Abstract
Hepatocellular adenoma (HA) is a benign hepatic lesion that predominantly occurs in young women. Most hepatocellular carcinomas (HCC) arise in a cirrhotic liver during the fifth or sixth decades. There have been several reported cases of HCC developing from HA in female patients. However, there are rare cases about HCC arising in HA in a non-cirrhotic male patient. We have recently encountered a 53-year-old man who had a liver mass in a non-cirrhotic liver, and the liver mass was compatible with HA on the pre-operative computed tomography. The mass was completely resected and the histopathology revealed a focus of HCC arising in HA. We report here on this case along with a brief review of the relevant literature.
Keywords: Adenoma, Hepatocellular, Hepatocellular carcinoma
INTRODUCTION
Hepatocellular adenoma (HA) is a rare benign liver tumor that most frequently occurs in women. This neoplasm makes up two percent of all hepatic tumors. It occurs in non-cirrhotic liver parenchyme and it has an estimated prevalence of 1-3 per 100,000 young women with a long-standing history of oral contraceptive use [1]. HA typically develops in the setting of hormonal or metabolic abnormalities that stimulate hepatocyte proliferation. There have been many reports about the relationship between oral contraceptives and HA, and this relationship was first described by Banum, et al. [2]. However, the precise mechanism for this relationship has not yet been determined [3,4]. Besides oral contraceptives, there are other predisposing factors for HA such as anabolic steroids, diabetes mellitus, beta-thalassemia and glycogen storage disease type 1 [5-7]. Although the pathophysiology is not yet fully known, malignant transformation of HA into hepatocellular carcinoma (HCC) has been reported [8-10]. There are about 12 reported cases of malignant transformation in women and one case in a man from other countries in the medical literature [11,12]. However, only one case of HCC arising in HA in a man has been reported in Korea [13]. We report here on a case of HCC within HA in a non-cirrhotic male patient.
CASE REPORT
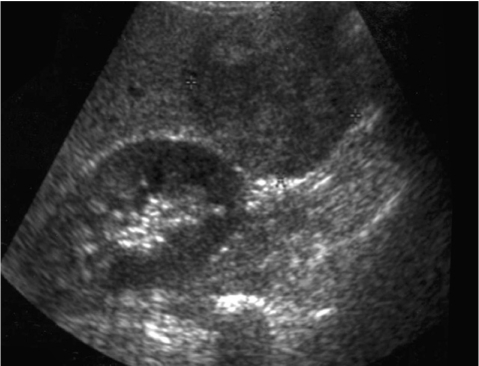
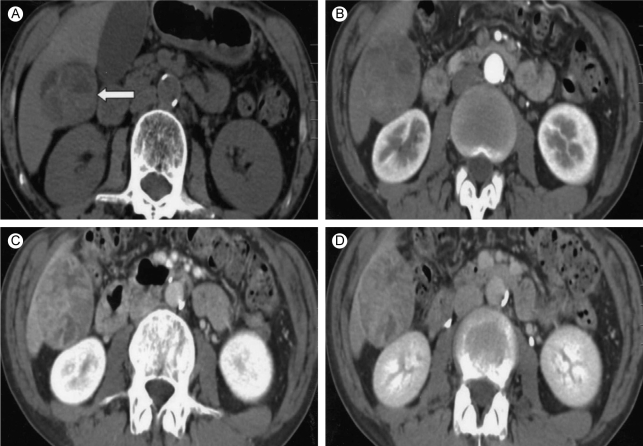
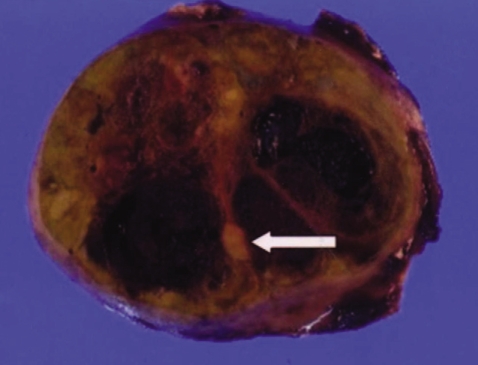
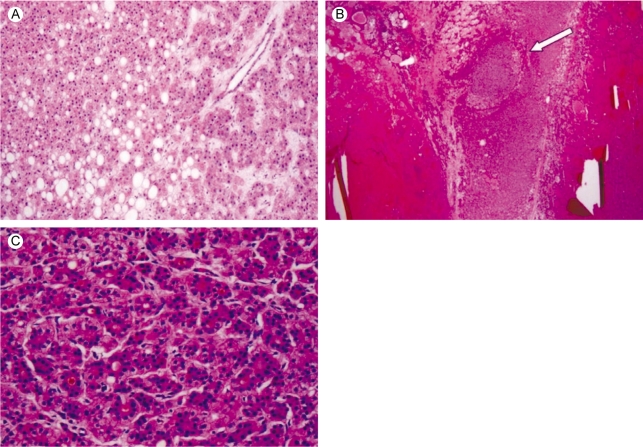
A 53-year-old man visited our hospital for evaluation of his vague abdominal discomfort. On the initial visit, the patient complained of having right upper quadrant discomfort and fatigue for the past 4 months. His body weight was the same over the recent years. His past medical history was unremarkable and he had no history of drug or alcohol abuse. The physical examination did not reveal any abnormal findings except for hoarseness due to heavy smoking (20 pack-years). The laboratory findings were as follows: white blood cell count 4,700/mm3, hemoglobin 13.8 g/dL, platelet count 