Abstract
The T1R2 and T1R3 proteins are expressed in taste receptor cells and form a heterodimer binding with compounds described as sweet by humans. We examined whether Polycose taste might be mediated through this heterodimer by testing T1R2 knockout (KO) and T1R3 KO mice and their wild-type (WT) littermate controls in a series of brief-access taste tests (25-min sessions with 5-s trials). Sucrose, Na-saccharin, and Polycose were each tested for three consecutive sessions with order of presentation varied among subgroups in a Latin-Square manner. Both KO groups displayed blunted licking responses and initiated significantly fewer trials of sucrose and Na-saccharin across a range of concentrations. KO mice tested after Polycose exposure demonstrated some degree of concentration-dependent licking of sucrose, likely attributable to learning related to prior postingestive experience. These results are consistent with prior findings in the literature, implicating the T1R2+3 heterodimer as the principal taste receptor for sweet-tasting ligands, and also provide support for the potential of postingestive experience to influence responding in the KO mice. In contrast, T1R2 KO and T1R3 KO mice displayed concentration-dependent licking responses to Polycose that tracked those of their WT controls and in some cases licked midrange concentrations more; the number of Polycose trials initiated overall did not differ between KO and WT mice. Thus, the T1R2 and T1R3 proteins are individually unnecessary for normal concentration-dependent licking of Polycose to be expressed in a brief-access test. Whether at least one of these T1R protein subunits is necessary for normal Polycose responsiveness remains untested. Alternatively, there may be a novel taste receptor(s) that mediates polysaccharide taste.
Keywords: C57BL/6 mice, gustatory system, Tas1r2, Tas1r3, sweet taste, sucrose, saccharin
the primary taste receptor that mediates the transduction of compounds that humans report as “sweet” and rodents treat as sucrose-like and rewarding in a variety of behavioral tests has been identified. This receptor is composed of two protein subunits, T1R2 and T1R3, encoded by the Tas1r2 and Tas1r3 genes, that combine to form a heterodimer that binds with sweet tasting ligands (2, 14, 20, 22, 25–27, 29, 30, 38, 53). Although it is widely accepted that the T1R2+3 heterodimer is critical for normal taste responsiveness to sugars and artificial sweeteners to be maintained, there has been some question about whether there are other taste receptors that can also bind with saccharides (see Refs. 1 and 44). Genetically engineered mice missing one of the two subunits, either T1R2 or T1R3, appear to be ageusic to artificial sweeteners, such as saccharin as measured electrophysiologically and behaviorally (54). Thus, if there are additional receptors, they are not sufficient to maintain any observable taste responsiveness to these ligands, at least in mice. However, T1R2 knockout (KO) and T1R3 KO mice do display some very weak concentration-dependent responses to sugars, such as sucrose, glucose, and maltose, as measured in brief-access taste tests and electrophysiologically (4, 54). The maintained responsiveness to sugars at high concentrations in T1R3 KO mice is even more evident in two-bottle intake tests (4), but such procedures can be influenced by postingestive factors supporting the development of learned preferences (e.g.. 43, 45). In this same study, electrophysiological recordings from the chorda tympani nerve of T1R3 KO mice showed abolished responses to artificial sweeteners and moderately diminished responses to sugars. Interestingly, there was no significant difference in the CT responses of T1R3 KO mice and WT mice to application of 0.5 M glucose to the tongue. Also, Delay et al. (5) has shown that T1R3 KO mice display normal sucrose detection thresholds as measured in a shock avoidance task. Although mice lacking one of the two subunits of the T1R2+3 receptor can display some degree of responsiveness to certain sugars, double KO mice that have both the Tas1r2 and the Tas1r3 gene deleted are entirely unresponsive to all sweet-tasting ligands that have been tested, whether natural or synthetic, as measured behaviorally or electrophysiologically (54).
On balance, the evidence is clear that the T1R2+3 heterodimer is the primary receptor for prototypical sweet-tasting compounds. However, on the basis of findings from behavioral and electrophysiological studies, it has been suggested that rodents may possess an additional type of receptor that binds with longer-chain polysaccharides (40, 44). Polysaccharides are glucose polymers of various chain lengths. Of the polysaccharides, Polycose has been the most studied in taste experiments and contains a small amount of glucose and maltose but primarily consists of glucose polymers of three or more glucose units with an average of ∼5 (19). In taste aversion generalization studies, rats, mice, and gerbils conditioned to avoid sucrose also avoid fructose, glucose, and maltose (8, 17, 31, 33, 34, 39). However, rats trained to avoid Polycose or sucrose show no or very weak cross-generalization (33, 36, 39), suggesting these two carbohydrate solutions have different taste qualities. Hamsters conditioned to avoid Polycose, sucrose, or a mixture of the two compounds display some degree of cross-generalization, but Polycose and sucrose, nonetheless, appear to have characteristics that make the compounds distinct from one another (11). Thus, it appears that Polycose generates a taste sensation in rodents that is discriminable from the prototypical sweetener, sucrose.
Interestingly, rodents appear to treat the taste quality of maltose (a disaccharide with two glucose moieties) somewhat falling between that of sucrose (a disaccharide with a fructose and a glucose moiety) and Polycose. Rats conditioned to avoid sucrose also display some avoidance of maltose (39), but it has also been shown by conditioned taste aversion generalization (49) or operant taste discrimination procedures in rats (50) and mice (7) that the taste quality of maltose and sucrose are distinguishable to some extent. Indeed, rats conditioned to avoid maltose also display some degree of avoidance of Polycose and vice-versa (33).
