reduced efficacy of antihypertensive therapy. This study also indicates that inhibition of central sympathetic outflow by PBA can abolish reflex sympathetic activation that may counteract the fall in arterial pressure during blockade of α1- and β1,2-adrenergic receptors.
While it is reasonable to speculate that the additional chronic blood pressure lowering effects of PBA during AB may be hormonally mediated, analogous to inhibition of ADH secretion during stimulation of baroreceptors, the abrupt fall in arterial pressure immediately following baroreflex activation, along with the attendant decrease in NE to control levels during chronic PBA, suggest that this added sustained fall in MAP was due to reduced release of NE and/or co-transmitters (such as NPY and ATP) from adrenergic nerve terminals. Because studies conducted in chronically instrumented dogs and in human subjects have demonstrated increased limb blood flow in response to localized arterial administration of α2-adrenergic receptor antagonists,6–7 we focused on the possibility that the differential blood pressure lowering effects of AB and PBA may be a result of the disparity in NE activating postjunctional vasoconstrictor α2-adrenergic receptors.
The contribution of postjunctional α2-adrenergic receptors to neural regulation of arterial pressure is unclear and difficult to assess. This is because antagonism of postjunctional α2-adrenergic receptors would be expected to cause vasodilation and thereby tend to decrease arterial pressure, while antagonism of central neuronal and prejunctional α2-adrenergic receptors would be expected to increase NE release and thereby tend to increase arterial pressure and heart rate by activation of postjunctional α1-and β-adrenergic receptors. In recognition of these opposing effects, we reasoned that the experimental design of the present study was optimal for determining whether the chronic physiological alterations in sympathetic activity associated with AB and AB+PBA might have differential tonic effects on arterial pressure due to activation of postjunctional α2-adrenergic receptors. Under the present experimental conditions, the prevailing blockade of α1-and β-adrenergic receptors would be expected to isolate any effects of acute α2-adrenergic receptor blockade to postjunctional α2-adrenergic receptors, particularly as the α2-adrenergic receptor antagonist employed (MK-467) does not enter the central nervous system and, as a result, cannot act centrally to increase sympathetic outflow.
Reductions in MAP in response to MK-467 were modest during AB+PBA (~7 mmHg) when circulating levels of NE were normal and considerably more robust during AB alone (~15mmHg). This suggests that the higher level of sympathetic activation during AB, as compared to AB+PBA, was sufficient to cause more intense constriction of the peripheral vasculature. As complete blockade of β-adrenergic receptors was confirmed by the absence of cardiovascular responses to isoproterenol after AB (Table), it is likely that the small increase in heart rate in response to MK-467 was secondary to reductions in parasympathetic activity as a result of the fall in MAP. In comparison, even though the confounding influence of blocking α2-adrenergic receptors on central neurons was circumvented with MK-467, the net effect of blocking peripheral prejunctional and postjunctional α2-adrenergic receptors under control conditions when α1-and β-adrenergic receptors were unblocked was a substantial increase in both MAP and heart rate, confirming previous observations.9
Because the fall in arterial pressure following MK-467 was appreciably smaller during AB+PBA than during AB, this is consistent with the interpretation that diminished activation of postjunctional α2-adrenergic receptors contributes to the long-term blood pressure lowering effects of PBA. However, there are two important caveats in extrapolating this acute response to the chronic differences in arterial pressure associated with AB and AB+PBA. First and foremost, the acute reductions in arterial pressure in response to MK-467 reflect only the tonic circulatory effects of stimulating postjunctional α2-adrenergic receptors. Importantly, these acute responses do not necessarily reveal the influence of postjunctional α2-adrenergic receptors on renal excretion of sodium. Unfortunately, despite the critical role of the kidneys in chronic regulation of arterial pressure, little is known about the physiological importance of renal postjunctional α2-adrenergic receptors in affecting chronic changes in renal excretory function. Therefore, the importance of renal postjunctional α2-adrenergic receptors in the chronic regulation of arterial pressure is undefined. A second issue is that compared to AB alone, basal levels of MAP were lower during AB+PBA prior to MK-467 administration. To what extent, if any, the lower basal levels of MAP may have diminished the subsequent fall in MAP to MK-467 during AB+PBA is unclear. Because of the above issues, an unambiguous elucidation of the undefined mechanisms that account for the chronic blood pressure lowering effects of PBA will require further investigation.
