Abstract
Obesity is a risk factor for type 2 diabetes in cats. The risk of developing diabetes is severalfold greater for male cats than for females, even after having been neutered early in life. The purpose of this study was to investigate the role of different metabolic pathways in the regulation of endogenous glucose production (EGP) during the fasted state considering these risk factors. A triple tracer protocol using 2H2O, [U-13C3]propionate, and [3,4-13C2]glucose was applied in overnight-fasted cats (12 lean and 12 obese; equal sex distribution) fed three different diets. Compared with lean cats, obese cats had higher insulin (P < 0.001) but similar blood glucose concentrations. EGP was lower in obese cats (P < 0.001) due to lower glycogenolysis and gluconeogenesis (GNG; P < 0.03). Insulin, body mass index, and girth correlated negatively with EGP (P < 0.003). Female obese cats had ∼1.5 times higher fluxes through phosphoenolpyruvate carboxykinase (P < 0.02) and citrate synthase (P < 0.05) than male obese cats. However, GNG was not higher because pyruvate cycling was increased 1.5-fold (P < 0.03). These results support the notion that fasted obese cats have lower hepatic EGP compared with lean cats and are still capable of maintaining fasting euglycemia, despite the well-documented existence of peripheral insulin resistance in obese cats. Our data further suggest that sex-related differences exist in the regulation of hepatic glucose metabolism in obese cats, suggesting that pyruvate cycling acts as a controlling mechanism to modulate EGP. Increased pyruvate cycling could therefore be an important factor in modulating the diabetes risk in female cats.
Keywords: nuclear magnetic resonance spectroscopy, diabetes mellitus, pyruvate cycling, thyroxine, polyunsaturated fatty acids
obesity is a significant risk factor for type 2 diabetes mellitus in humans (7, 28) and cats (16, 18). Obesity is typically associated with insulin resistance in humans (6, 27) and cats (18, 49) and can even be documented in some cats when they are still lean (49). In cats, an increase in body mass index (BMI) by ∼50% leads to a decrease in insulin sensitivity of about 60% (16). However, like humans, not all obese cats will convert from the obese, insulin-resistant state to type 2 diabetes. In cats, the risk for obese male cats to develop diabetes mellitus is severalfold greater than that for females, even when neutered early in their lives. This might be due in part to the fact that female cats continue to oxidize more fat than males when insulin is high, indicating sex-specific differences in the insulin sensitivity of fat metabolism (18).
Diet has been shown to influence insulin resistance in both humans and cats. In cats, the dietary content of protein and n–3 polyunsaturated fatty acids has beneficial effects on heat production and insulin sensitivity (49). There also is anecdotal and some scientific evidence (11) that a high-protein diet decreases insulin requirements in diabetic cats. Although we have shown that a high-protein/low-carbohydrate diet leads to an increase in energy expenditure compared with a low-protein/high-carbohydrate diet (19), it is not known how glucose metabolism is influenced by different diets.
Studies using nuclear magnetic resonance spectroscopy (NMR) have been performed in humans (24, 25), rats (20–23), and mice (3) to investigate metabolic pathways in glucose production by using a triple tracer method. By applying this method, it is possible to detect key steps in glucose metabolism noninvasively with a single blood sample using stable, nonradioactive isotopes. 13C-labeled glucose is used to measure glucose turnover by conventional indicator dilution; deuterium is used to measure the fractional contribution of glycogen, glycerol, and the tricarboxylic acid (TCA) cycle to endogenous glucose production (EGP), and 13C-labeled propionate is used as a gluconeogenic tracer to measure fluxes through pathways associated with the TCA cycle. We utilized this methodology to investigate the role of different metabolic pathways in the regulation of EGP in lean and obese neutered male and female cats during the fasted state.
MATERIALS AND METHODS
Animals and Diets
Twelve lean (6 female, 6 male) and twelve obese (6 female, 6 male) neutered adult purpose-bred cats were used for these studies. The age for the lean cats was 5 ± 2 yr, and that for the obese cats was 7 ± 1 yr (P = 0.144). The obese cats had been obese for >1 yr before the beginning of the study. Obesity was originally induced by allowing ad libitum food intake, whereas lean cats were fed only the amount needed to maintain their weight. Cats were maintained at the University of Georgia College of Veterinary Medicine Animal Care Facility under approved colony conditions. They were housed individually and were given free access to water. All animal studies were approved by the University of Georgia Animal Care and Use Committee and were conducted in accordance with guidelines established by the Animal Welfare Act and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animals were determined to be healthy based on the results of physical examination and clinical laboratory data. All cats were socialized with daily interactions. All 24 cats were on the same diet before the beginning of the study. They were then randomly allocated to one of three diets, and each cat received each of the three diets for 4 mo in a crossover design. All data were collected each time the triple tracer infusion experiment was performed, i.e., every 4 mo. The three diets included a high-protein regimen (HP), a high-carbohydrate /high-saturated fatty acid regimen (HCSAT), and a high-carbohydrate diet enriched in n–3 polyunsaturated fatty acids (HCPUFA). The diet composition (Table 1) was known to only one of the investigators (M. Waldron), who was not involved in execution of the experiments and data analyses.
Table 1.
