Abstract
The renal vasculature plays a major role in the regulation of renal blood flow and the ability of the kidney to control the plasma volume and blood pressure. Renal vascular dysfunction is associated with renal vasoconstriction, decreased renal blood flow, and consequent increase in plasma volume and has been demonstrated in several forms of hypertension (HTN), including genetic and salt-sensitive HTN. Several predisposing factors and cellular mediators have been implicated, but the relationship between their actions on the renal vasculature and the consequent effects on renal tubular function in the setting of HTN is not clearly defined. Gene mutations/defects in an ion channel, a membrane ion transporter, and/or a regulatory enzyme in the nephron and renal vasculature may be a primary cause of renal vascular dysfunction. Environmental risk factors, such as high dietary salt intake, vascular inflammation, and oxidative stress further promote renal vascular dysfunction. Renal endothelial cell dysfunction is manifested as a decrease in the release of vasodilatory mediators, such as nitric oxide, prostacyclin, and hyperpolarizing factors, and/or an increase in vasoconstrictive mediators, such as endothelin, angiotensin II, and thromboxane A2. Also, an increase in the amount/activity of intracellular Ca2+ concentration, protein kinase C, Rho kinase, and mitogen-activated protein kinase in vascular smooth muscle promotes renal vasoconstriction. Matrix metalloproteinases and their inhibitors could also modify the composition of the extracellular matrix and lead to renal vascular remodeling. Synergistic interactions between the genetic and environmental risk factors on the cellular mediators of renal vascular dysfunction cause persistent renal vasoconstriction, increased renal vascular resistance, and decreased renal blood flow, and, consequently, lead to a disturbance in the renal control mechanisms of water and electrolyte balance, increased plasma volume, and HTN. Targeting the underlying genetic defects, environmental risk factors, and the aberrant renal vascular mediators involved should provide complementary strategies in the management of HTN.
Keywords: blood pressure, dietary salt, oxidative stress, cytokines, kidney, endothelium, vascular smooth muscle, extracellular matrix, matrix metalloproteinases
the role of the kidney in the regulation of sodium and water balance and blood pressure (BP) is well recognized (53, 55, 128). A decrease in plasma volume and renal blood flow (RBF) stimulates renin release from the juxtaglomerular cells (JGCs) and activates the renin-angiotensin system (RAS). Renin transforms angiotensinogen to angiotensin I (ANG I), and angiotensin-converting enzyme (ACE) transforms ANG I to ANG II. ANG II stimulates the adrenal cortex to release aldosterone (Aldo), which acts on the renal collecting ducts, stimulates sodium and water absorption, and thereby restores plasma volume and RBF. The increased ANG II and intrarenal pressure then inhibit further renin release from JGCs (53). Other components of RAS, such as ANG III and IV (products of ANG II conversion by aminopeptidases), ANG 1-7 (product of ANG II conversion by ACE2), and (pro)renin receptor (in the kidney and other tissues), are potential participants in sodium balance and BP regulation.
In addition to renal control of water and electrolytes, regulation of renal vascular resistance (RVR) is critical to maintain RBF and kidney function. This involves rigorous control of blood flow through an intricate network of renal segmental, interlobar, arcuate, and interlobular arteries, down to afferent and efferent arterioles in the renal cortex, and vasa recta in the medulla. The kidney receives ∼20% of cardiac output. In the glomeruli, 20% of total renal plasma flow filters into Bowman's space, 70% goes through the efferent arterioles to the peritubular capillaries in the renal cortex, and 5–10% go to the medulla. To maintain salt and water balance, there is a need to control RBF and glomerular filtration rate (GFR). This is achieved via an array of local, paracrine, neural, endocrine, and systemic control mechanisms. For instance, renal autoregulation uses local and paracrine mechanisms to effectively control RBF. An increase in renal perfusion pressure activates renal autoregulation, leading to renal vasoconstriction and a reduction of RBF to a maintained normal level (103, 131). Renal autoregulation is partly achieved via a myogenic mechanism within the vascular smooth muscle (VSM) of preglomerular vessels. Another mechanism of renal autoregulation is tubuloglomerular feedback (TGF), in which the macula densa, a plaque of specialized tubular epithelial cells, senses the increases in filtered and tubular NaCl and sends a signal(s) to induce afferent arteriolar vasoconstriction, leading to a decrease in RBF and GFR, and thereby reduction of filtered NaCl. Thus localized release of vasodilators and vasoconstrictors in the kidney could act in a paracrine fashion to affect renal vascular tone (119). Renal vascular tone and TGF may also be modulated by additional neural, endocrine, and systemic agents. Circulating ANG II affects the cardiovascular centers in the brain to enhance sympathetic nerve firing to systemic and renal blood vessels. While sympathetic innervation of systemic vessels is largely involved in moment-to-moment restoration of BP in case of a sudden decrease, sympathetic innervation of renal vessels may affect renal function and long-term regulation of BP (100, 106). Also, circulating ANG II directly constricts blood vessels, leading to systemic and renal vasoconstriction and maintenance of BP.
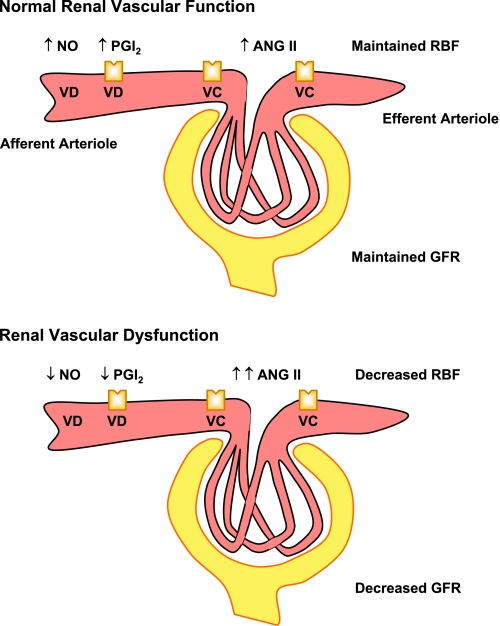
Thus BP regulation involves complex interaction between neural, hormonal, vascular, and renal control mechanisms. To maintain RBF and kidney function, the renal vessels are constantly adjusting their diameter and tone. In addition to renal autoregulation, short-term adjustment of renal vascular tone involves changes in endothelial cell function, neurohormonal drive, and VSM sensitivity to vasoconstrictor stimuli. Long-term adjustment involves vascular wall hypertrophy and structural changes in the vessel wall architecture and extracellular matrix (ECM). Excessive changes in endothelial cell function (decreased vasodilators, increased vasoconstrictors), VSM function (increased VSM tone and reactivity), and ECM composition (remodeling) could lead to renal vascular dysfunction and vasoconstriction (Fig. 1). Persistent renal vasoconstriction causes increases in RVR and could increase BP, in part, by contributing to total vascular resistance. Also, renal vasoconstriction in preglomerular arterioles decreases RBF and filtered NaCl, leading to activation of TGF and increased renin and ANG II release. The effects of ANG II and other vasoconstrictors are normally counterbalanced by vasodilators, such as nitric oxide (NO) and prostacyclin (PGI2). However, when the release of vasodilator mediators is compromised, ANG II acts unopposed in a paracrine and systemic fashion to induce further renal vasoconstriction. The released ANG II could also increase Aldo secretion and stimulate NaCl and water absorption. Additionally, ANG II-induced vasoconstriction of postglomerular efferent arterioles in the renal cortex, and vasa recta in the renal medulla, leads to further increases in sodium absorption and increased plasma volume and BP (Fig. 1). Long term, the increased BP causes renal vascular remodeling and further increases in renal vasoconstriction and RVR, leading to a vicious cycle, persistent increases in BP and hypertension (HTN).
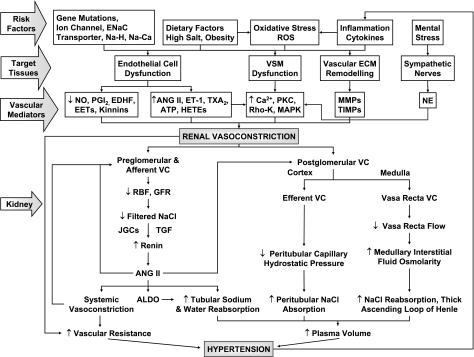
Fig. 1.
Mediators of renal vasoconstriction and hypertension (HTN). Genetic mutation of an ion channel or transporter, combined with dietary factors, oxidative stress, inflammatory response, and mental stress, cause vascular, renal, and neural dysfunction. Endothelial dysfunction causes a decrease in vasodilators (VDs) [nitric oxide (NO), prostacyclin (PGI2), endothelium-derived hyperpolarizing factor (EDHF)] and an increase in vasoconstrictors (VCs) [angiotensin II (ANG II), endothelin-1 (ET-1), thromboxane A2 (TxA2)]. Vascular smooth muscle (VSM) dysfunction is associated with increased Ca2+, PKC, Rho kinase, and MAPK and leads to VSM contraction and growth. Sympathetic nerves contribute to VSM hyperactivity. Changes in the expression/activity of matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs) in extracellular matrix (ECM) promote renal vascular remodeling. Excessive renal vasoconstriction increases renal vascular resistance (RVR) and contributes to total vascular resistance. Also, vasoconstriction of preglomerular arterioles decreases renal blood flow (RBF) and filtered NaCl, leading to activation of tubuloglomerular feedback (TGF) and increased release of renin and ANG II, which acts in a paracrine or systemic fashion to induce further vasoconstriction. ANG II also increases aldosterone (Aldo) secretion and stimulates tubular NaCl and water absorption. ANG II-induced vasoconstriction of postglomerular efferent arterioles in the cortex and vasa recta in the medulla further increases NaCl absorption, plasma volume, and blood pressure (BP). HTN, in turn, promotes vascular and renal inflammation and oxidative stress, leading to a vicious cycle and progression of HTN. Not shown, vascular agents could act in a paracrine fashion and affect tubular reabsorption, sympathetic nerves may affect renin release from juxtaglomerular cells (JGCs), and renal afferent nerves may send feedback signals to sympathetic centers. ENaC, epithelial Na channel; ROS, reactive oxygen species; EET, epoxyeicosatrienoic acid; HETE, hydroxyeicosatetraenoic acid; NE, norepinephrine; GFR, glomerular filtration rate.
Several reviews have provided detailed information on systemic vascular tone and renal tubular function as important control mechanisms of BP (16, 53, 55, 128, 138). However, the renal vessels are unique in that they are both anatomically and physiologically linked to renal glomerular filtration and tubular function. To highlight the close relationship between the vascular and renal mechanisms, this review will take a more integrative approach to discuss the potential predisposing factors and cellular mediators involved in renal vascular dysfunction and how they could affect the renal glomerular filtration and tubular function and lead to HTN. Because the amount of information could be enormous when describing all forms of HTN, and may be limited if discussing only one form of HTN, we will focus on two common forms, namely genetic and salt-sensitive (SS) HTN. The review will describe evidence of renal vascular dysfunction as a primary cause or consequence of HTN. The genetic predisposition, environmental and dietary risk factors, and the role of vascular inflammation and oxidative stress in HTN will be briefly described. The changes in renal hemodynamics, the vasodilatory and vasoconstrictive mediators involved, and the abnormalities in endothelium, VSM, and ECM will then be discussed. Throughout the review, we will discuss hemodynamic data in human then animal models, physiological evidence in large then small vessels, regional differences in the renal cortex then the medulla, followed by discussion of the cellular and molecular mechanisms involved. Future directions to further delineate the cellular mediators of renal vascular dysfunction and the clinical implications in treatment of HTN will also be discussed.
Renal Vascular Dysfunction as a Cause or Consequence of HTN
Although renal vascular dysfunction, increased RVR, and changes in kidney function are demonstrated in several forms of HTN, whether their relationship is causative or associative in nature has not been clearly established. The preglomerular vasculature are resistance vessels and therefore are likely to demonstrate the same structural and functional alterations that occur in other peripheral resistance vessels as a consequence of HTN. While this may be the case in some essential hypertensive patients, assessment of the renal hemodynamics in prehypertensive humans suggests that an increase in RVR may constitute an initial event that leads to elevation of total vascular resistance and BP (see Fig. 1). Studies have shown an increase in RVR but normal RBF and GFR in young people with a family history of HTN compared with control subjects, suggesting that an abnormal renal vasoconstriction exists in the prehypertensive state (135). Also, offspring of hypertensive parents demonstrate an exaggerated renal vasodilation in response to Ca2+ channel blockers, supporting the existence of functional renal vasoconstriction in subjects at risk of developing HTN (135). Borderline HTN is a condition in which a subject's BP is above normal, but not sufficiently high to require antihypertensive therapy, and may represent an early phase of essential HTN. Although RBF tends to be normal or slightly reduced in borderline HTN patients, RVR is elevated. Borderline hypertensive subjects also exhibit an enhanced renal vasoconstrictor response to infusion of norepinephrine. Thus, while increased preglomerular vascular tone may be due to a generalized disturbance in peripheral vascular resistance, it could also be due to localized alterations in the renal vasculature and could lead to HTN (135).
The primary importance of renal vascular alterations in the development of HTN is further supported by experimental studies. Experimental cross-transplantation studies suggest that the underlying pathology of HTN travels with the kidney from spontaneously hypertensive rat (SHR) donors to normotensive recipients, and kidneys from normotensive donors lower BP in transplanted SHR (27, 51). Measurement of the renal hemodynamics in SHR showed increased RVR as HTN develops, while GFR and glomerular capillary pressure are normal, and the unaltered glomerular function may be due, in part, to a renal autoregulatory adjustment to the increase in BP. However, in young SHR, an increase in RVR and renal vascular hypertrophy occurs as early as 4 wk of age, during which there is no established HTN (159). Also, in prehypertensive young SHR, there is increased reactivity of the renal vasculature to vasoconstrictors and decreased medullary blood flow, supporting a role in the development of HTN (139, 180). Furthermore, in SHR, preglomerular arterial wall hypertrophy is not reversed by antihypertensive treatment, which is not the case in other vascular beds (158). The presence of structural and functional changes in preglomerular and afferent arterioles before the development of HTN and the persistence of structural changes, despite normalization of BP, suggest that these renal vascular changes are not a mere consequence of elevated BP, but may be involved in the pathogenesis of HTN.
