Abstract
In addition to their well-known preference for sugars, mice and rats avidly consume starch-derived glucose polymers (e.g., Polycose). T1R3 is a component of the mammalian sweet taste receptor that mediates the preference for sugars and artificial sweeteners in mammals. We examined the role of the T1R3 receptor in the ingestive response of mice to Polycose and sucrose. In 60-s two-bottle tests, knockout (KO) mice preferred Polycose solutions (4–32%) to water, although their overall preference was lower than WT mice (82% vs. 94%). KO mice also preferred Polycose (0.5–32%) in 24-h two-bottle tests, although less so than WT mice at dilute concentrations (0.5–4%). In contrast, KO mice failed to prefer sucrose to water in 60-s tests. In 24-h tests, KO mice were indifferent to 0.5–8% sucrose, but preferred 16–32% sucrose; this latter result may reflect the post-oral effects of sucrose. Overall sucrose preference and intake were substantially less in KO mice than WT mice. However, when retested with 0.5–32% sucrose solutions, the KO mice preferred all sucrose concentrations, although they drank less sugar than WT mice. The experience-induced sucrose preference is attributed to a post-oral conditioned preference for the T1R3-independent orosensory features of the sugar solutions (odor, texture, T1R2-mediated taste). Chorda tympani nerve recordings revealed virtually no response to sucrose in KO mice, but a near-normal response to Polycose. These results indicate that the T1R3 receptor plays a critical role in the taste-mediated response to sucrose but not Polycose.
Keywords: preference, C57BL/6J mice, chorda tympani nerve, saccharin, post-oral conditioning
the taste of sugar is highly attractive to humans and many other animal species. Studies of inbred mouse strains led to the identification of the T1R2 and T1R3 receptor proteins that dimerize to form a sweet taste receptor (1). Selective elimination of these receptor proteins in knockout mice attenuates or completely blocks the behavioral and gustatory nerve responses to sugars and artificial sweeteners (7, 40). Further, allelic variation in the Tas1r3 gene, which codes for the T1R3 protein (3, 17–19, 24), contributes to strain differences in sensitivity (9), lick responsiveness (8, 10), peripheral taste nerve responsiveness (11), and daily intake and preference (11, 22) for sugars and artificial sweeteners.
Sugars are not the only carbohydrates that have an attractive taste to some nonhuman species. Twenty years ago, our laboratory published a series of papers demonstrating that rats, mice, hamsters, and gerbils are strongly attracted to the taste of starch-derived glucose polymers such as Polycose and other maltodextrins (26). Behavioral and electrophysiological evidence indicates that Polycose and sucrose have qualitatively distinct taste sensations in rodents. For example, aversions conditioned to Polycose or sucrose do not cross-generalize, and some taste inhibitors selectively reduce the peripheral gustatory response to sucrose or glucose polymers, but not both (20, 25, 34). Little is known about the taste receptor that mediates glucose polymer taste (“poly” taste), although some findings indicate a role for the T1R3 receptor. For instance, 129P3/J (129) and C57BL/6J (B6) mice, which express alternate alleles of Tas1r3, differ in their preference for Polycose and sucrose (4, 28). In 24-h two-bottle tests, 129 mice displayed weaker preferences for dilute (0.5–4%) Polycose and sucrose solutions and consumed less solution over a range of concentrations than did B6 mice (28). Consistent with these behavioral data, Polycose and sucrose elicited weaker chorda tympani (CT) nerve responses in 129 mice than in B6 mice (12). The CT nerve contains primary gustatory afferents that convey signals from taste cells in the anterior tongue to the nucleus of the solitary tract.
Recent data obtained with 129.B6-Tas1r3 congenic mouse strains, however, questioned the role of the T1R3 receptor in Polycose preference (11). Congenic 129.B6-Tas1r3 strains of mice have the same 129 genetic background but differ in their Tas1r3 alleles: B6/129 mice have a B6 allele, while the 129/129 mice have only 129 alleles. Despite displaying differences in behavioral and CT nerve responses to sucrose and other sweeteners, the B6/129 and 129/129 congenic strains did not differ in behavioral and CT nerve responses to Polycose. The authors concluded, therefore, that differences in the Polycose preferences of B6 and 129 mice are not due to polymorphisms in the T1R3 receptor. They did not, though, exclude the possibility that T1R3, in combination with another receptor protein, participates in Polycose taste detection.
The present study examined the role of the T1R3 receptor in the ingestive response to Polycose, using T1R3 knockout (KO) mice. These mice display attenuated ingestive and peripheral gustatory responses to sucrose and other sweeteners, although the magnitude of the behavioral deficit depends upon test method (e.g., brief vs. 24-h access) (7, 40). Here we compared preference and acceptance responses to Polycose and sucrose between KO and B6 wild-type (WT) controls. Preference refers to the percent intake of the saccharide solution relative to plain water, whereas acceptance refers to the absolute intake (in grams or milliliters) of the saccharide solution. Both measures are important because, as the present study revealed, KO and WT mice may display similar percent saccharide intakes (preference) yet greatly differ in their absolute intakes (acceptance).
In experiment 1, we examined short-term licking responses to minimize post-oral influences on avidity for the two saccharides. To this end, we used a brief access (60 s) two-bottle tests (saccharide vs. water). In experiment 2, we used standard 24-h two-bottle tests to determine whether the KO mice compensate for any taste deficits when the test session is prolonged and post-oral influences can operate. We also tested for experiential influences on saccharide preferences by giving KO and WT mice two series of preference tests with the saccharides. This was of interest because prior work revealed that the sucrose and Polycose preference differences between 129 and B6 mice disappeared with repeated testing. Whether experience has equally profound effects in KO mice is not known, although there is some evidence that prior experience influences sucrose preference in T1R3 KO mice (40). In experiment 3, we compared CT nerve responses of KO and WT mice to determine the contribution of the peripheral taste system to any differential intake. To this end, we measured CT nerve responses to lingual application of Polycose and sucrose in KO and WT mice.
MATERIALS AND METHODS
Subjects
T1R3 KO mice were derived from mice produced by homologous recombination in C57BL/6J embryonic stem cells and maintained on this background (7). C57BL/6J wild-type (B6 WT) mice were derived from mice obtained from the Jackson Laboratories (Bar Harbor, ME). Both male and female mice of each genotype were studied; they were 7 wk of age or older at testing. The animals were singly housed in plastic tub cages with ad libitum access to chow (5001, PMI Nutrition International, Brentwood, MO) and deionized water in a room maintained at 22°C with a 12:12-h light-dark cycle. Experimental protocols were approved by the Institutional Animal Care and Use Committee at Brooklyn College (experiments 1 and 2) and Columbia University (experiment 2) and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Test Solutions
In behavioral experiments, solutions were prepared using food-grade sucrose (Domino Foods, Yonkers, NY), Polycose (Ross Laboratories, Columbus, OH), saccharin (sodium saccharin, Sigma Chemical, St. Louis, MO), and deionized water. Polycose is a corn starch hydrolysate that contains, by weight, ∼2% glucose, 7% maltose, 55% maltooligosaccharides, and 36% maltopolysaccharides (Ross Laboratories, personal communication). The solutions were formulated on a wt/wt basis because intakes were measured by weight. A 1% soybean oil emulsion was prepared by diluting 20% Intralipid (Baxter, Deerfield, IL) with deionized water.
