Abstract
Medial thickening and vascular hypertrophy of resistance arteries can lead to cardiovascular complications associated with diabetes. While previous studies have established a role of Type 1 diabetes in vascular remodeling, we recently extended these observations to Type 2 diabetes and reported increased collagen deposition due to alterations in matrix metalloproteinase expression and activity in mesenteric resistance arteries. These studies also showed that remodeling response was mediated by endothelin-1 (ET-1) via activation of ETA receptors, whereas blockade of ETB receptors exacerbated the remodeling. However, the effectiveness of glycemic control strategies in preventing these vascular changes, including activation of the ET system still remained unclear. Also, very little is known about whether and to what extent reorganization of the extracellular matrix (ECM) affects vascular compliance and vasomotor tone. Accordingly, this study assessed structural remodeling of mesenteric microvessels, vascular compliance, and myogenic tone, as well as the role of matrix metalloproteinases (MMP) in mediating these processes. Spontaneously diabetic, non-obese Goto-Kakizaki (GK) rats, a model for Type 2 diabetes, and normoglycemic Wistar rats were used for the studies. A subset of GK rats were administered metformin to achieve euglycemia. Glycemic control normalized the increased media-to-lumen ratios (M/L) and myogenic tone seen in diabetes, as well as normalizing plasma ET-1 levels and mesenteric ETA receptor expression. There was increased collagen synthesis in diabetes paralleled by decreased collagenase MMP-13 activity, while glycemic control attenuated the process. These findings and our previous study taken together suggest that hyperglycemia-mediated activation of ET-1 and ETA receptors alter vascular structure and mechanics in Type 2 diabetes.
Keywords: vascular remodeling, vascular compliance, glycemic control, extracellular matrix, endothelin receptors
cardiovascular disease is the leading cause of mortality in the United States, and the presence of diabetes further increases the risk of morbidity and mortality by two- to four-fold (1, 3). Although mechanisms may vary between vascular beds, dysregulation of vascular function and structure is a common pathology in both macrovascular and microvascular complications associated with diabetes. Alterations in vessel morphology due to restructuring of extracellular matrix (ECM) components and vascular smooth muscle cells (VSMC) is referred to as vascular remodeling, and several groups have reported on it in various animal models of hypertension and diabetes (17, 19, 27). In the streptozotocin (STZ)-treated rat model of Type 1 diabetes, there is medial thickening, thereby increasing the functional media-to-lumen ratio (6, 7, 27, 39). These findings in Type 1 diabetes cannot be extrapolated due to extremely high blood glucose levels in STZ rats and the manual tissue fixation techniques employed thus far for morphometric evaluation of the vasculature. Several studies have shown that media thickness to lumen diameter ratio (M/L ratio) is increased in the small arteries of patients with Type 2 diabetes (26, 33). While this response has been attributed to remodeling and hypertrophy in the vessel wall, potential mechanisms underlying this hypertrophic remodeling were not fully understood. We recently demonstrated increased collagen deposition due to alterations in matrix metalloproteinase (MMP) expression and activity in mesenteric resistance arteries in GK rats, a lean, spontaneous model of Type 2 diabetes (30). These studies also showed that remodeling response was mediated by endothelin-1 (ET-1) via differential activation of ET receptors. Blockade of ETA receptors partially prevented this response, whereas blockade of ETB receptors exacerbated the remodeling (30). Another recent study by our group showed increased mesenteric ETA receptor expression in this model (28). However, whether glycemic control alone can prevent these changes in the ET system, as well as associated microvascular remodeling remains unknown.
Vascular compliance, defined as the ability of a vessel to distend and increase volume with increasing transmural pressure, may be affected by structural changes in the vessel wall. It has been reported that myogenic tone, which is an indicator of the vessel's ability to constrict in response to increasing intraluminal pressure, is increased in ophthalmic arteries in experimental models of Type 2 diabetes (20, 21). Schofield et al. (33) reported that distensibility of small arteries isolated from subcutaneous fat of gluteal biopsy specimens is increased in patients with Type 2 diabetes compared with control patients. These vessels also presented with significant wall hypertrophy and decreased myogenic reactivity in response to increasing intraluminal pressure. Impairment of vascular relaxation highly correlated with the degree of dyslipidemia in these patients (33). The effect of moderate hyperglycemia alone on myogenic reactivity and vessel mechanics in Type 2 diabetes remained to be established. Building on these past reports and our studies, which demonstrated that vascular remodeling in diabetes in both the mesenteric and cerebral circulations is due to upregulation of the endothelin system, our hypothesis was that glycemic control with metformin would prevent Type 2 diabetes-mediated impairment of the structure and compliance of mesenteric arteries, as well as the activation of the profibrotic ET-1/ETA receptor pathway.
METHODS
Animals.
All experiments were performed on male control Wistar (Harlan, Indianapolis, IN) and diabetic GK (in-house bred, derived from the Tampa colony) rats (35). The animals were housed at the Medical College of Georgia animal care facility, approved by the American Association for Accreditation of Laboratory Animal Care. All protocols were approved by the Institutional Animal Care and Use Committee. Animals were fed standard rat chow and tap water ad libitum until death, housed in individual cages, and maintained on a 12:12-h light-dark cycle. Metformin was administered in drinking water to a subset of GK rats, and dose was titrated for every animal starting from a dose of 150 mg·kg−1·day−1 to a maximum of 300 mg·kg−1·day−1 such that the minimum concentration necessary to achieve euglycemia (blood glucose <120 mg/dl) was used. The average dose used was (278 ± 16 mg·kg−1·day−1). Treatment was initiated after the onset of diabetes in GK rats (6 to 8 wk of age) and continued until death at 18 wk of age. Blood glucose was measured twice weekly from the tail vein using a commercial glucometer (Freestyle, Alameda, CA) for all of the groups. Long-term glucose control was assessed from glycosylated hemoglobin values (A1C, Metrika, Sunnyvale, CA). Blood pressure was monitored either by the tail cuff method, which we have previously validated on telemetry-implanted animals (10). At death, the animals were anesthetized with pentobarbital sodium and exsanguinated via cardiac puncture. Plasma was obtained from heparinized blood for measurement of insulin and ET-1 levels by enzyme-linked immunosorbant assay kits (Alpco Diagnostics, Windham, NH). Plasma triglycerides and cholesterol were measured using commercially available kits (Wako USA, Richmond, VA). The mesenteric bed was harvested and immersed in ice-cold (calcium-free) Krebs-HEPES buffer; third-order mesenteric arteries were isolated to study their structure and mechanical properties. The remaining arteries were processed for MMP zymography and immunoblotting.