270,000/mm3, aspartate aminotransferase 41 IU/L, alanine aminotransferase 38 IU/L, alkaline phosphatase 105 IU/L, gamma-glutamyl transpeptidase 46 IU/L and prothrombin time 100%. Serological tests for hepatitis B surface antigen (HBsAg), the antibody to the core antigen (anti-HBc), antibody to the hepatitis C virus (anti-HCV) and the markers for autoimmune liver disease were all negative and the serum levels of alpha-fetoprotein (AFP) and prothrombin induced by vitamin K deficiency or antagonist-II (PIVKA-II) were normal. Abdominal ultrasonography revealed an approximately 6.0 cm sized exophytic bulging mass that was focally cystic and heterogenous in the inferior portion of the right hepatic lobe (Fig. 1). The unenhanced image of multiphase helical computed tomography (CT) revealed a heterogenous solid exophytic hepatic mass with intratumoral hemorrhage (Fig. 2A). On the arterial and portal phases, a well-defined encapsulated mass with heterogenous contrast enhancement was noted (Fig. 2B and 2C). On the delayed phase, there was no definite appearance of washout (Fig. 2D). Based on the radiological findings, the patient was diagnosed with HA and so wedge resection of the hepatic mass was carried out. The resected specimen was a solitary bulging round mass that measured 4.5 cm in diameter. The cut section was well-demarcated, dark reddish and lobulated. Further, a partly hemorrhagic nodular feature was identified. A small round yellowish white nodule (about 1.0×0.9 cm) was noted within the mass (Fig. 3). The surrounding liver tissue revealed no fibrosis or cirrhotic change. Microscopic examination of the specimen revealed the typical feature of hepatocellular adenoma, including liver cell cords that were one to two cell thick, a slightly increased cell density and peliosis (Fig. 4A). In the adenoma, a small round well-demarcated lesion that showed the appearance of the typical trabecular type HCC was noted, and so this gave a pattern of a nodule within a nodule (Fig. 4B). The HCC area showed liver cell cords that were three or more cells thick, pseudoacinar formation and nuclear atypia (Edmondson-Steiner grade II) (Fig. 4C). The postoperative course was uneventful; the patient is now being followed-up and he is in a good condition.
Figure 1.
Abdominal ultrasonography revealed an approximately 6 cm sized exophytic bulging mass that was focally cystic and heterogenous in the inferior portion of the right lobe of the liver.
Figure 2.
Liver dynamic computed tomography findings. (A) On the non-contrast phase, the heterogenous density and fluid-fluid level suggested intratumoral hemorrhage (white arrow). Fat density is not evident. (B) On the arterial phase, heterogenous contrast enhancement was noted. (C) On the portal phase, the heterogenous contrast enhancement of the tumor persisted. (D) Some portion of the capsule was enhanced on the delayed phase.