In the nerve recording experiment, solutions were prepared with sucrose, sodium chloride, ammonium chloride (Sigma Chemical), Polycose (Ross Labs), and deionized water.
Experiment 1: 60-s Two-Bottle Tests
Rationale.
Saccharide acceptance (total intake) and preference (relative intake) are influenced by both taste and post-oral factors. To minimize post-oral factors, a brief-access (60 s) two-bottle test was used (10). Because intake volumes are very low in such short tests, licking responses to the saccharide and water sipper spouts rather than intakes were recorded. The animals were adapted to the test procedure by training them with a nutritive oil emulsion (1% Intralipid). They were first given a 24-h Intralipid vs. water test to familiarize them with the oil emulsion and were then trained to drink the oil emulsion in short-term tests. Pilot work indicated 1% Intralipid is palatable to both KO and WT mice.
Apparatus.
Brief access (60 s) two-bottle tests were conducted in clear plastic cages (10 × 23.5 × 27 cm) with a stainless-steel perforated floor. Fluid was available from one or two stainless-steel sipper spouts through slots (5 × 20 mm, 32 mm apart) in a stainless-steel plate at the front of the cage. The sipper spouts had a 1.5-mm hole designed for mice (Ancare, Belmore, NY) and were attached to 50-ml glass tubes with a rubber stopper. The tubes were mounted on motorized bottle holders (ENV-252M; Med Associates, Georgia, VT) that positioned the spouts 1 mm in front of the cage at the start of a trial and retracted them at the end of the trial. Licking behavior was monitored with electronic lickometers (ENV-250B, Med Associates) interfaced to a microcomputer.
Procedure.
The KO mice (10 male, 10 female) were given a 24-h two-bottle preference test with 0.2% saccharin vs. water in their home cages to confirm their phenotype (i.e., insensitivity to sweet taste); WT (10 male, 10 female) mice were not tested with saccharin, so that they would remain naïve to sweet taste prior to saccharide testing. All animals were then familiarized with the test cages by housing them overnight in the cages with ad libitum water and several 1-g Bio-Serv Chow pellets (F0173, Bio-Serv, Frenchtown, NJ), which were later used as food rations. The mice were then returned to their home cages and given a 24-h two-bottle choice test with 1% Intralipid vs. water. The 24-h choice tests were conducted over 2 days with the left-right positions of the tastant and water bottles alternating over days.
The mice were initially water-restricted and trained to drink in the test cages during brief daily sessions, but formal testing was conducted under food-restriction, which enhances intake of saccharide solutions. While water-restricted, the mice were given a two-bottle choice between 1% Intralipid and water for 5-m. They were then given 60-s two-bottle tests with Intralipid vs. water on the next two days. In these and subsequent 60-s choice tests, the mice were first given 5-s access to one sipper tube and then the other sipper tube to allow them to sample the contents of the left and right bottles. Throughout testing, the presentation order of the sipper tubes in the 5-s sessions varied in a LLRR pattern (left first for two sessions, followed by right first for two sessions), while the left-right position of the tastant and water sipper tubes in the 60-s session varied in a LRLR pattern. The timing of each session for each mouse began with the 10th lick of the mouse. If the mouse did not engage in 10 licks, the sipper tube was retracted after 60 s. The next 5- or 60-s session began for all mice ∼15 s after the last sipper tube was retracted. One hour after the end of the daily sessions, the animals were given 1-h access of water in their home cages.
After the last Intralipid test, the mice of each genotype were divided into Polycose and sucrose groups. The groups were equated for their 24-h saccharin (KO mice only) and 1% Intralipid intakes and preferences, 60-s Intralipid preferences, and body weight. While still water-restricted, the groups were given two 60 s/day choice tests with 4% Polycose or 4% sucrose vs. water. The mice were then given ad libitum access to water in their home cages but were food restricted and maintained at 85–90% of their free-feeding weight. A daily food ration (2–4 g) was presented 1 h after the test session. While food restricted, the mice were given a series of 60-s tests with Polycose or sucrose vs. water with the saccharides presented in ascending concentrations of 4, 8, 16, and 32%. Each concentration was presented on two successive 60 s/day sessions.
Data analysis.
The 60-s lick data were averaged for the two sessions at each saccharide concentration. Saccharide acceptance was evaluated by comparing the number of licks on the saccharide tube during the 60-s sessions. Saccharide preference was expressed as the percent of licks on the saccharide tube [saccharide licks/(saccharide + water licks) × 100]. If a mouse engaged in fewer than 20 total licks during a 60-s trial, it was assigned a preference score of 50% (i.e., no preference); this avoided spurious high or low preferences when there were few licks. Genotype differences in saccharide acceptance were evaluated using a mixed model ANOVA with genotype as a between-group factor and saccharide concentration as a within-group factor; separate ANOVAs compared the Polycose and sucrose groups. Additional ANOVAs compared Polycose vs. sucrose acceptance and preference within a genotype. The significance of the saccharide preference at each concentration was evaluated within each group by comparing 60-s saccharide and water licks using paired t-tests. To control for multiple comparisons, the alpha-level for the t-tests was corrected with the Bonferroni procedure. A preliminary analysis revealed no sex differences in Polycose and sucrose licks, and therefore the data for male and female mice were combined.
Experiment 2: 24-h Two-Bottle Tests
Rationale.
Long-term two-bottle tests (24 h/day, which are also referred to as 48-h tests when extended over 2 days as in the present experiment) are commonly used to compare genotype differences in taste preference and acceptance. These tests are particularly informative at low saccharide concentrations, which generate relatively low intakes (or licks) in brief access tests. At high concentrations, saccharide preferences may be influenced by post-oral nutritive effects that may alter the animal's response to dilute solutions in subsequent tests (28, 30). Experiential effects on sucrose and Polycose preferences in KO and WT mice were evaluated by giving the animals two series of 24-h saccharide vs. water tests.
Apparatus.
The 24-h two-bottle tests were conducted in the animal's home cage. Fluid was available through sipper spouts attached to 50- or 80-ml plastic tubes that were placed on the top of the cage. The sipper spouts were inserted through holes positioned 3.7 cm apart in a stainless-steel plate, and the drinking tubes were fixed in place with clips. Fluid intakes were measured to the nearest 0.1 g by weighing the drinking tubes on an electronic balance interfaced to a laptop computer. Daily fluid spillage was estimated by recording the change in weight of two drinking tubes that were placed on an empty cage. The estimated spill throughout the experiment was ∼0.2 g, and intake measures were corrected by this amount.