A recurring important observation from chronic studies during PBA is that PRA does not increase concomitantly with chronic reductions in MAP, even when PBA-induced reductions in MAP are substantial.1–4 This was particularly impressive in the present study during AB+PBA when MAP was ~ 30 mmHg below control. The absence of pressure-dependent renin release suggests that PBA has pronounced inhibitory effects on renin secretion, which are presumably mediated by suppression of renal sympathetic nerve activity and decreased activation of renal adrenergic receptors.12 Furthermore, because even small increases in plasma ANG II greatly attenuate the chronic blood pressure lowering effects of PBA,2 baroreflex suppression of renin secretion plays a critical role in permitting the long-term blood pressure lowering effects of PBA.
Perspectives
Increased sympathetic activity plays a critical role in the pathogenesis of primary hypertension and, in addition, promotes adverse cardiovascular events.17–18 Therefore, despite the obvious value of suppressing sympathetic activity, some commonly used antihypertensive agents, such as diuretics, chronically stimulate the sympathetic nervous system even further.15–16 Although only conducted in normotensive animals, the findings from the present study suggest that adrenergic blocking agents may also belong on the list of antihypertensive drugs that chronically activate the sympathetic nervous system by the baroreflex. Furthermore, the present study suggests that chronic reflex activation of the sympathetic nervous system may have an appreciable effect to attenuate the efficacy of antihypertensive therapy and that this adverse effect associated with drug therapy can be abolished by PBA. Presumably, central inhibition of sympathetic outflow accounts for the impressive sustained, non-pharmacological reduction in arterial pressure reported in patients with resistant hypertension treated for more than 2 years with baroreflex therapy while maintained on a constant number (4.8) of antihypertensive agents.19
Acknowledgements
The authors greatly appreciate the outstanding technical assistance provided by Mac Abernathy, Jamie Beckman and Bennie Harris. MK-467 was a generous gift from Merck & Co., Inc.
Sources of Funding
National Heart, Lung, and Blood Institute Grant HL-51971.
Footnotes
Conflicts of Interest/Disclosure
Thomas E. Lohmeier, Consultant fees, Scientific Advisory Board,-CVRx
Eric D. Irwin, Consultant fees- Scientific Advisory Board,-CVRx
Martin A. Rossing, Employment,-CVRx
Adam W. Cates, Employment,-CVRx
References
- 1.Lohmeier TE, Irwin ED, Rossing MA, Sedar DJ, Kieval RS. Prolonged activation of the baroreflex produces sustained hypotension. Hypertension. 2004;43:306–311. doi: 10.1161/01.HYP.0000111837.73693.9b. [DOI] [PubMed] [Google Scholar]
- 2.Lohmeier TE, Dwyer TM, Hildebrandt DA, Irwin ED, Rossing MA, Sedar DJ, Kieval RS. Influence of prolonged baroreflex activation on arterial pressure in angiotensin hypertension. Hypertension. 2005;46:1194–1200. doi: 10.1161/01.HYP.0000187011.44201.2e. [DOI] [PubMed] [Google Scholar]
- 3.Lohmeier TE, Hildebrandt DA, Dwyer TM, Barrett AM, Irwin ED, Rossing MA, Kieval RS. Renal denervation does not abolish sustained baroreflex-mediated reductions in arterial pressure. Hypertension. 2007;49:373–379. doi: 10.1161/01.HYP.0000253507.56499.bb. [DOI] [PubMed] [Google Scholar]
- 4.Lohmeier TE, Dwyer TM, Irwin ED, Rossing MA, Kieval RS. Prolonged activation of the baroreflex abolishes obesity-induced hypertension. Hypertension. 2007;49:1307–1314. doi: 10.1161/HYPERTENSIONAHA.107.087874. [DOI] [PubMed] [Google Scholar]