In rats, bilateral transections of peripheral taste nerves have also been shown to produce differential effects on Polycose and sucrose intake. For example, bilateral transection of the glossopharyngeal nerve has been shown to decrease intake of Polycose without affecting sucrose consumption (52). In the rat, chorda tympani nerve responses to sweeteners and Polycose are differentially affected by various “sweet-taste” inhibitors and enhancers (39). In gerbils, chorda tympani responses to sucrose have been shown to be inhibited by oral treatment of CuCl2, but Polycose responses are not affected (48). Collectively, these findings show that peripheral nerve responses to Polycose and sweeteners differ, suggesting that these stimuli may not bind with an identical set of taste receptors.
The findings in the peripheral gustatory system are supported by observations made centrally. In the rat, the response of single neurons in the rostral nucleus of solitary tract, which is the first central relay in the ascending gustatory system, to Polycose, were poorly correlated with that of sucrose and other prototypical basic taste representatives (12, 28). Recording from single neurons in the gustatory zone of the parabrachial nucleus of the rat, Nishijo and Norgren (32) found that responses to sucrose correlated more strongly with those to fructose, maltose, and glycine than with those to Polycose. These findings suggest Polycose and standard sweeteners do not elicit identical neural responses in taste-responsive cell populations in the brain stem.
As noted above, sweet taste is thought to be mediated through the T1R2+3 heterodimer. Whether Polycose taste perception is also mediated through this heterodimer taste receptor has yet to be explicitly investigated. If, as the behavioral and electrophysiological data suggest, sweeteners and Polycose generate distinct taste sensations, then it would follow that the T1R2+3 heterodimer that binds with sweet-tasting ligands may not play an exclusive role, if any, in taste responsiveness to Polycose. Hints of this possibility are already present in the literature in a study examining 129.B6-Tas1r3 congenic mice that have the C57BL/6 “sweet-sensitive” allele of the Tas1r3 taste receptor gene upon a genetic background of 129 mice, which normally have the “sweet-subsensitive” allele. It was shown that taste responses to Polycose measured behaviorally in a 48-h two-bottle preference test and electrophysiologically from the chorda tympani nerve of the 129.B6-Tas1r3 congenic mice and 129/129 mice did not significantly differ (16). It appears, then, that polymorphisms in the Tas1r3 gene influence responsiveness to some sweeteners but not to Polycose in mice.
In the present study, T1R2 KO and T1R3 KO mice and their wild-type (WT) littermate controls were tested in a brief-access taste test. The test compounds chosen were Polycose (glucose polysaccharide), sucrose (glucose-fructose disaccharide), and Na-saccharin (an artificial sweetener). On the basis of previous findings in the literature, responses to sucrose and saccharin should be severely blunted or eliminated in T1R2 KO or T1R3 KO mice. If Polycose taste is mediated through the heterodimer formed by T1R2 and T1R3 subunits, it would be expected that responsiveness to Polycose would also be reduced in T1R2 KO and T1R3 KO mice.
MATERIAL AND METHODS
Subjects.
Male and female breeding pairs of mice that were respectively homozygous null for Tas1r2 or Tas1r3 (derived initially from 129X1/SvJ and backcrossed with C57BL/6 mice for at least three generations) were provided by Dr. Charles Zuker (University of California, San Diego). Wild-type C57BL/6J (B6) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Homozygous null mice and wild-type mice were bred to obtain progeny that were heterozygous for Tas1r2 or Tas1r3. Mice heterozygous (−/+) for Tas1r2 or Tas1r3 were then mated resulting in heterozygous (−/+), homozygous null (-/-) and wild-type (+/+) mice. Homozygous null and wild-type same-sex littermate controls served as subjects in the behavioral tests.
The mice were housed in polycarbonate shoebox cages in a room with automatically controlled temperature, humidity, and a 12:12-h light-dark lighting cycle. Except where noted during behavioral testing, mice were provided ad libitum chow (Purina Laboratory Chow 5001, St. Louis, MO) and deionized reverse-osmosis water.
From at least 7 wk of age, the mice that served as behavioral test subjects were singly housed with ad libitum food and water for at least 2 days before behavioral testing began. During the 4 days of water training (Monday to Thursday), the mice were put on a restricted water-access schedule for which water was available only during the daily testing sessions. Water bottles were removed on Sunday, no more than 23 h before testing. Mice that fell below 85% of their free-drinking weight during the water-restriction schedule received 1 ml supplemental water 1 h after the end of the testing session. Water bottles were returned to their home cages after the last session on Thursday. All procedures were approved by the Florida State University Animal Care and Use Committee.
Taste stimuli.
All solutions were prepared daily with deionized reverse-osmosis water and presented at room temperature. Test stimuli consisted of six concentrations of Polycose (0.01, 0.02, 0.08, 0.16, 0.24, 0.32 M; calculated using the nominal average molecular weight of 1,000; Ross Laboratories, Columbus, OH), six concentrations of sucrose (0.03, 0.06, 0.1, 0.3, 0.6, 1.0 M; BDH Chemicals, West Chester, PA) and six concentrations of Na-saccharin (0.1, 0.5, 1.0, 5.0, 10.0, 50.0 mM; Sigma Chemical, St. Louis, MO). These concentrations span the dynamic range for mice, and concentrations within this range have been used in previous studies (e.g.. 6, 16).
Procedure.
Testing took place in a lickometer (Davis MS-160, DiLog Instruments, Tallahassee, FL), as described previously elsewhere (e.g., 13, 47). A mouse is placed in the test chamber of the apparatus. A motorized shutter opens presenting the mouse access to a single sipper tube containing a taste stimulus, recessed by ∼5 mm behind a slot. A small fan, positioned above the sample slot directs a current of air past the drinking spout to minimize potential olfactory cues from the stimulus. The mouse initiates a trial by licking the spout. Each trial is 5 s, followed by a 7.5-s intertrial interval during which time the tube is changed via a motorized block for the next trial. Concentration is presented in randomized order (without replacement) in blocks of trials. The mice are able to initiate as many trials as possible during the daily 25-min sessions.
Water training.