Diet compositions for diets
| Diet Composition | HP | HCSAT | HCPUFA |
|---|---|---|---|
| Protein, % | 44.2 | 33.8 | 35.1 |
| Fat, % | 14.7 | 16.9 | 16.6 |
| CHO, % (by subtraction) | 25.3 | 32.1 | 32.8 |
| Fiber (crude), % | 1.4 | 1.7 | 1.5 |
| Ash, % | 8.9 | 8.2 | 7.2 |
| Moisture, % | 5.5 | 7.3 | 6.8 |
| Caloric value, kcal/g (by calculation) | 4.1 | 4.2 | 4.2 |
| Fatty Acid Analysis, %fat | |||
| 14:0 | 1.6 | 2.01 | 2.61 |
| 14:1 | 0.4 | 0.4 | 0.3 |
| 16:0 | 21.3 | 22.3 | 21.4 |
| 16:1 n–7 | 3.6 | 3.7 | 4.4 |
| 18:0 | 10 | 11.8 | 10.2 |
| 18:1 n–9 | 32.9 | 34.2 | 30.3 |
| 18:1 n–7 | 1.54 | 1.53 | 1.77 |
| 18:1 n–9 T | 2.8 | 3.4 | 3.4 |
| 18:2 n–6 | 18.5 | 13.5 | 12.8 |
| 20:0 | 0.2 | 0.2 | 0.2 |
| 18:3 n–3 | 1.0 | 0.8 | 0.9 |
| 20:2 n–6 | < 0.1 | < 0.1 | 0.2 |
| 20:3n–6 | 0.1 | 0.10 | 0.1 |
| 20:4 n–6 | 0.5 | 0.4 | 0.5 |
| 24:0 | 0.12 | < 0.1 | < 0.1 |
| 20:5 n–3 | < 0.1 | < 0.1 | 1.9 |
| 22:5 n–3 | < 0.1 | < 0.1 | 0.4 |
| 22:6 n–3 | < 0.1 | < 0.1 | 1.5 |
| Total fatty acid, %fat | 92.5 | 91.8 | 89.9 |
HP, high-protein diet; HCSAT, high-carbohydrate/high-saturated fatty acid diet; HCPUFA, high-carbohydrate diet enriched in n–3 polyunsaturated fatty acids diet. Diet ingredients: whole chicken, poultry by-product meal, brewers rice, corn gluten meal, soybean meal (dehulled), whole corn (yellow), beef tallow, menhaden fish oil, corn bran, palatability coating, phosphoric acid, potassium chloride, mineral premix, NaCl, l-lysine, taurine, choline chloride.
Food intake was recorded at each feeding. The weight of the cats was monitored once weekly, and food intake was adjusted to maintain body weight within a narrow range. The weight of the lean cats was 3.5 ± 0.5 kg before and at the end of the study. In the obese cats, it was 7.2 ± 1.2 kg at the beginning and 7.1 ± 1.2 kg at the end of the study, and there were no differences in weight gained or lost on any of the diets. Anthropometric measurements were taken from all cats for the determination of BMI (expressed in kg/m2) and girth circumference before each infusion experiment (31, 32). All measurements were performed by the same person to minimize variability (S. Kley).
Infusion Experiment
To allow blood sampling, catheters were placed in the jugular vein of cats 48 h before each of the infusion experiments. Catheter patency was maintained by injection of 0.5 ml of 0.38% sterile citrate flush (citric acid, trisodium salt dihydrate; Sigma-Aldrich, St. Louis, MO) every 8 h. Cats were fasted overnight and then tranquilized with tiletamine/zolazepam (2 mg per cat intravenously; Fort Dodge Animal Health, Ft. Dodge, IA). We have previously shown that telazol has no effect on glucose tolerance and insulin sensitivity (18). A baseline blood sample (2.0 ml) was collected, and 6.0 ml/kg of a deuterium oxide (2H2O, 99.9%,)-sodium propionate ([U-13C3]propionate, 99%; Cambridge Isotopes, Andover, MA) solution (200:1) was administered via gastric tube. The cats then received a bolus intravenous infusion (3.69 mg/kg) of a 2 mg/ml solution of [3,4-13C2]glucose (99%; Omicron Biochemicals, South Bend, IN) in normal saline, immediately followed by a continuous intravenous infusion of 0.055 mg·kg−1·min−1 via the jugular vein catheter for 60 min. Another blood sample (12.0 ml) was collected at 60 min.
Validation of the NMR spectroscopy technique in cats was performed in a pilot study in four cats where triple tracer was infused for 60, 90, and 120 min to evaluated whether steady-state conditions could already be achieved at the 60-min time point. Performing the infusion experiment for 60 min instead of 90 or 120 min is superior because of practical and financial aspects of performing these stable isotope infusion experiments in larger mammals such as cats. An advantage to the validation of this technique in rats, we were able to confirm the steady-state metabolic conditions by taking multiple blood samples from each cat during infusion. We found that a primed 60-min infusion was sufficient to accurately measure EGP in cats compared with the results of primed 90- and 120-min infusion periods. EGP in cats at 90 and 120 min was 89 ±_4.6 and 97 ± 9.1% of the EGP at 60 min, respectively, indicating that steady state was achieved at 60 min. We also assumed isotopic steady state for the metabolism of [U-13C3]propionate in this study, because the results of the analysis of the multiplet pattern of carbon-2 (C2) of glucose (or monoacetone glucose, MAG) among the different time points were similar. The analysis of C2 depends solely on the multiplet pattern detected in the 13C NMR spectrum, not on the absolute 13C enrichment, as has been stated previously by Jin et al. (22). These findings are similar to observations from studies in rats (21, 22).
Sample Processing
For the collection of plasma, whole blood was placed into chilled tubes containing EDTA, and the samples were centrifuged immediately at 4°C and 890 g. Plasma samples were stored at −80°C until further processing. For analysis by NMR, plasma glucose from the 60-min postinfusion blood sample was isolated and converted to MAG as previously reported, with some minor modifications (20). Briefly, 5.0 ml of double-distilled water (ddH2O), followed by 1.5 ml of zinc sulfate solution (ZnSO4, 0.3 N; Sigma-Aldrich) and 1.5 ml of barium hydroxide standard solution [Ba(OH)2, 0.3 N; Sigma-Aldrich], were added to each milliliter of plasma following centrifugation at 25,000 g to precipitate plasma proteins. Glucose was separated from salt and purified by passing the resulting supernatant through a column containing 30 ml of Amberlite IRA-67 anion exchange and 15 ml of Dowex 50W-X8-200 cation exchange resins (both Sigma-Aldrich; prepared as described in the product literature) in series. The glucose was eluted with 100 ml of water; the pH was adjusted to 6–7 with the Amberlite anion exchange resin. The sample was freeze-dried, resuspended in 6.0 ml of acetone (Acetone Chromasolv for HPLC > 99.9%; Sigma-Aldrich) and 240 μl of sulfuric acid (95–98%, A.C.S. reagent; Sigma-Aldrich), and stirred for 4 h at room temperature to yield diacetone glucose. The volume was then doubled by the addition of ddH2O, and the pH was adjusted to 2.0 by the addition of 1.5 M Na2CO3. This solution was stirred for 24 h before the pH was adjusted to 8.0 by the addition of more 1.5 M Na2CO3 to convert diacetone glucose into MAG. The sample was then freeze-dried, and the MAG was extracted by dissolving it into boiling ethyl acetate (anhydrous, 99.8%; Sigma-Aldrich). Finally, the ethyl acetate was evaporated, and the dry MAG sample was stored before NMR analysis. The conversion from glucose to MAG has been reported to give a high yield of 80% in rats (5). It was assumed that the conversion of glucose to MAG would have a similar yield in cats, because the conversion from glucose to MAG is a chemical reaction performed on purified glucose extracted from plasma and not on whole plasma. In rats, the overall yield of glucose taken through the glucose purification process has been described as ∼80% (13). We found a similar yield (∼86%) for glucose purification from cat plasma when taking it through that same extraction process.