Procedure.
New mice were used in this experiment. The KO mice (10 male, 10 female) were given a 24-h two-bottle preference test with 0.2% saccharin vs. water in their home cages to confirm their phenotype; WT (10 male, 10 female) mice were not tested with saccharin, so that they would remain naïve to sweet taste prior to saccharide testing. The mice of each genotype were divided into Polycose and sucrose groups equated for saccharin intake (KO mice only), water intake and body weight. They were then given a series of two-bottle saccharide vs. water tests. In test 1 the Polycose groups were given 24-h Polycose vs. water tests at ascending concentrations of 0.5, 1, 2, 4, 8, 16, and 32%. The solutions were presented on two successive days at each concentration with the position of the bottles alternated daily. Following 4 days of water only, the test sequence was repeated a second time (test 2). The sucrose groups were tested like the Polycose groups except with sucrose vs. water. After another 4-day period of water only, all mice were tested with saccharin vs. water (test 3) at ascending sweetener concentrations of 0.2, 0.4, and 0.8%.
Data analysis.
Daily solution and water intakes were averaged over the 2 days at each solution concentration. Saccharide intakes were also expressed as kcal/day, and saccharide preferences were expressed as percent intakes [saccharide intake/(saccharide + water intakes) × 100]. Overall, WT mice weighed more than KO mice (25.1 vs. 23.1 g) and male mice weighed more than female mice (26.2 vs. 21.9 g) based on body weights averaged at the start and end of the study. Preliminary analyses of the saccharide intakes expressed as intake/mouse or intake/30 g body wt, as in previous studies (11, 28), produced very similar results except that female mice consumed more fluid per 30 g but not more per mouse compared with male mice. There were no interactions with sex and solution or concentration. Therefore, the data are reported as intake/mouse, and male and female data were combined.
Genotype differences in saccharide intakes and preferences were evaluated using separate mixed-model ANOVAs with genotype and saccharide concentration as between-group and within-group factors, respectively; separate ANOVAs compared the Polycose and sucrose groups. Additional ANOVAs compared Polycose vs. sucrose acceptance and preference within a genotype. The significance of the saccharide preference at each concentration was evaluated within each group by comparing saccharide and water intakes using paired t-tests corrected for multiple comparisons using the Bonferroni procedure.
Experiment 3: Chorda Tympani Nerve Responses
Rationale.
To determine how the T1R3 receptor contributes to the peripheral taste response to Polycose and sucrose, we compared responses of the CT nerve in KO and WT mice. We focused on the CT nerve because 1) it relays afferent signals from T1R3-expressing taste cells in fungiform papillae (on the anterior portion of the tongue) to the brain in WT mice (14); 2) B6 and 129 mice differ in their CT nerve response to Polycose, as well as sucrose (12); and 3) knocking out T1R3 receptor dramatically reduces the CT nerve response to sucrose (7, 40).
Procedure.
New mice were used in this experiment. Recordings were obtained from the right CT nerve of KO (5 male, 4 female) and WT (4 male, 4 female) mice as it passed through the middle-ear cavity, using a modified version of the technique described by Cheal (6). The mouse was anesthetized with 2–4% isoflurane, which was delivered initially through a nose-cone and then, following a tracheotomy, through a tracheal cannula. The mouse's head was secured in a nontraumatic head-holder, its right pinna and ear canal were removed, and the dorsal region of the tympanic membrane was lacerated. Using a dissecting microscope (at ×60 magnification), a small section of the CT nerve traversing the gap between the malleus head and posterior tympanic spine was localized (39), and all recordings were made from this exposed section of nerve. Throughout the surgery and nerve recordings, the mouse was maintained on a circulating-water heating pad set at 37°C.
Because the sheath of the CT nerve was so thin, recording of robust responses could be obtained simply by contacting the intact nerve with a sharpened tungsten electrode. The indifferent electrode was shunted to the ground electrode. The neural response was amplified (×10,000) with an optically coupled isolated bioamplifier (ISO-80; World Precision Instruments, Sarasota, FL), passed through a band-pass filter (40–3,000 Hz), and then rectified and integrated with a time constant of 1 s (Biopac Software, Goleta, CA). As the mouse breathed, its entire body moved. This movement caused the CT nerve to rub against the tungsten electrode, creating a breathing artifact in the neural responses. The data analysis was not impaired, however, because we could quantify the signal between each movement artifact.
The fungiform taste papillae were stimulated using a continuous-flow system (VC-6 Perfusion Valve Control System; Warner Instruments, Hamden, CT). Solutions (∼22°C) were delivered to the anterior surface of the tongue at a rate of 10 ml/m. Each chemical stimulation lasted 20 s and was followed by at least 40 s of water rinse. The mice were stimulated with four concentrations of sucrose (0.03, 0.1, 0.3, and 1 M), Polycose (4, 8 16 and 32%), and NaCl (0.03, 0.1, 0.3, 1.0 M) in an ascending order. Molar sucrose concentrations were used to be consistent with prior studies (12), and the sucrose concentration range (1, 3, 10, 34%) overlapped that used with Polycose. Each concentration series was bracketed by the application of ammonium chloride (100 mM).
To determine the absolute response magnitude for a given chemical stimulus, we measured the neural response 20 s immediately before (baseline response) and during the 20-s application of the tastant (taste response). Across each of these 20-s periods, we determined the mean neural response, excluding the breathing artifacts. The absolute response magnitude was defined as the difference between the baseline and taste responses.
Because the absolute magnitude of response can vary across a single recording session in one animal, and between different recording sessions with different animals, we used a normalization procedure. To this end, we divided the absolute magnitude of response to each test stimulus (e.g., 32% sucrose) by the absolute magnitude of response to 100 mM ammonium chloride. We selected 100 mM ammonium chloride as the reference stimulus so as to be consistent with prior studies (7, 11, 13).
Data analysis.
We analyzed the results for sucrose, Polycose, and NaCl separately. To test for strain differences in responsiveness to each taste stimulus, we used a mixed-model ANOVA. We treated concentration as a within factor, and strain as a between factor; the dependent measure was the normalized integrated response. In addition, we used a one-way repeated-measure ANOVA to determine whether the response to sucrose in KO (or WT) mice increased with concentration. Because preliminary analyses indicated no sex differences, the data from male and female mice were combined.
RESULTS
Experiment 1: 60-s Two-Bottle Tests
Pretests.
As expected, the KO mice failed to prefer 0.2% saccharin in the initial two-bottle test; in fact, they drank less saccharin than water [1.9 vs. 2.5 g/day, t(19) = 2.80, P < 0.05]. In the subsequent 1% Intralipid test, the KO and WT mice consumed substantially more oil emulsion than water (KO: 15.5 vs. 0.2 g/day; WT 13.8 vs. 0.3 g/day). When water deprived and given a 60-s choice test, the KO and WT mice did not significantly differ in their preference for 1% Intralipid (49% vs. 65%).
Saccharide test.