Mice were placed on a 23-h restricted water-access schedule during the 4 days of water training. On days 1 and 2, the mice were presented with a single stationary tube of water and trained to lick in the apparatus. The session began when the mouse licked the spout and lasted 25 min. On days 3 and 4, seven sipper tubes of water were prepared and presented one at a time in 5-s trials over a 25-min session.
Stimulus testing.
Each taste stimulus was tested for three daily sessions on Mondays, Wednesdays, and Fridays. Exposure to oral and postoral factors of sucrose and Polycose has been shown to increase saccharide preference in mice (41, 46). Thus, we assigned mice to one of three groups designed to account for potential carryover effects due to experience with the prior test stimuli. Each group was presented Polycose, saccharin, and sucrose in different orders as outlined in Table 1.
Table 1.
Stimulus testing order
| GROUP 1 | GROUP 2 | GROUP 3 | |||
|---|---|---|---|---|---|
| WEEK 1 | ———— Water training ———— | ||||
| WEEK 2 | Polycose | Sucrose | Saccharin | ||
| WEEK 3 | Saccharin | Polycose | Sucrose | ||
| WEEK 4 | Sucrose | Saccharin | Polycose | ||
Mice were maintained on a partial food and water restriction schedule by presenting them with 1 g of chow and 2 ml of water ∼23 h before testing (see Ref. 13). The order of testing of different genotypes was balanced as much as possible throughout the day. Recovery days during which food and water were available ad libitum were interjected between testing days.
Data analysis.
The mean number of licks at each concentration per trial was collapsed across the three test sessions. For each mouse, the mean number of licks to water was subtracted from the mean number of licks at each concentration, yielding a Licks to Tastant-Licks to Water value. This measure has also been successfully used in previous studies (18, 51) to produce concentration-response curves that are relative to a water baseline. The lick response (adjusted for water) for each concentration of a stimulus was compared using ANOVAs. The statistical rejection criterion of 0.05 was used for all analyses.
Curves were fit to mean data for each group by using the following logistic function
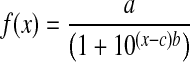 |
(1) |
where x is log10 stimulus concentration, a is asymptotic lick response adjusted for water, b is the slope, and c is the log10 concentration at the inflection point.
Lick data for a particular stimulus from an individual mouse were included for analysis only if the mouse initiated at least 2 trials at each concentration. However, data from each mouse were used to analyze the number of trials initiated to each of the three stimuli.
Genotyping.
Tail samples were obtained from mice to determine genotype by PCR. DNA was extracted, and PCR was performed in a final reaction mixture volume of 12.5 μl, including 1 μl of the isolated DNA.
The primers used for T1R3 were 5′-CCC CAC ACA CCC ATC TAT TGT TAG-3′ and 5′-GAC TTG AAT GCT TCT GCC CCC TAG-3′. PCR conditions were as follows: a preheating step for 30 s at 95°C followed by 40 cycles of 20 s at 94°C, 1 min at 65°C, and 3 min at 72°C and an autoextension step of 2 min at 72°C.
For T1R2, the primers used were 5′-TTG GAG GAG GGG GCA GTG GGA GTG-3′ and 5′-ATA ATC CTC TCC TGC CAC CCT AAC-3′ and 5′-CTG CCC CAA AGG CCT ACC CGC TTC-3′. PCR conditions were as follows: a preheating step for 30 s at 95°C followed by 34 cycles of 15 s at 95°C, 15 s at 68°C, and 30 s at 72°C and an autoextension step of 30 s at 72°C.
The PCR products were separated using electrophoresis on a 1% agarose gel.
RESULTS
Polycose.
No mice from the T1R2 WT or KO groups, 1 mouse from the T1R3 WT group, and 4 mice from the T1R3 KO group were discarded from the Polycose licking analysis due to an insufficient number of initiated trials.
Remarkably, both genotypes of KO mice had Polycose concentration-response functions that tracked those of their respective WT littermate controls (Table 2; Fig. 1). In the statistical comparison of licking responses between each KO group and their WT controls, there was a significant genotype × concentration interaction (Table 2), but it is clear from the curves that this difference was relatively small and due largely to the KO animals actually licking more vigorously to some midrange concentrations. Bonferroni-corrected post hoc t-tests conducted for each Polycose concentration revealed that T1R3 KO mice licked 0.08 M Polycose more than their WT controls [t(39) = 3.15, Bonferroni-corrected P = 0.019]. The T1R3 KO mice also had a tendency to lick 0.16 M Polycose more than WT mice [t(39) = 2.06, uncorrected P = 0.046], but this difference failed to survive the Bonferroni correction (P = 0.274). Interestingly, despite the significant interaction, there were no significant differences between the T1R2 KO and WT mice at any Polycose concentration tested regardless of whether a Bonferroni correction was used or not.
Table 2.
ANOVA values for tests of licking as a function of genotype and concentration for the three test stimuli
|
T1R2 |
T1R3
|
|||||
|---|---|---|---|---|---|---|
| Genotype | Concentration | Genotype × Concentration | Genotype | Concentration | Genotype × Concentration | |
| Polycose | F(1,40) = 0.040, P = 0.842 | F(5,200) = 391.589, P < 0.001 | F(5,200) = 5.115, P < 0.001 | F(1,39) = 1.382, P = 0.247 | F(5,195) = 342.153, P < 0.001 | F(5,195) = 6.466, P < 0.001 |
| Saccharin | F(1,37) = 109.693, P < 0.001 | F(5,185) = 90.891, P < 0.001 | F(5,185) = 90.465, P < 0.001 | F(1,37) = 164.895, P < 0.001 | F(5,185) = 102.458, P < 0.001 | F(5,185) = 107.907, P < 0.001 |
| Sucrose | F(1,35) = 45.388, P < 0.001 | F(5,175) = 104.961, P < 0.001 | F(5,175) = 25.088, P < 0.001 | F(1,31) = 30.907, P < 0.001 | F(5,155) = 96.671, P < 0.001 | F(5,155) = 31.852, P < 0.001 |
Fig. 1.