NMR Spectroscopy
All NMR spectra were collected using a Varian INOVA 14.1 T spectrometer (Varian Instruments, Palo Alto, CA) equipped with a 3 mm broadband probe (3-mm PFG dual broadband probe; Varian) as previously described (20). Spectral analysis was processed using the curve-fitting program MestRe-C (MestRe-C1; Mestrelab Research, Santiago de Compostela, Spain), a personal computer-based NMR spectral analysis program.
Metabolic Analysis
Glucose turnover was estimated from the dilution of infused [3,4-13C2]glucose by using 13C NMR of the MAG samples as previously described (21). The fluxes from glycogen, glycerol, and phosphoenolpyruvate (PEP) into plasma glucose were estimated from the deuterium enrichment at positions 2, 5, and 6s (H2, H5, and H6s, respectively) based on the 2H NMR spectra (24, 33). 13C NMR analysis of the multiplets of C2 of MAG yields the relative fluxes in the TCA cycle as described previously (20, 23).
Other Assays
Glucose measurements were performed using a colorimetric glucose oxidase method (DCL, Oxford, CT). Baseline adiponectin concentrations were measured with the mouse/rat ELISA kit obtained from B-Bridge International (Sunnyvale, CA). The assay has been previously validated for use in cats (19). Plasma insulin (15) and total thyroxine (TT4) (36) concentrations were measured as described previously, using assays validated for cats.
Statistical Analysis
All analyses were carried out using Systat 12 software (Systat, Richmond, CA) with a mixed-models analysis. When diet × weight differed significantly, pairwise comparisons were made using Bonferroni adjustment of P values (Systat 12). Results were pooled when no significant differences were found among the three different diets, and the model was thus reduced to a test of weight. Linear regression analysis was used to estimate associations among continuous variables in the data set. Data are means ± SD unless otherwise stated. Values of P < 0.05 were considered significant.
RESULTS
Body Weight, BMI, Girth, and Food Intake
The weight, BMI, girth, and food intake of the cats are shown in Table 2. The data are shown for the total study period because there were no diet differences. Weight, BMI, and girth were significantly lower in lean than in obese cats (P < 0.001). Lean cats had an ∼30% higher caloric intake (kcal/kg) than obese cats (P < 0.001).
Table 2.
Body weight, anthropometric measurements, and energy intake
| Measurements | BW, kg | BMI, kg/m2 | Girth, cm | Caloric Intake, kcal/kg |
|---|---|---|---|---|
| Lean cats | 3.5±0.5a | 31.5±3.3b | 36.3±2.1c | 53.6±8.3d |
| Obese cats | 7.2±1.2a | 59.0±7.7b | 54.9±4.4c | 37.6±4.0d |
Values are means ± SD for body weight (BW), body mass index (BMI), girth, and caloric intake in 12 lean and 12 obese cats fed 3 different diets. The results were combined because there were no differences among the diets.
Significant difference between lean and obese groups (P < 0.001).
Glucose Turnover and Metabolic Fluxes
Results from NMR analysis were available for a total of 27 lean (n = 6 for diet HP, n = 11 for diet HCSAT, n = 10 for diet HCPUFA) and 33 obese cats (n = 12 for diet HP, n = 12 for diet HCSAT, n = 9 for diet HCPUFA). Compared with that in obese cats, EGP was higher in lean cats and there were no diet differences (P < 0.001; Table 3).
Table 3.
Relative and absolute fluxes through pathways in glucose production
| Lean Cats | Obese Cats | |
|---|---|---|
| Endogenous glucose production, mg·kg−1·min−1 | 2.08±0.46a | 1.58±0.46a |
| 2H NMR: fractional sources of plasma glucose, % | ||
| Total gluconeogenesis | 62±16 | 65±13 |
| Flux from glycerol to glucose | 20±10 | 21±10 |
| Flux from PEP to glucose | 42±15 | 44±10 |
| Glycogenolysis | 38±16 | 35±13 |
| 13C NMR: fluxes relative to citrate synthase | ||
| Flux through PEPCK (PC/PEPCK)/CS | 4.69±1.47 | 4.53±1.60 |
| Flux through pyruvate kinase/CS | 3.68±1.22 | 3.61±1.26 |
| Flux from PEP to glucose/CS | 1.00±0.42 | 0.92±0.48 |
| Derived fluxes, mg·kg−1·min−1 | ||
| Flux from glycogen to glucose | 0.77±0.30b | 0.57±0.30b |
| Flux from glycerol to glucose | 0.81±0.39d* | 0.35±0.24d* |
| Flux from PEP to glucose | 1.8±0.87c | 1.29±0.35c |
| Flux through PEPCK (PC/PEPCK) | 7.71±3.2 | 7.77±3.91 |
| Flux through pyruvate kinase | 6.07±2.66 | 6.41±3.66 |
| Flux through OAA to citrate | 1.76±0.83 | 1.89±1.01 |
Values are means (± SD) for the combined values of the 12 lean and 12 obese cats.
P < 0.001; values with the same superscript differ significantly
(P < 0.001,
P < 0.02,
P < 0.03). *Diet differences was found between lean and obese cats
(P < 0.002).
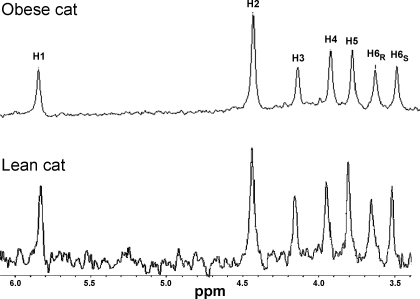
Origin of blood glucose by 2H NMR analysis.