Genetic Predisposing Factors in Renal Vascular Dysfunction
Essential HTN, a form of HTN with no clear cause, is believed to have a hereditary influence. Also, BP is similar in descendants of the same family, but is different between adopted and biological siblings, supporting a genetic influence on BP. Linkage studies utilizing whole genome scans have identified chromosomal regions with potential relation to BP regulation. Studying sibling pairs with discordant BP (one sibling with high BP and one with low BP) as well as concordant pairs (both with high BP or both with low BP) led to the identification of several regions on chromosomes 3, 11, 15, 16, and 17 that could be linked to BP regulation. Further studies suggested that chromosome 15 is the most likely region to harbor a HTN gene (189).
Genetic studies have identified 20 genes associated with changes in BP in humans. Also, gene-targeting experiments in mice have identified over 30 genes, for which inactivating or activating mutations trigger chronic changes in BP. Most of these gene mutations involve an ion channel, an ion transporter, or a regulatory enzyme that could affect the renal function (84, 110) (Fig. 2). Some of these channels, transporters, and enzymes are expressed in various blood vessels (9, 44, 73, 183, 194), and mutations in their genes in the renal vasculature could significantly affect renal vascular function. Examples of channels, transporters, and enzymes involved in BP regulation include epithelial Na channel (ENaC), RAS, Na+/H+ exchanger (NHE), and Na+/Ca2+ exchanger (NCX).
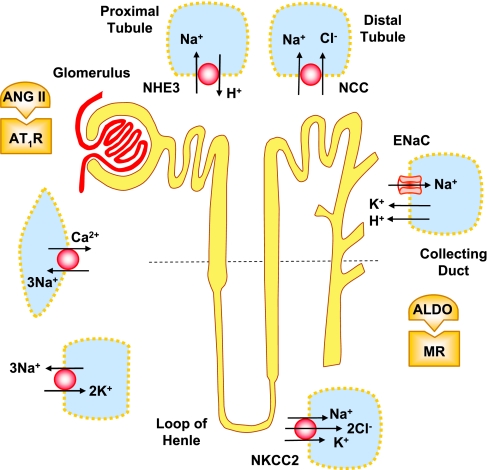
Fig. 2.
Genetic factors in renal and vascular dysfunction and HTN. Genetic mutation in the Na+/H+ exchanger (NHE), Na+-Cl− cotransporter (NCC), ENaC, Na+-K+-2Cl− cotransporter (NKCC2), and Na+-K+ pump in the nephron could lead to sodium and water retention. Changes in Aldo synthesis and mineralocorticoid receptor (MR), as well as ANG II and ANG II type 1 receptor (AT1R), contribute to sodium absorption. Mutations in the genes expressing ENaC, NHE, and Na+/K+/2Cl− and Na+/Ca2+ exchanger (NCX) in VSM could promote renal vasoconstriction and exacerbate HTN.
ENaC.
The amiloride-sensitive ENaC is responsible for sodium absorption in the cortical collecting tubules. Mutations in the genes encoding β- and γ-subunits of ENaC result in increased channel activity and sodium absorption. Given the impact of ENaC activity on H+ and K+ secretory mechanisms in the principal cells of the cortical collecting tubules, mutations in ENaC result in hypokalemic alkalosis, low renin SS-HTN, and low-plasma Aldo, all characteristics of Liddle's syndrome (110). Studies suggest the presence of β- and γ-ENaC in mouse renal interlobar VSM, where they may play a role in the renal vascular mechanosensitivity and the myogenic response to intravascular pressure (73). The regulation of renal vascular ENaC during high dietary sodium intake and in hypertensive compared with normotensive individuals is an important area for investigation.
RAS.
RAS plays a role in the regulation of sodium balance and BP, and ANG II acts via ANG II type 1 receptor (AT1R) to induce renal vasoconstriction, Aldo secretion, and sodium reabsorption. Molecular variants of individual components of RAS may contribute to genetic predisposition to HTN. Linkage studies have suggested a relation between the chromosomal region harboring the AT1R gene and HTN (80). At least 25 polymorphisms in AT1R gene have been described, and the A1166C polymorphism (adenine/cytosine substitution at position 1166) is associated with severe HTN (10). A link between functional gene copies of AT1R and BP is also observed in mice, and the expression of AT1R gene in the aorta, brain, and kidney is regulated by salt intake (110). Studies have suggested a role of AT2R gene (+1675 G/A) on left ventricular structure in humans (145). Also, the higher BP in male compared with female SHR has been related to decreased expression of ANG II type 2 receptor (AT2R) in the aorta and mesenteric microvessels of males (155). Whether changes in the relative expression of AT1R and AT2R also occur in the renal vasculature of SHR remains to be examined. With regard to ACE, linkage studies between I/D polymorphism of ACE gene (insertion/deletion of a 287 bp in intron 16) and HTN gave mixed results, but an association with salt sensitivity has been suggested (1, 49, 144). The relation between ACE and BP was also tested in mice having one, two, or three functional copies of ACE gene at its chromosomal location. Although serum ACE activity increased progressively from the one-copy to three-copy mice, BP did not differ, leading to the suggestion that I/D ACE polymorphism may affect BP only during salt loading (91). Studies in mice strains with functional copies of the angiotensinogen gene suggest an association between plasma angiotensinogen levels and BP. Also, the M235T polymorphism (methionine/threonine substitution at codon 235) in angiotensinogen gene has been associated with HTN; however, its association with salt sensitivity yielded mixed results (61, 74, 148). Additional information on the expression of ANG II receptors and other components of RAS in the kidney and renal vasculature and their role in HTN are described below under mediators of renal endothelial dysfunction.
NHE, Na+-Cl− and Na+-K+-2Cl− cotransporter.
The proximal tubules account for 60% of sodium reabsorption. NHE3 mediates a considerable fraction of proximal tubular sodium uptake and may play a role in HTN. NHE activity is increased in erythrocytes and lymphocytes of patients with essential HTN (110). Also, apical membrane vesicles from kidney of young prehypertensive SHR show increased sodium uptake via NHE (2). SS Dahl/Rapp rats have reduced ability to decrease their renal NHE3 exchange rate in response to high-salt diet compared with salt-resistant rats (95). Also, transgenic mice overexpressing the NHE in the renal tubules become hypertensive during salt loading (13). Gene mutations in the Na+-Cl− cotransporter in the distal tubules and Na+-K+-2Cl− cotransporter in the Loop of Henle could also affect sodium and water absorption and BP (Fig. 2). The NHE and Na+-K+-2Cl− have been identified in vascular tissues and could affect the intracellular pH and renal vascular function. This is supported by the report that inhibition of the Na+-K+-2Cl− cotransporter affects the myogenic and ANG II responses in the rat afferent arteriole (183).
NCX.
In VSM, NCX promotes Ca2+ extrusion and lowers intracellular Ca2+ concentration ([Ca2+]i) (9). NCX activity is greater in afferent than efferent arterioles (8). Protein kinase C (PKC) upregulates NCX in afferent and efferent arterioles and mesangial cells in Dahl salt-resistant, but not Dahl SS, rats (8). In SHR, Dahl SS, and Dahl/Rapp SS rats, NCX activity is suppressed mainly in afferent arterioles, leading to accumulation of intracellular Ca2+ and vasoconstriction (8).
The identification of these gene mutations supports the concept of genetic predisposition to HTN. Also, most single-gene disorders with an effect on BP involve a defect in renal sodium reabsorption, emphasizing the role of the kidney. The identification of some of these channels and transporters in the renal vasculature raises the possibility that their genetic mutation could affect renal vascular function. However, studies of the human genome did not find an association between a particular locus or single-gene defect and the large variation in BP seen in HTN, suggesting multiple loci, each of which can contribute to HTN.
Role of Dietary Salt Intake, Vascular Inflammation, and Oxidative Stress
In genetically predisposed individuals, other factors, such as dietary salt intake, vascular inflammation, and oxidative stress, may promote renal vascular dysfunction and HTN (Fig. 1).
Dietary salt intake.
SS-HTN is defined as an increase in BP to an increase in dietary salt intake. In humans with SS-HTN and in rat models of SS-HTN, a high-salt diet is associated with renal and vascular inflammation, oxidative stress, increased reactive oxygen species (ROS), and endothelial cell dysfunction that eventually lead to renal vasoconstriction and increased BP (Fig. 1). SS-HTN involves a genetic defect, combined with subtle acquired renal injury. Genetic abnormalities in the renal transport mechanisms in proximal tubules and Loop of Henle cause increased NaCl delivery to the macula densa, leading to suppression of renin release, and thereby impair the kidney's ability to suppress renin release during high sodium intake and cause SS-HTN (55). Renal abnormalities that cause loss of functional kidney mass and inability to modulate RAS could also lead to SS-HTN. For example, renal vascular dysfunction, arteriolopathy, and tubulo-interstitial injury could lead to renal hypoxia-ischemia, inflammation, and loss of functional nephrons (78). With loss of functional nephrons, surviving nephrons attempt to maintain balance and excrete greater amounts of sodium and water by increasing GFR and decreasing tubular sodium reabsorption, resulting in increased NaCl concentration in distal tubules and macula densa, and leading to suppression of renin release and impaired kidney's ability to decrease renin secretion during high sodium intake. Long term, the loss of functional nephrons and persistent compensatory vascular and renal changes eventually damage surviving nephrons, leading to glomerulosclerosis, decreased glomerular capillary filtration coefficient, and SS-HTN (12).
Vascular inflammation.
There is a potential link between vascular inflammation and HTN. Cross-sectional studies in hypertensive individuals have shown increased plasma and vascular tissue levels of C-reactive protein, cytokines such as TNF-α and IL-6, chemokines such as monocyte chemoattractant protein-1 and plasminogen activator inhibitor-1, and adhesion molecules such as P-selectin and soluble ICAM-1 (20). However, whether inflammation causes structural or functional changes in the renal vasculature and lead to HTN, or is just a consequence of HTN, is unclear. Studies with ANG II have supported a relationship between vascular inflammation, renal vascular dysfunction, and HTN. ANG II stimulates TNF-α production by human monocytes and the adherence of monocytes to endothelium (54). Also, ANG II infusion for 3 days in rats is associated with increased TNF-α production by renal glomerular endothelial cells and tubular and vascular cells (136). ANG II also stimulates renal and vascular IL-6 production and activates transcription factor NF-κB which further increases the production of cytokines, chemokines, and adhesion molecules (136). Interestingly, the HTN associated with ANG II infusion is attenuated in IL-6 knockout mice (94). The role of IL-6 in HTN partly involves increased AT1R density in proximal tubules, leading to increased sodium and water reabsorption and ANG II-dependent HTN (94). IL-6 may also upregulate AT1R and enhance the effects of ANG II on vascular inflammation, oxidative stress, and vasoconstriction (184). ANG II increases ROS, which further promotes the inflammatory process. The role of ROS in ANG II responses is supported by the observation that ANG II-induced cytokine production is inhibited by free-radical scavengers (90). In HTN, the mechanical stress on arterial wall and the release of proinflammatory humoral stimuli, such as ANG II and ET-1, are thought to induce oxidative stress, which could cause vascular inflammation (50, 172). The inflammatory factors stimulate ECM deposition and promote hypertrophy/hyperplasia of VSM cells (174). Inflammation, in turn, increases oxidative stress, leading to vicious cycle, chronic vascular inflammation, and atherosclerosis. Collectively, in HTN, inflammation is associated with endothelial dysfunction and vascular remodeling, either directly or indirectly through oxidative stress (24).
Oxidative stress.
Oxidative stress is an important factor in the regulation of renal vascular function and BP. Oxidative stress is an imbalance between ROS production and the ability of biological system to scavenge or metabolize ROS. Most animal models of HTN have oxidative stress, and antioxidants decrease BP in hypertensive animals. Among ROS, superoxide (O2•−) has high reactivity and ability to scavenge NO. Also, H2O2 reacts with metals to form highly reactive hydroxyl radical (OH−). OH− and O2•− react with NO to form peroxynitrite (ONOO−). O2•− oxidizes the endothelial NO synthase (eNOS) cofactor tetrahydrobiopterin to dihydrobiopterin, causing uncoupling of eNOS and more O2•− production. NADPH oxidase produces O2•− in the renal cortex. In the medulla, NADH oxidase, NADPH oxidase, and mitochondrial respiratory enzymes produce O2•− (196). NADPH oxidase is also widely expressed in the renal vasculature.
ROS can cause renal vasoconstriction by decreasing NO bioavailability (146). O2•− may also mediate the acute effects of ANG II, endothelin (ET)-1, and catecholamines on renal vasoconstriction by a mechanism independent of NO (79). ROS also exert vasoconstriction in the renal microcirculation indirectly by stimulating the release of F2 isoprostane and adenosine (23). In some vascular beds, ROS may promote vasoconstriction directly by increasing VSM [Ca2+]i (101), or indirectly by inhibiting the production of other vasodilators, such as PGI2 (197), and the role of these mechanisms in the renal microcirculation remains to be defined.
In the renal cortex, basal O2•− production plays a role in TGF. Increased NaCl delivery and flow-associated mechanical factors induce NADPH oxidase in macula densa to produce O2•−. O2•− enhances TGF directly by constricting afferent arterioles, indirectly by scavenging NO in the macula densa, which tonically inhibits TGF (98), and by increasing the production and vasoconstrictor action of adenosine (23) (Fig. 3). Under basal conditions, the renal medulla produces more O2•− than the cortex, owing to its lower Po2, and O2•− may participate in the regulation of renal medullary blood flow (196). Oxidative stress and O2•− levels are also affected by vascular mediators, such as ANG II and ET. ANG II induces oxidative stress and upregulation of NADPH oxidase and increases ROS formation in mitochondria, and some of these effects are mediated by Aldo (48, 137). ANG II-induced renal interstitial influx of phagocytes and cytokines also increases O2•− production. O2•−, in turn stimulates NF-κB, which activates cytokine-encoding genes and proinflammatory response in the kidney, leading to a vicious cycle. Thus ROS modulate the renal hemodynamics, both directly, by causing vasoconstriction and sodium reabsorption and indirectly, by inducing renal inflammation.
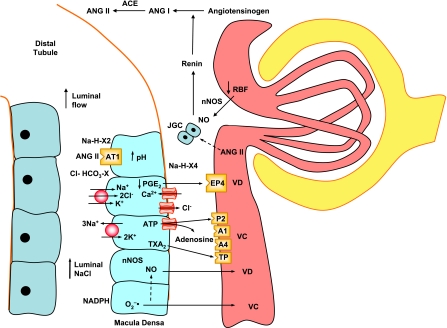
Fig. 3.