Means ± SE, licks to Polycose adjusted for licks to water for T1R2 wild-type (WT) (top, •) and T1R2 knockout (KO) (top, ○), T1R3 WT (bottom, •) and T1R3 KO (bottom, ○).
To determine whether there were stimulus test order effects, we analyzed the concentration-response data as a function of week (1, 2, or 3), during which Polycose was tested. For both genotypes of KO and WT mice, licking responses to Polycose increased in a concentration-dependent manner regardless of the week during which Polycose was presented (Figs. 2–5). A repeated-measures one-way ANOVA to test for the effect of concentration was significant for each KO (Table 3) and WT group (Table 4) during each week of presentation.
Fig. 2.
Means ± SE, licks (adjusted for water licks) to Polycose (top row), sucrose (middle row) and Na-saccharin (bottom row) for individual T1R2 KO mice. Responses to stimuli presented during the first week of testing (left column), second week (middle column) and third week (right column). Mean for each taste stimulus and week shown by bold plot.
Fig. 5.
Means ± SE, licks (adjusted for water licks) to Polycose (top row), sucrose (middle row), and Na-saccharin (bottom row) for individual T1R3 WT mice. Responses to stimuli presented during the first week of testing (left column), second week (middle column), and third week (right column). Mean for each taste stimulus and week shown by bold plot.
Table 3.
ANOVA values for tests of licking in KO mice as a function of concentration and week of testing for each of the three test stimuli
|
T1R2 KO |
T1R3 KO
|
|||||
|---|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 1 | Week 2 | Week 3 | |
| Polycose | F(5,30) = 61.368, P < 0.001 | F(5,30) = 137.918, P < 0.001 | F(5,30) = 45.944, P < 0.001 | F(5,30) = 174.087, P < 0.001 | F(5,20) = 150.825, P < 0.001 | F(5,30) = 89.945, P < 0.001 |
| Saccharin | F(5,20) = 1.987, P = 0.389 | F(5,30) = 1.006, P = 0.432 | F(5,30) = 0.680, P = 0.642 | F(5,15) = 1.192, P = 0.359 | F(5,30) = 0.658, P = 0.658 | F(5,25) = 0.488, P = 0.782 |
| Sucrose | F(5,25) = 1.780, P = 0.153 | F(5,20) = 1.112, P = 0.386 | F(5,25) = 12.134, P < 0.001 | F(5,30) = 5.409, P < 0.001 | ||
KO, knockout.
Table 4.
ANOVA values for tests of licking in WT mice as a function of concentration and week of testing for each of the three test stimuli
|
T1R2 WT |
T1R3 WT
|
|||||
|---|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 1 | Week 2 | Week 3 | |
| Polycose | F(5,25) = 109.830, P < 0.001 | F(5,30) = 73.466, P < 0.001 | F(5,35) = 47.701, P < 0.001 | F(5,30) = 963.605, P < 0.001 | F(5,35) = 125.477, P < 0.001 | F(5,30) = 51.652, P < 0.001 |
| Saccharin | F(5,25) = 51.323, P < 0.001 | F(5,30) = 107.950, P < 0.001 | F(5,30) = 136.117, P < 0.001 | F(5,30) = 69.876, P < 0.001 | F(5,30) = 181.877, P < 0.001 | F(5,35) = 328.484, P < 0.001 |
| Sucrose | F(5,30) = 55.204, P < 0.001 | F(5,25) = 93.508, P < 0.001 | F(5,30) = 207.087, P < 0.001 | F(5,35) = 101.701, P < 0.001 | F(5,30) = 102.933, P < 0.001 | F(5,30) = 214.660, P < 0.001 |
WT, wild-type.
There were no significant differences between the T1R2 KO and T1R2 WT mice [F(1,40) = 0.006, P = 0.940] or between the T1R3 KO and T1R3 WT mice [F(1,44) = 0.003, P = 0.960] in the number of trials initiated to Polycose. Analysis of the number of trials that KO mice initiated to Polycose by week of presentation was also conducted. Regardless of which week Polycose was presented, there were no significant differences among T1R2 KO mice in the number of trials initiated [F(2,18) = 0.381, P = 0.688]. There was, however, a significant difference in the number of Polycose trials initiated by T1R3 KO as a function of the week of testing [F(2,20) = 3.753, P = 0.041]. It is evident in Fig. 4 that this was primarily due to a greater number of trials initiated in the group that was tested with Polycose in week 1 (Fig. 6).
Fig. 4.
Means ± SE, licks (adjusted for water licks) to Polycose (top row), sucrose (middle row) and Na-saccharin (bottom row) for individual T1R2 WT mice. Responses to stimuli presented during the first week of testing (left column), second week (middle column), and third week (right column). Mean for each taste stimulus and week shown by bold plot.
Fig. 6.
Means ± SE, total number of trials initiated by T1R2 WT, T1R2 KO (top), T1R3 WT and T1R3 KO (bottom) mice during Polycose, saccharin, and sucrose sessions for mice presented the taste stimulus during week 1 (1), week 2 (2), week 3 (3), and the overall mean (x̄).
Sucrose.
On the basis of a low number of initiated trials, four mice from the T1R2 KO group, 1 mouse from the T1R2 WT group, 12 mice from the T1R3 KO group, and 1 mouse from the T1R3 WT group were not included in the sucrose licking analysis. As expected, both T1R2 KO and T1R3 KO mice displayed severely blunted responses to sucrose relative to their WT littermate controls (Table 2, Fig. 7). There was, however, a significant genotype × concentration interaction when each KO group was compared with their respective WT group. A repeated-measures one-way ANOVA to test for simple effects of concentration was significant for T1R2 KO mice (P < 0.001) and T1R3 KO mice (P = 0.007). This indicates that both KO genotypes displayed some increased responsiveness at higher concentrations of sucrose, albeit severely attenuated.