Vascular structure.
Vascular structure was assessed by morphometric analysis of Masson trichrome-stained mesenteric artery cross sections. Third-order mesenteric microvessels were fixed in formalin using the quick-transfer freezing chamber (Living Systems Instrumentation, Burlington, VT), wherein, they were maintained at a constant intraluminal pressure in calcium-free Krebs-HEPES buffer (50 mmHg for 30 min) and frozen immediately. This procedure corrected for variations in vascular structure due to inconsistencies in manual perfusion. Images were captured and wall thickness, lumen, and outer diameter were measured from Masson stained cross sections using SPOT software (Diagnostic Instruments, Sterling Heights, MI).
Collagen deposition patterns were evaluated in mesenteric artery cross sections stained with picrosirius red captured under polarized light, as previously described (31). Mature collagen stained red, whereas newly formed collagen stained green. Collagen turnover was quantified using Metamorph software (Molecular Devices, Sunnyvale, CA) by measuring the intensities of green- and red-stained regions. Collagen type 1 (Rabbit polyclonal antibody, Calbiochem, San Diego, CA) levels were evaluated by slot-blot analysis to confirm picrosirius staining results, as previously described (10). Protein levels were normalized using β-actin (A3854; Sigma Aldrich, St. Louis, MO) as a loading control.
Vascular mechanics.
Third-order mesenteric artery segments were mounted on two glass cannulas (100–150 μm in diameter) and secured using 10–0 ophthalmic sutures in a small vessel arteriograph (Living Systems Instrumentation, Burlington, VT). The distal cannula was closed off for a blind-sac experiment in conditions of zero-flow, and the system was maintained at 5 mmHg (vessels collapse at 0 mmHg), placed under an inverted video microscope, and equilibrated for 30 min. Medial thickness, lumen, and outer diameters at different pressures ranging from 5 to 120 mmHg were measured using a video dimension analyzer at 20-mmHg pressure increments. The time between each recording was between 5 and 10 min. Upon completion, the system was then equilibrated in Krebs-HEPES buffer free of calcium to study passive vessel mechanics. This allowed the measurement of vessel morphometry under a fully relaxed state. Lumen and outer diameter measurements obtained under active and passive conditions were then used for calculation of myogenic tone, stress, strain, and stiffness (beta-coefficient) using earlier reports (25). Myogenic tone was calculated using the formula: 1 − (active diameter/passive diameter)·100 and expressed as % tone. Circumferential wall stress (σ) and circumferential wall strain (ε) were calculated as (intraluminal pressure·lumen diameter)/(2·wall thickness) and (lumen diameter − lumen diameter at 5 mmHg pressure)/lumen diameter at 5 mmHg pressure, respectively. Stress-strain curves were plotted using KaleidaGraph ver. 4.0 (Synergy Software, Reading, PA). Stiffness coefficient β was obtained from the slope of the stress vs. strain curve using the equation y = αeβx, where α is the intercept and β is the slope of the exponential fit. All vessels were mounted, pressurized, and studied within the same time frame to avoid overestimation of the myogenic response as compliance of vessels may increase with time on the arteriograph chamber.
MMP activity.
Snap-frozen mesenteric artery segments were placed in modified radioimmunoprecipitation assay buffer (50 mmol/l Tris·HCl, 1% Nonidet P-40, 0.25% Na-deoxycholate, 150 mmol/l NaCl, 1 mmol/l phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 mmol/l sodium orthovanadate, and 1 mmol/l sodium fluoride) and sonicated at room temperature for 8- to 10-s bursts. Samples were placed on ice between sonications. Total protein was measured using the Bradford method (Bio-Rad, Richmond, CA). Vascular collagenase (MMP-13) and gelatinase (MMP-2 and 9) activities were determined using fluorescein-conjugated collagen or gelatin assay kits, as we have previously described (15, 30). Briefly, homogenates (20 μg total protein) were incubated with the substrate. Increased fluorescence that is directly proportional to the proteolytic activity of MMP enzymes was measured at 24 h using a microplate fluorometer. Tissue inhibitor of metalloproteinase-2 levels (TIMP-2) were obtained using ELISA kits (Amersham Biosciences, Piscataway, NJ).
Immunoblotting.
Vascular extracts (20 μg) were separated on 10% SDS gels and transferred to a nitrocellulose membrane in Tris-glycine transfer buffer supplemented with 20% methanol. The immunoblots were blocked for 1 h in 5% powdered goat milk diluted in 0.2 M Tris-base, 1.4 M NaCl, 0.1% Tween 20 and 0.02% NaN3. The membranes were then incubated overnight with the primary antibodies for MMP-13 (Calbiochem) or ETA receptors as recommended by the manufacturer (Alomone Laboratories, Jerusalem, Israel). Specificity of the receptor bands was confirmed by using increasing concentrations of competing peptide for the ETA antibody. Bands were visualized using ChemiGlow (Alpha Innotech, San Leandro, CA). All densitometric measurements were normalized using an antibody against β-actin (Sigma Aldrich) as a loading control.
Data analysis.
A one-way ANOVA was done to determine group differences coupled with a post hoc Tukey analysis. GraphPad Prism 5.0 was used for these analyses (GraphPad Software, San Diego, CA). Significance was considered at P < 0.05. All results are reported as unadjusted means ± SE.
RESULTS
Metabolic parameters.
GK rats were originally derived from selective breeding of glucose-intolerant Wistar rats, which serve as the control for this model (4, 35). The colony used in this study is neither hyperlipidemic nor hypertensive, allowing the opportunity to determine hyperglycemia effects in a spontaneous model of diabetes (4, 9, 14, 29). Metabolic parameters summarized in Table 1. Blood glucose was significantly elevated in diabetes, while metformin treatment normalized blood glucose (118 ± 6 mg/dl). Body weight was lower in diabetic rats compared with controls. The hyperinsulinemia seen early on in diabetes has been reported in GK rats (13) and proceeds to low plasma insulin levels with diabetes progression. However, studies from our laboratory show that these animals remained insulin resistant in spite of not having hyperinsulinemia and were glucose intolerant (15). Metformin treatment decreased plasma insulin levels in diabetes (0.7 ± 0.1 ng/ml). Blood glucose and hemoglobin A1C (Hb A1C) measures correlated tightly with changes in insulin levels. (Table 1). There was no difference in mean arterial pressure or lipid profile (total cholesterol and triglycerides) between the groups.