Representative 2H NMR spectra of a MAG sample from a lean and an obese cat are shown in Fig. 1. The ratio of H6s to H2 in the 2H NMR spectra is a direct measurement of the fraction of blood glucose produced by gluconeogenesis from the TCA cycle (24), which was, as expected, the major contributor of EGP in all of the fasted lean and obese cats (∼ 40%). The percentage of total gluconeogenesis was almost identical between lean (62 ± 16%) and obese cats (65 ± 13%) and was unrelated to sex or diet, so the data were combined (Table 3). The relative amount of gluconeogenesis coming from either glycerol or PEP (TCA cycle) was similar in lean (20 ± 10 and 42 ± 15%, respectively) and obese cats (21 ± 10 and 44 ± 10%, respectively), and the percentage of glycogenolysis contributing to EGP was also similar (38 ± 16 and 35 ± 13%, for lean and obese cats, respectively; Table 3). Absolute fluxes were obtained by combining the relative fluxes with the measured EGP (20). The absolute flux from glycogen was significantly higher in lean than obese cats, and there were no diet differences within the groups, so the data were combined (Table 3). Similarly, the absolute flux from PEP to glucose was significantly higher in lean compared with obese cats (P < 0.03; Table 3), and there were no diet differences, so the data were combined.
Fig. 1.
Resonances from 2H NMR spectra of monoacetone glucose (MAG) derived from plasma glucose from an overnight fasted obese and a lean cat, respectively, after infusion with [3,4-13C2]glucose and oral administration of 2H2O and [U-13C2]propionate. The spectrum shows 7 aliphatic hydrogens derived from plasma glucose.
The absolute flux from glycerol to glucose was similar between lean and obese cats fed the HP diet (0.74 ± 0.36 and 0.57 ± 0.26, respectively, P > 0.05) but was higher in lean compared with obese cats fed either the HCSAT (0.84 ± 0.41 and 0.24 ± 0.1, respectively, P < 0.002) or the HCPUFA diet (0.83 ± 0.42 and 0.23 ± 0.12, respectively, P < 0.002).
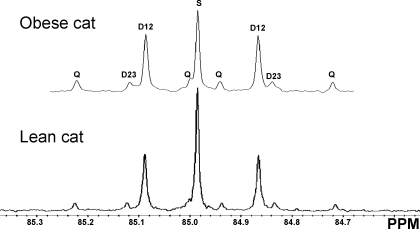
TCA cycle and related fluxes measured by 13C NMR analysis.
The fluxes through the TCA cycle and related fluxes measured by 13C NMR are shown in Table 3. Analyzing the C2 multiplets (Fig. 2) of the 13C NMR spectrum of MAG provides an estimate of fluxes through pyruvate carboxylase/phosphoenolpyruvate carboxykinase (PC/PEPCK)- and pyruvate kinase/malic enzyme (PK/ME)-mediated pyruvate exchange with TCA cycle intermediates (pyruvate cycling), as well as gluconeogenesis from the TCA cycle, all relative to citrate synthase flux. Neither weight nor diet influenced the anaplerotic/cataplerotic fluxes through PC/PEPCK, pyruvate cycling, or gluconeogenesis from the TCA cycle relative to the flux through citrate synthase (Table 3). Combining the results of the measured EGP, fractional sources of glucose production by 2H NMR, and TCA cycle fluxes obtained by 13C NMR yields the absolute fluxes through multiple pathways of the TCA cycle. There were no effects of diet or weight on the absolute fluxes through PC/PEPCK, pyruvate cycling, and flux from oxaloacetate (OAA) to citrate (citrate synthase) (Table 3). The only difference identified was comparing female with male obese cats, where the absolute fluxes through PC/PEPCK and citrate synthase were all significantly higher, which was consistent for all diets (Table 4).
Fig. 2.
13C NMR spectrum of carbon-2 resonance of MAG derived from plasma glucose. Fluxes of MAG were derived from plasma glucose from an overnight fasted obese and a lean cat, respectively, after infusion with [3,4-13C2]glucose and oral administration of 2H2O and [U-13C2]propionate. The spectrum shows a doublet from coupling of carbon-1 with carbon-2 (D12), a doublet from coupling of carbon-2 with carbon-3 (D23), a doublet of doublets (quartet; Q) arising from coupling of carbon-2 with both carbon-1 and -3, and a singlet resonance (S).
Table 4.
Sex-related differences in absolute fluxes through PC/PEPCK, pyruvate kinase (pyruvate cycling), and from OAA to PEP in obese cats
| Absolute Fluxes, mg·kg−1·min−1 | Obese Males | Obese Females |
|---|---|---|
| Flux through PC/PEPCK | 6.17±2.24a | 9.28±4.57a |
| Flux through pyruvate kinase | 4.94±2.07b | 7.80±4.32b |
| Flux from OAA to citrate | 1.53±0.78c | 2.22±1.10c |
Values are means ± SD for the combined results of 6 female and 6 male obese cats. PC/PEPCK, pyruvate carboxylase/phosphoenolpyruvate carboxykinase; OAA, oxaloacetate; PEP, phosphoenolpyruvate. Values with the same superscript differ significantly
(P < 0.02,
P < 0.03,
P < 0.05).
Other Assays
The results for plasma glucose and insulin concentrations are shown in Table 5. There was no significant difference of plasma glucose concentrations before and after infusion in the fasted lean and obese cats (Table 5). Lean cats had significantly lower baseline plasma insulin concentrations than obese cats (P < 0.001; Table 5). This was unrelated to sex or diet differences.
Table 5.
Blood concentrations for glucose, insulin, TT4, and adiponectin
| Measurements | Lean Cats | Obese Cats |
|---|---|---|
| Baseline glucose, mmol/l | 4.99±0.74 | 5.51±0.69 |
| 1-h Postinfusion glucose, mmol/l | 4.77±0.61 | 5.59±1.20 |
| Baseline insulin, pmol/l | 82.21±42.87a | 156.10±88.39a |
| Baseline TT4, μg/dl | 2.20±0.46b | 2.65±0.55b |
| Baseline adiponectin, μg/ml | 5.86±3.09c | 1.34±0.70c |
Values are means ± SD for the 12 lean and 12 obese cats fed 3 different diets. TT4, total thyroxine.