Fig. 7.
Means ± SE, licks to sucrose adjusted for licks to water for T1R2 WT (top, •) and T1R2 KO (top, ○), T1R3 WT (bottom, •), and T1R3 KO (bottom, ○).
For WT mice, licking responses to sucrose increased in a concentration-dependent manner regardless of the week during which sucrose was presented (Figs. 4 and 5). A repeated-measures one-way ANOVA to test for the effect of concentration was significant for WT group (Table 4) during each week of presentation. Further analyses of T1R2 KO and T1R3 KO licking responses to sucrose was conducted by repeated-measures one-way ANOVAs to test for effects of concentration during each week (Figs. 2 and 3). Analysis of licking responses of T1R3 KO mice presented sucrose during weeks 1 and 2 were not conducted due to the low sample size. Statistical analysis revealed the effect of concentration was significant for each KO group when sucrose was presented during week 3 (Table 3). With the exception of two subjects, mice from both KO genotypes displayed virtually flat concentration-response functions if sucrose were tested during week 1 or 2. In contrast, when sucrose was tested during week 3 after prior experience with Polycose testing, the KO mice displayed some degree of increased responsiveness as a function of concentration.
Fig. 3.
Means ± SE, licks (adjusted for water licks) to Polycose (top row), sucrose (middle row), and Na-saccharin (bottom row) for individual T1R3 KO mice. Responses to stimuli presented during the first week of testing (left column), second week (middle column) and third week (right column). Mean for each taste stimulus and week shown by bold plot.
As shown in Fig. 6, T1R2 KO mice initiated significantly fewer trials to sucrose than T1R2 WT mice [F(1,40) = 19.261, P < 0. 001] and similarly T1R3 KO mice initiated significantly fewer trials to sucrose than T1R3 WT mice [F(1,44) = 31.170, P < 0.001]. As Fig. 6 illustrates, T1R2 KO and T1R3 KO mice that were presented sucrose during week 3 of testing, after prior experience with Polycose testing, initiated more trials than KO mice presented sucrose during week 1 or 2. Statistical analysis confirmed this with a difference between trials initiated by T1R2 KO mice [(2,18) = 5.016, P = 0.019] and by T1R3 KO mice [(2,20) = 7.377, P = 0.004] depending on week of presentation.
Saccharin.
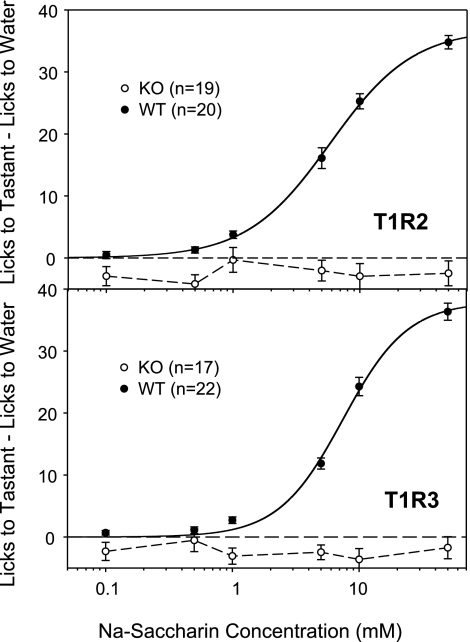
On the basis of the low number of initiated trials, two mice from the T1R2 KO group, 1 mouse from the T1R2 WT group, 6 mice from the T1R3 KO group and 1 mouse from the T1R3 WT group were not included for the saccharin lick analysis. The statistics confirm what is evident in Fig. 8; T1R2 KO and T1R3 KO mice displayed virtually no responsiveness to saccharin compared with the concentration-dependent licking observed in WT mice (Table 2).
Fig. 8.
Means ± SE, licks to Na-saccharin adjusted for licks to water for T1R2 WT (top, •), T1R2 KO (top, ○), T1R3 WT (bottom, •), and T1R3 KO (bottom, ○). A curve could not be fit to the data for T1R2 KO and T1R3 KO mice.
Regardless of the week of Na-saccharin presentation for both KO genotypes, repeated-measures one-way ANOVAs revealed no significant effect of concentration (Table 3). This indicates that irrespective of presentation order, T1R2 KO and T1R3 KO mice show no concentration-dependent responsiveness to saccharin. For WT mice, repeated-measures, one-way ANOVAs revealed a significant effect of concentration during each week of presentation (Table 4).
As shown in Fig. 6, T1R2 KO mice initiated significantly fewer trials to Na-saccharin than did T1R2 WT mice [F(1,40) = 30.312, P < 0. 001]. Similarly, T1R3 KO mice initiated significantly fewer trials to Na-saccharin than T1R3 WT mice [F(1,44) = 32.457, P < 0. 001]. Statistical analysis revealed a difference in the number of trials initiated as a function of the week Na-saccharin was presented to T1R2 KO [F(2,18) = 4.738, P = 0.022] and T1R3 KO [F(2,20) = 6.338, P = 0.007] mice. As is evident from Fig. 6, both KO genotypes that were presented Na-saccharin during week 1 initiated fewer trials that KO mice presented Na-saccharin during weeks 2 or 3.
Analysis by sex.
A two-way ANOVA (sex × concentration) of licking responses for each genotype group and compound were conducted and revealed a main effect of sex for sucrose (P = 0.025) and saccharin (P = 0.009) for T1R2 WT mice with males displaying increased licking. It is clear from Fig. 9 that these differences were relatively small. For all other groups, there was no main effect of sex. No groups showed a sex × concentration interaction effect.
Fig. 9.