Table 1.
Metabolic parameters of control Wistar, diabetic GK, and euglycemic GK rats
| Control (n = 10) | Diabetic (n = 10) | Euglycemic (n = 6) | |
|---|---|---|---|
| Weight, g | 481.9±14.9 | 349.6±11.3* | 314.3±7.2* |
| Glucose, mg/dl | 99.1±9.4 | 158.7±12.9* | 118.0±6.4 |
| Hb A1C, % | 5.43±0.06 | 7.61±0.57* | 5.56±0.07 |
| Insulin, ng/ml | 1.5±0.3 | 1.2±0.2 | 0.7±0.1† |
| Total cholesterol, mg/dl | 78.3±6.9 | 88.6±3.5 | 83.2±4.7 |
| Triglycerides, mg/dl | 56.5±5.1 | 48.1±6.3 | 50.8±7.3 |
| Mean arterial pressure, mm Hg | 105.6±2.0 | 109.0±1.3 | 111.2±2.7 |
GK, Goto-Kakizaki.
P < 0.05 vs. control,
P < 0.05 vs. control or diabetic.
Vascular remodeling.
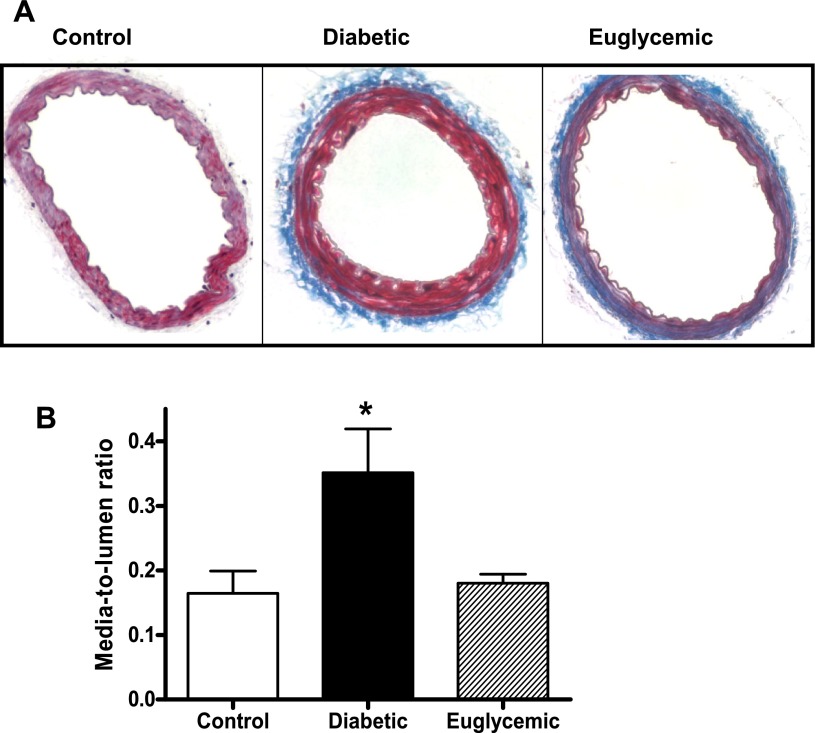
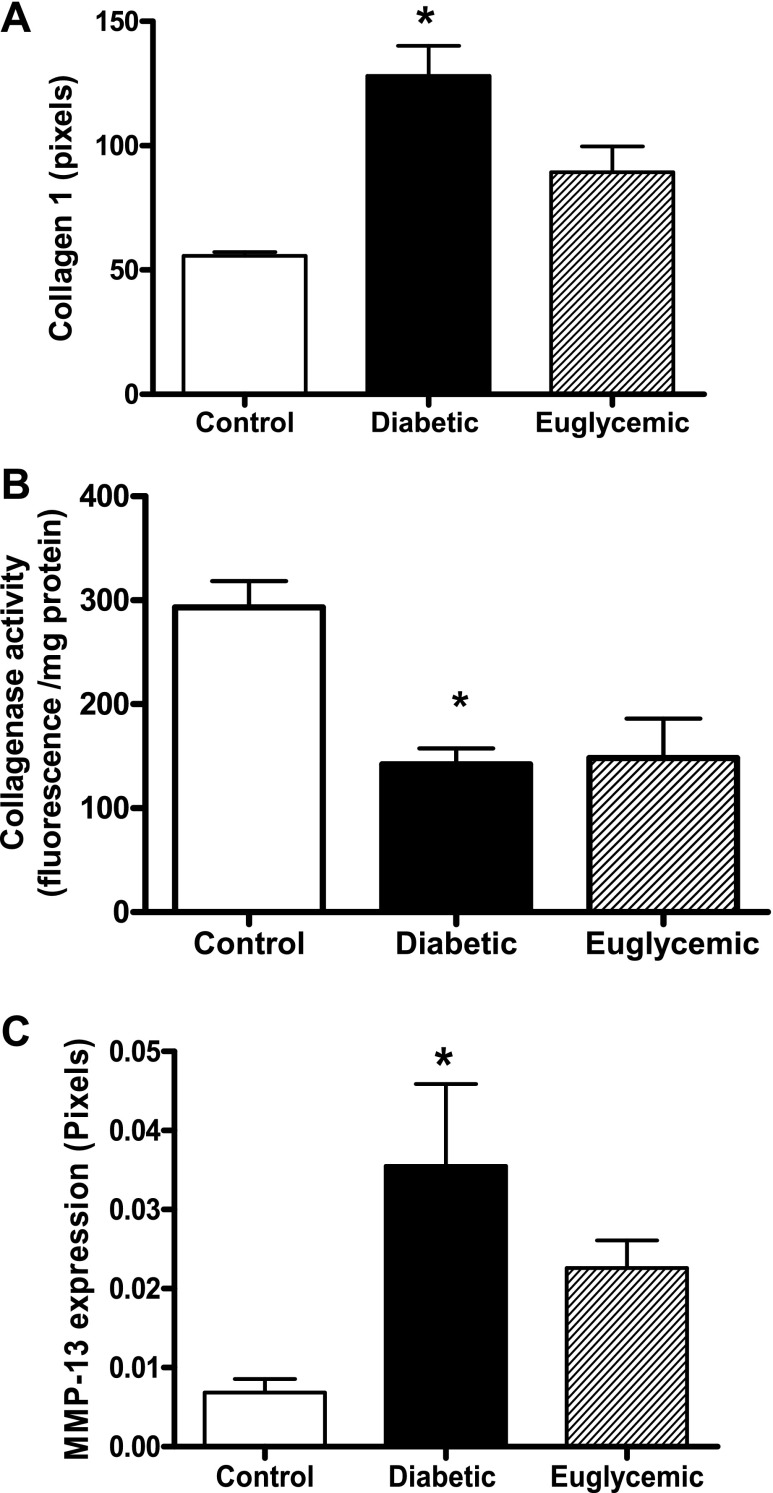
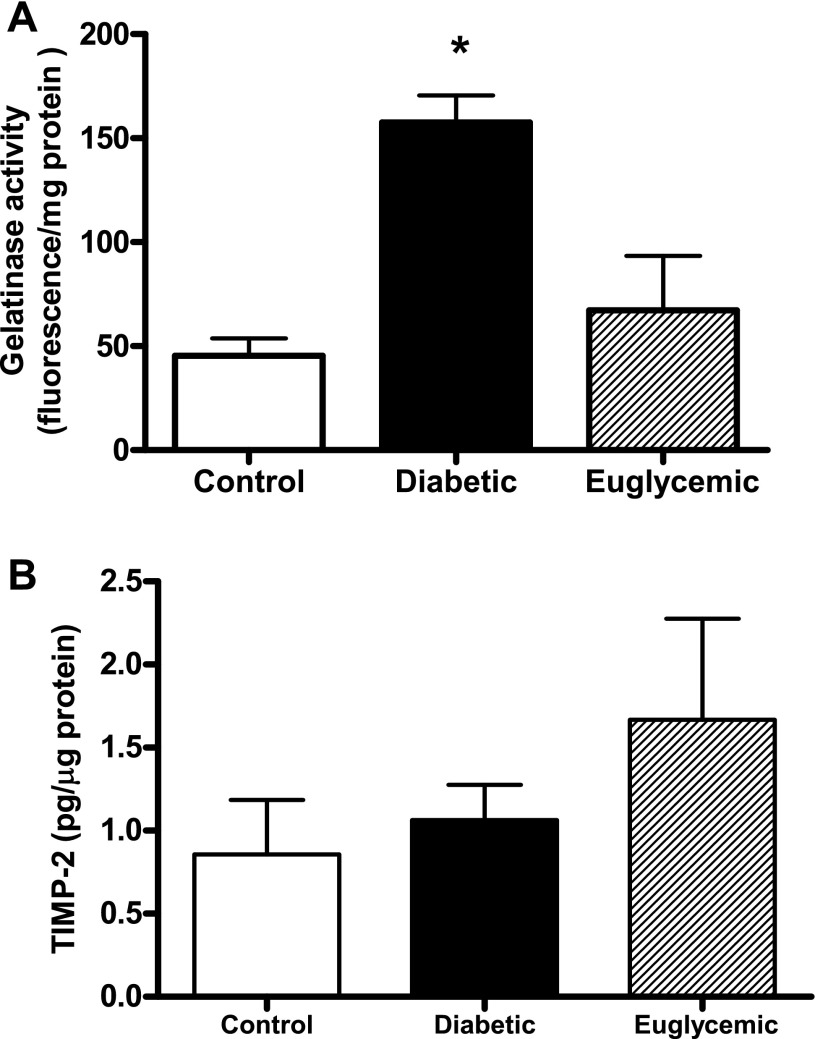
Masson staining of mesenteric artery cross sections showed that diabetic rats had medial thickening compared with controls (red-stained regions) at 18 wk of age, although total vessel size was not different. This denotes encroachment of the media into the lumen, thereby causing higher M/L ratios. Metformin treatment markedly attenuated vascular remodeling in diabetes, with M/L values comparable to controls (Fig. 1). Further, increased collagen staining was seen in the adventitia in diabetes, indicated by the blue regions in Masson trichrome-stained sections (Fig. 1). This correlated patterns observed with picrosirius red staining (Fig. 2, A and B). There was a small increase in mature thick collagen, but the main difference appeared to be in newly formed collagen, indicative of increased synthesis in diabetes. Metformin treatment, which started at the onset of diabetes, resulted in decreased staining for new collagen. Total collagen levels assessed by slot-blot analysis provided further evidence for augmented collagen deposition in diabetic animals (Fig. 3A). While picrosirius staining results pointed to increased synthesis, since collagen deposition may arise from increased synthesis or decreased degradation, we next studied collagenase (MMP-13) and gelatinase (MMP-2 and -9) activity. Cleavage of the fluorogenic substrate was significantly blunted in collagenase assay, indicating decreased MMP-13 activity in diabetes (Fig. 3B). Interestingly, metformin treatment did not normalize collagenase activity. Protein levels on the other hand were significantly elevated in diabetes, and glycemic control did not reduce MMP-13 protein levels (Fig. 3C). Gelatinase MMP activity was augmented in diabetes, and regulation of blood glucose prevented this increase (Fig. 4A). To determine whether increased gelatinase activity is due to a suppression of regulatory proteins of MMP activity, we measured tissue TIMP-2 levels, but there was no difference between the groups (Fig. 4B).
Fig. 1.
Formalin-fixed mesenteric artery cross sections were analyzed for morphological changes and collagen deposition by Masson staining. Diabetes induced nearly a two-fold increase in M/L ratio, and glycemic control with metformin prevented this increase. Representative images (A) and combined analysis (B) are shown. *P < 0.05 vs. control or euglycemic; n = 6/group.
Fig. 2.
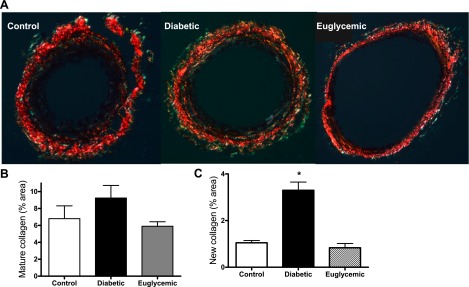
Collagen deposition patterns were observed from picrosirius red-stained cross sections, viewed under polarized light. There was increased new collagen in diabetes compared with control (increased green staining), and metformin treatment prevented this effect. Representative images (A) and combined analyses are shown of mature (B) and new collagen (C) for all animals. *P < 0.05 vs. control or euglycemic; n = 5/group.
Fig. 3.
Expression of type 1 collagen (A), collagenase (B), and MMP-13 (C) were determined by slot-blotting and immunoblotting, respectively. Densitometric analysis showed increased expression of type 1 collagen and MMP-13 in diabetic animals. B: increased fluorescence that is directly proportional to the proteolytic cleavage of FITC-collagen by MMP-13 was measured at 24 h, and it was decreased in diabetic animals. *P < 0.05 vs. control, n = 6–8/group.