- 5.Lohmeier TE, Hildebrandt DA, Hood WA. Renal nerves promote sodium excretion during long-term increases in salt intake. Hypertension. 1999;33(part II):487–492. doi: 10.1161/01.hyp.33.1.487. [DOI] [PubMed] [Google Scholar]
- 6.Buckwalter JB, Clifford PS. α-Adrenergic vasoconstriction in active skeletal muscles during dynamic exercise. Am J Physiol Heart Circ Physiol. 1999;277:H33–H39. doi: 10.1152/ajpheart.1999.277.1.H33. [DOI] [PubMed] [Google Scholar]
- 7.Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Post-junctional αadrenoceptors and basal limb vascular tone in healthy men. J Physiol. 2002;540(3):1103–1110. doi: 10.1113/jphysiol.2001.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szemeredi K, Stull R, Kopin IJ, Goldstein DS. Effects of a peripherally acting α2-adrenoceptor antagonist (L-659,066) on hemodynamics and plasma levels of catechols in conscious rats. Eur J Pharmacol. 1989;1089:53–59. doi: 10.1016/0014-2999(89)90133-7. [DOI] [PubMed] [Google Scholar]
- 9.Pagel PS, Proctor LT, Devcic A, Hettrick DA, Kersten JR, Tessmer JP, Farber NE, schmeling WT, Warltier DC. A novel alpha 2-adrenoceptor antagonist attenuates the early, but preserves the late cardiovascular effects of intravenous effects of intravenous dexmedetomidine in conscious dogs. J Cardiothorac Vasc Anesth. 1998;12:429–434. doi: 10.1016/s1053-0770(98)90197-5. [DOI] [PubMed] [Google Scholar]
- 10.Guyton AC. Arterial Pressure and hypertension. Philadelphia: Saunders; 1980. [Google Scholar]
- 11.Lohmeier TE, Hildebrandt DA, Warren S, May PJ, Cunningham JT. Recent insights into the interactions between the baroreflex and the kidneys in hypertension. Am J Physiol Regulatory Integrative Comp Physiol. 2005;288:R828–R836. doi: 10.1152/ajpregu.00591.2004. [DOI] [PubMed] [Google Scholar]
- 12.Lohmeier TE, Drummond HA. The baroreflex in the pathogenesis of hypertension. In: Lip GYH, Hall JE, editors. Comprehensive Hypertension. Philadelphia, PA: Elsevier; 2007. pp. 265–279. [Google Scholar]
- 13.Malpas SC. What sets the long-term level of sympathetic nerve activity: is there a role for arterial baroreceptors? Am J Physiol Regulatory Integrative Comp Physiol. 2003;286:R1–R12. doi: 10.1152/ajpregu.00496.2003. [DOI] [PubMed] [Google Scholar]
- 14.Thrasher TN. Effects of chronic baroreceptor unloading on blood pressure in the dog. Am J Physiol Regulatory Integrative Comp Physiol. 2005;288:R863–R871. doi: 10.1152/ajpregu.00489.2004. [DOI] [PubMed] [Google Scholar]
- 15.Fu Q, Witkowski S, Arbab-Zadeh A, Prasad A, Okazaki K, Levine BD. Persistent sympathetic activation during chronic antihypertensive therapy. A potential mechanism for long term morbidity? Hypertension. 2005;45:513–521. doi: 10.1161/01.HYP.0000158312.63381.c1. [DOI] [PubMed] [Google Scholar]
- 16.Vongpatanasin W, Menon DV, Arbique D, Wang Z, Auchus RJ. Differential effects of chlorthalidone vs. spironolactone on muscle sympathetic nerve activity and insulin resistance in hypertensive patients. Hypertension. 2008;52:e-43. [Google Scholar]
- 17.Esler M, Stranznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48:787–796. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- 18.Shibao C, Gamboa A, Diedrich A, Ertl AC, Chen KY, Byrne DW, Farley G, Paranjape SY, Davis SN, Biaggioni Autonomic contribution to blood pressure and metabolism in obesity. Hypertension. 2007;49:27–33. doi: 10.1161/01.HYP.0000251679.87348.05. [DOI] [PubMed] [Google Scholar]
- 19.Scheffers I, Schmidli J, Kroon AA, Tordoir J, Mohaupt M, Allemann Y, Jordan J, Engeli S, Liebeskind U, Luft FC, Eckert S, Hansky B, Baal T, de Leeuw PW. Sustained blood pressure reduction by baroreflex hypertension therapy with a chronically implanted system: 2-year data from the RHEOS DEBUT-HT Study in patients with resistant hypertension. J Hypertens. 2008;26 Suppl 1:S19. [Google Scholar]