Means ± SE, licks (adjusted for water licks) to Polycose (left), sucrose (middle), and Na-saccharin (right) of male WT (▴), female WT (•), male KO (▵), and female KO (○) mice for the T1R2 (top) and T1R3 (bottom) genotypes.
DISCUSSION
The findings of this study demonstrate that, individually, the T1R2 and T1R3 proteins are unnecessary for mice to express normal affective taste responses to Polycose as measured in a brief-access test. Both T1R2 KO and T1R3 KO mice displayed concentration-dependent increases in licking to varying concentrations of Polycose in a manner similar to their WT littermate controls. If anything, the KO mice actually licked midrange concentrations more than their WT controls. There were also no genotype differences in the overall number of trials initiated to Polycose. Whether at least one of these T1R subunits is necessary for normal responsiveness to Polycose remains unclear. Alternatively, as suggested in the literature, there may be a novel taste receptor(s) that mediates polysaccharide taste (16, 40, 44).
As expected, both T1R2 KO and T1R3 KO mice displayed severely blunted responses to and initiated significantly fewer trials of saccharin and sucrose. Because the brief-access test is dependent on the hedonic component of the stimulus to drive a behavioral response, the blunted responses in the KO mice suggest the affective value of saccharin and sucrose is markedly reduced or eliminated. These results are consistent with prior findings in the literature implicating the T1R2+3 heterodimer as the principal taste receptor for ligands described by humans as sweet (2, 14, 20, 22, 25–27, 29, 30, 38, 53).
Both T1R2 KO and T1R3 KO mice that were tested with sucrose after experience with Polycose, a caloric compound, initiated significantly more trials and displayed increased licking at the higher sucrose concentrations compared with KO mice that had no previous caloric compound exposure. Others using various KO models have shown that, although initially reduced, behavioral responsiveness to saccharides can be partially restored by prolonged testing (42, 54). The effect of prior testing experience seen in this and other studies can possibly be explained by learned associations between some oral sensory cue and the positive postingestive consequences of caloric intake. A number of studies in rats (e.g., 10, 43) and mice (45) have shown that a strong preference can be conditioned by pairing a flavored nonnutritive solution with an intragastric infusion of a caloric sugar.
It is true that because the brief-access test involves the presentation of trials of varying concentrations across a single session, associations of particular concentrations with postingestive events are minimized. Nevertheless, this procedure does not preclude an animal from associating the overall taste or other orosensory aspects of the stimulus with the overall postoral consequences of ingestion throughout the session. With this in mind, we designed our experiment with a Latin-Square order of stimulus presentation such that each stimulus was presented in the first, second, or third week of testing in different subgroups. Indeed, because of this, we were able to see the expression of the effect of prior testing experience. It was interesting that, with a couple exceptions, the KO animals that were tested with sucrose before Polycose exposure displayed no evidence of any orderly concentration-dependent licking. This is likely because the animals did not initiate a sufficient number of trials for orosensory-postingestive associations to be formed.
What are the potential oral sensory cues that could be serving as the conditioned stimuli if postingestive learning is indeed occurring in the KO animals? Whatever the relevant signals are, they must be shared by Polycose and sucrose, but not with saccharin. KO mice that were tested with saccharin after Polycose exposure did not show any tendency to increase their licking in a concentration-dependent manner. Possible cues that Polycose might share with sucrose but not saccharin, especially at the higher concentrations, include viscosity and smell (see Ref. 37). In addition, the fact that saccharin, at higher concentrations, has an aversive quinine-like taste quality to rodents (34) and a bitter taste quality to humans (3, 15), presumably caused by its ability to bind with a T2R (21, 35), may have also influenced responding. One way to address this issue would be to employ a procedure in which cues such as postingestive feedback, olfaction, and viscosity are further minimized and see if the presentation order effects disappear. It is also worth noting that whatever the postingestive events are that support such conditioning, they apparently do not depend on the individual presence of either the T1R2 or T1R3 proteins in the gut (e.g., 9, 23, 24).
Despite the appearance of some degree of postingestive learning occurring in some of the KO animals with respect to sucrose responsiveness, it is quite clear that the deletion of either the Tas1r2 or Tas1r3 gene severely blunts or eliminates unconditioned affective responses to the disaccharide and also to the artificial sweetener saccharin. In striking contrast, concentration-dependent responses to Polycose remain relatively intact. Our findings are consistent with an independent study concurrently conducted by Zukerman et al. (55). In that study, the investigators used very brief, as well as longer-term, two-bottle preference tests (vs. water) to examine the licking responses of T1R3 KO mice to Polycose and sucrose. In general, they found that T1R3 KO mice displayed slightly but significantly reduced preference to Polycose especially at lower concentrations compared with WT mice, but like in the present study, licking of sucrose was severely impaired by the gene deletion. Moreover, whole nerve chorda tympani (innervating taste buds of the anterior tongue) responses to a range of Polycose concentrations did not differ between WT and T1R3 KO mice. The difference between the lack of decreases in licking responses to Polycose by T1R3 KO mice relative to WT controls (and an actual increase in Polycose responsiveness at 0.08 M) in our experiment compared with the slight deficit observed in the Zukerman et al. (55) study remains to be understood but may potentially relate to the specific methodological aspects of the behavioral assays employed, differences in genetic background, or the procedure used to generate the gene deletion. Nevertheless, on the whole, the findings from the two studies regarding responsiveness of T1R3 KO mice to Polycose generally concur notwithstanding the disparities noted above.
In summary, our findings are consistent with studies in the literature that implicate the T1R2+3 heterodimer as the primary taste receptor for sweet-tasting compounds. In contrast, the T1R2 and T1R3 proteins individually appear unnecessary to maintain normal affective taste responses to Polycose in a brief-access test. It is possible that the presence of only one of these subunits is necessary for Polycose responsiveness or, alternatively, that there are novel taste receptors that bind with polysaccharides. To distinguish between these two possibilities, the measurement of responsiveness to Polycose in mice missing both the T1R2 and T1R3 subunits would be instructive.