Figure 3.
Macroscopic findings. The cut surface shows a well-demarcated round, lobulated and dark reddish mass with a partly hemorrhagic degenerated area, and the mass measured 4.5 cm in diameter. A small round yellowish white nodule (arrow) is noted within the mass, giving the pattern of a nodule within a nodule.
Figure 4.
Microscopic findings. (A) The liver cell cords are one to two cells thick and the cell density is slightly increased compared to the surrounding liver. Portal tracts are absent and focal steatosis is noted (haematoxylin-eosin stain, ×100). (B) A low power microscopic view reveals hepatocellular carcinoma (white arrow) arising in the hepatocellular adenoma (haematoxylin-eosin stain, ×12.5). (C) Microscopic findings of the hepatocelllular carcinoma area. Note the typical trabecular features are more than three cell in thickness (Edmondson-Steiner grade II), and pseudoacinar formation is also noted (haematoxylin-eosin stain, ×200).
DISCUSSION
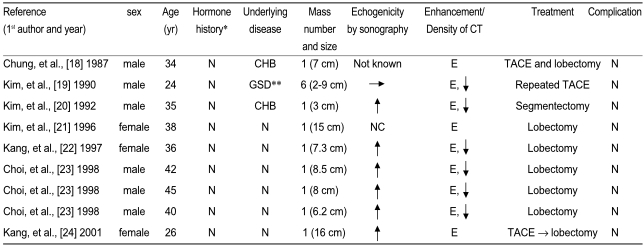
HA is a rare benign hepatic tumor [1], and there have been only a few reports of HA in Koreans. The characteristics of the reported cases are summarized in Table 1. HA has been reported to usually regress after cessation of hormonal stimulation [11]; however, it does have the potential of malignant transformation. This might represent an adenoma-carcinoma progression sequence in hepatocarcinogenesis, which is similar to that seen for colorectal cancer. However, such a transformation of HA into HCC is very unusual because most HAs are resected upon discovery. Therefore, only a few cases regarding HCC arising in HA have been reported in the medical literature.
Table 1.
The characteristics of the cases of hepatocellular adenomas reported in Korea
*Male-anabolic steroid, Female-Oral contraceptives; **not confirmed; N, none; CHB, chronic hepatitis B; GSD, glycogen storge disease; ↑, Increased; ↓, Decreased; →, isoechoic; E, enhanced; NC, not checked.
For most cases of HCC arising in HA, there is a medication history of oral contraceptives or other anabolic steroids [8-10] and in one previously reported case, a male patient had a history of nephrotic syndrome that was treated with oral prednisolone for 15 years [12]. However, it is interesting that the patient in our case hadn't any risk factors such as metabolic disease or a medication history, including steroid hormone.
The clinical symptoms of HA include abdominal pain and distension due to the presence of a mass, but in many instances the tumor is asymptomatic and it is diagnosed incidentally [4]. The liver function tests are generally normal, but a slight elevation of serum transaminases or alkaline phosphatase may be observed. AFP and PIVKA-II is usually not elevated in adenoma, but the tumor markers can be elevated for the cases of malignant transformation [9]. In this present case, both the AFP and PIVKA-II remained within the normal ranges.
The imaging modalities that are used for the diagnosis of HA include abdominal ultrasound, CT and magnetic resonance imaging. A percutaneous liver biopsy is controversial because the histology is often non-diagnostic and the procedure carries the risk of acute hemorrhage [4,5,8,14]. Although HA is first detected by ultrasound in many cases, the sonographic features are usually non-specific and they may mimic other benign or malignant hepatic lesions. Typically, contrast-enhanced CT scans may show peripheral enhancement during the early phase with subsequent centripetal flow during the portal venous phase in adenoma; in the late phase, the lesion may become isodense and then hypodense [14]. For the current case, the CT findings are somewhat atypical; on the arterial phase, the mass did not show peripheral enhancement, but rather, it showed heterogenous enhancement. On the portal and delayed phase, the lesion became isodense or slightly enhanced. In particular, on the delayed phase, some capsular portion of the mass appeared to retain contrast material. This might be due to old intratumoral hemorrhage, necrosis or the fat contents. For the pathologic finding, adenomas are composed of large plates of liver cells, which are typically larger than normal hepatocytes and they contain glycogen and lipid. The nuclei are small and regular, and mitoses are seldom seen. There is an absence of normal hepatic architecture and the cells are arranged in normal or thickened trabeculae interspersed with prominent arteries, thin walled blood vessels and sinusoids [14].