Values with the same superscript differ significantly (a,b,cP < 0.001).
The TT4 concentrations were significantly lower in lean compared with obese cats (P < 0.001; Table 5). Adiponectin concentrations were significantly higher in lean than in obese cats (P < 0.001, Table 5).
Correlation Between Results of Glucose Turnover and Metabolic Fluxes With Anthropometric Measurements and Assay Results
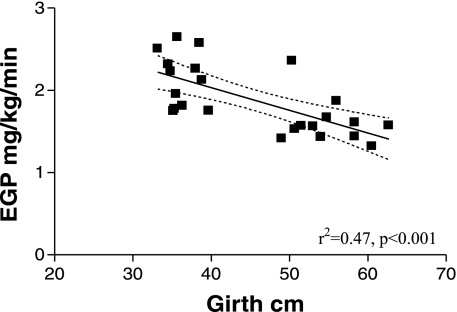
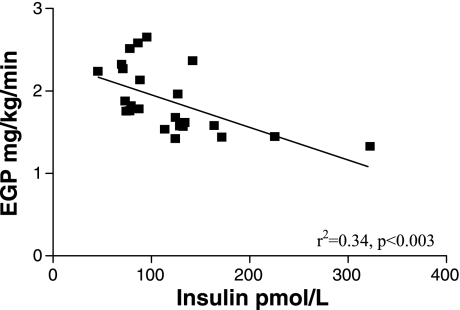
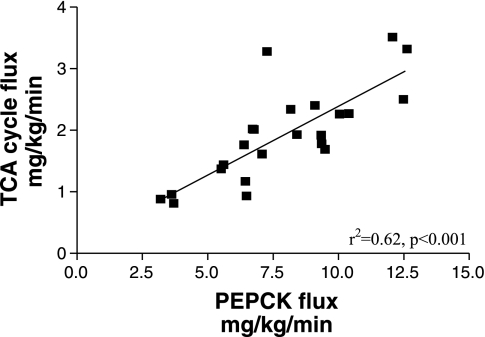
There was a significant inverse correlation between the EGP and either girth or BMI in lean and obese cats (r2 = 0.47, P < 0.001 and r2 = 0.44, P < 0.001, respectively; Fig. 3). The EGP also showed a significant inverse relationship to the baseline plasma insulin concentrations (r2 = 0.34, P < 0.003; Fig. 4). Interestingly, in addition, the TCA cycle flux was positively correlated to the PC/PEPCK flux (r2 = 0.62, P < 0.001; Fig. 5).
Fig. 3.
Relationship between endogenous glucose production (EGP) and girth in the 12 lean and 12 obese cats.
Fig. 4.
Relationship between EGP and baseline insulin concentrations in the 12 lean and 12 obese cats.
Fig. 5.
Relationship between the absolute flux through pyruvate carboxylase/phosphoenolpyruvate carboxykinase (PC/PEPCK) and the tricarboxylic acid (TCA) cycle flux (citrate synthase) in the 12 lean and 12 obese cats.
DISCUSSION
This study is the first noninvasive comprehensive analysis of glucose metabolism in cats using NMR spectroscopy in combination with a nonradioactive isotope tracer technique. We have demonstrated that EGP is unexpectedly lower in long-term obese cats compared with lean cats and shown a sex-related difference between obese male and female cats in their TCA cycle activity and related fluxes.
These results are important new information regarding glucose metabolism in cats. So far, only a few studies have examined intermediary metabolism in cats. Glucokinase (GK) activity and GK gene expression were found to be low or absent in feline liver; however, it has been shown that activities of hexokinase, fructokinase, pyruvate kinase, glucose-6-phosphate dehydrogenase, fructose-1,6-bisphosphatase, and glucose-6-phosphatase were significantly higher in cats than in dogs, who are thought to have normal GK activity (44, 47). From these findings, it was concluded that the higher activity of hexokinases may compensate for the lack of GK in cats. It also has been proposed that cats, in contrast to other mammals, cannot adapt glucose metabolism to dietary alterations and that gluconeogenesis is always high (39). However, the results of this study and other, more recent studies (17, 19, 40, 41) have refuted previous findings and shown that cats can adapt to variations in dietary macronutritients and regulate gluconeogenesis and EGP when necessary.
Glucose homeostasis depends mainly on coordination among β-cell function, glucose utilization by the peripheral tissue, and EGP by the liver and is strongly associated with fat metabolism. In the fasted state, the liver rather than the peripheral tissue is thought to be the target organ achieving glucose homeostasis (10). The regulation of EGP by the liver is controlled by sinusoidal glucose and insulin concentrations (8, 35, 46) as well as by the liver sinusoidal glucagon-to-insulin ratio (38). In the fasting state, plasma glucagon levels have been shown to be elevated in the presence of decreased plasma insulin concentrations, synergistically increasing hepatic EGP (1). Because in this study EGP was decreased rather than increased in the obese cats, and insulin concentrations were elevated in obese compared with lean cats, lower plasma glucagon concentrations might have been expected. We had measured glucagon concentrations in an earlier (unpublished) study in 10 lean and 10 obese fasted cats (equal sex distribution) and failed to demonstrate any significant difference in glucagon concentrations between lean and obese cats. The average glucagon concentration in that study was 79.3 ± 3.4 pg/ml in the lean cats and 82.4 ± 4.2 pg/ml in the obese cats (means ± SE). From these former results and the fact that the obese cats had increased plasma insulin concentrations compared with the lean cats, we assumed that insulin rather than glucagon might play a more important role in decreasing EGP in obese cats.