Perspectives and Significance
Although it is quite clear on the basis of findings in the literature and confirmed by the present report that the T1R2+3 heterodimer is essential for normal responsiveness to sweeteners to be maintained, some still question whether sugars might stimulate other receptors as well. Certainly, in the case of polysaccharide stimuli such as Polycose, the findings presented here give vitality to this possibility. However, this should not be construed as a challenge to the primacy of the T1R2+3 heterodimer as the principal mediator of what is referred to as “sweet” taste by human subjects. There is evidence that Polycose and sucrose do not have an identical taste quality, and the findings presented here and by Zukerman et al. (55) might reflect the underlying neural basis for the distinction between the two stimuli. Thus, although both Polycose and sucrose are apparently highly preferred solutions by rodents, their respective taste qualities might be quite different, to at least these animals.
The current set of results demonstrating that KO mice can show concentration-dependent responsiveness to sucrose depending on their testing history highlights the methodological complexities and interpretive nuances involved in the use of even simple behavioral techniques to make inferences on taste perception in general. Consistent with this view, Sclafani and colleagues (46) have found that with prolonged training or testing with caloric compounds, mice that are initially ageusic or hypogeusic as a result of other types of taste-related gene deletions can begin to show increased taste responsiveness in behavioral assays likely due to a learned association between the remaining orosensory signals of the stimulus with the positive consequences of its ingestion.
There is no perfect behavioral assay that is without some interpretive limitations. It is, therefore, important for the consequences of any manipulation of the gustatory system, genetic or otherwise, on taste-related behavior to be assessed through the use of a variety of complementary testing procedures. Such a strategy can lead to a comprehensive delineation of phenotypical outcomes and ultimately advance our understanding of taste function and its underlying neural mechanisms.
GRANTS
This study was supported by National Institutes of Health R01-DC004574 (A. C. Spector).
Acknowledgments
We would like to express our thanks to Steven Janasik and Melissa Isaacs for their technical help in this experiment and to Dr. Carrie Haskell-Luevano and Dr. Francois Rouzaud for their initial advice in establishing the breeding colonies and genotyping. We are grateful for the assistance that we received from Dr. Taimour Langaee and Ben Burkley in designing our genotyping protocols. We would also like to thank Dr. Charles Zuker for generously supplying the T1R2 and T1R3 knockout breeding pairs.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bachmanov AA, Beauchamp GK. Taste receptor genes. Annu Rev Nutr 27: 387–412, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, Tordoff MG, de Jong PJ, Wu C, West DB, Chatterjee A, Ross DA, Beauchamp GK. Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses 26: 925–933, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartoshuk LM Bitter taste of saccharin related to the genetic ability to taste the bitter substance 6-n-propylthiouracil. Science 205: 934–935, 1979. [DOI] [PubMed] [Google Scholar]
- 4.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301: 850–853, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Delay ER, Hernandez NP, Bromley K, Margolskee RF. Sucrose and monosodium glutamate taste thresholds and discrimination ability of T1R3 knockout mice. Chem Senses 31: 351–357, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Dotson CD, Roper SD, Spector AC. PLCβ2-independent behavioral avoidance of prototypical bitter-tasting ligands. Chem Senses 30: 593–600, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dotson CD, Spector AC. Behavioral discrimination between sucrose and other natural sweeteners in mice: Implications for the neural coding of T1R ligands. J Neurosci 27: 11242–11253, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dugas du Villard X, Her C, Mac Leod P. Qualitative discrimination of sweet stimuli: behavioural study on rats. Chem Senses 6: 143–148, 1981. [Google Scholar]
- 9.Dyer J, Salmon KSH, Zibrik L, Shirazai-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans 33: 302–305, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Elizalde G, Sclafani A. Flavor preferences conditioned by intragastric Polycose infusions: A detailed analysis using an electronic esophagus preparation. Physiol Behav 47: 63–77, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Formaker BK, Kearns CE, Frank ME. The taste of polycose in hamsters. Chem Senses 23: 675–682, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Giza BK, Scott TR, Sclafani A, Antonucci RF. Polysaccharides as taste stimuli: their effect in the nucleus tractus solitarius of the rat. Brain Res 555: 1–9, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Glendinning JI, Gresack J, Spector AC. A high-throughput screening procedure for identifying mice with abberant taste and oromotor function. Chem Senses 27: 461–474, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJP, Zuker CS. Putative mammalian taste receptors: A class of taste-specific GPCRs with distinct topographic selectivity. Cell 96: 541–551, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Horne J, Lawless HT, Speirs W, Sposato D. Bitter taste of saccharin and acesulfame-K. Chem Senses 27: 31–38, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Inoue M, Glendinning JI, Theodorides ML, Harkness S, Li X, Bosak N, Beauchamp GK, Bachmanov AA. Allelic variation of the Tas1r3 taste receptor gene selectively affects taste responses to sweeteners: evidence from 129. B6-Tas1r3 congenic mice. Physiol Genomics 32: 82–94, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakinovich W Taste aversion to sugars by the gerbil. Physiol Behav 28: 1065–1071, 1982. [DOI] [PubMed] [Google Scholar]
- 18.Jiang E, Blonde G, Garcea M, Spector AC. Greater superficial petrosal nerve transection in rats does not change unconditioned licking responses to putatively sweet taste stimuli. Chem Senses 33: 709–723, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy JF, Knill CJ, Taylor DW. Maltodextrins In: Handbook of Starch Hydrolysis Products and Their Derivatives, edited by Kearsley MW and Dziedzic SZ. London: Blackie Academic & Professional, 1995, p. 65–82.
