Abstract
Females have a lower incidence of heart failure and improved survival after myocardial ischemia-reperfusion (I/R) compared with males. Although estrogen-suppressed cardiomyocyte apoptosis may be mediated through the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway, it is unclear whether this action is mediated via estrogen receptor β (ERβ). Therefore, we hypothesized that ERβ mediates estrogen-induced cardioprotection through PI3K/Akt and antiapoptotic signaling in females but not in males. Isolated male and female hearts from ERβ knockout (ERβKO) and wild-type (WT) mice (n = 5 mice/group) were subjected to 20-min ischemia followed by 60-min reperfusion (Langendorff). Ablation of ERβ significantly decreased postischemic recovery of left ventricular developed pressure in female, but not male, hearts. Reduced activation of PI3K and Akt was noted in female ERβKO hearts, which was associated with increased expression of caspase-3 and -8, as well as decreased Bcl-2 levels compared with WT. However, myocardial STAT3, SOCS3 (suppressor of cytokine signaling 3), VEGF, and TNF receptors 1 and 2 levels did not change in ERβKO of either sex following I/R. Furthermore, deficiency of ERβ increased myocardial JNK activation in females but increased ERK1/2 activity in males during acute I/R. We conclude that ERβ mediates myocardial protection via upregulation of PI3K/Akt activation, decreased caspase-3 and -8, and increased Bcl-2 in female hearts following I/R. These findings provide evidence of ERβ-mediated PI3K/Akt and antiapoptotic signaling in the myocardium and may lend insight into the mechanistic pathways behind the observed variation in clinical outcomes between males and females after myocardial infarction.
Keywords: ischemia-reperfusion, apoptotic signaling, cardioprotection
myocardial ischemia-reperfusion (I/R) injury occurs during cardiac surgery and leads to production of inflammatory cytokines, as well as cardiomyocyte apoptosis and necrosis, all of which exacerbate postischemic myocardial dysfunction. Although there have been many studies focused on minimizing the adverse effects of I/R injury, few have resulted in significant clinical benefits. Sex differences have been noted in the myocardial response to ischemic injury with females exhibiting improved cardiac function, diminished inflammatory response, and reduced apoptotic signaling (17, 19). Studies from our group (25) and others (10) have further demonstrated that estrogen mediates cardioprotection in females following acute I/R. However, this notion was recently challenged by controversial results from clinical trials that did not demonstrate a cardioprotective effect of hormone replacement therapy on postmenopausal females (16). This led to a recognition that estrogen-mediated cardioprotection appears more complicated than originally thought and requires more research.
The effects of estrogen are mediated mostly through estrogen receptor α (ERα) and/or estrogen receptor β (ERβ), both of which are expressed in the heart and have been involved in regulating cardioprotection (9, 22). In fact, deficiency of the ERβ gene worsens cardiac dysfunction following myocardial ischemic injury (2, 6, 13). In addition, a selective ERβ agonist, 2,3-bis(4-hydroxyphenyl)-propionitrile (DPN), protects the myocardium from acute ischemia in ovariectomized (OVX) females (11). Furthermore, 17β-estradiol (E2) has been shown to suppress cardiomyocyte apoptosis via activation of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway during myocardial infarction (12, 15). However, it is unknown whether this action is mediated through ERβ. On the other hand, our group has previously demonstrated TNF receptor 1 (TNFR1) signaling resistance as well as upregulated signal transduction and activator of transcription 3 (STAT3)/suppressor of cytokine signaling 3 (SOCS3)/VEGF cascade in female hearts following I/R (23, 24). It is important to elucidate whether ERβ plays a role in these pathways.
Therefore, with the use of mice containing a targeted deletion of ERβ, this study aimed to determine 1) whether ERβ mediates protection of cardiac function through PI3K/Akt and antiapoptotic signaling in females, but not males, following acute I/R injury and 2) whether ERβ may facilitate this cardioprotection via TNFR/STAT3/SOCS3/VEGF-mediated cascades.
MATERIALS AND METHODS
Animals.
A total of 20 male and female C57BL/6J mice (16 ± 4 wk) with and without ERβ deficiency (ERβKO; Taconic Farms, Hudson, NY) were fed a standard diet and acclimated in a quiet quarantine room for more than 1 mo before the experiments. The animal protocol was reviewed and approved by the Indiana Animal Care and Use Committee of Indiana University. All animals received humane care in compliance with the “Guide for the Care and Use of Laboratory Animals” [DHEW Publication No. (NIH) 85-23, Revised 1996, Office of Science and Health Reports, DRR/NIH, Bethesda, MD 20205].