The inhibitory effect of insulin on hepatic gluconeogenesis has been thought to be mainly mediated by insulin binding directly to insulin receptors (IRs) on the liver as well as on other peripheral tissues providing gluconeogenic substrates for EGP (i.e., muscle, adipose tissue) (8). However, a more recent model for insulin regulating glucose metabolism proposed that insulin also binds and activates IRs in the central nervous system on agouti-related peptide (AgRP)-expression neurons, leading to the activation of hepatic IL-6, which decreases hepatic gluconeogenesis (29, 30). Insulin suppresses hepatic glucose production (37) by reducing glycogenolysis and gluconeogenesis (8). Both were found to be significantly decreased in obese compared with lean cats in our study, leading to an ∼30% lower EGP in the obese cats. In the fasted state, the obese cats were hyperinsulinemic, indicated by approximately doubled baseline peripheral insulin concentrations and no difference in glucose concentrations compared with lean cats, and there was a significant inverse correlation between plasma insulin concentration and EGP. Together, these results might be interpreted as strong evidence that in the fasting state, insulin is still the main controller of hepatic EGP in obese cats.
The results suggest that the liver of obese cats is still insulin sensitive and that hepatic autoregulation is intact despite the fact that peripheral insulin resistance and impaired glucose clearance have been well documented in obese cats (16–19). The decreased EGP in obese cats might be a compensatory mechanism and could explain the fact that baseline glucose concentrations and glycosylated hemoglobin concentrations remain normal and that impaired fasting glucose is not evident in obese cats despite decreased expression of insulin-dependent GLUT4 in muscle and decreased glucose clearance during intravenous glucose tolerance testing (2). This is in contrast to humans, where obese individuals with normal fasting glucose but impaired glucose tolerance show less suppressed EGP during high insulin concentrations compared with healthy subjects (48), implying that hepatic insulin resistance is already present in obese humans even when fasting glucose concentrations are still normal.
A limitation of the study is that it was only performed in the fasted state and that peripheral insulin resistance was not tested with the euglycemic hyperinsulinemic clamps (EHC). However, we have performed EHC in obese cats before, and all showed peripheral insulin resistance. In fact, we calculated that insulin sensitivity decreases by ∼30% for every kilogram of weight gain (17–19). When the results of these previous studies were compared, no differences between insulin sensitivity (SI) were shown among the studies, and SI in obese cats on average was ∼30% of the value in lean cats. We also found lower adiponectin levels in the obese compared with lean cats and demonstrated in an earlier study that adiponectin levels are positively correlated with insulin sensitivity (19) as also has been shown in humans (43).
Gluconeogenesis is mainly dependent on supply from glycerol, gluconeogenic amino acids, pyruvate, and lactate and is promoted by hepatic fat oxidation (50). Gluconeogenesis and fluxes through PEPCK have been shown to be impaired when hepatic TCA cycle activity (i.e., fat oxidation) is decreased (4), whereas induction of hepatic fat oxidation has been shown to stimulate hepatic gluconeogenesis (42), indicating an important link between both processes. In agreement with these findings, we demonstrated in this study that, in cats, fluxes through PC/PEPCK are strongly linked to TCA cycle activity. During fasting, β-oxidation will produce a large amount of acetyl-CoA, which stimulates pyruvate carboxylase (PC) and citrate synthase and at the same time inhibits pyruvate dehydrogenase (PDH), thereby favoring the gluconeogenic pathway (9). It is conceivable that fluxes through PC/PEPCK are actually regulated by PC activity, and this might explain why PC/PEPCK fluxes are so tightly correlated to TCA fluxes, since both PC and citrate synthase activities are controlled by acetyl-CoA levels (45). Although we did not find that hepatic TCA cycle fluxes were impaired in fasted obese compared with lean cats, we did find that the absolute gluconeogenic fluxes from glycerol to glucose were lower in obese compared with lean cats when fed a diet high in carbohydrates and fat, suggesting diet-dependent alteration in fat metabolism in obese cats. This was not seen when cats were fed the HP diet, likely because more gluconeogenic amino acids were available in the fasting state, sparing fat/glycerol mobilization. Glycerol mobilization is dependent on the activity of hepatic glycerol kinase and glycerol 3-phosphate dehydrogenase, as well as peroxisome proliferator-activated receptor-α (PPARα) (34). PPARα stimulates fatty acid oxidation and ketogenesis (34) and increases the gluconeogenic flux from glycerol to glucose (42). Obese cats have lower PPARα expression levels in adipose tissue compared with lean cats, but this has not been examined in liver tissue (14). Lower hepatic PPARα activities could be in part responsible for the decreased gluconeogenic flux from glycerol seen in the obese cats. Decreased PPARα levels would also lead to increased free fatty acids. Although nonesterified fatty acid (NEFA) concentrations were not measured at the same time as glucose fluxes were measured, baseline NEFA concentrations were found to be higher in the same obese cats compared with the lean cats despite significantly higher plasma insulin concentrations when lipid profiles were measured after 2 mo on the same diets (26).
Sex-related differences in glucose and fat metabolism have been documented in obese cats: female obese cats have been reported to have reduced glucose oxidation, glycogenesis, and lipogenesis during EHC compared with lean and male obese cats (18). Interestingly, we found in our study that female obese cats had ∼1.5 times higher fluxes through PC/PEPCK and citrate synthase accompanied by a 1.5-fold increased pyruvate cycling compared with male obese cats. This suggests that the hepatic TCA cycle (i.e., fat oxidation) is significantly more active in female obese than male obese cats but does not increase EGP from gluconeogenesis, because pyruvate cycling acts as a controlling mechanism to regulate EGP. Pyruvate cycling has previously been described as a “futile cycle” through OAA → PEP → pyruvate → OAA or from malate → pyruvate → OAA → malate (12, 23). The cycle may indeed not be futile, because increased pyruvate cycling with higher fluxes through PEPCK and TCA cycle fluxes has been reported in fasted Diabetic Zucker (fa/fa) rats and has been suggested to act as a controller in modulating gluconeogenesis from the level of the TCA cycle whenever glucose is available via glycogenolysis (22). It might be hypothesized that female obese cats are able to compensate their overnutrition state by enhancing PEPCK and TCA fluxes as well as “futile” pyruvate cycling. This would decrease hepatic oxidative stress and permit greater fatty acid oxidation and could be a protective mechanism for female obese cats against the development of diabetes mellitus. Because these animals were neutered at an early age, it also suggests that sex differences are programmed very early in life.