TGF and autoregulation of RBF and renin release. A decrease in RBF is associated with increased neuronal NO synthase (nNOS) activity, NO-mediated renin release by JGC, and formation of ANG II. ANG II inhibits further renin release. Paracrine signaling by macula densa also controls RVR and renin release. Increased luminal NaCl alters macula densa cell Na+ concentration, pH, volume, basolateral membrane potential, and intracellular Ca2+ concentration ([Ca2+]i). Luminal ANG II via AT1R and consequent changes in Na/H/X2 exchanger and Cl/HCO3−/X at the apical membrane and Na/H/X4 at the basolateral membrane affect macula densa Na+ transport, cell alkalinization, and volume. NaCl entry via apical NKCC2 and Cl exit through basolateral channel lead to cell depolarization and increased [Ca2+]i. Moderate increases in luminal NaCl prompt macula densa cells to release the VCs TxA2 and ATP and to decrease vasodilation by prostaglandin E2 (PGE2). ATP diffuses through ATP-permeable maxi-anion channels and stimulates P2 receptors and vasoconstriction. ATP is also dissociated to adenosine, which binds to A1 and A4 receptors, causing further afferent arteriolar constriction and decreased renin release from JGCs. Macula densa intracellular Na+ is also regulated by apical Na+-K+-ATPase. Normally, large increases in luminal NaCl stimulate the release of NO, which prevents excessive TGF-mediated vasoconstriction. An increase in oxidative stress and superoxide (O2•−) decreases NO bioactivity and antagonizes its inhibitory effect on TGF. ACE, angiotensin-converting enzyme.
Urinary excretion of oxidative stress biomarkers, such as 8-iso-PGF2α and thiobarbituric acid reactive substances, is increased in HTN (151). During the development of HTN in SHR, increased ROS inactivates NO and leads to diminished buffering by NO in JGC and enhanced TGF and preglomerular vasoconstriction (33, 185). An increase in AT1R-mediated formation of O2•− appears to diminish NO signaling in JGCs of SHR (185). In the renal medulla of SHR, ANG II-induced vasoconstriction is reversed by intramedullary infusion of the NO synthase (NOS) substrate l-arginine, suggesting that there is less NO to buffer the actions of ANG II, possibly due to increased O2•− and NO scavenging (35). Also, in SHR, treatment with the O2•− dismutase mimetic tempol decreases RVR and BP, largely by increasing NO bioavailability in the renal vasculature and JGCs (146).
O2•− also plays a role in SS-HTN. In Dahl-SS rats, high-salt diet is associated with rapid decline in medullary blood flow and increased BP, which can be prevented by infusing l-arginine in the renal medulla, suggesting reduced capacity to generate NO (114). In Dahl SS-HTN, there is decreased GFR and RBF, increased O2•− production, BP, renal tubulointerstitial damage, and glomerular necrosis, which are reversed by antioxidants (172). Also, O2•− is elevated in sympathetic neurons of DOCA-salt hypertensive rats via activation of NADPH oxidase (28). Increased salt intake is also associated with enhanced renal expression/activity of NADPH oxidase, generation of O2•−, and diminished expression of SOD (86).
The increased oxidative stress, combined with the aforementioned genetic predisposition, dietary salt intake, and vascular inflammation adversely affect the renal vascular endothelial cell function, VSM, and ECM and lead to significant elevation of RVR, BP, and HTN (Fig. 1).
Renal Endothelial Cell Dysfunction in HTN
There is a debate whether endothelial cell dysfunction is a cause or a consequence of HTN. The debate toward the consequence side stems from the observations that endothelial dysfunction is found in all forms of HTN, increases with increases in BP, and is partly reversed by antihypertensive therapy. Other studies have demonstrated that adult SHR have both endothelial dysfunction and overt HTN, while young SHR have endothelial dysfunction but normal BP, suggesting that endothelial dysfunction may be a cause rather than a consequence of HTN (124). Endothelial cell dysfunction involves an imbalance in the release of vasodilatory mediators, such as NO, PGI2, and endothelium-derived hyperpolarizing factor (EDHF), and vasoconstrictive mediators, such as ET, ANG II, and thromboxane A2 (TxA2) (Fig. 4).
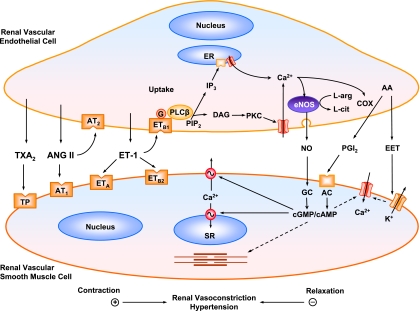
Fig. 4.
Endothelium-derived renal VDs and VCs in HTN. In renal vascular endothelial cells, Ca2+ release from the endoplasmic reticulum (ER) increases endothelial NO synthase (eNOS) activity and NO production. NO diffuses into renal VSM cells, activates guanylate cyclase (GC), and increases cGMP. cGMP causes VSM relaxation by inhibiting Ca2+ influx and stimulating Ca2+ extrusion mechanisms. Activation of cyclooxygenases (COX) increases PGI2 production, which activates adenylate cyclase (AC) and increases cAMP in VSM. cAMP causes VSM relaxation by mechanisms similar to those of cGMP. Renal endothelial release of EDHF, such as certain EETs, activates K+ channels and causes hyperpolarization of VSM and inhibition of Ca2+ influx through Ca2+ channels. The endothelium also releases ET-1, ANG II, and TxA2, which act on specific receptors in VSM to cause renal vasoconstriction. Activation of endothelial ETB1R and AT2R is coupled to increased release of VDs and renal VSM relaxation. A decrease in endothelium-derived VDs and an increase in endothelium-derived VCs are associated with renal vasoconstriction, increased RVR, and HTN. IP3, inositol trisphosphate; PIP2, phosphatidylinositol 4,5-bisphosphate; DAG, diacyglycerol; TP, thromboxane-prostanoid; SR, sarcoplasmic reticulum.
NO.
NO is a major vasodilator and regulator of the renal hemodynamics and BP (7). NO is produced by NOS using l-arginine as substrate. Three NOS isoforms, NOS1 [neuronal NOS (nNOS)], NOS2 [inducible NOS (iNOS)], and NOS3 (eNOS), are widely distributed in the kidney (7). Constitutive NOS3 (eNOS) in microvascular endothelium and NOS1 (nNOS) in the macula densa produce a large portion of the basal NO release required to maintain RBF and GFR (185). However, the cellular source of NO may not be restricted to the endothelium and macula densa. As NOS isoforms are constitutively expressed by many cell types of the nephron, it is possible that NO produced by neighboring epithelial cells, such as proximal tubules or renal connecting tubules, could diffuse to microvascular smooth muscle cells and, consequently, influence vascular tone. NOS inhibition in rats by intravenous infusion of Nω-nitro-l-arginine methyl ester (l-NAME) at a dose that does not affect systemic BP, increases RVR and decreases RBF, GFR, diuresis, and natriuresis, suggesting that NO exerts a greater tonic vasodilator effect in the renal vasculature than in other systemic vascular beds (93, 154). NO controls sodium excretion via tubular action, regulation of medullary vascular tone, and pressure-natriuresis. NO also causes vasodilation of afferent and efferent arterioles (32); blunting of TGF-mediated constriction of afferent arterioles in response to high NaCl delivery at macula densa (188); counterbalance of renal vasoconstriction caused by ANG II, ET-1, and TxA2 (Fig. 5); and inhibition of sodium absorption in proximal and distal nephron (29, 163).
Fig. 5.
Regulation of RBF by VCs and VDs. Decreased RBF is associated with increased renin and ANG II release. The vasoconstrictive effects of ANG II and other VCs are normally counterbalanced by VDs, such as NO and PGI2, leading to maintained RBF and GFR. When the release of VD mediators is compromised, ANG II acts unopposed to induce renal vasoconstriction, leading to decreased RBF and GFR, which, in turn, promote renin release, further activate renin-angiotensin system, and lead to progression of HTN.
Impaired endothelial NO production is observed in offsprings of essential hypertensive patients (165). Also, normally, high dietary salt intake is associated with increased NO production to increase salt excretion and maintain normal BP (153). This may explain the observation that, in normotensive rats, high salt diet blunts TGF, and NOS blockade is associated with enhanced TGF. This response to NOS blockade during HS intake is lost in SS-Dahl/Rapp rats, supporting reduced NO production in SS-HTN (188). The renal myogenic autoregulatory mechanism is also impaired in Dahl-SS rats during high-salt diet (81). Also, afferent arteriolar vasodilatation by NO is impaired in Dahl-SS rats due to increased free radical (123). Studies in dogs have also shown that NOS inhibition by l-NAME increases O2•− in the renal vasculature, indicating a protective role of NO against the effects of O2•− (105). Endothelial cell dysfunction may also involve increased arginase activity, which converts l-arginine to l-ornithine and urea, thus decreasing the substrate for NO production (76). H2O2 upregulates arginase activity and thereby may impair renal vasodilatation (170).
PGI2.
Eicosanoids may play a role in the regulation of renal vascular function and BP. Eicosanoids are synthesized from arachidonic acid in a compartmentalized fashion, such that afferent arteriolar endothelial cells produce mainly PGI2 by cyclooxgenase (COX) (161), glomeruli mainly PGE2 and TxA2, and interstitial cells and collecting ducts mainly PGE2 (115). PGI2 diffuses through the intima, increases cAMP in VSM, and promotes vascular relaxation. Increased renal perfusion pressure induces endothelial cells to release PGI2 (71). On the other hand, increased medullary BP or flow induces the renal medullary interstitium and collecting ducts to release PGE2. Young humans with a family history of HTN have defective vasodilator prostaglandin system and thereby increased renal vasoconstriction sensitivity to ANG II (124).
EDHF.
The endothelium may influence the renal vascular tone by releasing EDHF. EDHF hyperpolarizes endothelial cells by activating Ca2+-activated K+ channels. Hyperpolarization is transferred to VSM cells via myoendothelial coupling and gap junctions (connexins), or the efflux of K+ ions from hyperpolarized endothelial cells may accumulate in the extracellular space and act on the inward rectifier potassium channel and Na+-K+ pump to cause VSM hyperpolarization. The nature and mechanism of action of EDHF vary in different tissues and species. EDHF include K+ ion, epoxyeicosatrienoic acids (EETs), H2O2, and C-type natriuretic peptide (38). EDHF significantly affects RBF in human interlobar arteries (14). Also, in the rat renal microcirculation, even during inhibition of NOS and COX, there is residual vasodilatory response to acetylcholine, indicating the presence of EDHF (30). Administration of 40Gap 27, an inhibitory peptide homologous to the second extracellular loop of connexin-40, abolishes the residual acetylcholine vasodilatation, supporting a role for gap junctions in EDHF-mediated renal vasodilation. Also, administration of the 40Gap 27 inhibitory peptide, even in the absence of l-NAME and indomethacin, is associated with decreased RBF and increased BP, suggesting that tonic EDHF may maintain basal renal vascular tone and RBF (30). Cx40-deficient mice are hypertensive, suggesting that impaired EDHF-mediated renal vasodilatation may play a role in HTN (31). Also, while EDHF-mediated renal vascular relaxation is normal in young SHR, it is reduced in aged SHR and associated with depolarized resting membrane potential of the renal artery VSM (15), suggesting that decreased EDHF may be involved in the progression of HTN.
EETs and hydroxyeicosatetraenoic acids.
Epoxy and 20-hydroxy derivatives of arachidonic acid oxidation by cytochrome P-450 monooxygenase may also play a role in the regulation of renal vascular function, BP, and the pathogenesis of HTN. EETs are produced in the endothelium, proximal tubules, and collecting ducts, and some of the EETs are potent vasodilators and natriuretic compounds. Increasing the renal levels of EETs by inhibiting soluble epoxide-hydrolase reduces BP in SHR (193). Also, high-salt diet induces the expression of renal epoxygenases in Dahl salt resistant, but not hypertensive Dahl-SS rats. Furthermore, in mice, CYP4A10 deletion causes a decrease in EET urinary excretion and promotes SS-HTN (64). These studies have raised interest in cytochrome P-450-derived EETs as EDHFs in the kidney and as potential targets for antihypertensive therapies (64). It is important to note that the vascular effects of EETs may vary. For example, 11,12-EET is a stereoselective renal vasodilator, and 11,12(R,S)-EET, but not 11,12(S,R)-EET, increases the diameters of the interlobular and afferent arterioles. Also, the response to 11,12-EET is not affected by removal of the endothelium or by inhibition of COX with indomethacin. In contrast, 5,6-EET elicits vasoconstriction in the interlobular and afferent arterioles, and the vasoconstrictor response is abolished by removal of the endothelium or COX inhibition. These findings indicate that the preglomerular vasodilation to 11,12-EET is stereoselective and is the result of direct action of the epoxide on the preglomerular VSM, whereas the vasoconstriction to 5,6-EET is COX dependent and requires an intact endothelium (65).
20-Hydroxyeicosatetraenoic acid (HETE) may play a role in renal myogenic autoregulation, TGF control of afferent arterioles, and vasoconstrictor actions of ANG II and ET-1 (3, 104). In addition to its potent renal vasoconstrictor effects, 20-HETE is also natriuretic and, therefore, has counteracting effects in the control of BP. The natriuretic action involves inhibition of Na+-K+-ATPase in the proximal tubules and Na+-K+-2Cl− cotransporter in thick ascending limb (60). The vasoconstrictor action involves blockade of Ca2+-activated K+ channels in VSM. Renal expression of CYP4A2, which produces 20-HETE, is increased in SHR, and CYP4A2 inhibitors attenuate HTN in SHR and DOCA SS-HTN (150). Also, in mice, deletion of CYP4A14 increases expression of CYP4A2, resulting in increased 20-HETE and HTN (64).