All isolated mouse hearts were subjected to the same I/R protocol as described previously (23, 24). Mouse hearts were divided into four experimental groups: normal males (n = 5), ERβKO males (n = 5), normal females (n = 5), and ERβKO females (n = 5).
Isolated heart preparation (Langendorff).
Experiments were performed with the use of a Langendorff apparatus as described previously for mouse hearts (24). The left ventricular developed pressure (LVDP) was continuously recorded using a PowerLab 8 preamplifier/digitizer (AD Instruments, Milford, MA) and an Apple G4 PowerPC computer (Apple Computer, Cupertino, CA).
Western blotting.
Western blot analysis was performed to measure PI3K/Akt signaling, STAT3 activation, SOCS3 expression, apoptotic proteins (caspase-3 and -8 and Bcl-2), and mitogen-activated protein kinases [MAPKs: p38 MAPK, c-Jun NH2-terminal kinase (JNK), and extracellular signal-regulated protein kinase p42/p44 (ERK1/2)]. Heart tissue was homogenized in cold radioimmunoprecipitation assay (RIPA) buffer (product no. R 0278; Sigma, St. Louis, MO) and was centrifuged at 12,000 rpm for 10 min. The protein extracts (15 μg/lane) were subjected to electrophoresis on a 4–12% Bis-Tris protein gel (Invitrogen, Carlsbad, CA) and transferred to a nitrocellulose membrane. The membranes were incubated in 5% dry milk for 1 h and then incubated with the following primary antibodies: PI3K, p-PI3K, Akt, p-Akt, STAT3, p-STAT3 (Tyr705), p38 MAPK, p-p38 MAPK, JNK, p-JNK (Thr183/Tyr185), ERK1/2, and p-ERK1/2 (Thr180/Tyr182) (1:1,000 dilution; Cell Signaling Technology, Beverly, MA); caspase-3 and -8 and SOCS3 (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA); Bcl-2 (Oncogene Research Products, San Diego, CA); and GAPDH (1:5,000 dilution, Biodesign International, Saco, ME). Membranes were then incubated with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG secondary antibody (Pierce, Rockford, IL), and detection was performed using SuperSignal West Pico stable peroxide solution (Pierce). Films were scanned using an Epson Perfection 3200 scanner (Epson America, Long Beach, CA), and band density was analyzed using ImageJ software (NIH, Bethesda, MD).
Enzyme-linked immunosorbent assay.
Myocardial VEGF levels in the cardiac tissue were determined by enzyme-linked immunosorbent assay (ELISA) using a commercially available ELISA set (Duo Set ELISA development system; R&D Systems, Minneapolis, MN). ELISA was performed according to the manufacturer's instructions. All samples and standards were measured in duplicate.
Presentation of data and statistical analysis.
All reported values are means ± SE. Data were compared using one-way ANOVA with post hoc Tukey's test or Student's t-test (male WT vs. female WT, male WT vs. male ERβKO, and female WT vs. female ERβKO). A two-tailed P value of <0.05 was considered statistically significant.
RESULTS
Myocardial function.
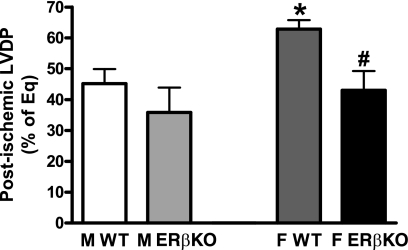
LVDP at the end of reperfusion was exhibited as a percentage of equilibration. I/R injury decreased myocardial LVDP in both WT and ERβKO mice (Fig. 1). Deficiency of the ERβ gene did not significantly affect myocardial recovery of LVDP in male hearts following I/R (35.9 ± 8 vs. WT 45.2 ± 4.7%), whereas ERβKO markedly decreased LVDP recovery in female hearts (43 ± 6.3%) compared with female WT (62.9 ± 2.9%, P < 0.05). In addition, sex difference existed in I/R-depressed myocardial function with improved LVDP in female WT hearts compared with male WT. However, ablation of the ERβ gene neutralized this difference following I/R.