In this study, weight was strictly controlled to avoid influences of changes in weight on metabolic parameters. Diet did not seem to have a significant impact on glucose metabolism in lean and obese cats. There were no diet differences in caloric intake during the study period, likely because all three diets were isocaloric. The diet differences also were not extreme. Long-term obese cats had an ∼30% lower food intake (kcal/kg) than lean cats to maintain their body weight through the study. This might be explained by the fact that obese cats have significantly lower heat production per metabolic size (18, 19).
In conclusion, we have demonstrated that long-term obese cats have a lower fasting EGP compared with lean cats. These results might explain in part why long-term obese cats are still able to maintain normal baseline glucose concentrations and glycosylated hemoglobin concentrations despite the fact that they show peripheral insulin resistance and decreased glucose clearance. Our data further suggest that sex-related differences exist in the regulation of hepatic glucose metabolism in long-term obese cats, suggesting that pyruvate cycling acts as a controlling mechanism to modulate EGP in females, which could be an important factor in the decreased risk of female obese cats to progress to diabetes.
Perspectives and Significance
This study demonstrated that a triple tracer infusion technique in combination with NMR analysis is a useful noninvasive tool to analyze glucose metabolism in cats. We were able to demonstrate with this study that certain metabolic events are still normal even in long-term obese cats, allowing them to maintain euglycemia, and that upregulation of certain futile cycles may explain in part the sex difference in the risk of developing diabetes that is seen in male cats. The cat could provide an excellent model to further examine differences in metabolic pathways responsible for the progression from the obese nondiabetic to the obese diabetic state.
GRANTS
This study was supported in part by a grant from the Nestle Purina Petcare, St. Louis, MO (to M. Hoenig) and by National Center for Research Resources Grant P41 RR005351 “Research in Integrated Glycotechnology” (to J. H. Prestegard).
Present address of S. Kley: Royal Veterinary College, University of London, Hawkshead Lane, North Mymms, Hertfordshire AL9 7TA, UK.
Acknowledgments
We thank Dr. David Schaeffer for the statistical analysis.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aguilar-Parada E, Eisentraut AM, Unger RH. Effects of starvation on plasma pancreatic glucagon in normal man. Diabetes 18: 717–723, 1969. [DOI] [PubMed] [Google Scholar]
- 2.Brennan CL, Hoenig M, Ferguson DC. GLUT4 but not GLUT1 expression decreases early in the development of feline obesity. Domest Anim Endocrinol 26: 291–301, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Burgess SC, Jeffrey FM, Storey C, Milde A, Hausler N, Merritt ME, Mulder H, Holm C, Sherry AD, Malloy CR. Effect of murine strain on metabolic pathways of glucose production after brief or prolonged fasting. Am J Physiol Endocrinol Metab 289: E53–E61, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Burgess SC, Leone TC, Wende AR, Croce MA, Chen Z, Sherry AD, Malloy CR, Finck BN. Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha)-deficient mice. J Biol Chem 281: 19000–19008, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgess SC, Weis B, Jones JG, Smith E, Merritt ME, Margolis D, Dean Sherry A, Malloy CR. Noninvasive evaluation of liver metabolism by 2H and 13C NMR isotopomer analysis of human urine. Anal Biochem 312: 228–234, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes 45: 633–638, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 17: 961–969, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Cherrington AD, Edgerton D, Sindelar DK. The direct and indirect effects of insulin on hepatic glucose production in vivo. Diabetologia 41: 987–996, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Eaton S, Pourfarzam M, Bartlett K. The effect of respiratory chain impairment of beta-oxidation in rat heart mitochondria. Biochem J 319: 633–640, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felig P, Wahren J. Influence of endogenous insulin secretion on splanchnic glucose and amino acid metabolism in man. J Clin Invest 50: 1702–1711, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank G, Anderson W, Pazak H. Use of a high protein diet in the management of feline diabetes mellitus. Vet Ther 2: 238–246, 2001. [PubMed] [Google Scholar]
- 12.Freidmann B, Goodman EH Jr, Saunders HL, Kostos V, Weinhouse S. An estimation of pyruvate recycling during gluconeogenesis in the perfused rat liver. Arch Biochem Biophys 143: 566–578, 1971. [DOI] [PubMed] [Google Scholar]
- 13.Hausler N, Browning J, Merritt M, Storey C, Milde A, Jeffrey FM, Sherry AD, Malloy CR, Burgess SC. Effects of insulin and cytosolic redox state on glucose production pathways in the isolated perfused mouse liver measured by integrated 2H and 13C NMR. Biochem J 394: 465–473, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoenig M, Caffall Z, Ferguson DC. Triiodothyronine differentially regulates key metabolic factors in lean and obese cats. Domest Anim Endocrinol 34: 229–237, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Hoenig M, Ferguson DC. Impairment of glucose tolerance in hyperthyroid cats. J Endocrinol 121: 249–251, 1989. [DOI] [PubMed] [Google Scholar]
- 16.Hoenig M, Hall G, Ferguson D, Jordan K, Henson M, Johnson K, O'Brien T. A feline model of experimentally induced islet amyloidosis. Am J Pathol 157: 2143–2150, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoenig M, Thomaseth K, Brandao J, Waldron M, Ferguson DC. Assessment and mathematical modeling of glucose turnover and insulin sensitivity in lean and obese cats. Domest Anim Endocrinol 31: 373–389, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Hoenig M, Thomaseth K, Waldron M, Ferguson DC. Fatty acid turnover, substrate oxidation, and heat production in lean and obese cats during the euglycemic hyperinsulinemic clamp. Domest Anim Endocrinol 32: 329–338, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Hoenig M, Thomaseth K, Waldron M, Ferguson DC. Insulin sensitivity, fat distribution, and adipocytokine response to different diets in lean and obese cats before and after weight loss. Am J Physiol Regul Integr Comp Physiol 292: R227–R234, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Jin ES, Burgess SC, Merritt ME, Sherry AD, Malloy CR. Differing mechanisms of hepatic glucose overproduction in triiodothyronine-treated rats vs. Zucker diabetic fatty rats by