Figure 1 presents the results of the 60-s two-bottle saccharide vs. water tests. When water deprived, the KO and WT mice did not prefer 4% Polycose or 4% sucrose to water, and the genotypes did not differ in the number of licks emitted during the 60-s tests. When food deprived, however, the KO and WT mice preferred Polycose to water. Both genotypes increased their licks to Polycose as concentration increased [F(1,18) = 90.8, P < 0.001], and there was no genotype × concentration interaction (Fig. 1). Overall, the KO mice licked less for Polycose than did the WT mice [165.9 vs. 218.6 licks, F(1,18) = 7.9, P < 0.05]. Within-group analyses revealed that WT mice preferred all Polycose concentrations to water. KO mice licked significantly more (P < 0.05) for Polycose than water at 8–32% concentrations. Overall, Polycose preference was less in KO mice than in WT mice [82 vs. 94%, F(1,18) = 38.9; P < 0.001]. Both genotypes increased their Polycose preference [F(3,54) = 5.3, P < 0.01] with concentration, and there was no genotype × concentration interaction.
Fig. 1.
Saccharide licks (± SE) (top) and percent saccharide preference over water (bottom) in T1R3 KO and B6 WT mice during 60-s two-bottle tests. Licks of water are not shown. Significant (P < 0.05) differences between KO and WT genotypes are indicated by an asterisk (*). Overall genotype difference is denoted by a number sign (#).
In the sucrose vs. water two-bottle tests, only the WT mice significantly preferred sucrose to water. Overall, the KO mice licked much less for sucrose than did WT mice [24.9 vs. 224.9 licks, F(1,18) = 246.2, P < 0.001]. Both genotypes increased their licking with sucrose concentration, but this effect was greater in the WT mice [genotype × concentration interaction, F(3,54) = 6.5, P < 0.001]. Within-group analyses revealed WT significantly preferred sucrose to water at all concentrations, while KO mice did not prefer sucrose at any concentration. At the 16% concentration, only 3 of 10 KO mice responded to sucrose with more than 20 licks; at the 32% concentration, 6 of 10 KO mice responded. Overall, percent sucrose licks were lower for KO mice than WT mice [57 vs. 96%, F(1,18) = 112.8, P < 0.0001]. Percent licks increased with sucrose concentration [F(3,54) = 8.3, P < 0.001], and the genotype × concentration interaction was not significant.
Across all concentrations, the KO mice licked much more for Polycose than for sucrose [165.9 vs. 24.9, F(1,18) = 71.4, P < 0.001]. KO mice also showed a stronger preference for Polycose than for sucrose [82 vs. 57%, F(1,18) = 40.0, P < 0.001]. In contrast, the WT mice did not differ in total licks for Polycose and sucrose (218.6 vs. 224.9) or in Polycose and sucrose preferences (94 vs. 96%).
Experiment 2: 24-h Two-Bottle Tests
Polycose vs. water tests.
In the first Polycose test series (Fig. 2), the KO mice consumed less Polycose overall than did the WT mice [7.9 vs. 10.5 g/day, F(1,18) = 11.9, P < 0.01]. Both genotypes increased and then decreased their solution intake as concentration increased [F(6,108) = 184.0, P < 0.001], and there was no genotype × concentration interaction. Polycose energy intake increased with concentration [F(6,108) = 529.7, P < 0.001], and the KO mice consumed less than WT mice at 8% to 32% concentrations [genotype × concentration interaction, F(6,108) = 6.6, P < 0.001]. With respect to Polycose preference, the KO mice significantly preferred Polycose to water at 2–32% concentrations, whereas WT mice significantly preferred Polycose at 1–32% concentrations. Polycose preferences of the KO mice were less than those of the WT at 0.5–4% concentrations, but both genotypes displayed near-total preferences for 8–32% Polycose solutions [genotype × concentration interaction, F(6,108) = 11.4, P < 0.001].
Fig. 2.
Polycose solution intake (± SE) (top), percent Polycose preference over water (middle) and Polycose energy intake (bottom) in T1R3 KO and B6 WT mice during 24-h two-bottle Polycose vs. water tests 1 and 2. Water intakes are not shown. Significant (P < 0.05) genotype differences at individual concentrations are indicated by an asterisk (*) and significant overall genotype differences are indicated by number sign (#). The lowest concentration at which Polycose was significantly (P < 0.05) preferred to water is indicated by a plus sign (+).
In the second Polycose test (Fig. 2), there was no overall genotype difference in solution intake, although there was a genotype × concentration interaction [F(6,108) = 3.6, P < 0.01]. The KO mice consumed slightly more 0.5–8% Polycose, while the WT mice consumed more 16–32% Polycose, but these individual differences were not significant. In terms of energy intake, however, the KO mice consumed significantly less Polycose than did WT mice at 16% and 32% concentrations [genotype × concentration interaction, F (6,108) = 12.5, P < 0.001]. The percent Polycose intakes of the KO and WT mice were very similar, and both genotypes displayed near-total preferences for Polycose at all concentrations. Within- genotype analyses indicated that both the KO and WT mice increased their Polycose intakes and preferences from test 1 to 2, with the differences being most pronounced at the 0.5–8% concentrations [text × concentration interactions, F(6,54) > 9.5, P < 0.001].
Sucrose vs. water tests.
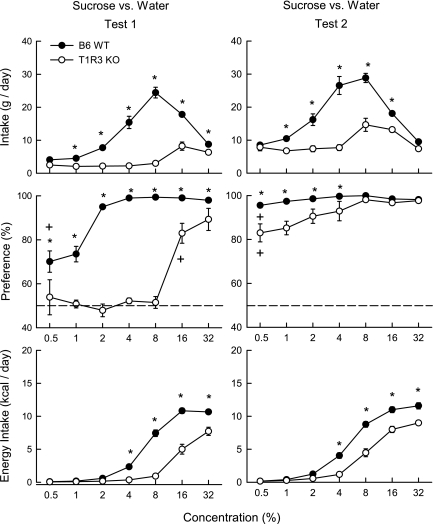
In the first sucrose test (Fig. 3), the KO mice consumed considerably less sugar overall than did the WT mice [3.8 vs. 11.8 g/day, F(1,18) = 140.5, P < 0.001], and the genotypic differences were significant at all concentrations except 0.5% [genotype × concentration interaction, F(6,108) = 55.3, P < 0.001]. With respect to energy intake, the KO mice consumed less sucrose than did the WT mice at 4–32% concentrations [F(6,108) = 42.9, P < 0.001]. Analysis of the sucrose preference data indicated that KO mice were indifferent to 0.5–8% sucrose and only at 16% and 32% concentrations did they drink more (P < 0.05) sugar than water. In contrast, WT mice preferred all sucrose solutions to water. The percent sucrose intakes of KO mice were less than WT mice at all concentrations, although the genotype difference decreased at the two highest concentrations [genotype × concentration interaction, F(6,108) = 15.72, P < 0.001].
Fig. 3.