Fig. 1.
Myocardial left ventricular developed pressure (LVDP) at the end of ischemia-reperfusion (I/R), represented as a percentage of equilibration (% of Eq), in hearts of wild-type (WT) and estrogen receptor β knockout (ERβKO) mice of both sexes. M, male; F, female. Deficiency of the ERβ gene decreased postischemic myocardial LVDP in female, but not male, hearts. Results are means ± SE; n = 5 mice/group. *P < 0.05 vs. male WT. #P < 0.05 vs. female WT.
Effects of ERβ on myocardial PI3K/Akt signaling during acute I/R.
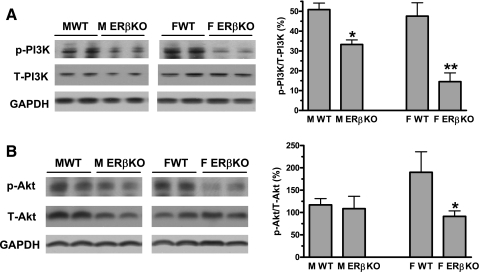
Myocardial ischemia increases activation of the PI3K/Akt pathway, which in turn mediates cardiomyocyte survival in response to I/R injury. Ablation of the ERβ gene significantly decreased myocardial PI3K/Akt activation in females after I/R. ERβKO reduced activation of PI3K from 47.6% (percentage of p-PI3K/total PI3K) in WT to 14.6% (Fig. 2A) and Akt from 190% (percentage of p-Akt/total Akt) to 91.3% in female hearts (Fig. 2B). Although deficiency of ERβ decreased PI3K activation, the KO did not affect myocardial Akt activation in males (Fig. 2).
Fig. 2.
Activation of myocardial phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling in WT and ERβKO hearts of both sexes following I/R. A: ablation of ERβ reduced PI3K activation in both sexes, but with relatively greater effect in female hearts. B: ERβKO decreased myocardial Akt activity only in female, not male, hearts. Representative immunoblots (2 lanes/group) are shown at left. Densitometry data [percentages of phosphorylated (p) to total (T) protein] are shown at right. Results are means ± SE. *P < 0.05; **P < 0.01 vs. corresponding WT. Experiments were repeated in 2 different sessions.
Effects of ERβ on myocardial apoptotic proteins after I/R injury.
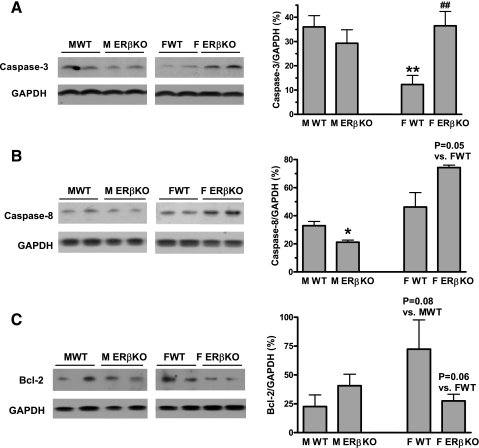
Given that the experimental period in this study is too brief (20-min ischemia followed by 60-min reperfusion) to detect significant apoptosis, we measured proapoptotic signaling by examining caspase-3 and -8 and antiapoptotic protein Bcl-2 expression instead. ERβKO significantly increased myocardial expression of caspase-3 and -8 and reduced Bcl-2 in females following I/R (Fig. 3). This suggests that ERβ may mediate antiapoptotic signaling and improve cardiomyocyte survival in female hearts. However, ablation of ERβ appeared to have an opposite effect in males, since a trend of decreased caspase-3 and -8 and enhanced Bcl-2 levels were noted in male ERβKO hearts (Fig. 3). It is possible that sex differences exists in ERβ-mediated apoptotic signaling following acute I/R.
Fig. 3.
Expression of myocardial caspase-3, caspase-8, and Bcl-2 in ERβKO and WT hearts of both sexes after I/R. A: increased caspase-3 activity was noted in female, but not male, ERβKO hearts. B: deficiency of the ERβ gene increased caspase-8 activity in female hearts but decreased its activity in male hearts following I/R. C: ERβKO decreased antiapoptotic protein Bcl-2 in female hearts. Representative immunoblots (2 lanes/group) are shown at left and densitometry bar graphs (%GAPDH) at right. Results are means ± SE. *P < 0.05; **P < 0.01 vs. male WT. ##P < 0.01 vs. female WT. Experiments were repeated in 2 different sessions.