NMR analysis of plasma glucose. Am J Physiol Endocrinol Metab 288: E654–E662, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Jin ES, Jones JG, Merritt M, Burgess SC, Malloy CR, Sherry AD. Glucose production, gluconeogenesis, and hepatic tricarboxylic acid cycle fluxes measured by nuclear magnetic resonance analysis of a single glucose derivative. Anal Biochem 327: 149–155, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Jin ES, Park BH, Sherry AD, Malloy CR. Role of excess glycogenolysis in fasting hyperglycemia among pre-diabetic and diabetic Zucker (fa/fa) rats. Diabetes 56: 777–785, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Jones JG, Naidoo R, Sherry AD, Jeffrey FM, Cottam GL, Malloy CR. Measurement of gluconeogenesis and pyruvate recycling in the rat liver: a simple analysis of glucose and glutamate isotopomers during metabolism of [1,2,3-13C3]propionate. FEBS Lett 412: 131–137, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Jones JG, Solomon MA, Cole SM, Sherry AD, Malloy CR. An integrated 2H and 13C NMR study of gluconeogenesis and TCA cycle flux in humans. Am J Physiol Endocrinol Metab 281: E848–E856, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Jones JG, Solomon MA, Sherry AD, Jeffrey FM, Malloy CR. 13C NMR measurements of human gluconeogenic fluxes after ingestion of [U-13C]propionate, phenylacetate, and acetaminophen. Am J Physiol Endocrinol Metab 275: E843–E852, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Jordan E, Kley S, Le NA, Waldron M, Hoenig M. Dyslipidemia in obese cats. Domest Anim Endocrinol 35: 290–299, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Kahn SE The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 46: 3–19, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP, Porte D Jr. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 42: 1663–1672, 1993. [DOI] [PubMed] [Google Scholar]
- 29.Koch L, Wunderlich FT, Seibler J, Konner AC, Hampel B, Irlenbusch S, Brabant G, Kahn CR, Schwenk F, Bruning JC. Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest 118: 2132–2147, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Bruning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 5: 438–449, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Laflamme DP Development and validation of a body condition score system for cats: a clinical tool. Feline Pract 25: 13–18, 1997. [Google Scholar]
- 32.Laflamme DP, Hume E, Harrison J. Evaluation of zoometric measures as an assessment of body composition of dogs and cats. Compend Contin Educ Pract Vet 23: 88, 2001. [Google Scholar]
- 33.Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC. Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest 98: 378–385, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patsouris D, Mandard S, Voshol PJ, Escher P, Tan NS, Havekes LM, Koenig W, Marz W, Tafuri S, Wahli W, Muller M, Kersten S. PPARalpha governs glycerol metabolism. J Clin Invest 114: 94–103, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen KF, Laurent D, Rothman DL, Cline GW, Shulman GI. Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans. J Clin Invest 101: 1203–1209, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterson ME, Kintzer PP, Cavanagh PG, Fox PR, Ferguson DC, Johnson GF, Becker DV. Feline hyperthyroidism: pretreatment clinical and laboratory evaluation of 131 cases. J Am Vet Med Assoc 183: 103–110, 1983. [PubMed] [Google Scholar]
- 37.Rizza RA, Mandarino LJ, Gerich JE. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol Endocrinol Metab 240: E630–E639, 1981. [DOI] [PubMed] [Google Scholar]
- 38.Roden M, Perseghin G, Petersen KF, Hwang JH, Cline GW, Gerow K, Rothman DL, Shulman GI. The roles of insulin and glucagon in the regulation of hepatic glycogen synthesis and turnover in humans. J Clin Invest 97: 642–648, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogers QR, Morris JG, Freedland RA. Lack of hepatic enzymatic adaptation to low and high levels of dietary protein in the adult cat. Enzyme 22: 348–356, 1977. [DOI] [PubMed] [Google Scholar]
- 40.Russell K, Lobley GE, Millward DJ. Whole-body protein turnover of a carnivore, Felis silvestris catus. Br J Nutr 89: 29–37, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Russell K, Murgatroyd PR, Batt RM. Net protein oxidation is adapted to dietary protein intake in domestic cats (Felis silvestris catus). J Nutr 132: 456–460, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Satapati S, He T, Inagaki T, Potthoff M, Merritt ME, Esser V, Mangelsdorf DJ, Kliewer SA, Browning JD, Burgess SC. Partial resistance to peroxisome proliferator-activated receptor-alpha agonists in ZDF rats is associated with defective hepatic mitochondrial metabolism. Diabetes 57: 2012–2021, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schondorf T, Maiworm A, Emmison N, Forst T, Pfutzner A. Biological background and role of adiponectin as marker for insulin resistance and cardiovascular risk. Clin Lab 51: 489–494, 2005. [PubMed] [Google Scholar]
- 44.Tanaka A, Inoue A, Takeguchi A, Washizu T, Bonkobara M, Arai T. Comparison of expression of glucokinase gene and activities of enzymes related to glucose metabolism in livers between dog and cat. Vet Res Commun 29: 477–485, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Utter MF, Keech DB, Scrutton MC. A possible role for acetyl CoA in the control of gluconeogenesis. Adv Enzyme Regul 2: 49–68, 1964. [DOI] [PubMed] [Google Scholar]
- 46.Vella A, Reed AS, Charkoudian N, Shah P, Basu R, Basu A, Joyner MJ, Rizza RA. Glucose-induced suppression of endogenous glucose production: dynamic response to differing glucose profiles. Am J Physiol Endocrinol Metab 285: E25–E30, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Washizu T, Tanaka A, Sako T, Washizu M, Arai T. Comparison of the activities of enzymes related to glycolysis and gluconeogenesis in the liver of dogs and cats. Res Vet Sci 67: 205–206, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Weyer C, Bogardus C, Pratley RE. Metabolic characteristics of individuals with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 48: 2197–2203, 1999. [DOI] [PubMed] [Google Scholar]
- 49.Wilkins C, Long RC Jr, Waldron M, Ferguson DC, Hoenig M. Assessment of the influence of fatty acids on indices of insulin sensitivity and myocellular lipid content by use of magnetic resonance spectroscopy in cats. Am J Vet Res 65: 1090–1099, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Williamson JR, Kreisberg RA, Felts PW. Mechanism for the stimulation of gluconeogenesis by fatty acids in perfused rat liver. Proc Natl Acad Sci USA 56: 247–254, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]