Sucrose solution intake (± SE) (top), percent sucrose preference over water (middle), and sucrose energy intake (bottom) in T1R3 KO and B6 WT mice during 24-h two-bottle sucrose vs. water tests 1 and 2. Water intakes are not shown. Significant (P < 0.05) genotype differences at individual concentrations are indicated by an asterisk (*). The lowest concentration at which sucrose was significantly (P < 0.05) preferred to water is indicated by a plus sign (+).
In the second sucrose test (Fig. 3), KO mice continued to consume less sucrose overall than did WT mice [9.3 vs. 16.9 g/day, F(1,18) = 31.3, P < 0.001], and the genotype differences were significant from 1% to 16% concentrations [genotype × concentration interaction, F(6,108) = 30.5, P < 0.001]. In terms of energy intake, KO mice consumed significantly less sucrose than WT mice at 4–32% concentrations. A key finding was that the KO mice, like the WT mice, now preferred sucrose to water at all concentrations tested [t(9) > 5.2, P < 0.05]. However, sucrose preference of KO mice was less than that of WT mice at 0.5–4% concentrations [genotype × concentration interaction, F(6,108) = 5.4, P < 0.001]. Within-genotype analyses indicated that both the KO and WT mice increased their sucrose intakes, and preferences from test 1 to 2, with the differences being most pronounced at the 0.5 to 4 or 8% concentrations [test × concentration interactions, F(6,54) > 17.5, P < 0.001].
Polycose vs. sucrose comparisons.
In the first test series, the KO mice consumed more Polycose than sucrose overall [7.9 vs. 3.8 g/day, F(1,18) = 46.1, P < 0.001]; the differences were significant at 4–16% concentrations [saccharide × concentration interaction, F(6,108) = 36.9, P < 0.001]. The KO mice also displayed a stronger preference for Polycose than for sucrose [80% vs. 61%, F(1,18) = 41.5, P < 0.001]; the differences were significant at 2–16% concentrations [saccharide × concentration interaction, F(6,108) = 18.1, P < 0.001]. In the second test series, the KO mice again consumed more Polycose than sucrose [16.7 vs. 9.3 g/day, F(1,18) = 28.2, P < 0.001]; the differences were significant at 0.5–8% concentrations [saccharide × concentration interaction, F(6,108) = 15.2, P < 0.001]. The KO mice also showed a greater preference for Polycose than sucrose [98 vs. 92%, F(1,18) = 9.1, P < 0.01]; the differences were significant at 0.5–4% concentrations [F(1,18) = 7.04, P < 0.001].
With respect to the WT mice, overall intakes of Polycose and sucrose did not differ in test 1 (10.5 vs. 11.8 g/day), although the mice consumed more sucrose at 4 and 8% concentrations [saccharide × concentration interaction, F(6,108) = 5.3, P < 0.01]. The WT mice showed a somewhat stronger preference for sucrose than Polycose in test 1 [90.6% vs. 88.0%, F(1,18) = 4.9, P < 0.05]; the differences were significant at 0.5 and 2% concentrations [saccharide × concentration interaction F(6,108) = 4.3, P < 0.001]. In test 2, WT mice consumed similar amounts of Polycose and sucrose (16.5 vs. 16.9 g/day), although sucrose intake was higher at the 4% concentration [saccharide × concentration interaction, F(6,108) = 5.1, P < 0.001]. The WT mice also did not differ in their preferences for Polycose and sucrose in test 2 (99 vs. 98%).
Saccharin vs. Water
The saccharin intakes and preferences of the mice tested with sucrose and Polycose were very similar, and therefore the groups were combined for statistical analysis. In the preliminary test with 0.2% saccharin, the KO mice consumed slightly less saccharin than water, and their percent saccharin intake was 44%. Their 0.2% saccharin preference declined to 36% [t(19) = 2.1, P < 0.05] in test 3 after the mice had been tested with sucrose or Polycose (Fig. 4). In contrast to the WT mice, which showed strong and stable preferences for 0.2–0.8% saccharin solutions in test 3, the KO mice decreased their preference from 36% to 22% as saccharin concentration increased [genotype × concentration interaction, F(2,76) = 10.7, P < 0.001]. The WT mice consumed substantially more saccharin than did the KO mice [19.8 vs. 2.3 g/day, F(1,38) = 273.9, P < 0.001], although the absolute intake of the sweetener declined in both genotypes as concentration increased [F(2,76) = 99.9, P < 0.001].
Fig. 4.
Saccharin solution intake (± SE) (top) and percent saccharin preference (bottom) over water in T1R3 KO and B6 WT mice during 24-h two-bottle saccharin vs. water tests. Water intakes are not shown. Significant (P < 0.05) differences genotype differences are indicated by an asterisk (*).
Experiment 3: Chorda Tympani Nerve Responses
For sucrose, the WT and KO mice exhibited highly divergent responses (Fig. 5). There was a significant main effect of genotype [F(1,45) = 30.7, P < 0.001], concentration [F(3,45) = 17.5, P < 0.001], and interaction of genotype and concentration [F(1,45) = 13.7, P < 0.001]. The significant interaction reflects the fact that the CT nerve response increased significantly with concentration in WT mice [F(3,21) = 14.4, P < 0.001] but not in KO mice. This finding confirms that knocking out T1R3 virtually eliminates the CT nerve response to sucrose.
Fig. 5.
Chorda tympani nerve responses (normalized to 100 mM NH4Cl) of T1R3 KO and WT mice to a range of concentrations of sucrose (A), Polycose (B), and NaCl (C). Values are means ± SE; n = 8 or 9 per strain.
For Polycose and NaCl, on the other hand, the WT and KO mice responded similarly (Fig. 5). The main effect of concentration [in both cases, F(3,45) > 15, P < 0.001] was significant, revealing a robust effect of concentration on the response of the CT nerve to both stimuli. The main effect of genotype and the interaction of genotype and concentration were not significant. While there was a trend for the response to 32% Polycose to be lower in the KO mice, this difference was not significant. These results show that knocking out T1R3 had no systematic effect on the CT nerve response to Polycose or NaCl.
DISCUSSION
This study examined the role of the T1R3 sweet taste receptor in the taste response to Polycose, a glucose polymer mixture that is very attractive to rodents. The findings revealed that KO mice lacking T1R3 displayed near-normal ingestive and CT nerve responses to Polycose. In contrast, the KO mice showed substantially impaired ingestive and CT nerve responses to sucrose, confirming prior reports (7, 40). Thus, while critical for sweet taste signaling, the T1R3 receptor is not essential for the detection of glucose polymers. These findings are consistent with prior evidence that sucrose and Polycose generate qualitatively different taste sensations in rodents (20, 25). The present study further revealed that prior consumption of Polycose largely eliminated the small deficits in Polycose preference and acceptance displayed by KO mice. Sucrose experience had an even more profound effect on the KO mice. Whereas sugar-naïve KO mice were completely indifferent to dilute sucrose solutions, sugar-experienced KO mice significantly preferred all sucrose solutions tested, although they continued to underconsume sucrose compared with WT mice. These experiential effects can be attributed to the post-oral reinforcing actions of the carbohydrates.