Effects of ERβ on myocardial TNFR/STAT3/SOCS3/VEGF cascade after I/R injury.
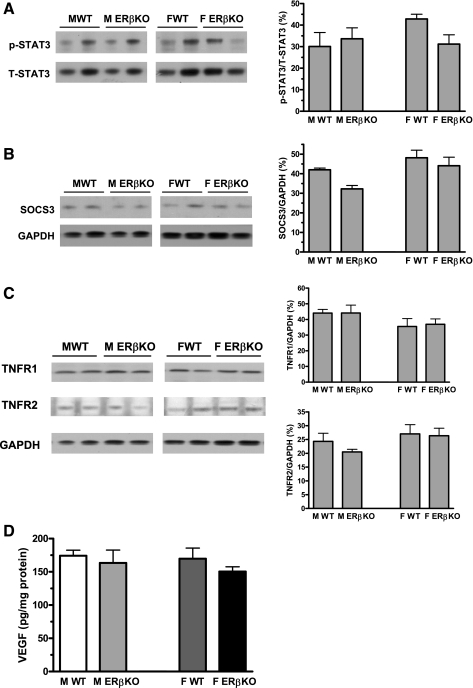
Although a trend of decreased STAT3 activation was observed in female ERβKO hearts, deficiency of ERβ did not significantly change myocardial STAT3 activation, TNFR1 and TNFR2 expression, SOCS3 levels, or VEGF production in either sex after acute I/R (Fig. 4).
Fig. 4.
Activation of myocardial STAT3 (A), expression of suppressor of cytokine signaling 3 (SOCS3; B), TNF receptor 1 and 2 (TNFR1 and TNFR2; C), and VEGF (D) in ERβKO and WT hearts of both sexes after I/R. ERβKO did not affect myocardial TNFR/STAT3/SOCS3/VEGF cascades following I/R. Representative immunoblots (2 lanes/group) are shown at left and densitometry bar graphs at right in A–C. ELISA data for VEGF are shown in D. Results are means ± SE.
Effects of ERβ on myocardial activation of MAPKs after I/R injury.
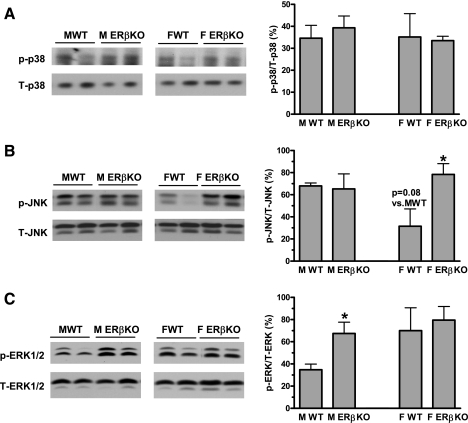
MAPK pathways (p38 MAPK, JNK, and ERK1/2) play important roles in myocardial inflammation, cardiac function, and cardiomyocyte death following I/R. In males, ablation of ERβ did not affect myocardial activation of p38 MAPK and JNK, whereas increased ERK1/2 activation was noted in the KO hearts (Fig. 5). Interestingly, enhanced JNK activation was observed in female ERβKO myocardium in response to I/R, whereas activation of p38 MAPK and ERK1/2 was not changed by deficiency of ERβ in females (Fig. 5). This finding suggests that specific sex differences exist in ERβ-mediated MAPK signaling.
Fig. 5.
Activation of myocardial p38 MAPK (A), JNK (B), and ERK1/2 (C) in ERβKO and WT hearts of both sexes after I/R. Ablation of ERβ did not affect myocardial p38 MAPK activation in either sex (A). However, ERβKO significantly increased JNK activation in female hearts (B) and elevated ERK1/2 activity in males following I/R (C). Representative immunoblots (2 lanes/group) are shown at left and densitometry bar graphs at right. Results are means ± SE. *P < 0.05 vs. corresponding WT. Experiments were repeated in 2 different sessions.