Fig. 4.
A: mesenteric artery homogenates from diabetic animals show increased gelatinolytic activity when incubated with FITC-gelatin, which was decreased to control levels in metformin-treated rats. B: TIMP-2 levels measured by ELISA were similar in all groups. *P < 0.05 vs. control or euglycemic; n = 6–8/group.
Mechanical properties.
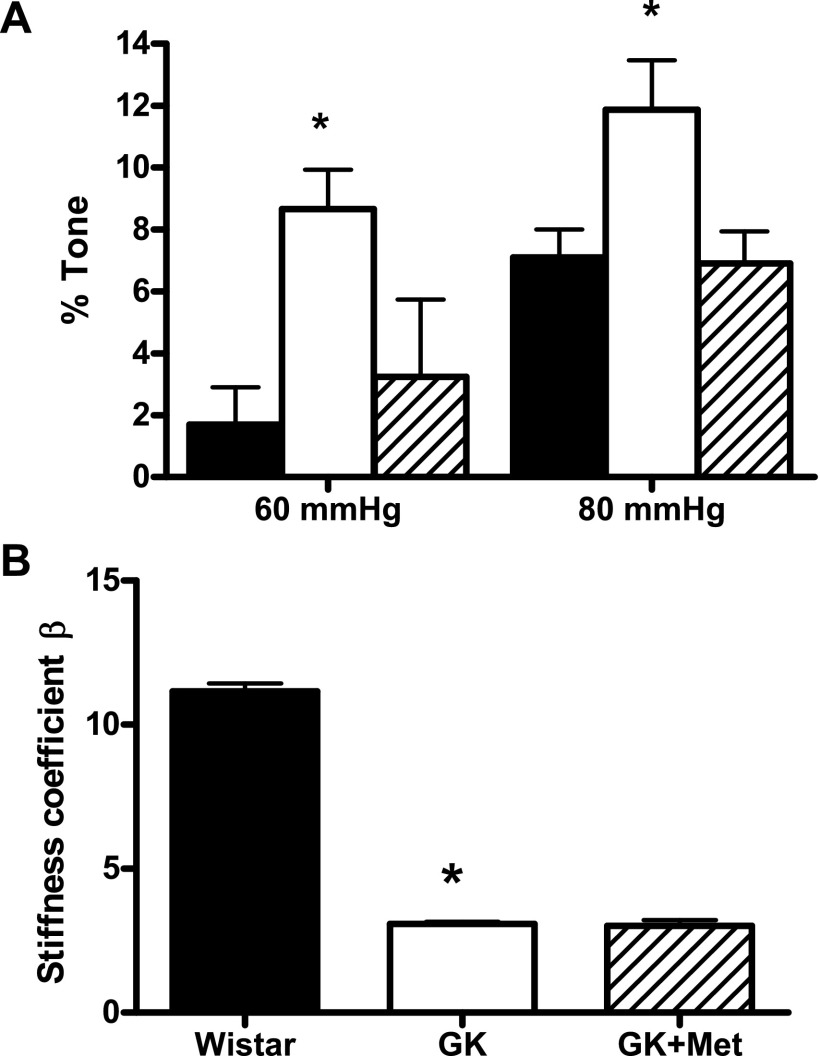
Myogenic tone was assessed from active and passive diameters of the vessel measured across the pressure range using the arteriograph. Hyperglycemia caused increased tone at 60 and 80 mmHg pressure points in the diabetic rats, whereas metformin treatment protected against the increase in myogenic tone (Fig. 5A). Vessel stiffness (β-coefficient) was calculated from the slopes of stress vs. strain curves for each individual animal, which was smaller in diabetic rats, indicating less stiffness. Metformin did not have an effect on vessel stiffness (Fig. 5B).
Fig. 5.
A: microvascular tone at 60 and 80 mmHg intraluminal pressure was increased in diabetes and glycemic control with metformin prevented this phenomenon. B: β-coefficient, a measure of vessel stiffness, was decreased in diabetes and metformin treatment did not have any effect on vessel stiffness. *P < 0.05 vs. control or euglycemic; n = 6–8/group.
Endothelin system.
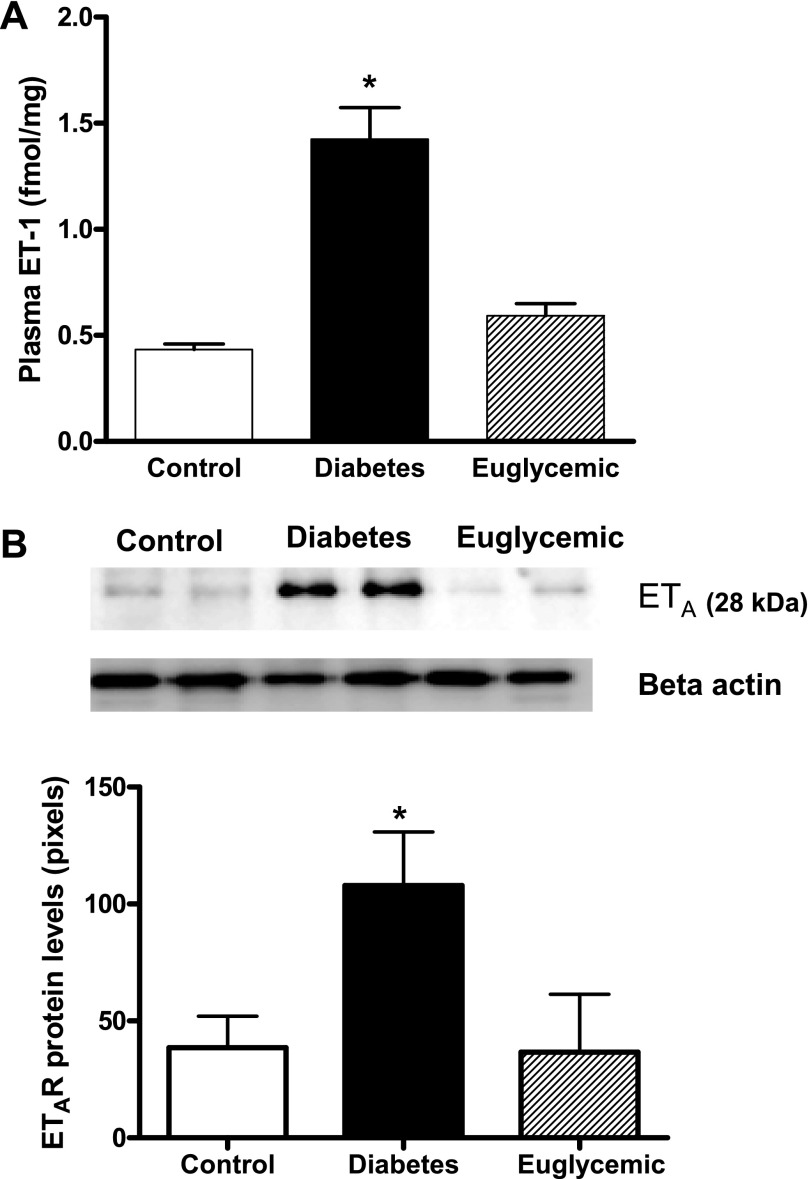
Plasma ET-1 levels, as well as mesenteric artery ETA receptor density, were significantly higher in diabetic animals confirming our previous results (15, 29). Glycemic control decreased both plasma ET-1 and ETA levels to control levels (Fig. 6, A and B).