Polycose preference.
The two-bottle preference results and the CT nerve data demonstrate that genetic deletion of the T1R3 receptor produces only small deficits in responsiveness to Polycose. For instance, in the 60-s choice tests, the overall difference in Polycose preference between the KO and WT mice was ∼12%, and in the 24-h choice test, the genotypes differed in preference by ∼13% at the low concentrations (0.5–4%). The preferences of the KO and WT mice for the higher Polycose concentrations (8–32%) differed in 60-s tests, but not the 24-h tests. This finding may reflect the fact that only the 24-h tests provided sufficient time for the reinforcing post-oral effects of the Polycose solutions to enhance intake in both KO and WT mice.
The lower preference for Polycose in the KO mice was surprising given that their CT nerve responses to Polycose were statistically indistinguishable from those of the WT mice. We can propose two explanations for these seemingly contradictory findings. One stems from the fact that the CT nerve only relays signals from taste cells in the front of the tongue. Taste fields in the back of the tongue are innervated by the glossopharyngeal (GL) nerve, and those on the palate are innervated by the greater superficial petrosal (GSP) nerve. It is possible that the GL and/or GSP nerve displays a deficit in Polycose responsiveness, and that this deficit was responsible for the attenuated Polycose preference in KO mice. In support of this possibility, a nerve transection study in rats indicated that input from the GL nerve is more important than input from the CT nerve in stimulating Polycose intake (37). A second explanation is that the Polycose preference deficit displayed by the KO mice is too subtle to be revealed by whole nerve recordings.
It is possible that Polycose solutions are more attractive to WT than KO mice because the solutions, in addition to having a “poly” taste, have a slightly sweet taste to WT (but not to KO) mice due to a small amount of free glucose and maltose. To test this idea, naïve KO and WT mice were given 24-h two-bottle tests with dilute solutions (0.5–4%) of a maltooligosaccharide mixture (M-138, Pfanstiehl Laboratories, Waukegan, IL) that contained polymers of 4–8 glucose units and virtually no glucose or maltose (Zukerman and Sclafani, unpublished study). Overall, the KO mice displayed a weaker preference for the maltooligosaccharide solutions over water than did the WT mice (77% vs. 87%, P < 0.01), although this difference disappeared at the 4% concentration (96% vs. 97%). This finding is similar to the preference differences displayed by KO and WT mice for 0.5–4% Polycose solutions in experiment 2. Thus, the attenuated Polycose preference of the KO mice is not explained by an insensitivity to the taste of the sugar in Polycose. Further, the strong preferences displayed by KO and WT mice for 1% Intralipid in the 24-h test of experiment 1 indicate that KO mice do not have a generalized deficit in nutrient preferences.
In an experiment conducted independently of the present study, Treesukosol et al. (35) compared the licking responses of T1R3 KO and WT mice to Polycose and sucrose in 5-s one-bottle tests. Consistent with the present findings, the T1R3 KO mice licked considerably more for Polycose than for sucrose over a range of concentrations. However, in their study the Polycose licking responses of the T1R3 KO and WT mice were very similar. It remains to be determined why the T1R3 KO mice in the two studies differed somewhat in their avidity for Polycose. In addition to the different behavioral test procedures used (two-bottle vs. one-bottle), the T1R3 KO mice in the two studies differed both in the method used to delete the Tas1R3 gene and in their genetic background (C57BL/6 vs. C57BL/6 × 129) (7, 40). The background difference may be important because of the different Polycose preferences displayed by C57BL/6 and 129 mice (28). Nevertheless, the two studies agree in showing that the behavioral response to Polycose, unlike that to sucrose, is largely if not completely spared in T1R3 KO mice. The Treesukosol et al. (35) study further revealed that T1R2 KO mice are also normal in their licking response to Polycose solutions. Thus, deletion of either the T1R2 or T1R3 component of the sweet taste receptor largely or completely spares the taste response to glucose polymers.
Sucrose preference.
The failure of the KO mice to prefer 4–32% sucrose in the 60-s two-bottle tests is consistent with an early report that KO mice show little or no stimulation of drinking, above water baseline, in 5-s one-bottle tests (40). The KO mice did show a small licking response to a concentrated sucrose solution (1 M = 34%), which was attributed to the activity of the remaining T1R2 receptor (40). Supporting this interpretation, double knockout mice missing both T1R2 and T1R3 receptor elements showed no detectable licking responses to concentrated sucrose solutions (40) A residual T1R2-mediated taste response may account for the 32% sucrose preference displayed by some KO mice in experiment 1.
In contrast to their minimal sucrose response in the 60-s test, the KO mice significantly preferred concentrated (16–32%) sucrose solutions in the first 24-h test series. The 83% preference observed for 16% sucrose is consistent with the ∼80% and ∼75% preferences observed for 0.4 M (14%) and 0.65 M (22%) sucrose solutions in earlier studies of T1R3 KO mice in 24-h tests with ascending sugar concentrations (7, 40). At these high concentrations, sucrose has potent post-oral reinforcing actions in mice (30). It may be that the post-oral effects of sucrose conditioned the KO mice to prefer the orosensory properties of the sugar solution (i.e., its residual T1R2-mediated taste, its odor, and/or texture). This interpretation is supported by the finding that T1R3 KO mice, like WT mice, learn to prefer a distinctively flavored (e.g., grape) solution that is paired with intragastric (IG) infusions of 16% sucrose over a different flavored solution (e.g., cherry) paired with IG water infusions during 24-h test sessions (29).
Saccharide experience effect.
The exposure to saccharide solutions in the first 24-h two-bottle test series had a significant effect on subsequent saccharide preferences of the KO mice and, to a lesser extent, the WT mice. In the case of Polycose, the KO mice increased their preference for dilute solutions from the first to second tests so that, like WT mice, they displayed near-total preferences for Polycose at all concentrations in test 2. For example, the KO and WT mice increased their preferences for 0.5% Polycose from 50% and 57% in test 1 to 97% and 98%, respectively, in test 2. Furthermore, whereas the KO mice underconsumed dilute Polycose solutions in test 1, they drank somewhat more 0.5–4% Polycose than did the WT mice in test 2. However, in test 2 the KO mice continued to consume less Polycose (in kcal) at high concentrations compared with WT mice. The reduced intake of concentrated Polycose solutions by KO mice may have been due to an impaired ability to absorb the saccharide (as glucose) in the gastrointestinal tract. Recent studies indicate that T1R3 receptors localized in intestinal enteroendocrine cells respond to ingested carbohydrates by promoting the expression of glucose transporters, thereby enhancing glucose absorption (15, 16). KO mice, which lack T1R3 receptors in the gut as well as in the mouth, display reduced glucose uptake compared with WT mice when fed a high-carbohydrate diet (16). This attenuated glucose absorption may increase the satiating action of concentrated Polycose solutions in KO mice and thereby limit consumption.