DISCUSSION
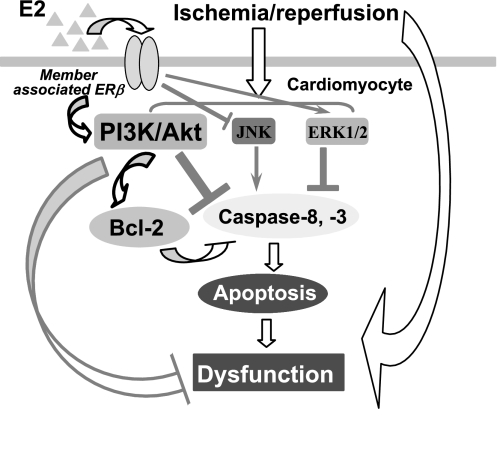
Accumulating evidence has demonstrated that ERβ is involved in mediating E2-induced cardioprotection in females following I/R combined with isoproterenol-induced hypercontractile condition (2, 11). In the present study, by utilizing mice lacking ERβ, we found that ERβ mediates acute myocardial functional protection likely through activation of PI3K/Akt pathway and decreased apoptotic signaling following I/R in females (presumably having a higher baseline levels of endogenous estrogen compared with males) but not in males (Fig. 6).
Fig. 6.
Simplified schematic illustrates how the ERβ mediates activation of PI3K/Akt and apoptotic pathway and thus protects the female myocardium in response to I/R. ERβ-regulated JNK and ERK1/2 signaling is also shown.
ERβ is a member of the nuclear receptor gene family of transcription factors and may play an important role in protecting against myocardial dysfunction caused by I/R injury. Previously, we indicated that E2, a nonselective ER ligand, improved functional recovery, reduced myocardial inflammation, and diminished proapoptotic signaling following acute ischemia in males as well as OVX females (25). Recently, deficiency of ERβ was shown to worsen postischemic myocardial dysfunction in female hearts with hypercontractile condition (2). In addition, DPN, a selective ERβ agonist, restored E2-mediated cardiac protection in OVX females under conditions of enhanced contractility following I/R injury (11). In our study, significantly decreased recovery of LVDP was noted in female ERβKO hearts compared with female WT after acute I/R, whereas ablation of ERβ did not affect postischemic myocardial functional recovery in males. This result further confirms that ERβ mediates protection of myocardial function not only in female hearts associated with isoproterenol-enhanced contractility (2, 11) but also in normal females during acute I/R.
ERβ has been shown to mediate E2-induced cardioprotection through genomic mechanisms including regulation of metabolic gene expression (2, 11) and modulation of ion channel expression and calcium-handling protein (6, 13). However, it is also possible that ERβ may be located in the plasma membrane and mediates nongenomic events trigged by E2 (18). Akt and its upstream signal, PI3K, play critical roles in mediating cell survival and apoptosis (12). It is evident that the E2-activated PI3K/Akt pathway functions as one of the acute nongenomic actions of E2 in various types of cells (18). Upregulation of PI3K/Akt by administration of E2 results in endothelial nitric oxide synthase activation via a transcription-independent mechanism (3). In addition, activation of the PI3K/Akt pathway is required for E2-suppressed apoptosis and E2-protected myocardial function in the heart following ischemia (12). Although most studies on the interaction of estrogen receptor with the PI3K/Akt pathway have indicated a crucial role for ERα as a membrane-associated receptor engaged in this action (18), little information exists regarding the importance of ERβ in the E2-mediated nongenomic effect of PI3K/Akt activation. In this study, we found significantly reduced activation of the PI3K/Akt pathway in female ERβKO hearts, but not in males, following acute I/R. This provides evidence that ERβ is also involved in PI3K/Akt-regulated cardioprotection in females. In fact, ERβ has been shown to mediate E2-induced antiapoptotic effects through the PI3K/Akt pathway in skeletal muscle cells (21). In addition, administration of E2 protects cardiac H9c2 cells from oxidative stress-induced apoptosis through ERβ-activated Akt signaling (20). Consistent with these observations, we have demonstrated that deficiency of the ERβ gene significantly increases levels of proapoptotic proteins caspase-3 and -8 while reducing antiapoptotic protein Bcl-2 levels in female hearts in response to acute I/R.