Fig. 6.
Plasma ET-1 and ET receptor expression in mesenteric arteries. A: ET-1 levels are increased three-fold in diabetes, which was prevented by glycemic control. B: representative immunoblot showing ETA protein levels and densitometric analysis indicated an increase in ETA in diabetes and metformin treatment normalized receptor expression to control values. Densitometry values reported have been normalized to β-actin levels in all samples to account for differences in loading. *P < 0.05 vs. control or euglycemic; n = 6/group.
DISCUSSION
The effects of Type 1 diabetes on vascular remodeling have been fairly well studied (6–8, 12). However, the extent of structural remodeling of microvessels in Type 2 diabetes and the associated mechanics by which these occur are not clearly mapped out, partly because of the lack of a relevant animal model. Insulin resistance oftentimes clusters with Type 2 diabetes, either in the absence or presence of the other confounding factors, such as dyslipidemia, obesity, and hypertension that together form the metabolic syndrome or “syndrome X”. The Goto-Kakizaki rat, which is Type 2 spontaneously diabetic, nonobese, and normotensive, was thus a fitting animal model to address the effects of hyperglycemia alone in mediating vascular complications (2). Building upon our previous study, which demonstrated that after a relatively short period (12 wk) of moderate hyperglycemia, GK rats display mesenteric remodeling, which can be prevented by ETA receptor antagonism, the current study addressed the following questions: 1) Does vascular remodeling characterized by increased collagen deposition and medial thickening affect myogenic tone and compliance of resistance arteries?, 2) Is regulation of blood glucose sufficient to prevent the remodeling response and associated functional changes?, and if so, 3) Does glycemic control offer vasculoprotection via modulation of the ET system? Our results provide evidence that increased collagen deposition due to augmented collagen synthesis and suppression of degradation as a result of decreased collagenase activity are associated with increased tone of mesenteric vessels. We provide further evidence that glycemic control prevents remodeling and improves vascular mechanics via downregulation of the ET-1/ETA pathway.
Vascular remodeling is an extensively researched concept in hypertension, whereas our knowledge of vascular restructuring in diabetes is not as deep. Fukuda et al. (11) established the presence of diabetes-induced vascular hypertrophy and remodeling in aortas of a drug-induced rat model of Type 1 diabetes. Similarly, the mesenteric microvasculature has also been shown to undergo hypertrophic remodeling due to increased medial mass and media-lumen ratios (7, 12). Studies using small subcutaneous arteries isolated from gluteal fat biopsies showed hypertrophic remodeling and increased collagen-to-elastin ratio in diabetic subjects (26). Interestingly, Schofield et al. (33) reported that small arteries from individuals with diabetes also present with increased distensibility and reduced myogenic tone. Intengan et al. (18) also reported this paradoxical increase in compliance despite an increase in collagen-to-elastin ratio in the small arteries from hypertensive individuals. Building on these past observations, the current study evaluated both structural and mechanical properties of microvessels in the mesenteric circulation in an experimental model of Type 2 diabetes. Our results showed that there is remodeling and fibrosis as evidenced by increased M/L ratios and collagen synthesis. Similar to findings obtained from diabetic individuals, mesenteric arteries from diabetic rats also displayed decreased stiffness yet increased collagen synthesis in this group. One potential explanation may be the collagen deposition patterns. There is no difference in mature collagen, but mainly new collagen levels are increased in diabetic animals, and at the time point that we studied, this does not affect vascular compliance. Our study, however, differs from the results reported by Schofield et al. (33) in that there is increased as opposed to decreased myogenic tone reported in diabetic individuals. In human subjects, dyslipidemia was a significant factor affecting vascular function, whereas in our experimental model, there is no difference in total cholesterol or triglyceride levels compared with control. Aside from species difference, this may have contributed to the opposing differences in myogenic tone. Another aspect of the relationship between vessel wall structure and mechanics is the role of MMPs. Su et al. (36) demonstrated a direct correlation between MMP-9 and resistance artery mechanics, wherein the absence of the MMP-9 gene was associated with decreased myogenic tone and improved endothelial-dependent vasodilation. Although it is known that MMP enzymes are upregulated in diabetes, the effect of Type 2 diabetes-mediated changes in vascular mechanics are not well understood. However, it has to be recognized that we did not directly study the effect of MMP inhibition on myogenic tone in our model, and as such, whether upregulation of gelatinase activity contributes to enhanced vascular tone in diabetes remains to be tested.
Plasma ET-1 levels are elevated in both Type 1 and Type 2 diabetes as well as in experimental models of this disease (5, 37, 38). Gilbert et al. (12) reported that ETA receptor antagonism prevents mesenteric vascular hypertrophy in STZ-induced diabetes by inhibiting macrophage infiltration and epidermal growth factor signaling (12). We have recently shown that ETA antagonism prevents mesenteric MMP activation and remodeling in GK rats, whereas ETB blockade exacerbates diabetes-mediated restructuring of resistance arteries (30). We also showed GK rats present with elevated ET-1 levels and increased ETA receptor expression (15, 29). Because glucose stimulates ET-1 production in vitro (16), we questioned whether moderate elevations in blood glucose are responsible for stimulating ET-1/ETA pathway and thus remodeling. Current findings that metformin treatment normalizes blood glucose, vascular remodeling, and ET-1 and ETA receptor expression strongly suggest that this is the case. These results are also important to show that glycemic control alone can prevent diabetes-induced changes in vascular structure and mechanics.