Hydroxy derivatives of arachidonic acid 12- and 15-HETE are produced by 12/15-lipooxygenase in the glomeruli, mesangial cells, and renal microvessel endothelial and VSM cells (195). 12- and 15-HETE constrict renal vessels and glomerular mesangial cells. 12-HETE induces VSM growth and partly mediates ANG II-induced afferent arteriolar vasoconstriction, as well as TGF-β and ANG II-induced mesangial cell hypertrophy and ECM accumulation (192). 12/15-Lipooxygenase inhibition or gene deletion blunts the pressor response to ANG II and blocks ANG II-induced hypertrophy and ECM accumulation in rat mesangial cells (85). Also, 12/15-lipooxygenase deficiency is associated with increased eNOS expression/activity, suggesting a relation between the two pathways in the control of vascular function (5). Urinary excretion of 12-HETE is increased in essential HTN. Also, 12-HETE production is increased in SHR, and 12-lipooxygenase inhibitors decrease BP in these rats (140).
Kinins and kallikriens.
Kinins (bradykinin, lysyl-bradykinin) have been implicated in the regulation of RBF, renal function, and BP (132). The vasodilator, diuretic, and natriuretic effects of kinins may be mediated by the release of NO, PGI2, and EDHF. RAS tonically stimulates renal kinin production via a non-AT1R pathway, possibly involving endothelial AT2R (156). Kallikrein mRNA and protein are also expressed in vascular tissues. Renal kallikrein excretion is increased in humans and rats with mineralocorticoid/salt-dependent HTN, but is decreased in essential HTN (18).
ET.
Renal endothelial dysfunction may involve an increase in vasoconstrictive mediators, such as ET-1. ET is a potent vasoconstrictor peptide produced by the renal endothelial, mesangial, and tubular epithelial cells (88). Compared with other organs, the kidney expresses low levels of ET isopeptides (43). ET-1 is produced in the afferent and efferent arterioles, while ET-3 is produced in the proximal convoluted tubule, part of the distal tubule, and the cortical and outer medullary collecting duct. The inner medullary collecting duct produces both ET-1 and ET-3 (175). ET production is triggered by physical stimuli, such as shear stress, hypoxia, and osmolarity, by hormones such as insulin, adrenaline, arginine-vasopressin (AVP), and ANG II, and by peptides such as cytotoxin, thrombin, endotoxin, and TGF-β (107). However, the plasma level of ET is very low, and ET acts mainly locally in an autocrine or paracrine fashion.
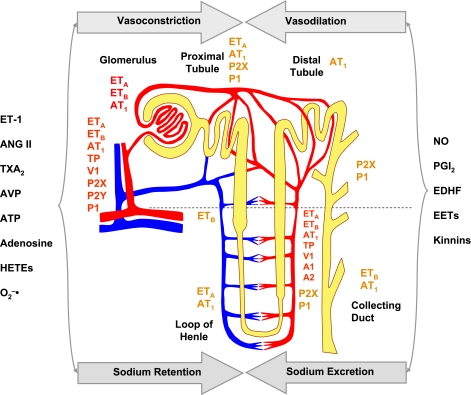
ET acts via two main receptor subtypes, ETAR and ETBR. Both ETAR and ETBR are expressed in the afferent and efferent arterioles and the glomerulus (Fig. 6). Activation of ETAR and ETB2R in VSM induces vasoconstriction, while activation of ETB1R in endothelial cells induces the release of NO and PGI2 and vasodilation. ETAR mediates vasoconstrictor actions of ET-1 in both afferent and efferent arteriole. ETB2R mediates vasoconstriction in afferent arteriole, whereas ETB1R mediates vasodilatation in efferent arteriole (67). ETAR is more predominant in renal VSM, proximal convoluted tubule, and renal medullary interstitial cells. ETBR are present in proximal straight tubule, collecting duct, and upper thick ascending limb (167). ETAR recognizes ET-1 and ET-2, whereas ETBR recognizes ET-1, -2, and -3 (62).
Fig. 6.
Differential distribution of receptors and mediators in the renal vasculature and nephron. Renal vascular receptors (shown in red) are activated by VCs to regulate RBF in afferent and efferent arterioles and vasa recta. VDs prevent excessive renal vasoconstriction. Decreased VDs and increased VCs lead to renal vasoconstriction and HTN. Receptors in tubules, loop of Henle, and collecting ducts (shown in brown) are modulated by substances that promote sodium retention or excretion and thereby maintain water and electrolyte balance, plasma volume, and BP. Increased sodium and water retention lead to increased plasma volume and HTN. AVP, arginine-vasopressin.
At low doses, ET-1 and ET-3 may activate mainly ETBR and stimulate the release of NO and PGI2 and thereby increase RBF and sodium and water excretion. In contrast, high doses of ET-1 and ET-3 activate mainly ETAR and promote renal vasoconstriction and sodium and water absorption (83, 162). ET-1 induces vasoconstriction of afferent and, to a lesser extent, efferent arterioles, increases RVR, and decreases RBF (83). ET-1 also causes contraction of mesangial cells and decreases glomerular filtration surface area and GFR (6). ET may also mediate some of the renal actions of ANG II (130). ET-1 via ETAR and ETBR may also regulate eNOS and nNOS isoforms in the renal inner medulla (164).
ET-1 production is increased in the endothelium and the kidney of rat models of SS-HTN. ET-1 increases oxidative stress, vascular inflammatory response, and vascular remodeling. In rats, CYP450 hydroxylase and COX arachidonic acid metabolites may contribute to ET-1-induced afferent arteriolar vasoconstriction and VSM [Ca2+]i (66). Also, chronic ET-1 infusion in rats is associated with vasoconstriction of afferent and efferent arterioles and increased urinary excretion of the oxidative stress biomarkers 8-iso-PGF2α and thiobarbituric acid reactive substances, and these effects are attenuated by the O2•− dismutase mimetic tempol. ET stimulates O2•− formation in rat VSM, possibly via PKC-mediated increase in NADPH oxidase activity. The increased ET production in SS-HTN is also associated with increased oxidative stress (151). Also, chronic ET-1 infusion in rats on a high-salt diet is associated with enhanced vascular reactivity and Ca2+ influx into VSM (160). Interestingly, the renal vascular reactivity to ET-1 is greater in SHR than Wistar-Kyoto rats (173), but is blunted in DOCA SS hypertensive rats (143). Also, while the plasma levels of ET-1 may be low in SHR, neutralization of ET-1 by specific antibodies or ETAR blockade decreases BP and RVR, increases renal plasma flow and GFR, and improves the renal hemodynamics and sodium excretion (82, 122, 173). ETAR antagonists also reduce BP and vascular hypertrophic remodeling in ET-dependent rat models of SS-HTN (143).
ANG II.
ANG II is a major mediator of renal vascular dysfunction and vasoconstriction. ANG I and ANG II levels are higher in the kidney than the plasma, suggesting that the intrarenal ANG II levels are not due to simple equilibration with circulating ANG II. Studies on rat have demonstrated that the concentrations of total immunoreactive angiotensin (reflecting ANG II) in glomerular filtrate and early, mid-, and late proximal tubule fluid averaged 29–40 nM, compared with 32 pM in systemic plasma. Also, angiotensin concentrations in the plasma of star vessel (end of efferent arteriole) exceeded systemic levels by a factor of 1,000. These observations have suggested that substantial amounts of angiotensin peptides are released into or generated within intrarenal fluid compartments, that the components necessary for ANG II formation are present in these sites, and that local ANG II is likely to affect the regulation of renal function independently of systemic ANG II (118, 152).
ANG II stimulates both AT1R and AT2R. AT1R is more widely expressed in the kidney than AT2R, and AT1R density is greater in the medulla than the cortex. The high ANG II levels and AT1R density in the medulla support a major role in regulating hemodynamic and tubular function. AT1R are also localized on VSM cells of the renal vasculature, including afferent and efferent arterioles (Fig. 6). ANG II contributes to regulation of salt and water balance directly by activating the NHE3 transporter and stimulating sodium reabsorption in the proximal tubules and indirectly by stimulating Aldo secretion in the adrenals (97, 182). Also, renin secretion in JGCs is controlled by negative feedback loop involving ANG II and intrarenal pressure. Communication between endothelial cells and renin secreting JGCs through connexin 40-dependent gap junctions may mediate the Ca2+-dependent inhibitory effects of ANG II and intrarenal pressure on renin synthesis (181).
Some of the components of the RAS are upregulated in certain forms of HTN. The intrarenal angiotensinogen mRNA is increased in Dahl SS rats on a high-salt diet (87). Increased systemic and renal vascular sensitivity to ANG II is observed in normotensive men with a family history of HTN (187), and in SHR even in the developmental phase of HTN (21, 180). Also, sympathetic nerves enhance the renovascular responses to ANG II in SHR (34). The ability of PGI2 to attenuate ANG II-induced vasoconstriction is also reduced in the kidney of SHR (72). Interestingly, low-dose infusion of ANG II for 6–10 days in rabbits, rats, or mice causes increases in RVR, BP, oxidative stress, urinary 8-iso-PGF2α, ONOO− generation, and nitrotyrosine deposition. ANG II induces ET-1 production, which increases O2•− and decreases NO bioavailability. ANG II also promotes the renal production of TxA2, which, in turn, causes renal vasoconstriction (177). The effects of ANG II on multiple systems and vascular mediators may explain the elevated BP in essential and renovascular HTN where the ANG II levels may be normal (133).
The net effects of ANG II on the vascular and renal mechanisms and BP may also depend on the duration of increased ANG II activity. Short-term infusion of ANG II in rats causes an elevation in BP that returns to normal on cessation of ANG II. In contrast, prolonged ANG II infusion for 6–10 days causes long-lasting elevation in BP, afferent and efferent arteriolar constriction, and reduced GFR that persists after cessation of ANG II infusion (46). Long-term ANG II may cause injury to the renal microvessels and peritubular capillary and tubulointerstitium, leading to structural changes, such as afferent arteriolar wall thickening and loss of peritubular capillaries, as well as functional changes, such as decreased NO and increased ET-1. The ANG II-induced tubulointerstitial injury also promotes the release of proinflammatory chemokines and leukocyte adhesion proteins and infiltration of mononuclear cells, which express ANG II at the site of injury and thereby further augment intrarenal ANG II (77). The increased ANG II leads to further renal vasoconstriction, microvascular wall thickening, decreased glomerular filtration, sodium retention, and SS-HTN (45).
TxA2 and PGE2.
TxA2 is another prostanoid produced by COX and an important mediator of the renal hemodynamic and pressor effects of ANG II. TxA2 is a potent vasoconstrictor in the renal vessels and afferent arterioles, acting via thromboxane-prostanoid (TP) receptors to increase renal VSM [Ca2+]i (Fig. 7). TxA2 may also cause eNOS activation and NO production in endothelial cells by increasing [Ca2+]i due to activation of endothelial TP receptor or through gap junctions from VSM (147). In isolated, microperfused rabbit afferent arterioles, TP receptor activation produces ROS, which promotes vasoconstriction, but also produces NO, which counteracts vasoconstriction (147). An increase in endogenous levels of TxA2 may contribute to the enhanced TGF activity in young SHR, leading to a rightward shift in the pressure-natriuresis curve and HTN (11). TP receptors may also mediate the effects of the F2 isoprostane 8-iso-PGF2α on preglomerular vasoconstriction. The renal vascular reactivity to TxA2 is increased in SHR, possibly due to increased affinity/number of renal TP receptors (21).
Fig. 7.
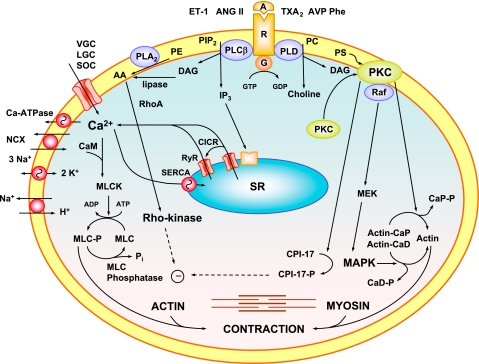
Mechanisms of renal VSM contraction in HTN. Increased VC agonists, such as ET-1, ANG II, or phenylephrine (Phe), activate their receptor (R), stimulate PLC-β, and increase IP3 and DAG. IP3 stimulates Ca2+ release from the SR. Ca2+ stimulates ryanodine-sensitive receptors (RyR) in SR and further releases intracellular Ca2+ [Ca2+-induced Ca2+ release (CICR)]. Agonists also stimulate Ca2+ influx through voltage-gated (VGC), ligand-gated (LGC), and store-operated channels (SOC). Ca2+ binds calmodulin (CAM), activates myosin light chain (MLC) kinase (MLCK), causes MLC phosphorylation, and initiates renal VSM contraction. Ca2+ mobilization mechanisms are normally counterbalanced by Ca2+ and Na+ extrusion via plasmalemmal Ca2+-ATPase, Na+-K+-ATPase, and NCX and NHE to maintain [Ca2+]i and intracellular pH. DAG activates PKC. PKC induces phosphorylation (P) of CPI-17, which inhibits MLC phosphatase and enhances myofilament Ca2+ sensitivity. PKC-induced phosphorylation of calponin (Cap) allows more actin to bind myosin. PKC also activates protein kinase cascades involving Raf, MAPK kinase (MEK), and MAPK, leading to phosphorylation of the actin-binding protein caldesmon (CaD). Also, RhoA/Rho kinase inhibits MLC phosphatase and further enhances the Ca2+ sensitivity of the contractile proteins. Significant increases in renal VSM [Ca2+]i and the Ca2+-sensitization pathways of VSM contraction cause renal vasoconstriction, increased RVR, and HTN. AA, arachidonic acid; G, GTP-binding protein; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PS, phosphatidylserine.
PGE2 elicits both vasodilatory and vasoconstrictor actions in afferent arterioles. In rats, vasodilation is mediated by EP4 receptors and Gαs protein, increased cAMP, and decreased VSM [Ca2+]i (126). The vasoconstriction of afferent arterioles and interlobular arteries is mediated by EP3 receptors and Gαi (166, 178). In mice, EP2 receptors partly mediate PGE2-induced afferent arteriolar vasodilation and buffer the vasoconstriction mediated by EP1 and EP3 receptors and ET-1 (63). ANG II via AT1R also tonically stimulates renal PGE2 production (156). Spontaneous increase in tone above initial tension is observed in renal arteries of SHR, likely due to reduced NO production and large-conductance, calcium- and voltage-activated potassium channel activity, leaving the actions of endogenous COX products that activate TxA2/TP and PGE2/EP1 receptors unopposed (111).
ATP and adenosine.