E2-regulated Akt activation also may mediate myocardial protection via additional mechanisms. Previous studies have demonstrated, for example, that OVX-reduced Akt activation occurs with decreased myocyte contractile function and impaired intracellular calcium handling, whereas E2-upregulated Akt is associated with restored cardiac contractility and intracellular calcium homeostasis (15). This suggests that Akt signaling may play a role in E2-mediated protection of myocardial function in addition to reduction of apoptosis. Indeed, activation of the Akt pathway also has been linked to a lower incidence of arrhythmias after myocardial infarction (14). In addition, delivery of a constitutively active Akt mutant gene markedly improves myocardial function in vivo following acute I/R and protects hypoxia-induced cardiomyocyte dysfunction in vitro, likely by preventing hypoxia-induced abnormalities in calcium transients and shortening (7). Therefore, it is possible that decreased activation of Akt may lead to reduced myocardial recovery of LVDP in female ERβKO hearts following acute I/R in our study.
TNFR1 signaling has been shown to mediate detrimental effects of TNF on the myocardium (8, 24), whereas the TNFR2 pathway conducts protective effects (4, 23). Evidence suggests that the balance of TNFR1 and TNFR2 signaling shifts in favor of TNFR2 and its beneficial effects in female hearts during I/R (23, 24). However, it is unclear whether ERβ is engaged in the regulatory balance of the TNFR1 and TNFR2 pathways. In addition, TNFR2-induced cardioprotection appears to be mediated through STAT3, SOCS3, and VEGF in female hearts. STAT3 has been shown to be a direct target gene for estradiol (1); administration of E2 upregulates STAT3 activation, which partially mediates nongenomic effects of E2 (1). Therefore, the question arises whether ERβ is involved in regulating myocardial STAT3/SOCS3/VEGF cascades in females. In this study, we found that ablation of ERβ did not affect myocardial expression of TNFR1 or TNFR2. In addition, ERβKO did not change myocardial STAT3/SOCS3/VEGF signaling following I/R. However, further investigation is required to determine whether the myocardial TNFR/STAT3/SOCS3/VEGF cascade is related to E2-mediated cardiac protection and, if so, whether ERα plays a role in this action.
MAPKs (p38 MAPK, JNK, and ERK1/2) have been shown to mediate myocardial responses during injury. Activity of p38 MAPK and JNK is related to myocardial dysfunction (5), whereas ERK1/2 activation improves cardiac functional recovery following I/R (22). In addition, E2 has been reported to block hypoxia-induced activation of p38 MAPK and JNK, thus protecting the myocardium (5, 25). Conversely, E2-increased activation of ERK1/2 has been shown to inhibit cardiomyocyte apoptosis during myocardial ischemia (22). It is evident that both ERα and ERβ are involved in regulating myocardial MAPKs (5, 22). In fact, ERα has been shown to upregulate activation of the protective ERK1/2, decrease the proapoptotic JNK activation, and improve myocardial function in females during acute ischemia (22). In the present study, we further found significantly increased JNK activation in female ERβKO hearts but elevated ERK1/2 activation in male ERβKO following I/R. This suggests that sex differences exist in ERβ-mediated MAPK signaling in the hearts in response to acute ischemic injury.
Perspectives and Significance
This study provides the direct evidence of ERβ-mediated cardioprotection in female hearts following acute I/R. Improved myocardial function is associated with ERβ-activated PI3K/Akt pathway and subsequently decreased apoptotic signaling in female hearts in response to I/R. Further investigation is required to elucidate the detailed mechanisms on the specific function of ER subtypes in the heart. Understanding effects of estrogen and estrogen receptors may help in advancing therapeutic manipulations in menopausal females and, potentially, males.