Although factors such as chronic liver disease in male patients or elevated tumor markers (AFP or PIVKA II) strongly indicate that a hepatic neoplasm is malignant, it is difficult to distinguish HA from well-differentiated HCC only by examining small biopsied tissues and occasionally even by examining the resected tumor specimens. Recently, gene analysis is one of the useful methods for distinguishing these tumors, although it is not practical as an ordinary diagnostic procedure. Multiple chromosomal aberrations detected by performing comparative genomic hybridization (CGH), including gains or losses in one or more than six chromosomes (1q, 4q, 8p, 8q, 16p and 19p), have been reported in HCC, but not in HA [17].
Until now, an established treatment modality for HA does not exist due to the small number of HA cases and the small number of cases with HCC arising in HA. Some investigators believe that that HA may regress over time, especially after cessation of hormonal contraception in females, and these researchers recommend resection only for adenomas larger than 5 cm in diameter or for a tumor that is increasing in size [11,25,26]. Yet other case reports have described female patients with hepatocellular adenoma that was confirmed by biopsy, and their tumor didn't regress after discontinuation of oral contraception, but instead it progressed into HCC within 3 to 7 years of follow-up. Furthermore, malignant transformation of HA may occur without an increase of tumor size or increased AFP levels. With this possibility of malignant transformation, there is also a high chance of rupture that can cause bleeding and mortality. Therefore, many investigators suggest that hepatic adenomas should be removed regardless of their size [9,26].
In conclusion, our case suggests that HCC can develop from HA in a non-cirrhotic male patient who was without any risk factors for HCC. Therefore, complete resection of HA might be required for properly treating this type of tumor.
References
- 1.Rooks JB, Ory HW, Ishak KG, et al. Epidemiology of hepatocellular adenoma: the role of oral contraceptive use. JAMA. 1979;242:644–688. [PubMed] [Google Scholar]
- 2.Baum JK, Bookstein JJ, Holtz F, Klein EW. Possible association between benign hepatomas and oral contraceptives. Lancet. 1973;2:926–929. doi: 10.1016/s0140-6736(73)92594-4. [DOI] [PubMed] [Google Scholar]
- 3.Edmonson H, Henderson B, Benton B. Liver-cell adenomas associated with use of oral contraceptives. N Engl J Med. 1976;294:470–472. doi: 10.1056/NEJM197602262940904. [DOI] [PubMed] [Google Scholar]
- 4.Klatskin G. Hepatic tumors: possible relationship to use of oral contraceptives. Gastroenterology. 1977;73:386–394. [PubMed] [Google Scholar]
- 5.Kerlin P, Davis GL, McGill DB, Weiland LH, Adson MA, Sheedv PF., 2nd Hepatic adenoma and focal nodular hyperplasia: clinical, pathologic, and radiologic features. Gastroenterology. 1983;84:994–1002. [PubMed] [Google Scholar]
- 6.Coire CI, Qizilbash AH, Castelli MF. Hepatic adenoma in type Ia glycogen storage disease. Arch Pathol Lab Med. 1987;111:166–169. [PubMed] [Google Scholar]
- 7.Leese T, Farges O, Bismuth H. Liver cell adenomas: a 12-year surgical experience from a specialist hepato-biliary unit. Ann Surg. 1988;208:558–564. doi: 10.1097/00000658-198811000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon SC, Reddy KR, Livingstone AS, Jeffers LJ, Schiff ER. Resolution of a contraceptive-steroid-induced hepatic adenoma with subsequent evolution into hepatocellular carcinoma. Ann Intern Med. 1986;105:547–549. doi: 10.7326/0003-4819-105-4-547. [DOI] [PubMed] [Google Scholar]