Sucrose experience had a remarkable effect on the sugar preference of KO mice. The KO mice went from being completely indifferent to 0.5–8% sucrose in test 1 to displaying 83% to 98% preferences in test 2. Their preference for 16 and 32% sucrose solutions also increased from the first to the second test (∼86% to ∼97%). The WT mice also increased their preference for dilute sucrose (0.5–2%) from the first to second tests series, although this effect was less dramatic. As noted above, the preference KO mice displayed for 16–32% sucrose in the initial 24-h test series can be attributed to post-oral conditioning. Their subsequent preference for dilute sucrose solutions in test 2 may represent a generalization of this learned response. What orosensory stimuli mediated the sucrose preference of the KO mice is not certain. Texture or T1R2-mediated taste cues would appear unlikely candidates at low sugar concentrations. Olfactory cues may be involved in view of reports that mice and rats can smell sucrose solutions (23, 36). This question is currently under investigation.
While sucrose experience greatly enhanced the sucrose preferences of KO mice, it had much less effect on sugar acceptance. As in the first test, KO mice consumed substantially less sucrose solution and energy than did WT mice over a wide range of concentrations (1–32%) in test 2. Also, whereas the WT mice increased their sucrose solution intake threefold as the concentration increased from 0.5 to 4%, the KO mice showed no change in the actual quantity of sucrose ingested. Only at 8% and 16% concentrations did the KO mice show some increase in sugar solution intake. The fact that sucrose preference was enhanced more than sucrose acceptance is consistent with a post-oral conditioning process. Mice acquire significant preferences for flavored solutions paired with IG sucrose infusions and this conditioning occurs with initially unpreferred flavors (e.g., sour grape) and initially preferred flavors (e.g., sweet grape) (30). Mice also increase their absolute intake of a nutrient-paired flavor, but this conditioned acceptance is most pronounced when the flavor is already preferred. In particular, B6 mice overconsumed a sweetened flavored solution much more than an unsweetened flavored solution paired with IG sucrose infusions (30). Thus, the finding that the KO mice strongly preferred the sucrose solutions in test 2 but yet did not drink very much sucrose indicates that they did not sense the sweet taste of the sugar but were responding to other orosensory properties (odor, texture).
Although the sucrose KO mice displayed strong sugar preferences in test 2, they, like the Polycose KO mice, failed to prefer saccharin in test 3. In fact, their 0.2% saccharin preference declined from initial test (before saccharide tests) to test 3, and the KO mice strongly avoided 0.8% saccharin in test 3. Saccharin avoidance by KO mice has been attributed to the mice responding to the bitter taste component of the artificial sweetener (5). The saccharin avoidance by the KO mice in the last test clearly demonstrates that their sucrose preference in the second test does not represent a recovery of sweet taste sensitivity.
The enhanced Polycose and sucrose preferences displayed by KO and WT mice in test 2 is likely due, in large part, to an increase in the reward value of the flavor (taste, odor, texture) of the saccharide solutions conditioned by the saccharide's post-oral reinforcing actions. Another contributing factor could be the increased familiarity of the saccharide flavors (a “mere exposure effect”). In a recent study (28), B6 mice displayed experience-induced enhancements in sweetener preferences when given repeated tests with nutritive (sucrose) and nonnutritive (saccharin) solutions, although the effect was more pronounced with the sugar solutions. Also, prior sucrose experience increased saccharin intake more than did prior saccharin experience (28). Rats also show experience-induced increases in sucrose preference, which are related to increased expression of the transcription factor ΔFosB in the nucleus accumbens, an important component of the brain reward system (38). This same factor is implicated in the sensitization to other natural rewards (sexual behavior), as well as drugs of abuse (38). The present finding that T1R3 KO mice show a profound experience-induced increase in sucrose preference demonstrates that this phenomenon does not depend upon an intact sweet taste system.
Conclusions.
A role for the T1R3 receptor in Polycose preference was hypothesized based on the different behavioral and neural responses observed in B6 and 129 mice for both Polycose and sucrose (28). However, the present finding that KO mice show a much greater deficit in sucrose taste than in Polycose taste refutes the hypothesis that strain differences in T1R3 sensitivity account for the different preference profiles displayed by B6 and 129 mice for Polycose and sucrose. A similar conclusion is indicated by the observations that congenic 129.B6-Tas1r3 strains differ in both the magnitude of preference and CT nerve response to sucrose, but not Polycose (11). It should also be noted that 129 mice underconsume Polycose, relative to B6 mice, much more than do KO mice (28). Taken together, these data indicate that genetic differences other than that in the T1R3 receptor are responsible for the differential response of 129 and B6 mice to Polycose (see also Ref. 2).
The taste receptor for Polycose has yet to be identified. Behavioral data suggest that the receptor preferentially binds to short-chain glucose polymers. In brief two-choice tests, rats preferred a maltooligosaccharide solution containing polymers of 4 to 8 glucose units to solutions containing shorter (maltose, maltotriose) or longer (maltopolysaccharides) glucose polymers (31). The present findings along with those of Treesukosol et al. (35) indicate that the presence of either T1R3 or T1R2 is not critical for the taste response to Polycose. Conceivably, either protein alone may function as a glucose polymer receptor. If so, then T1R2/T1R3 double knockout mice should be insensitive to Polycose taste. Alternately, Polycose may bind to an as yet unidentified taste receptor. Whatever receptor mediates glucose polymer taste, it appears to share some of the same intracellular signaling proteins as the T1R2/T1R3 sweet receptor. Both α-gustducin and Trpm5 knockout mice display impaired preferences for Polycose and sucrose (33). Thus, the glucose polymer receptor is likely to be expressed in the same subset of type II taste cells that express these downstream signaling elements.
Perspectives and Significance
It has long been assumed that sugars are the only carbohydrates that stimulate the mammalian taste system. While this may be true for humans, the present findings along with other data (26, 27) indicate that some mammalian species have separate taste receptors for simple (mono- and disaccharides) and complex (maltosaccharides and polysaccharides) carbohydrates. In fact, multiple carbohydrate receptors may exist because rats and mice appear to distinguish between maltooligosaccharides (e.g., Polycose) and polysaccharides (e.g., pure corn starch) (21, 32, 33). Although the taste transduction pathways for sugars are now well understood, those for complex carbohydrates remain terra incognita.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-031135 (to A. Sclafani) and National Institute of Deafness and Other Communications Disorders Grants DC-03055 and DC-03155 (R. F. Margolskee).
DISCLOSURES
R. F. Margolskee has a personal financial interest in the form of stock ownership in the Redpoint Bio company, receives consulting fees from the Redpoint Bio company, and is an inventor on patents and patent applications that have been licensed to the Redpoint Bio company.