GRANTS
This work was supported in part by National Institutes of Health Grants R01 GM070628, R01 HL085595, and K99/R00 HL0876077.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bjornstrom L, Sjoberg M. Signal transducers and activators of transcription as downstream targets of nongenomic estrogen receptor actions. Mol Endocrinol 16: 2202–2214, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Gabel SA, Walker VR, London RE, Steenbergen C, Korach KS, Murphy E. Estrogen receptor beta mediates gender differences in ischemia/reperfusion injury. J Mol Cell Cardiol 38: 289–297, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res 87: 677–682, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Higuchi Y, McTiernan CF, Frye CB, McGowan BS, Chan TO, Feldman AM. Tumor necrosis factor receptors 1 and 2 differentially regulate survival, cardiac dysfunction, and remodeling in transgenic mice with tumor necrosis factor-alpha-induced cardiomyopathy. Circulation 109: 1892–1897, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Kim JK, Pedram A, Razandi M, Levin ER. Estrogen prevents cardiomyocyte apoptosis through inhibition of reactive oxygen species and differential regulation of p38 kinase isoforms. J Biol Chem 281: 6760–6767, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Korte T, Fuchs M, Arkudas A, Geertz S, Meyer R, Gardiwal A, Klein G, Niehaus M, Krust A, Chambon P, Drexler H, Fink K, Grohe C. Female mice lacking estrogen receptor beta display prolonged ventricular repolarization and reduced ventricular automaticity after myocardial infarction. Circulation 111: 2282–2290, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation 104: 330–335, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Meldrum DR Tumor necrosis factor in the heart. Am J Physiol Regul Integr Comp Physiol 274: R577–R595, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science 308: 1583–1587, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 340: 1801–1811, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Nikolic I, Liu D, Bell JA, Collins J, Steenbergen C, Murphy E. Treatment with an estrogen receptor-beta-selective agonist is cardioprotective. J Mol Cell Cardiol 42: 769–780, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Patten RD, Pourati I, Aronovitz MJ, Baur J, Celestin F, Chen X, Michael A, Haq S, Nuedling S, Grohe C, Force T, Mendelsohn ME, Karas RH. 17beta-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ Res 95: 692–699, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Pelzer T, Loza PA, Hu K, Bayer B, Dienesch C, Calvillo L, Couse JF, Korach KS, Neyses L, Ertl G. Increased mortality and aggravation of heart failure in estrogen receptor-beta knockout mice after myocardial infarction. Circulation 111: 1492–1498, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Ravingerova T, Matejikova J, Neckar J, Andelova E, Kolar F. Differential role of PI3K/Akt pathway in the infarct size limitation and antiarrhythmic protection in the rat heart. Mol Cell Biochem 297: 111–120, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Ren J, Hintz KK, Roughead ZK, Duan J, Colligan PB, Ren BH, Lee KJ, Zeng H. Impact of estrogen replacement on ventricular myocyte contractile function and protein kinase B/Akt activation. Am J Physiol Heart Circ Physiol 284: H1800–H1807, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 288: 321–333, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Simon T, Mary-Krause M, Funck-Brentano C, Jaillon P. Sex differences in the prognosis of congestive heart failure: results from the Cardiac Insufficiency Bisoprolol Study (CIBIS II). Circulation 103: 375–380, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407: 538–541, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tunstall-Pedoe H, Morrison C, Woodward M, Fitzpatrick B, Watt G. Sex differences in myocardial infarction and coronary deaths in the Scottish MONICA population of Glasgow 1985 to 1991. Presentation, diagnosis, treatment, and 28-day case fatality of 3991 events in men and 1551 events in women. Circulation 93: 1981–1992, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Urata Y, Ihara Y, Murata H, Goto S, Koji T, Yodoi J, Inoue S, Kondo T. 17beta-Estradiol protects against oxidative stress-induced cell death through the glutathione/glutaredoxin-dependent redox regulation of Akt in myocardiac H9c2 cells. J Biol Chem 281: 13092–13102, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Vasconsuelo A, Milanesi L, Boland R. 17beta-Estradiol abrogates apoptosis in murine skeletal muscle cells through estrogen receptors: role of the phosphatidylinositol 3-kinase/Akt pathway. J Endocrinol 196: 385–397, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Wang M, Crisostomo P, Wairiuko GM, Meldrum DR. Estrogen receptor-α mediates acute myocardial protection in females. Am J Physiol Heart Circ Physiol 290: H2204–H2209, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Crisostomo PR, Markel TA, Wang Y, Meldrum DR. Mechanisms of sex differences in TNFR2-mediated cardioprotection. Circulation 118: S38–S45, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang M, Tsai BM, Crisostomo PR, Meldrum DR. Tumor necrosis factor receptor 1 signaling resistance in the female myocardium during ischemia. Circulation 114: I282–289, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Wang M, Tsai BM, Reiger KM, Brown JW, Meldrum DR. 17-beta-Estradiol decreases p38 MAPK-mediated myocardial inflammation and dysfunction following acute ischemia. J Mol Cell Cardiol 40: 205–212, 2006. [DOI] [PubMed] [Google Scholar]