- 20.Kitagawa M, Kusakabe Y, Miura H, Ninomiya Y, Hino A. Molecular genetic identification of a candidate receptor gene for sweet taste. Biochem Biophys Res Commun 283: 263–242, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn C, Bufe B, Winnig M, Hofmann T, Frank O, Behrens M, Lewtchenko T, Slack JP, Ward C, Meyerhof W. Bitter taste receptors for saccharin and acesulfame K. J Neurosci 24: 10260–10265, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA 99: 4692–4696, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol 582: 379–392, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Margolskee RF, Dryer J, Kokrashvili Z, Salmon KSH, Ilegems E, Daly K, Maillet E, Ninomiya Y, Mosinger B, and Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 104: 15075–15080, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet 28: 58–63, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci 4: 492–497, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Montmayeur JP, Matsunami H. Receptors for bitter and sweet taste. Curr Opin Neurobiol 12: 366–371, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura K, Norgren R. Taste responses of neurons in the nucleus of the solitary tract of awake rats: An extended stimulus array. J Neurophysiol 70: 879–891, 1993. [DOI] [PubMed] [Google Scholar]
- 29.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJP, Zuker CS. Mammalian sweet taste receptors. Cell 106: 381–390, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Nie Y, Vigues S, Hobbs JR, Conn GL, Munger SD. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr Biol 15: 1948–1952, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Ninomiya Y, Higashi T, Katsukawa H, Mizukoshi T, Funakoshi M. Qualitative discrimination of gustatory stimuli in three different strains of mice. Brain Res 322: 83–92, 1984. [DOI] [PubMed] [Google Scholar]
- 32.Nishijo H, Norgren R. Parabrachial neural coding of taste stimuli in awake rats. J Neurophysiol 78: 2254–2268, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Nissenbaum JW, Sclafani A. Qualitative differences in polysaccharide and sugar tastes in the rat: A two-carbohydrate taste model. Neurosci Biobehav Rev 11: 187–196, 1987. [DOI] [PubMed] [Google Scholar]
- 34.Nowlis GH, Frank ME, Pfaffman C. Specificity of acquired aversions to taste qualities in hamsters and rats. J Comp Physiol Psychol 94: 932–942, 1980. [DOI] [PubMed] [Google Scholar]
- 35.Pronin AN, Xu H, Tang H, Zhang L, Li Q, Li X. Specific alleles of bitter receptor genes influence human sensitivity to the bitterness of aloin and saccharin. Curr Biol 17: 1403–1408, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Ramirez I Thresholds for starch and Polycose are lower than for sucrose in rats. Physiol Behav 50: 699–703, 1991. [DOI] [PubMed] [Google Scholar]
- 37.Rhinehart-Doty JA, Schumm J, Smith JC, Smith GP. A non-taste cue of sucrose in short-term taste tests in rats. Chem Senses 19: 425–431, 1994. [DOI] [PubMed] [Google Scholar]
- 38.Sainz E, Korley JN, Battey JF, Sullivan SL. Identification of a novel member of the T1R family of putative taste receptors. J Neurochem 77: 896–903, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Sako N, Shimura T, Komure M, Mochizuki R, Matsuo R, Yamamoto T. Differences in taste responses to Polycose and common sugars in the rat as revealed by behavioral and electrophysiological studies. Physiol Behav 56: 741–745, 1994. [DOI] [PubMed] [Google Scholar]
- 40.Sclafani A Carbohydrate taste, appetite, and obesity: An overview. Neurosci Biobehav Rev 11: 131–153, 1987. [PubMed] [Google Scholar]
- 41.Sclafani A Enhanced sucrose and Polycose preference in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice after experience with these saccharides. Physiol Behav 87: 745–756, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Sclafani A Fat and sugar flavor preference and acceptance in C57BL/6J and 129 mice: Experience attenuates strain differences. Physiol Behav 90: 602–611, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Sclafani A Oral and postoral determinants of food reward. Physiol Behav 81: 773–779, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Sclafani A The sixth taste? Appetite 43: 1–3, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: oral and postoral interactions. Am J Physiol Regul Integr Comp Physiol 289: R712–R720, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Sclafani A, Zukerman S, Glendinning JI, Margolskee RF. Fat and carbohydrate preferences in mice: the contribution of alpha-gustducin and Trpm5 taste-signaling proteins. Am J Physiol Regul Integr Comp Physiol 293: R1504–R1513, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith JC The history of the “Davis Rig”. Appetite 36: 93–98, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Somenarain L, Jakinovich W Jr. Antagonism of the gerbil's sweetener and Polycose gustatory responses by copper chloride. Brain Res 522: 83–89, 1990. [DOI] [PubMed] [Google Scholar]
- 49.Spector AC, Grill HJ. Differences in the taste quality of maltose and sucrose in rats: issues involving the generalization of conditioned taste aversions. Chem Senses 13: 95–113, 1988. [Google Scholar]
- 50.Spector AC, Markison S, St. John SJ, Garcea M. Sucrose vs maltose taste discrimination by rats depends on the input of the seventh cranial nerve. Am J Physiol Regul Integr Comp Physiol 272: R1210–R1218, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Spector AC, Redman R, Garcea M. The consequences of gustatory nerve transection on taste-guided licking of sucrose and maltose in the rat. Behav Neurosci 110: 1096–1109, 1996. [PubMed] [Google Scholar]
- 52.Vigorito M, Sclafani A, Jacquin MF. Effects of gustatory deafferentation on Polycose and sucrose appetite in the rat. Neurosci Biobehav Rev 11: 201–209, 1987. [DOI] [PubMed] [Google Scholar]
- 53.Xu H, Straszewski L, Tang H, Adler E, Zoller M, Li X. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc Natl Acad Sci USA 101: 14258–14263, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJP, Zuker CS. The receptors for mammalian sweet and umami taste. Cell 115: 255–266, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am J Physiol Regul Integr Comp Physiol (December 17, 2008). doi: 10.1152/ajpregu.90870.2008. [DOI] [PMC free article] [PubMed]