ATP and adenosine play a role in the regulation of renal function through their action on the renal microcirculation and tubular function (69). Purinoceptors P1 and P2 are widely distributed in the kidney (52, 68) (Fig. 6). P1 receptor has more affinity to adenosine, whereas P2 receptor is more toward ATP. ATP released from the macula densa in response to increased NaCl delivery is broken down to AMP in the extracellular space, then acted upon by ecto-5′-nucleotidases to form adenosine, a mediator of TGF (89, 129) (Fig. 3). O2•− may also increase the production and renal vasoconstrictor actions of adenosine (23).
Both P2X and P2Y receptors increase VSM [Ca2+]i; P2X induces Ca2+ influx, while P2Y stimulates intracellular Ca2+ mobilization (42). The renal vascular responsiveness to ATP is increased in SHR, possibly due to nonspecific functional changes in the renal vasculature, rather than specific alterations in the renal purinoceptors (42).
It is important to note that the vasoconstrictor stimuli vary along the renal arterial arcade. ANG II mainly constricts small resistance renal arterioles, while TxA2 and AVP act on the interlobar and arcuate arteries, causing variable degrees of renal vasoconstriction. Adenosine affects TGF, while ATP acts on P2X receptors in the renal vascular, glomerular, mesangial, and tubular epithelial cells to regulate the renal microvascular function, RBF, and autoregulation, as well as the renal hemodynamics and tubular function (68, 69, 171, 186).
Also, although the medullary microcirculation receives only ∼10% of RBF, it plays a critical role in long-term regulation of BP. Pressure diuretic-natriuretic responses are associated with changes in the medullary blood flow and the renal interstitial hydrostatic pressure. Also, pericytes are contractile smooth muscle-like cells that surround descending vasa recta and thereby affect medullary blood flow. Known vasoconstrictors of descending vasa recta include ANG II, ET-1, norepinephrine, TxA2, AVP, and adenosine. Vasodilators include NO, PGE2, acetylcholine, and bradykinin (Fig. 6). The vasa recta blood flow is subjected to tonic vasoconstrictor influence of ANG II and vasodilator influence of prostaglandins (26). NO produced from eNOS regulates baseline blood flow in the mouse renal medulla, and NO produced by nNOS is an important buffer of the constrictor actions of ANG II (109). The specific role of NO in the medulla is supported by the observation that acute and chronic NOS inhibition in the renal medulla decreases medullary blood flow and reduces sodium and water excretion in the absence of changes in cortical blood flow (108).
Renal VSM Dysfunction in HTN
Renal VSM dysfunction involves an increase in basal tone and VSM reactivity to vasoconstrictors and increased signaling pathways of VSM contraction. In VSM, agonists, such as ANG II, ET-1, and norepinephrine, act via G protein-coupled receptors to activate phospholipase C, which stimulates the hydrolysis of phosphatidyinositol bisphosphate into inositol trisphosphate (IP3) and diacyglycerol (DAG) (Fig. 7). IP3 stimulates Ca2+ release from the sarcoplasmic reticulum, and DAG activates PKC. Vasoconstrictor agonists also increase Ca2+ influx from the extracellular space (116). Ca2+ binds calmodulin to form a complex that activates myosin light chain kinase, causes myosin phosphorylation, and initiates actin-myosin interaction and VSM contraction. PKC, Rho-kinase, and mitogen-activated protein kinase (MAPK) contribute to VSM contraction by increasing the [Ca2+]i sensitivity of contractile proteins (59, 138).
[Ca2+]i.
VSM [Ca2+]i is a major determinant of the myogenic mechanism of renal vascular autoregulation and agonist-induced vasoconstriction (116, 131). The renal microcirculation has heterogeneous distribution of Ca2+ channels and differential Ca2+ influx in various vascular segments. The renal cortical preglomerular afferent arteriole, juxtamedullary efferent arteriole, and outer medullary vasa recta have L- and T-type voltage-gated Ca2+ channels (VGCCs), whereas the mid-to-outer cortical efferent arteriole does not (57). ANG II-induced vasoconstriction of afferent arteriole involves depolarization and VGCCs, whereas, in efferent arteriole, receptor-operated and store-operated Ca2+ channels are involved (102). The vasoconstriction mechanisms in the renal medulla are similar to the preglomerular vasculature and involve depolarization and VGCCs (57).
In the afferent arteriole, ANG II, ET-1, or norepinephrine causes an increase in [Ca2+]i that is augmented by activation of ryanodine receptor and Ca2+-induced Ca2+ release in the sarcoplasmic reticulum. Cyclic adenosine diphosphate ribose (ADPR), produced from β-nicotinamide adenine dinucleotide by membrane-bound ADPR cyclase, sensitizes ryanodine receptor to further amplify Ca2+-induced Ca2+ release and augment [Ca2+]i and renal vasoconstriction (96, 168). ET-1 increases [Ca2+]i by stimulating the release of intracellular Ca2+ and Ca2+ influx from the extracellular medium (149). In the afferent arteriole, ET-1 also increases the formation of O2•−, which activates ADPR cyclase and accounts for 60% of subsequent Ca2+ signaling (39). Also, in the afferent arteriole, epidermal growth factor receptor (EGFR) tyrosine kinase may contribute to ANG II-induced [Ca2+]i by promoting Ca2+ influx (22).
Renal vascular reactivity to AVP and ANG II is increased in SHR (56). AVP-induced vascular reactivity and RVR are attributed to increased density of V1 receptor in the renal interlobular arteries and augmented VSM [Ca2+]i due to Ca2+ mobilization from the intracellular stores and Ca2+ influx through plasma membrane (37). In SHR, the enhanced vascular reactivity to ANG II is due to increased number/affinity of AT1R and may also involve increased basal [Ca2+]i, PKC activity and sensitivity of myosin light chain to Ca2+, and reduced cAMP generation due to ANG II-induced increase in formation of vasoconstrictive prostaglandins from arachidonic acid (134, 142, 180). Ca2+ signaling and store-operated Ca2+ entry are exaggerated in preglomerular VSM of young SHR (40). Also, diminished NCX and Ca2+ sequestering/extrusion mechanisms via sarcoplasmic reticulum and plasmalemmal Ca2+-ATPase could contribute to increased [Ca2+]i in afferent arteriolar VSM of SHR (8, 120).
PKC.
Phospholipase C isoforms-β, -γ, and -δ are upregulated in systemic and renal vessels of SHR, supporting their role in enhancing vascular reactivity in genetic HTN (125). Activation of phospholipase C increases DAG production, which activates PKC. Classical α-, βI-, βII-, and γ-PKC isoforms are activated by DAG, phorbol esters, and Ca2+. Novel δ-, ɛ-, η-, θ-, and μ-PKCs are activated by DAG/phorbol esters, but are Ca2+ independent. Atypical ζ- and λ/ι-PKCs are independent of DAG or phorbol esters, but activated by other lipid mediators (138). The role of Ca2+ release, Ca2+ influx, and PKC varies between agonists, with ANG II depends on the three factors, TxA2 independent of Ca2+ and AVP has intermediate dependence (19).
PKC plays a role in the regulation of renal vascular function and tubular transport systems. In rabbit, both the afferent and efferent arterioles express NCX, which is activated by PKC (44, 113). PKC is also involved in the regulation of Na+-K+-ATPase by ANG II, dopamine, ET, and norepinephrine. PKC-mediated activation of Na+-K+-ATPase in basolateral membrane of the renal tubular cells drives sodium reabsorption throughout the nephron and thereby affects extracellular fluid volume and BP (41).
Studies in systemic vessels have shown that α-PKC enhances Ca2+-dependent VSM contraction and is overexpressed in HTN (138). Ca2+-independent ɛ-PKC may increase the myofilament sensitivity to [Ca2+]i in VSM. δ-PKC is mainly associated with the cytoskeleton and may play a role in vascular remodeling. ζ-PKC is localized in the nucleus and may promote VSM growth and vascular hypertrophic changes associated with HTN (138). Studies in cannulated rat femoral arterial branches have shown that high pressure induces O2•− production via PKC-dependent activation of NADPH oxidase (176). It has also been shown that the increased NADPH oxidase-mediated O2•− production in renovascular HTN involves PKC (58). Further studies are needed to investigate whether increased PKC expression/activity in VSM enhances renal vasoconstriction and trophic vascular changes and leads to increased RVR and HTN.
RhoA/Rho kinase.
RhoA/Rho kinase signaling plays a role in maintaining VSM contraction in response to stretch and receptor-mediated activation. NO causes vasodilation partly through inhibition of Rho kinase, and maintenance of renal basal vascular tone depends on a balance between NO production and Rho/Rho kinase signaling. This is supported by the observation that RhoA/Rho kinase signaling is amplified in eNOS knockout mouse (141).
Rho kinase is involved in the renal basal vascular tone, the myogenic contraction of preglomerular and afferent arterioles, and the vasoconstriction to ANG II, TxA2, and AVP (117). Also, the effect of ROS on vascular tone and VSM contraction may be mediated by Rho/Rho kinase. Increased NADPH-dependent ROS and reduced NO bioavailability activate Rho kinase, which, in turn, inhibits myosin light chain phosphatase, leading to augmented myosin light chain phosphorylation and VSM contraction (75).
While the expression of Rho guanine nucleotide exchange factor, a positive regulator of Rho kinase, is similar in Wistar-Kyoto and young 4-wk-old SHR, it is increased in SHR with established HTN, suggesting that increased Rho/Rho kinase signaling is likely a consequence of HTN (191). On the other hand, RhoA/Rho kinase may participate in the pathogenesis of nephrosclerosis in SHR, partly by upregulation of gene expressions of oxidative stress, ECM, adhesion molecules, and antifibrinolysis (121).
MAPK.
MAPK is one of several kinases that plays a role in the transduction of extracellular mitogenic signals to the nucleus. MAPK is a Ser/Thr protein kinase that is fully activated by dual phosphorylation at Thr and Tyr residues and in turn activates nuclear transcription factors and VSM growth and proliferation. In growth-arrested human VSM cells, MAPK is mainly cytosolic, but translocates into the nucleus during activation by mitogens (112). In cultured VSM cells, ANG II-induced activation of MAPK and mitogenic effects requires Ca2+-dependent transactivation of EGFR, because it provides docking sites for the upstream tyrosine kinase c-Src and the downstream adaptors Shc and Grb2 at the plasma membrane, which are essential for MAPK activation (36). MAPK, in turn, phosphorylates and activates several kinases and transcription factors and induces the nuclear protooncogene c-fos (25). Tyrosine phosphorylation events involving EGFR tyrosine kinase and rapid c-Src activation also contribute to the renal microvascular and afferent arteriolar vasoconstriction and increased [Ca2+]i and Ca2+ influx in response to ANG II (22). ET-1-induced activation of PKC and MAPK also plays a role in VSM contraction and growth (17). Also, in differentiated VSM cells, MAPK causes phosphorylation of the actin-binding protein caldesmon, thus allowing more actin to interact with myosin, and thereby enhances VSM contraction (138) (Fig. 7).
MAPK-mediated VSM growth may underlie the vascular hypertrophic changes in HTN. MAPK activity is enhanced in the vasculature of SHR (92). Also, the renal VSM expression/activity of EGFR is increased in Dahl-SS rats before the onset and in established HTN. EGFR mediates the fibrogenic and vasoconstricting actions of ANG II and ET-1, and inhibition of EGFR attenuates the HTN caused by ANG II and the renal vascular fibrosis in l-NAME-induced HTN (47, 190).
ECM and Renal Vascular Remodeling in HTN
The renal vasculature undergoes significant remodeling, fibrotic changes, and structural alterations in HTN (157). Renal vascular remodeling is associated with VSM growth and proliferation and increased deposition of ECM proteins, especially collagen types I, III, and IV in resistance arteries, glomerulus, and interstitium. NO inhibits collagen I gene activation, and chronic NOS inhibition is associated with activated collagen I gene and accumulation of ECM in the renal vasculature. Also, ANG II causes collagen gene I activation and fibrogenic action in the renal vasculature, independent of hemodynamics, and likely mediated by ET-1, MAPK, and TGF-β (169). The fibrogenic action of ET-1 involves EGFR-mediated activation of MAPK p42/44 and collagen I gene (47). ANG II-induced ET-1 expression in adventitial fibroblasts is partly mediated by NADPH oxidase, and ET-1 in turn stimulates collagen formation, implicating oxidative stress in vascular remodeling (4).
During vascular remodeling, matrix metalloproteinases (MMPs) degrade ECM proteins and adhesion molecules, thus enabling VSM cells to migrate and proliferate, and inflammatory cells to infiltrate the vessel wall (179). Endogenous tissue inhibitors of MMPs provide a balancing mechanism and prevent excessive ECM degradation (99). Several MMPs are released in the vasculature in response to inflammatory cytokines and transcription factors and contribute to vascular remodeling by promoting ECM degradation. Additional effects of MMPs on the endothelium and VSM have also been described (127). The role of MMPs in the regulation of the renal vasculature and kidney function needs to be thoroughly examined.
In SHR, all renal arteries except preglomerular arterioles exhibit increased media thickness and VSM cell number and volume. Prehypertensive SHR also exhibit increased cross-sectional arterial media (159). In the vasculature of adult SHR, a decrease in MMP-1, -2, and -3 may contribute to remodeling of resistance arteries and the setting of HTN (70). Also, the expression of integrins is abnormal in blood vessels of SHR. Integrins act as physical “joints” between ECM and cytoskeletal components and as signal-transducing receptors. Changes in the adhesion receptor integrins lead to abnormal interaction between VSM cells and ECM proteins and changes in the vessel structure. Also, the interaction of arginine-glycine-aspartate (RGD) containing peptides and fibronectin with α5β1-integrin promotes VSM growth and is enhanced in SHR vessels. Osteopontin, a glycoprotein that interacts with integrin and increases VSM cell proliferation, is also overexpressed in HTN (127).