MMPs have been shown to play an important role in vascular dynamics by controlling the turnover of ECM components such as collagen and fibronectin. Our group recently established that differential MMP regulation resulted in altered ECM turnover patterns in the Type 2 diabetic GK rat (30). In the current study, we observed a difference in collagen turnover patterns, which reflect ECM dynamics. Collagen deposition was evaluated by three different methods and showed that although there is a small increase in mature thick collagen filaments, the increased total collagen in diabetes appears to be as a result of increased synthesis as evidenced by more new collagen in picrosirius staining. Collagenase MMP-13 activity, which degrades fibrillar collagen, was attenuated in diabetes despite an increase in protein levels, suggesting regulation at the posttranslational or inhibitory checkpoints of MMP-13 activity. On the other hand, gelatinase activity, which is based on the cleavage of FITC-gelatin by MMP-2 and MMP-9, was augmented, confirming our past results of increased mesenteric MMP-2 activity determined by zymographic methods (30). Because recent studies suggest that MMP-2 and MMP-9 are involved in VSMC migration and activation of profibrotic membrane-bound proteins (22, 32, 34), increased MMP-2 activity may indeed contribute to increased collagen synthesis observed in this study.
This study employed metformin as a means to achieve euglycemia in the diabetic animals since GK rats present with insulin resistance, and we started the treatment right after the onset of diabetes to assess effect of glucose control on the ET system. Metformin was successful in preventing impairment in vascular structure, mechanics and compliance manifested by Type 2 diabetes. It has to be recognized that metformin has pleiotropic effects, including antihypertensive, anti-inflammatory (24), vasculoprotective (23), and advanced glycation end-product formation inhibitory properties (40). Because there was no effect of metformin treatment on mean arterial blood pressure, we believe that prevention of vascular remodeling is not due to antihypertensive effects of metformin. Although we cannot rule out that improvement of insulin sensitivity alone or these pleiotropic effects promoted the observed improvements, our results are important to show that glycemic control can prevent activation of growth-promoting and profibrotic vasoactive factors and thus prevent vascular remodeling and dysfunction. Another limitation of the current study is that metformin treatment was designed as a preventive strategy rather than as an intervention strategy to determine whether vascular remodeling can be reversed if treatment were started in established diabetes. While we do not have direct evidence with metformin treatment, our previous studies showed that treatment with ET-1 receptor antagonist after the onset of diabetes (6–8 wk after the onset of diabetes) restored vascular structure (30), suggesting that inhibition of profibrotic and growth-promoting factors can reverse vascular remodeling. This study showed that metformin treatment can prevent activation of the ET system.
Perspectives and Significance
Complications of Type 2 diabetes are associated with both macrovascular and microvascular pathologies. This study demonstrated that glycemic control started shortly after the onset of diabetes is effective in preventing the activation of the ET system, as well as the remodeling that occurs in the mesenteric arteries of diabetic rats. Whether glucose regulation in established diabetes can prevent and/or reverse vascular remodeling and dysfunction remains to be determined.
GRANTS
This work was supported by grants from National Institutes of Health (DK074385), Philip Morris, and Philip Morris International to Adviye Ergul.
Acknowledgments
We also thank Dr. Christine S. Rigsby for guidance with arteriograph techniques and Matthew Socha for assistance with the picrosirius red staining.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.American Diabetes Association. National diabetes fact sheet. American Diabetes Association [article online at http://www.diabetes.org/diabetes-statistics.jsp], 2008.
- 2.Bitar MS, Al-Saleh E, Al-Mulla F. Oxidative stress-mediated alterations in glucose dynamics in a genetic animal model of type II diabetes. Life Sci 77: 2552–2573, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control, and Prevention. National Center for Health Statistics [article online at http://www.cdc.gov/nchs/fastats/lcod.htm], 2008.
- 4.Cheng ZJ, Vaskonen T, Tikkanen I, Nurminen K, Ruskoaho H, Vapaatalo H, Muller D, Park JK, Luft FC, Mervaala EMA. Endothelial dysfunction and salt-sensitive hypertension in spontaneously diabetic Goto-Kakizaki rats. Hypertension 37: 433–439, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Collier A, Leach JP, McLellan A, Jardine A, Morton JJ, Small M. Plasma endothelin-like immunoreactivity levels in IDDM patients with microalbuminuria. Diabetes Care 15: 1038–1040, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Cooper ME, Gilbert RE, Jerums G. Diabetic vascular complications. Clin Exp Pharmacol Physiol 24: 770–775, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Cooper ME, Rumble J, Komers R, Du HC, Jandeleit K, Chou ST. Diabetes-associated mesenteric vascular hypertrophy is attenuated by angiotensin-converting enzyme inhibition. Diabetes 43: 1221–1228, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Dharmani M, Mustafa MR, Achike FI, Sim MK. Effect of des-aspartate-angiotensin I on the actions of angiotensin II in the isolated renal and mesenteric vasculature of hypertensive and STZ-induced diabetic rats. Regul Pept 129: 213–219, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Elgebaly MM, Kelly A, Harris AK, Elewa H, Portik-Dobos V, Ketsawatsomkron P, Marrero M, Ergul A. Impaired insulin-mediated vasorelaxation in a nonobese model of type 2 diabetes: role of endothelin-1. Can J Physiol Pharmacol 86: 358–364, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elgebaly MM, Portik-Dobos V, Sachidanandam K, Rychly D, Malcom D, Johnson MH, Ergul A. Differential effects of ET(A) and ET(B) receptor antagonism on oxidative stress in type 2 diabetes. Vasc Pharmacol 47: 125–130, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda G, Khan ZA, Barbin YP, Farhangkhoee H, Tilton RG, Chakrabarti S. Endothelin-mediated remodeling in aortas of diabetic rats. Diabetes Metab Res Rev 21: 367–375, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert RE, Rumble JR, Cao Z, Cox AJ, van Eeden P, Allen TJ, Kelly DJ, Cooper ME. Endothelin receptor antagonism ameliorates mast cell infiltration, vascular hypertrophy, and epidermal growth factor expression in experimental diabetes. Circ Res 86: 158–165, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Goto Y, Kakizaki M, Masaki N. Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J Exp Med 119: 85–90, 1976. [DOI] [PubMed] [Google Scholar]