Acknowledgments
The authors thank Karen Ackroff for helpful comments on this paper, and Jade Gieseke and Heather Spain for helping collect the chorda tympani nerve data.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bachmanov AA, Beauchamp GK. Taste receptor genes. Annu Rev Nutr 27: 389–414, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmanov AA, Beauchamp GK. Amino acid and carbohydrate preferences in C57BL/6ByJ and 129P3/J mice. Physiol Behav 93: 37–43, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, Tordoff MG, de Jong PJ, Wu C, West DB, Chatterjee A, Ross DA, Beauchamp GK. Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses 26: 925–933, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Nutrient preference and diet-induced adiposity in C57BL/6ByJ and 129P3/J mice. Physiol Behav 72: 603–613, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav 7: 1–13, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheal M Taste responses of the chorda tympani nerve in the mouse. Physiol Behav 19: 175–177, 1977. [DOI] [PubMed] [Google Scholar]
- 7.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301: 850–853, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Dotson CD, Spector AC. The relative affective potency of glycine, L-serine and sucrose as assessed by a brief-access taste test in inbred strains of mice. Chem Senses 29: 489–498, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Eylam S, Spector AC. Stimulus processing of glycine is dissociable from that of sucrose and glucose based on behaviorally measured taste signal detection in Sac ‘Taster’ and ‘Non-taster’ mice. Chem Senses 29: 639–649, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Glendinning JI, Chyou S, Lin I, Onishi M, Patel P, Zheng KH. Initial licking responses of mice to sweeteners: effects of Tas1R3 polymorphisms. Chem Senses 30: 601–614, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Inoue M, Glendinning JI, Theodorides ML, Harkness S, Li X, Bosak N, Beauchamp GK, Bachmanov AA. Allelic variation of the Tas1r3 taste receptor gene selectively affects taste responses to sweeteners: evidence from 129. B6-Tas1r3 congenic mice. Physiol Genomics 32: 82–94, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue M, McCaughey SA, Bachmanov AA, Beauchamp GK. Whole nerve chorda tympani responses to sweeteners in C57BL/6ByJ and 129P3/J mice. Chem Senses 26: 915–923, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue M, Reed DR, Li X, Tordoff MG, Beauchamp GK, Bachmanov AA. Allelic variation of the Tas1r3 taste receptor gene selectively affects behavioral and neural taste responses to sweeteners in the F2 hybrids between C57BL/6ByJ and 129P3/J mice. J Neurosci 24: 2296–2303, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim MR, Kusakabe Y, Miura H, Shindo Y, Ninomiya Y, Hino A. Regional expression patterns of taste receptors and gustducin in the mouse tongue. Biochem Biophys Res Commun 312: 500–506, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Mace OJ, Affleck JA, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol 582: 379–392, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KSH, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, and Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1.Proc Natl Acad Sci USA 104: 15075–15080, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet 28: 58–63, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci 4: 492–498, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell 106: 381–390, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Nissenbaum JW, Sclafani A. Qualitative differences in polysaccharide and sugar tastes in the rat: A two-carbohydrate taste model. Neurosci Biobehav Rev 11: 187–196, 1987. [DOI] [PubMed] [Google Scholar]
- 21.Ramirez I Does starch taste like Polycose? Physiol Behav 50: 389–392, 1991. [DOI] [PubMed] [Google Scholar]
- 22.Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, Bachmanov AA. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. J Neurosci 24: 938–946, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhinehart-Doty JA, Schumm J, Smith JC, Smith GP. A non-taste cue of sucrose in short-term taste tests in rats. Chem Senses 19: 425–431, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Sainz E, Korley JN, Battey JF, Sullivan SL. Identification of a novel member of the T1R family of putative taste receptors. J Neurochem 77: 896–903, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Sako N, Shimura T, Komure M, Mochizuki R, Matsuo R, Yamamoto T. Differences in taste responses to Polycose and common sugars in the rat as revealed by behavioral and electrophysiological studies. Physiol Behav 56: 741–745, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Sclafani A Carbohydrate taste, appetite, obesity: An overview. Neurosci Biobehav Rev 11: 131–153, 1987. [PubMed] [Google Scholar]
- 27.Sclafani A The sixth taste. Appetite 43: 1–3, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Sclafani A Enhanced sucrose and Polycose preference in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice after experience with these saccharides. Physiol Behav 87: 745–756, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Sclafani A, Glass DS, Glendinning JI, Margolskee RF. T1R3 knockout mice learn to prefer flavors paired with intragastric sucrose infusions (Abstract). Chem Senses 33: 2008.
- 30.Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: Oral and postoral interactions. Am J Physiol Regul Integr Comp Physiol 289: R712–R720, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Sclafani A, Hertwig H, Vigorito M, Sloan H, Kerzner B. Influence of saccharide length on polysaccharide appetite in the rat. Neurosci Biobehav Rev 11: 197–200, 1987. [DOI] [PubMed] [Google Scholar]
- 32.Sclafani A, Nissenbaum JW, Vigorito M. Starch preference in rats. Neurosci Biobehav Rev 11: 253–262, 1987. [DOI] [PubMed] [Google Scholar]
- 33.Sclafani A, Zukerman S, Glendinning JI, Margolskee RF. Fat and carbohydrate preferences in mice: The contribution of α-gustducin and Trpm5 taste signaling proteins. Am J Physiol Regul Integr Comp Physiol 293: R1504–R1513, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Somenarain L, Jakinovich W Jr. Antagonism of the gerbil's sweetener and Polycose gustatory responses by copper chloride. Brain Res 522: 83–89, 1990. [DOI] [PubMed] [Google Scholar]
- 35.Treesukosol Y, Blonde G, Spector AC. The T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to Polycose: implications for saccharide taste receptors in mice. Am J Physiol Regul Integr Comp Physiol (January 21, 2009). doi: 10.1152/ajpregu.90869.2008. [DOI] [PMC free article] [PubMed]
- 36.Uebayashi H, Hatanaka T, Kanemura F, Tonosaki K. Acute anosmia in the mouse: behavioral discrimination among the four basic taste substances. Physiol Behav 72: 291–296, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Vigorito M, Sclafani A, Jacquin MF. Effects of gustatory deafferentation on Polycose and sucrose appetite in the rat. Neurosci Biobehav Rev 11: 201–209, 1987. [DOI] [PubMed] [Google Scholar]
- 38.Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, Graham DL, Green TA, Kirk A, Iniguez SD, Perrotti LI, Barrot M, DiLeone RJ, Nestler EJ, and Bolanos-Guzman CA. The influence of ΔFosB in the nucleus accumbens on natural reward-related behavior. J Neurosci 28: 10272–10277, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weijnen JA, Surink S, Verstralen MJ, Moerkerken A, De Bree GJ, Bleys RL. Main trajectories of nerves that traverse and surround the tympanic cavity in the rat. J Anat 197: 247–262, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJP, Zuker CS. The receptors for mammalian sweet and umami taste. Cell 115: 255–266, 2003. [DOI] [PubMed] [Google Scholar]