- 9.Gyorffy EJ, Bredfeldt JE, Black WC. Transformation of hepatic cell adenoma to hepatocellular carcinoma due to oral contraceptive use. Ann Intern Med. 1989;110:489–490. doi: 10.7326/0003-4819-110-6-489. [DOI] [PubMed] [Google Scholar]
- 10.Closset J, Veys I, Peny MO, et al. Retrospective analysis of 29 patients surgically treated for hepatocellular adenoma or focal nodular hyperplasia. Hepatogastroenterology. 2000;47:1382–1384. [PubMed] [Google Scholar]
- 11.Colovic R, Grubor N, Micev M, Radak V. Hepatocellular adenoma with malignant alteration. Hepatogastroenterology. 2007;54:386–388. [PubMed] [Google Scholar]
- 12.Chuang WY, Chen TC, Hsu HL, Lee WC, Jeng LB, Huang SF. Liver cell adenoma with concomitant hepatocellular carcinoma: report of two cases. J Formos Med Assoc. 2002;101:798–802. [PubMed] [Google Scholar]
- 13.Choe MS, Yu E. Hepatocellular carcinoma arising in hepatocellular adenoma. Korean J Hepatol. 2002;8:107–109. [PubMed] [Google Scholar]
- 14.Grazioli L, Federle MP, Brancatelli G, Ichikawa T, Olivetti L, Blacher A. Hepatic adenomas: imaging and pathologic findings. Radiographics. 2001;21:877–892. doi: 10.1148/radiographics.21.4.g01jl04877. [DOI] [PubMed] [Google Scholar]
- 15.Ito M, Sasaki M, Wen CY, et al. Liver cell adenoma with malignant transformation: a case report. World J Gastroenterol. 2003;9:2379–2381. doi: 10.3748/wjg.v9.i10.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anthony PP, Vogel CL, Barker LF. Liver cell dysplasia:a premalignant condition. J Clin Pathol. 1973;26:217–223. [PMC free article] [PubMed] [Google Scholar]
- 17.Nolte M, Werner M, Nasarek A, et al. Expression of proliferation associated antigens and detection of numerical chromosome aberrations in primary human liver tumours: relevance to tumour characteristics and prognosis. J Clin Pathol. 1998;51:47–51. doi: 10.1136/jcp.51.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung WJ, Lim PS, Lee HS, et al. A case of hepatocellular adenoma. Korean J Gastroenterol. 1987;19:644–649. [Google Scholar]
- 19.Kim CM, Lee JH, Lee HS, Kim CY, Park JH, Kim YI. A case of multiple hepatocellular adenoma in adult male. Korean J Gastroenterol. 1990;22:477–483. [Google Scholar]
- 20.Kim SH, Han NI, Han SW, et al. A case of pleomorphic hepatic adenoma in healthy carrier of hepatitis type B. Korean J Gastroenterol. 1992;24:381–387. [Google Scholar]
- 21.Han HJ, Yoon SK, Kim MH, et al. A case of large hepatocellular adenoma in adult female with no history of steroid use. Korean J Med. 1996;51:701–705. [Google Scholar]
- 22.Yeon JE, Park SH, Kim JH, et al. A case of hepatocellular adenoma. Korean J Med. 1997;52:131–136. [Google Scholar]
- 23.Choi NS, Kim BH, Lee SB, et al. Clinical review of hepatic adenoma reported in Korea. Korean J Med. 1998;54:627–632. [Google Scholar]
- 24.Kang SJ, Lee OJ, Jung KW, et al. A case of hepatocellular adenoma not associated with oral contraceptive in woman. Korean J Gastroenterol. 2001;38:444–448. [Google Scholar]
- 25.Charny CK, Jarnagin WR, Schwartz LH, et al. Management of 155 patients with benign liver tumors. Br J Surg. 2001;88:808–813. doi: 10.1046/j.0007-1323.2001.01771.x. [DOI] [PubMed] [Google Scholar]
- 26.Burri E, Steuerwald M, Cathomas G, et al. Hepatocellular carcinoma in a liver-cell adenoma within a non-cirrhotic liver. Eur J Gastroenterol Hepatol. 2006;18:437–441. doi: 10.1097/00042737-200604000-00020. [DOI] [PubMed] [Google Scholar]