- 14.Harris AK, Elgebaly MM, Li W, Sachidanandam K, Ergul A. Effect of chronic endothelin receptor antagonism on cerebrovascular function in type 2 diabetes. Am J Physiol Regul Integr Comp Physiol 294: R1213–R1219, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris AK, Hutchinson JR, Sachidanandam K, Johnson MH, Dorrance AM, Stepp DW, Fagan SC, Ergul A. Type 2 diabetes causes remodeling of cerebrovasculature via differential regulation of matrix metalloproteinases and collagen synthesis: role of endothelin-1. Diabetes 54: 2638–2644, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Hattori Y, Kasai K, Nakamura T, Emodo T, Shimoda SI. Effects of glucose and insulin on immunoreactive endothelin-1 release from cultured bovine endothelial cells. Metabolism 40: 165–169, 1991. [DOI] [PubMed] [Google Scholar]
- 17.Heagerty AM, Aalkjaer C, Bund SJ, Korsgaard N, Mulvany MJ. Small artery structure in hypertension. Dual processes of remodeling and growth. Hypertension 21: 391–397, 1993. [DOI] [PubMed] [Google Scholar]
- 18.Intengan HD, Deng L, Li J, Schiffrin EL. Mechanics and composition of human subcutaneous resistance arteries in essential hypertension. J Hypertens 33: 569–574, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension 38: 581–587, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Ito I, Jarajapu YP, Grant MB, Knot HJ. Characteristics of myogenic tone in the rat ophthalmic artery. Am J Physiol Heart Circ Physiol 292: H360–H368, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Ito I, Jarajapu YP, Guberski DL, Grant MB, Knot HJ. Myogenic tone and reactivity of rat ophthalmic artery in acute exposure to high glucose and in a type II diabetic model. Invest Ophthalmol Vis Sci 47: 683–692, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Johnson JL, van Eys GJ, Angelini GD, George SJ. Injury induces dedifferentiation of smooth muscle cells and increased matrix-degrading metalloproteinase activity in human saphenous vein. Arterioscler Thromb Vasc Biol 21: 1146–1151, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Kraemer de Aguiar LG, Laflor CM, Bahia L, Villela NR, Wiernsperger N, Bottino DA, Bouskela E. Metformin improves skin capillary reactivity in normoglycaemic subjects with the metabolic syndrome. Diabet Med 24: 272–279, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Lund SS, Tarnow L, Stehouwer CD, Schalkwijk CG, Teerlink T, Gram J, Winther K, Frandsen M, Smidt UM, Pedersen O, Parving HH, Vaag AA. Impact of metformin versus repaglinide on non-glycaemic cardiovascular risk markers related to inflammation and endothelial dysfunction in non-obese patients with type 2 diabetes. Eur J Endocrinol 158: 631–641, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Rigsby CS, Pollock DM, Dorrance AM. Spironolactone improves structure and increases tone in the cerebral vasculature of male spontaneously hypertensive stroke-prone rats. Microvasc Res 73: 198–205, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizzoni D, Porteri E, Guelfi D, Muiesan ML, Valentini U, Cimino A, Girelli A, Rodella L, Bianchi R, Sleiman I, Rosei E. Structural alterations in subcutaneous small arteries in normotensive and hypertensive patients with non-insulin-dependent diabetes mellitus. Circulation 103: 1238–1244, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Rumble JR, Cooper ME, Soulis T, Cox A, Wu L, Youssef S, Jasik M, Jerums G, Gilbert RE. Vascular hypertrophy in experimental diabetes. Role of advanced glycation end products. J Clin Invest 99: 1016–1027, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sachidanandam K, Elgebaly MM, Harris AK, Hutchinson JR, Mezzetti EM, Portik-Dobos V, Ergul A. Effect of chronic and selective endothelin receptor antagonism on microvascular function in Type 2 diabetes. Am J Physiol Heart Circ Physiol 294: H2743–H2749, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sachidanandam K, Elgebaly MM, Harris AK, Hutchinson JR, Mezzetti EM, Portik-Dobos V, Ergul A. Effect of chronic and selective endothelin receptor antagonism on microvascular function in Type 2 diabetes. Am J Physiol Heart Circ Physiol 294: H2743–H2749, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sachidanandam K, Portik-Dobos V, Harris AK, Hutchinson JR, Muller E, Johnson MH, Ergul A. Evidence for vasculoprotective effects of ETB receptors in resistance artery remodeling in diabetes. Diabetes 56: 2753–2758, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Said N, Motamed K. Absence of host-secreted protein acidic and rich in cysteine (SPARC) augments peritoneal ovarian carcinomatosis. Am J Pathol 167: 1739–1752, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito Y, Berk BC. Transactivation: a novel signaling pathway from angiotensin II to tyrosine kinase receptors. J Mol Cell Cardiol 33: 3–7, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Schofield I, Malik R, Izzard A, Austin C, Heagerty A. Vascular structural and functional changes in type 2 diabetes mellitus: evidence for the roles of abnormal myogenic responsiveness and dyslipidemia. Circulation 106: 3037–3043, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Shah BH, Catt KJ. A central role of EGF receptor transactivation in angiotensin II -induced cardiac hypertrophy. Trends Pharmacol Sci 24: 239–244, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Standaert ML, Sajan MP, Miura A, Kanoh Y, Chen HC, Farese RV Jr, and Farese RV. Insulin-induced activation of atypical protein kinase C, but not protein kinase B, is maintained in diabetic (ob/ob and Goto-Kakazaki) liver. Contrasting insulin signaling patterns in liver versus muscle define phenotypes of type 2 diabetic and high fat-induced insulin-resistant states. J Biol Chem 279: 24929–24934, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Su J, Palen DI, Lucchesi PA, Matrougui K. Mice lacking the gene encoding for MMP-9 and resistance artery reactivity. Biochem Biophys Res Commun 349: 1177–1181, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi K, Ghatei MA, Lam HC, O'Halloran DJ, Bloom SR. Elevated plasma endothelin in patients with diabetes mellitus. Diabetologia 33: 306–310, 1990. [DOI] [PubMed] [Google Scholar]
- 38.Takeda Y, Miyamori I, Yoneda T, Takeda R. Production of endothelin-1 from the mesenteric arteries of streptozotocin-induced diabetic rats. Life Sci 48: 2553–2556, 1991. [DOI] [PubMed] [Google Scholar]
- 39.Vranes D, Cooper ME, Dilley RJ. Cellular mechanisms of diabetic vascular hypertrophy. Microvasc Res 57: 8–18, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Jia X, Chang T, Desai K, Wu L. Attenuation of hypertension development by scavenging methylglyoxal in fructose-treated rats. J Hypertens 26: 765–772, 2008. [DOI] [PubMed] [Google Scholar]