Perspectives and Significance
Research into the renal vascular mechanisms of HTN demonstrates complex interaction between multiple genetic, environmental, and dietary factors and numerous cellular mediators of renal vascular dysfunction. Gene mutations/polymorphism in ion channel, transporter, or enzyme, combined with an adverse environmental or dietary factor, could lead to renal vascular dysfunction, renal tissue injury, and HTN. Multiple gene mutations are associated with HTN, and advanced genetic engineering should help to delineate more specific gene targets. Gene therapy designed to target the specific gene defect may provide new antihypertensive treatment. Successful management of HTN may also require rigorous control of environmental risk factors, particularly dietary salt intake and the associated increases in oxidative stress and vascular inflammation. Other factors related to age, ethnic background, sex, body weight, and comorbidities, such as diabetes, should also be considered in the design of antihypertensive therapy. Reduction of oxidative stress using antioxidants and management of vascular inflammation using cytokine antagonists may be useful. ACE inhibitors remain a major antihypertensive therapy to reduce ANG II production, salt and water retention, and ANG II-dependent renal vasoconstriction and HTN. ANG II, ET-1, TxA2 receptor blockers, Ca2+ channel blockers, and inhibitors of PKC, Rho kinase, or MAPK could reduce VSM mechanisms of renal vasoconstriction. Modulators of MMPs and tissue inhibitors of MMP activity could reduce ECM accumulation and renal vascular remodeling. A combination of these treatment strategies should be most effective in HTN.
GRANTS
This work was supported by grants from the National Heart, Lung, and Blood Institute (HL-65998 and HL-70659).
REFERENCES
- 1.Agarwal A, Williams GH, Fisher ND. Genetics of human hypertension. Trends Endocrinol Metab 16: 127–133, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Aldred KL, Harris PJ, Eitle E. Increased proximal tubule NHE-3 and H+-ATPase activities in spontaneously hypertensive rats. J Hypertens 18: 623–628, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Alonso-Galicia M, Maier KG, Greene AS, Cowley AW Jr, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol 283: R60–R68, 2002. [DOI] [PubMed] [Google Scholar]
- 4.An SJ, Boyd R, Zhu M, Chapman A, Pimentel DR, Wang HD. NADPH oxidase mediates angiotensin II-induced endothelin-1 expression in vascular adventitial fibroblasts. Cardiovasc Res 75:702–709, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Anning PB, Coles B, Bermudez-Fajardo A, Martin PE, Levison BS, Hazen SL, Funk CD, Kühn H, O'Donnell VB. Elevated endothelial nitric oxide bioactivity and resistance to angiotensin-dependent hypertension in 12/15-lipoxygenase knockout mice. Am J Pathol 166: 653–662, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badr KF, Murray JJ, Breyer MD, Takahashi K, Inagami T, Harris RC. Mesangial cell, glomerular and renal vascular responses to endothelin in the rat kidney. Elucidation of signal transduction pathways. J Clin Invest 83: 336–342, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baylis C, Qiu C. Importance of nitric oxide in the control of renal hemodynamics. Kidney Int 49: 1727–1731, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Bell PD, Mashburn N, Unlap MT. Renal sodium/calcium exchange; a vasodilator that is defective in salt-sensitive hypertension. Acta Physiol Scand 168: 209–214, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev 79: 763–854, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Bonnardeaux A, Davies E, Jeunemaitre X, Féry I, Charru A, Clauser E, Tiret L, Cambien F, Corvol P, Soubrier F. Angiotensin II type 1 receptor gene polymorphisms in human essential hypertension. Hypertension 24: 63–69, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Brännström K, Arendshorst WJ. Thromboxane A2 contributes to the enhanced tubuloglomerular feedback activity in young SHR. Am J Physiol Renal Physiol 276: F758–F766, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Brenner BM Nephron adaptation to renal injury or ablation. Am J Physiol Renal Fluid Electrolyte Physiol 249: F324–F337, 1985. [DOI] [PubMed] [Google Scholar]
- 13.Brooks HL, Sorensen AM, Terris J, Schultheis PJ, Lorenz JN, Shull GE, Knepper MA. Profiling of renal tubule Na+ transporter abundances in NHE3 and NCC null mice using targeted proteomics. J Physiol 530: 359–366, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Büssemaker E, Popp R, Binder J, Busse R, Fleming I. Characterization of the endothelium-derived hyperpolarizing factor (EDHF) response in the human interlobar artery. Kidney Int 63: 1749–1755, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Büssemaker E, Popp R, Fisslthaler B, Larson CM, Fleming I, Busse R, Brandes RP. Aged spontaneously hypertensive rats exhibit a selective loss of EDHF-mediated relaxation in the renal artery. Hypertension 42: 562–568, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Cain AE, Khalil RA. Pathophysiology of essential hypertension: role of the pump, the vessel, and the kidney. Semin Nephrol 22: 3–16, 2002. [PubMed] [Google Scholar]
- 17.Cain AE, Tanner DM, Khalil RA. Endothelin-1-induced enhancement of coronary smooth muscle contraction via MAPK-dependent and MAPK-independent [Ca2+]i sensitization pathways. Hypertension 39: 543–549, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Carretero OA Vascular remodeling and the kallikrein-kinin system. J Clin Invest 115: 588–591, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavarape A, Bauer J, Bartoli E, Endlich K, Parekh N. Effects of angiotensin II, arginine vasopressin and tromboxane A2 in renal vascular bed: role of rho-kinase. Nephrol Dial Transplant 18: 1764–1769, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension 38: 399–403, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Chatziantoniou C, Daniels FH, Arendshorst WJ. Exaggerated renal vascular reactivity to angiotensin and thromboxane in young genetically hypertensive rats. Am J Physiol Renal Fluid Electrolyte Physiol 259: F372–F382, 1990. [DOI] [PubMed] [Google Scholar]
- 22.Che Q, Carmines PK. Src family kinase involvement in rat preglomerular microvascular contractile and [Ca2+]i responses to ANG II. Am J Physiol Renal Physiol 288: F658–F664, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen YF, Li PL, Zou AP. Oxidative stress enhances the production and actions of adenosine in the kidney. Am J Physiol Regul Integr Comp Physiol 281: R1808–R1816, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Clapp BR, Hingorani AD, Kharbanda RK, Mohamed-Ali V, Stephens JW, Vallance P, MacAllister RJ. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res 64: 172–178, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Cobb MH, Goldsmith EJ. How MAP kinases are regulated. J Biol Chem 270: 14843–14846, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Cupples WA, Sakai T, Marsh DJ. Angiotensin II and prostaglandins in control of vasa recta blood flow. Am J Physiol Renal Fluid Electrolyte Physiol 254: F417–F424, 1988. [DOI] [PubMed] [Google Scholar]
- 27.Curtis JJ, Luke RG, Dustan HP, Kashgarian M, Whelchel JD, Jones P, Diethelm AG. Remission of essential hypertension after renal transplantation. N Engl J Med 309: 1009–1015, 1983. [DOI] [PubMed] [Google Scholar]
- 28.Dai X, Cao X, Kreulen DL. Superoxide anion is elevated in sympathetic neurons in DOCA-salt hypertension via activation of NADPH oxidase. Am J Physiol Heart Circ Physiol 290: H1019–H1026, 2006. [DOI] [PubMed] [Google Scholar]
- 29.De Nicola L, Blantz RC, Gabbai FB. Nitric oxide and angiotensin II. Glomerular and tubular interaction in the rat. J Clin Invest 89: 1248–1256, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Vriese AS, Van de Voorde J, Lameire NH. Effects of connexin-mimetic peptides on nitric oxide synthase- and cyclooxygenase-independent renal vasodilation. Kidney Int 61: 177–185, 2002. [DOI] [PubMed] [Google Scholar]
- 31.de Wit C, Roos F, Bolz SS, Kirchhoff S, Krüger O, Willecke K, Pohl U. Impaired conduction of vasodilation along arterioles in connexin 40-deficient mice. Circ Res 86: 649–655, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Deng A, Baylis C. Locally produced EDRF controls preglomerular resistance and ultrafiltration coefficient. Am J Physiol Renal Fluid Electrolyte Physiol 264: F212–F215, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Dilley JR, Arendshorst WJ. Enhanced tubuloglomerular feedback activity in rats developing spontaneous hypertension. Am J Physiol Renal Fluid Electrolyte Physiol 247: F672–F679, 1984. [DOI] [PubMed] [Google Scholar]
- 34.Dubinion JH, Mi Z, Jackson EK. Role of renal sympathetic nerves in regulating renovascular responses to angiotensin II in spontaneously hypertensive rats. J Pharmacol Exp Ther 317: 1330–1336, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Dukacz SA, Feng MG, Yang LF, Lee RM, Kline RL. Abnormal renal medullary response to angiotensin II in SHR is corrected by long-term enalapril treatment. Am J Physiol Regul Integr Comp Physiol 280: R1076–R1084, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Eguchi S, Numaguchi K, Iwasaki H, Matsumoto T, Yamakawa T, Utsunomiya H, Motley ED, Kawakatsu H, Owada KM, Hirata Y, Marumo F, Inagami T. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J Biol Chem 273: 8890–8896, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Fallet RW, Ikenaga H, Bast JP, Carmines PK. Relative contributions of Ca2+ mobilization and influx in renal arteriolar contractile responses to arginine vasopressin. Am J Physiol Renal Physiol 288: F545–F551, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Félétou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol 26: 1215–1225, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Fellner SK, Arendshorst W. Endothelin-A and -B receptors, superoxide, and Ca2+ signaling in afferent arterioles. Am J Physiol Renal Physiol 292: F175–F184, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Fellner SK, Arendshorst WJ. Store-operated Ca2+ entry is exaggerated in fresh preglomerular vascular smooth muscle cells of SHR. Kidney Int 61: 2132–2141, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Féraille E, Doucet A. Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control. Physiol Rev 81: 345–418, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Fernández O, Wangensteen R, Osuna A, Vargas F. Renal vascular reactivity to P2-purinoceptor activation in spontaneously hypertensive rats. Pharmacology 60: 47–50, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Firth JD, Ratcliffe PJ. Organ distribution of the three rat endothelin messenger RNAs and the effects of ischemia on renal gene expression. J Clin Invest 90: 1023–1031, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fowler BC, Carmines PK, Nelson LD, Bell PD. Characterization of sodium-calcium exchange in rabbit renal arterioles. Kidney Int 50: 1856–1862, 1996. [DOI] [PubMed] [Google Scholar]
- 45.Franco M, Martínez F, Quiroz Y, Galicia O, Bautista R, Johnson RJ, Rodríguez-Iturbe B. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 293: R251–R256, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Franco M, Tapia E, Santamaría J, Zafra I, García-Torres R, Gordon KL, Pons H, Rodríguez-Iturbe B, Johnson RJ, Herrera-Acosta J. Renal cortical vasoconstriction contributes to development of salt-sensitive hypertension after angiotensin II exposure. J Am Soc Nephrol 12: 2263–2271, 2001. [DOI] [PubMed] [Google Scholar]
- 47.François H, Placier S, Flamant M, Tharaux PL, Chansel D, Dussaule JC, Chatziantoniou C. Prevention of renal vascular and glomerular fibrosis by epidermal growth factor receptor inhibition. FASEB J 18: 926–928, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal 8: 1597–1607, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Giner V, Poch E, Bragulat E, Oriola J, González D, Coca A, De La Sierra A. Renin-angiotensin system genetic polymorphisms and salt sensitivity in essential hypertension. Hypertension 35: 512–517, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Griendling KK, Sorescu D, Lassègue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol 20: 2175–2183, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Grisk O, Rettig R. Renal transplantation studies in genetic hypertension. News Physiol Sci 16: 262–265, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Guan Z, Osmond DA, Inscho EW. P2X receptors as regulators of the renal microvasculature. Trends Pharmacol Sci 28: 646–652, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Guyton AC Blood pressure control–special role of the kidneys and body fluids. Science 252: 1813–1816, 1991. [DOI] [PubMed] [Google Scholar]
- 54.Hahn AW, Jonas U, Bühler FR, Resink TJ. Activation of human peripheral monocytes by angiotensin II. FEBS Lett 347: 178–180, 1994. [DOI] [PubMed] [Google Scholar]
- 55.Hall JE, Guyton AC, Brands MW. Pressure-volume regulation in hypertension. Kidney Int Suppl 55: S35–S41, 1996. [PubMed] [Google Scholar]
- 56.Hansen FH, Vågnes ØB, Iversen BM. Enhanced response to AVP in the interlobular artery from the spontaneously hypertensive rat. Am J Physiol Renal Physiol 288: F1023–F1031, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Hansen PB, Jensen BL, Andreasen D, Skøtt O. Differential expression of T- and L-type voltage-dependent calcium channels in renal resistance vessels. Circ Res 89: 630–638, 2001. [DOI] [PubMed] [Google Scholar]
- 58.Heitzer T, Wenzel U, Hink U, Krollner D, Skatchkov M, Stahl RA, MacHarzina R, Bräsen JH, Meinertz T, Münzel T. Increased NAD(P)H oxidase-mediated superoxide production in renovascular hypertension: evidence for an involvement of protein kinase C. Kidney Int 55: 252–260, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Hilgers RH, Webb RC. Molecular aspects of arterial smooth muscle contraction: focus on Rho. Exp Biol Med (Maywood) 230: 829–835, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Hoagland KM, Flasch AK, Roman RJ. Inhibitors of 20-HETE formation promote salt-sensitive hypertension in rats. Hypertension 42: 669–673, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Hunt SC, Geleijnse JM, Wu LL, Witteman JC, Williams RR, Grobbee DE. Enhanced blood pressure response to mild sodium reduction in subjects with the 235T variant of the angiotensinogen gene. Am J Hypertens 12: 460–466, 1999. [DOI] [PubMed] [Google Scholar]
- 62.Hynynen MM, Khalil RA. The vascular endothelin system in hypertension–recent patents and discoveries. Recent Pat Cardiovasc Drug Discov 1: 95–108, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Imig JD, Breyer MD, Breyer RM. Contribution of prostaglandin EP2 receptors to renal microvascular reactivity in mice. Am J Physiol Renal Physiol 283: F415–F422, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Imig JD Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol 289: F496–F503, 2005. [DOI] [PubMed] [Google Scholar]
- 65.Imig JD, Navar LG, Roman RJ, Reddy KK, Falck JR. Actions of epoxygenase metabolites on the preglomerular vasculature. J Am Soc Nephrol 7: 2364–2370, 1996. [DOI] [PubMed] [Google Scholar]
- 66.Imig JD, Pham BT, LeBlanc EA, Reddy KM, Falck JR, Inscho EW. Cytochrome P450 and cyclooxygenase metabolites contribute to the endothelin-1 afferent arteriolar vasoconstrictor and calcium responses. Hypertension 35: 307–312, 2000. [DOI] [PubMed] [Google Scholar]
- 67.Inscho EW, Imig JD, Cook AK, Pollock DM. ETA and ETB receptors differentially modulate afferent and efferent arteriolar responses to endothelin. Br J Pharmacol 146: 1019–1026, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Inscho EW, Schroeder AC, Deichmann PC, Imig JD. ATP-mediated Ca2+ signaling in preglomerular smooth muscle cells. Am J Physiol Renal Physiol 276: F450–F456, 1999. [DOI] [PubMed] [Google Scholar]
- 69.Inscho EW Modulation of renal microvascular function by adenosine. Am J Physiol Regul Integr Comp Physiol 285: R23–R25, 2003. [DOI] [PubMed] [Google Scholar]
- 70.Intengan HD, Schiffrin EL. Structure and mechanical properties of resistance arteries in hypertension: role of adhesion molecules and extracellular matrix determinants. Hypertension 36: 312–318, 2000. [DOI] [PubMed] [Google Scholar]
- 71.Jackson EK, Gerkens JF, Brash AR, Branch RA. Acute renal artery constriction increases renal prostaglandin I2 biosynthesis and renin release in the conscious dog. J Pharmacol Exp Ther 222: 410–413, 1982. [PubMed] [Google Scholar]
- 72.Jackson EK, Herzer WA. Angiotensin II/prostaglandin I2 interactions in spontaneously hypertensive rats. Hypertension 22: 688–698, 1993. [DOI] [PubMed] [Google Scholar]
- 73.Jernigan NL, Drummond HA. Myogenic vasoconstriction in mouse renal interlobar arteries: role of endogenous beta and gamma ENaC. Am J Physiol Renal Physiol 291: F1184–F1191, 2006. [DOI] [PubMed] [Google Scholar]
- 74.Jeunemaitre X, Soubrier F, Kotelevtsev YV, Lifton RP, Williams CS, Charru A, Hunt SC, Hopkins PN, Williams RR, Lalouel JM, Corvol P. Molecular basis of human hypertension: role of angiotensinogen. Cell 71: 169–180, 1992. [DOI] [PubMed] [Google Scholar]
- 75.Jin L, Ying Z, Hilgers RH, Yin J, Zhao X, Imig JD, Webb RC. Increased RhoA/Rho-kinase signaling mediates spontaneous tone in aorta from angiotensin II-induced hypertensive rats. J Pharmacol Exp Ther 318: 288–295, 2006. [DOI] [PubMed] [Google Scholar]
- 76.Johnson FK, Johnson RA, Peyton KJ, Durante W. Arginase inhibition restores arteriolar endothelial function in Dahl rats with salt-induced hypertension. Am J Physiol Regul Integr Comp Physiol 288: R1057–R1062, 2005. [DOI] [PubMed] [Google Scholar]
- 77.Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J, Schwartz SM. Renal injury from angiotensin II-mediated hypertension. Hypertension 19: 464–474, 1992. [DOI] [PubMed] [Google Scholar]
- 78.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med 346: 913–923, 2002. [DOI] [PubMed] [Google Scholar]
- 79.Just A, Olson AJ, Whitten CL, Arendshorst WJ. Superoxide mediates acute renal vasoconstriction produced by angiotensin II and catecholamines by a mechanism independent of nitric oxide. Am J Physiol Heart Circ Physiol 292: H83–H92, 2007. [DOI] [PubMed] [Google Scholar]
- 80.Kainulainen K, Perola M, Terwilliger J, Kaprio J, Koskenvuo M, Syvänen AC, Vartiainen E, Peltonen L, Kontula K. Evidence for involvement of the type 1 angiotensin II receptor locus in essential hypertension. Hypertension 33: 844–849, 1999. [DOI] [PubMed] [Google Scholar]
- 81.Karlsen FM, Leyssac PP, Holstein-Rathlou NH. Tubuloglomerular feedback in Dahl rats. Am J Physiol Regul Integr Comp Physiol 274: R1561–R1569, 1998. [DOI] [PubMed] [Google Scholar]
- 82.Kato T, Kassab S, Wilkins FC Jr, Kirchner KA, Keiser J, Granger JP. Endothelin antagonists improve renal function in spontaneously hypertensive rats. Hypertension 25: 883–887, 1995. [DOI] [PubMed] [Google Scholar]
- 83.Katoh T, Chang H, Uchida S, Okuda T, Kurokawa K. Direct effects of endothelin in the rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 258: F397–F402, 1990. [DOI] [PubMed] [Google Scholar]
- 84.Khalil RA Dietary salt and hypertension: new molecular targets add more spice. Am J Physiol Regul Integr Comp Physiol 290: R509–R513, 2006. [DOI] [PubMed] [Google Scholar]
- 85.Kim YS, Xu ZG, Reddy MA, Li SL, Lanting L, Sharma K, Adler SG, Natarajan R. Novel interactions between TGF-β1 actions and the 12/15-lipoxygenase pathway in mesangial cells. J Am Soc Nephrol 16: 352–362, 2005. [DOI] [PubMed] [Google Scholar]
- 86.Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol 14: 2775–2782, 2003. [DOI] [PubMed] [Google Scholar]
- 87.Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension 41: 592–597, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kohan DE Endothelin synthesis by rabbit renal tubule cells. Am J Physiol Renal Fluid Electrolyte Physiol 261: F221–F226, 1991. [DOI] [PubMed] [Google Scholar]
- 89.Komlosi P, Fintha A, Bell PD. Current mechanisms of macula densa cell signalling. Acta Physiol Scand 181: 463–469, 2004. [DOI] [PubMed] [Google Scholar]
- 90.Kranzhöfer R, Schmidt J, Pfeiffer CA, Hagl S, Libby P, Kübler W. Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 19: 1623–1629, 1999. [DOI] [PubMed] [Google Scholar]
- 91.Krege JH, Kim HS, Moyer JS, Jennette JC, Peng L, Hiller SK, Smithies O. Angiotensin-converting enzyme gene mutations, blood pressures, and cardiovascular homeostasis. Hypertension 29: 150–157, 1997. [DOI] [PubMed] [Google Scholar]
- 92.Kubo T, Ibusuki T, Saito E, Kambe T, Hagiwara Y. Vascular mitogen-activated protein kinase activity is enhanced via angiotensin system in spontaneously hypertensive rats. Eur J Pharmacol 372: 279–285, 1999. [DOI] [PubMed] [Google Scholar]
- 93.Lahera V, Salom MG, Miranda F, Moncada S, Romero JC. Effects of N-nitro-l-arginine methyl ester on renal function and blood pressure. Am J Physiol Renal Fluid Electrolyte Physiol 261: F1033–F1037, 1991. [DOI] [PubMed] [Google Scholar]
- 94.Lee DL, Sturgis LC, Labazi H, Osborne JB Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol 290: H935–H940, 2006. [DOI] [PubMed] [Google Scholar]
- 95.Lewis JL, Warnock DG. Renal apical membrane sodium-hydrogen exchange in genetic salt-sensitive hypertension. Hypertension 24: 491–498, 1994. [DOI] [PubMed] [Google Scholar]
- 96.Li N, Teggatz EG, Li PL, Allaire R, Zou AP. Formation and actions of cyclic ADP-ribose in renal microvessels. Microvasc Res 60: 149–159, 2000. [DOI] [PubMed] [Google Scholar]
- 97.Liu FY, Cogan MG. Angiotensin II stimulation of hydrogen ion secretion in the rat early proximal tubule. Modes of action, mechanism, and kinetics. J Clin Invest 82: 601–607, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu R, Ren Y, Garvin JL, Carretero OA. Superoxide enhances tubuloglomerular feedback by constricting the afferent arteriole. Kidney Int 66: 268–274, 2004. [DOI] [PubMed] [Google Scholar]
- 99.Liu YE, Wang M, Greene J, Su J, Ullrich S, Li H, Sheng S, Alexander P, Sang QA, Shi YE. Preparation and characterization of recombinant tissue inhibitor of metalloproteinase 4 (TIMP-4). J Biol Chem 272: 20479–20483, 1997. [DOI] [PubMed] [Google Scholar]
- 100.Lohmeier TE, Hildebrandt DA, Warren S, May PJ, Cunningham JT. Recent insights into the interactions between the baroreflex and the kidneys in hypertension. Am J Physiol Regul Integr Comp Physiol 288: R828–R836, 2005. [DOI] [PubMed] [Google Scholar]
- 101.Lounsbury KM, Hu Q, Ziegelstein RC. Calcium signaling and oxidant stress in the vasculature. Free Radic Biol Med 28: 1362–1369, 2000. [DOI] [PubMed] [Google Scholar]
- 102.Loutzenhiser K, Loutzenhiser R. Angiotensin II-induced Ca2+ influx in renal afferent and efferent arterioles: differing roles of voltage-gated and store-operated Ca2+ entry. Circ Res 87: 551–557, 2000. [DOI] [PubMed] [Google Scholar]
- 103.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol 290: R1153–R1167, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ma YH, Gebremedhin D, Schwartzman ML, Falck JR, Clark JE, Masters BS, Harder DR, Roman RJ. 20-Hydroxyeicosatetraenoic acid is an endogenous vasoconstrictor of canine renal arcuate arteries. Circ Res 72: 126–136, 1993. [DOI] [PubMed] [Google Scholar]
- 105.Majid DS, Nishiyama A, Jackson KE, Castillo A. Inhibition of nitric oxide synthase enhances superoxide activity in canine kidney. Am J Physiol Regul Integr Comp Physiol 287: R27–R32, 2004. [DOI] [PubMed] [Google Scholar]
- 106.Malpas SC, Ramchandra R, Guild SJ, McBryde F, Barrett CJ. Renal sympathetic nerve activity in the development of hypertension. Curr Hypertens Rep 8: 242–248, 2006. [DOI] [PubMed] [Google Scholar]
- 107.Marsen TA, Schramek H, Dunn MJ. Renal actions of endothelin: linking cellular signaling pathways to kidney disease. Kidney Int 45: 336–344, 1994. [DOI] [PubMed] [Google Scholar]
- 108.Mattson DL, Lu S, Nakanishi K, Papanek PE, Cowley AW Jr. Effect of chronic renal medullary nitric oxide inhibition on blood pressure. Am J Physiol Heart Circ Physiol 266: H1918–H1926, 1994. [DOI] [PubMed] [Google Scholar]
- 109.Mattson DL, Meister CJ. Renal cortical and medullary blood flow responses to l-NAME and ANG II in wild-type, nNOS null mutant, and eNOS null mutant mice. Am J Physiol Regul Integr Comp Physiol 289: R991–R997, 2005. [DOI] [PubMed] [Google Scholar]
- 110.Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev 85: 679–715, 2005. [DOI] [PubMed] [Google Scholar]
- 111.Michel FS, Man RY, Vanhoutte PM. Increased spontaneous tone in renal arteries of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 293: H1673–H1681, 2007. [DOI] [PubMed] [Google Scholar]
- 112.Mii S, Khalil RA, Morgan KG, Ware JA, Kent KC. Mitogen-activated protein kinase and proliferation of human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 270: H142–H150, 1996. [DOI] [PubMed] [Google Scholar]
- 113.Mitsuka M, Berk BC. Long-term regulation of Na+-H+ exchange in vascular smooth muscle cells: role of protein kinase C. Am J Physiol Cell Physiol 260: C562–C569, 1991. [DOI] [PubMed] [Google Scholar]
- 114.Miyata N, Cowley AW Jr. Renal intramedullary infusion of l-arginine prevents reduction of medullary blood flow and hypertension in Dahl salt-sensitive rats. Hypertension 33: 446–450, 1999. [DOI] [PubMed] [Google Scholar]
- 115.Morrison AR Biochemistry and pharmacology of renal arachidonic acid metabolism. Am J Med 80, Suppl 1A: 3–11, 1986. [DOI] [PubMed] [Google Scholar]
- 116.Murphy JG, Herrington JN, Granger JP, Khalil RA. Enhanced [Ca2+]i in renal arterial smooth muscle cells of pregnant rats with reduced uterine perfusion pressure. Am J Physiol Heart Circ Physiol 284: H393–H403, 2003. [DOI] [PubMed] [Google Scholar]
- 117.Nakamura A, Hayashi K, Ozawa Y, Fujiwara K, Okubo K, Kanda T, Wakino S, Saruta T. Vessel- and vasoconstrictor-dependent role of rho/rho-kinase in renal microvascular tone. J Vasc Res 40: 244–251, 2003. [DOI] [PubMed] [Google Scholar]
- 118.Navar LG, Imig JD, Zou L, Wang CT. Intrarenal production of angiotensin II. Semin Nephrol 17: 412–422, 1997. [PubMed] [Google Scholar]
- 119.Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev 76: 425–536, 1996. [DOI] [PubMed] [Google Scholar]
- 120.Nelson LD, Mashburn NA, Bell PD. Altered sodium-calcium exchange in afferent arterioles of the spontaneously hypertensive rat. Kidney Int 50: 1889–1896, 1996. [DOI] [PubMed] [Google Scholar]
- 121.Nishikimi T, Koshikawa S, Ishikawa Y, Akimoto K, Inaba C, Ishimura K, Ono H, Matsuoka H. Inhibition of Rho-kinase attenuates nephrosclerosis and improves survival in salt-loaded spontaneously hypertensive stroke-prone rats. J Hypertens 25: 1053–1063, 2007. [DOI] [PubMed] [Google Scholar]
- 122.Ohno A, Naruse M, Kato S, Hosaka M, Naruse K, Demura H, Sugino N. Endothelin-specific antibodies decrease blood pressure and increase glomerular filtration rate and renal plasma flow in spontaneously hypertensive rats. J Hypertens 10: 781–785, 1992. [PubMed] [Google Scholar]
- 123.Ozawa Y, Hayashi K, Kanda T, Homma K, Takamatsu I, Tatematsu S, Yoshioka K, Kumagai H, Wakino S, Saruta T. Impaired nitric oxide- and endothelium-derived hyperpolarizing factor-dependent dilation of renal afferent arteriole in Dahl salt-sensitive rats. Nephrology (Carlton) 9: 272–277, 2004. [DOI] [PubMed] [Google Scholar]
- 124.Palmgren E, Widgren B, Aurell M, Herlitz H. Increased renal vascular sensitivity to angiotensin II in hypertension is due to decreased response to prostaglandins. J Hypertens 21: 969–976, 2003. [DOI] [PubMed] [Google Scholar]
- 125.Peng Z, Dang A, Arendshorst WJ. Increased expression and activity of phospholipase C in renal arterioles of young spontaneously hypertensive rats. Am J Hypertens 20: 38–43, 2007. [DOI] [PubMed] [Google Scholar]
- 126.Purdy KE, Arendshorst WJ. EP(1) and EP(4) receptors mediate prostaglandin E(2) actions in the microcirculation of rat kidney. Am J Physiol Renal Physiol 279: F755–F764, 2000. [DOI] [PubMed] [Google Scholar]
- 127.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol 75: 346–359, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Reinhardt HW, Seeliger E. Toward an integrative concept of control of total body sodium. News Physiol Sci 15: 319–325, 2000. [DOI] [PubMed] [Google Scholar]
- 129.Ren Y, Arima S, Carretero OA, Ito S. Possible role of adenosine in macula densa control of glomerular hemodynamics. Kidney Int 61: 169–176, 2002. [DOI] [PubMed] [Google Scholar]
- 130.Riggleman A, Harvey J, Baylis C. Endothelin mediates some of the renal actions of acutely administered angiotensin II. Hypertension 38: 105–109, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Roman RJ, Harder DR. Cellular and ionic signal transduction mechanisms for the mechanical activation of renal arterial vascular smooth muscle. J Am Soc Nephrol 4: 986–996, 1993. [DOI] [PubMed] [Google Scholar]
- 132.Roman RJ, Kaldunski ML, Scicli AG, Carretero OA. Influence of kinins and angiotensin II on the regulation of papillary blood flow. Am J Physiol Renal Fluid Electrolyte Physiol 255: F690–F698, 1988. [DOI] [PubMed] [Google Scholar]
- 133.Romero JC, Reckelhoff JF. Role of angiotensin and oxidative stress in essential hypertension. Hypertension 34: 943–949, 1999. [DOI] [PubMed] [Google Scholar]
- 134.Ruan X, Arendshorst WJ. Role of protein kinase C in angiotensin II-induced renal vasoconstriction in genetically hypertensive rats. Am J Physiol Renal Fluid Electrolyte Physiol 270: F945–F952, 1996. [DOI] [PubMed] [Google Scholar]
- 135.Ruilope LM, Lahera V, Rodicio JL, Carlos Romero J. Are renal hemodynamics a key factor in the development and maintenance of arterial hypertension in humans? Hypertension 23: 3–9, 1994. [DOI] [PubMed] [Google Scholar]
- 136.Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, Egido J. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl 82: 12–22, 2002. [DOI] [PubMed] [Google Scholar]
- 137.Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol 18: 2439–2446, 2007. [DOI] [PubMed] [Google Scholar]
- 138.Salamanca DA, Khalil RA. Protein kinase C isoforms as specific targets for modulation of vascular smooth muscle function in hypertension. Biochem Pharmacol 70: 1537–1547, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sasajima H, Shima H, Toyoda Y, Kimura K, Yoshikawa A, Hano T, Nishio I. Increased Ca2+ sensitivity of contractile elements via protein kinase C in alpha-toxin permeabilized SMA from young spontaneously hypertensive rats. Cardiovasc Res 36: 86–91, 1997. [DOI] [PubMed] [Google Scholar]
- 140.Sasaki M, Hori MT, Hino T, Golub MS, Tuck ML. Elevated 12-lipoxygenase activity in the spontaneously hypertensive rat. Am J Hypertens 10: 371–378, 1997. [PubMed] [Google Scholar]
- 141.Sauzeau V, Rolli-Derkinderen M, Marionneau C, Loirand G, Pacaud P. RhoA expression is controlled by nitric oxide through cGMP-dependent protein kinase activation. J Biol Chem 278: 9472–9480, 2003. [DOI] [PubMed] [Google Scholar]
- 142.Schiffrin EL Intracellular signal transduction for vasoactive peptides in hypertension. Can J Physiol Pharmacol 72: 954–962, 1994. [DOI] [PubMed] [Google Scholar]
- 143.Schiffrin EL Vascular endothelin in hypertension. Vascul Pharmacol 43: 19–29, 2005. [DOI] [PubMed] [Google Scholar]
- 144.Schmidt S, van Hooft IM, Grobbee DE, Ganten D, Ritz E. Polymorphism of the angiotensin I converting enzyme gene is apparently not related to high blood pressure: Dutch Hypertension and Offspring Study. J Hypertens 11: 345–348, 1993. [DOI] [PubMed] [Google Scholar]
- 145.Schmieder RE, Erdmann J, Delles C, Jacobi J, Fleck E, Hilgers K, Regitz-Zagrosek V. Effect of the angiotensin II type 2-receptor gene (+1675 G/A) on left ventricular structure in humans. J Am Coll Cardiol 37: 175–182, 2001. [DOI] [PubMed] [Google Scholar]
- 146.Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension 32: 59–64, 1998. [DOI] [PubMed] [Google Scholar]
- 147.Schnackenberg CG, Welch WJ, Wilcox CS. TP receptor-mediated vasoconstriction in microperfused afferent arterioles: roles of O2- and NO. Am J Physiol Renal Physiol 279: F302–F308, 2000. [DOI] [PubMed] [Google Scholar]
- 148.Schorr U, Blaschke K, Beige J, Distler A, Sharma AM. Angiotensinogen M235T variant and salt sensitivity in young normotensive Caucasians. J Hypertens 17: 475–479, 1999. [DOI] [PubMed] [Google Scholar]
- 149.Schroeder AC, Imig JD, LeBlanc EA, Pham BT, Pollock DM, Inscho EW. Endothelin-mediated calcium signaling in preglomerular smooth muscle cells. Hypertension 35: 280–286, 2000. [DOI] [PubMed] [Google Scholar]
- 150.Schwartzman ML, da Silva JL, Lin F, Nishimura M, Abraham NG. Cytochrome P450 4A expression and arachidonic acid omega-hydroxylation in the kidney of the spontaneously hypertensive rat. Nephron 73: 652–663, 1996. [DOI] [PubMed] [Google Scholar]
- 151.Sedeek MH, Llinas MT, Drummond H, Fortepiani L, Abram SR, Alexander BT, Reckelhoff JF, Granger JP. Role of reactive oxygen species in endothelin-induced hypertension. Hypertension 42: 806–810, 2003. [DOI] [PubMed] [Google Scholar]
- 152.Seikaly MG, Arant BS Jr, Seney FD Jr. Endogenous angiotensin concentrations in specific intrarenal fluid compartments of the rat. J Clin Invest 86: 1352–1357, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shultz PJ, Tolins JP. Adaptation to increased dietary salt intake in the rat. Role of endogenous nitric oxide. J Clin Invest 91: 642–650, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sigmon DH, Carretero OA, Beierwaltes WH. Renal versus femoral hemodynamic response to endothelium-derived relaxing factor synthesis inhibition. J Vasc Res 30: 218–223, 1993. [DOI] [PubMed] [Google Scholar]
- 155.Silva-Antonialli MM, Tostes RC, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, Fortes ZB, Nigro D. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res 62: 587–593, 2004. [DOI] [PubMed] [Google Scholar]
- 156.Siragy HM, Jaffa AA, Margolius HS, Carey RM. Renin-angiotensin system modulates renal bradykinin production. Am J Physiol Regul Integr Comp Physiol 271: R1090–R1095, 1996. [DOI] [PubMed] [Google Scholar]
- 157.Skov K, Mulvany MJ. Structure of renal afferent arterioles in the pathogenesis of hypertension. Acta Physiol Scand 181: 397–405, 2004. [DOI] [PubMed] [Google Scholar]
- 158.Smeda JS, Lee RM, Forrest JB. Prenatal and postnatal hydralazine treatment does not prevent renal vessel wall thickening in SHR despite the absence of hypertension. Circ Res 63: 534–542, 1988. [DOI] [PubMed] [Google Scholar]
- 159.Smeda JS, Lee RM, Forrest JB. Structural and reactivity alterations of the renal vasculature of spontaneously hypertensive rats prior to and during established hypertension. Circ Res 63: 518–533, 1988. [DOI] [PubMed] [Google Scholar]
- 160.Smith L, Payne JA, Sedeek MH, Granger JP, Khalil RA. Endothelin-induced increases in Ca2+ entry mechanisms of vascular contraction are enhanced during high-salt diet. Hypertension 41: 787–793, 2003. [DOI] [PubMed] [Google Scholar]
- 161.Smith WL Prostaglandin biosynthesis and its compartmentation in vascular smooth muscle and endothelial cells. Annu Rev Physiol 48: 251–262, 1986. [DOI] [PubMed] [Google Scholar]
- 162.Stier CT, Quilley CP, McGiff JC. Endothelin-3 effects on renal function and prostanoid release in the rat isolated kidney. J Pharmacol Exp Ther 262: 252–256, 1992. [PubMed] [Google Scholar]
- 163.Stoos BA, Garcia NH, Garvin JL. Nitric oxide inhibits sodium reabsorption in the isolated perfused cortical collecting duct. J Am Soc Nephrol 6: 89–94, 1995. [DOI] [PubMed] [Google Scholar]
- 164.Sullivan JC, Goodchild TT, Cai Z, Pollock DM, Pollock JS. EndothelinA (ETA) and ETB receptor-mediated regulation of nitric oxide synthase 1 (NOS1) and NOS3 isoforms in the renal inner medulla. Acta Physiol (Oxf) 191: 329–336, 2007. [DOI] [PubMed] [Google Scholar]
- 165.Taddei S, Virdis A, Mattei P, Ghiadoni L, Sudano I, Salvetti A. Defective l-arginine-nitric oxide pathway in offspring of essential hypertensive patients. Circulation 94: 1298–1303, 1996. [DOI] [PubMed] [Google Scholar]
- 166.Tang L, Loutzenhiser K, Loutzenhiser R. Biphasic actions of prostaglandin E(2) on the renal afferent arteriole: role of EP(3) and EP(4) receptors. Circ Res 86: 663–670, 2000. [DOI] [PubMed] [Google Scholar]
- 167.Terada Y, Tomita K, Nonoguchi H, Marumo F. Different localization of two types of endothelin receptor mRNA in microdissected rat nephron segments using reverse transcription and polymerase chain reaction assay. J Clin Invest 90: 107–112, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Thai TL, Fellner SK, Arendshorst WJ. ADP-ribosyl cyclase and ryanodine receptor activity contribute to basal renal vasomotor tone and agonist-induced renal vasoconstriction in vivo. Am J Physiol Renal Physiol 293: F1107–F1114, 2007. [DOI] [PubMed] [Google Scholar]
- 169.Tharaux PL, Chatziantoniou C, Fakhouri F, Dussaule JC. Angiotensin II activates collagen I gene through a mechanism involving the MAP/ER kinase pathway. Hypertension 36: 330–336, 2000. [DOI] [PubMed] [Google Scholar]
- 170.Thengchaisri N, Hein TW, Wang W, Xu X, Li Z, Fossum TW, Kuo L. Upregulation of arginase by H2O2 impairs endothelium-dependent nitric oxide-mediated dilation of coronary arterioles. Arterioscler Thromb Vasc Biol 26: 2035–2042, 2006. [DOI] [PubMed] [Google Scholar]
- 171.Thomson S, Bao D, Deng A, Vallon V. Adenosine formed by 5′-nucleotidase mediates tubuloglomerular feedback. J Clin Invest 106: 289–298, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tian N, Moore RS, Braddy S, Rose RA, Gu JW, Hughson MD, Manning RD Jr. Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 293: H3388–H3395, 2007. [DOI] [PubMed] [Google Scholar]
- 173.Tomobe Y, Miyauchi T, Saito A, Yanagisawa M, Kimura S, Goto K, Masaki T. Effects of endothelin on the renal artery from spontaneously hypertensive and Wistar Kyoto rats. Eur J Pharmacol 152: 373–374, 1988. [DOI] [PubMed] [Google Scholar]
- 174.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev 52: 639–672, 2000. [PubMed] [Google Scholar]
- 175.Ujiie K, Terada Y, Nonoguchi H, Shinohara M, Tomita K, Marumo F. Messenger RNA expression and synthesis of endothelin-1 along rat nephron segments. J Clin Invest 90: 1043–1048, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation 108: 1253–1258, 2003. [DOI] [PubMed] [Google Scholar]
- 177.Vågnes ØB, Iversen BM, Arendshorst WJ. Short-term ANG II produces renal vasoconstriction independent of TP receptor activation and TxA2/isoprostane production. Am J Physiol Renal Physiol 293: F860–F867, 2007. [DOI] [PubMed] [Google Scholar]
- 178.van Rodijnen WF, Korstjens IJ, Legerstee N, Ter Wee PM, Tangelder GJ. Direct vasoconstrictor effect of prostaglandin E2 on renal interlobular arteries: role of the EP3 receptor. Am J Physiol Renal Physiol 292: F1094–F1101, 2007. [DOI] [PubMed] [Google Scholar]
- 179.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92: 827–839, 2003. [DOI] [PubMed] [Google Scholar]
- 180.Vyas SJ, Jackson EK. Angiotensin II: enhanced renal responsiveness in young genetically hypertensive rats. J Pharmacol Exp Ther 273: 768–777, 1995. [PubMed] [Google Scholar]
- 181.Wagner C, de Wit C, Kurtz L, Grünberger C, Kurtz A, Schweda F. Connexin 40 is essential for the pressure control of renin synthesis and secretion. Circ Res 100: 556–563, 2007. [DOI] [PubMed] [Google Scholar]
- 182.Wang T, Chan YL. Mechanism of angiotensin II action on proximal tubular transport. J Pharmacol Exp Ther 252: 689–695, 1990. [PubMed] [Google Scholar]
- 183.Wang X, Breaks J, Loutzenhiser K, Loutzenhiser R. Effects of inhibition of the Na+/K+/2Cl− cotransporter on myogenic and angiotensin II responses of the rat afferent arteriole. Am J Physiol Renal Physiol 292: F999–F1006, 2007. [DOI] [PubMed] [Google Scholar]
- 184.Wassmann S, Stumpf M, Strehlow K, Schmid A, Schieffer B, Böhm M, Nickenig G. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res 94: 534–541, 2004. [DOI] [PubMed] [Google Scholar]
- 185.Welch WJ, Tojo A, Wilcox CS. Roles of NO and oxygen radicals in tubuloglomerular feedback in SHR. Am J Physiol Renal Physiol 278: F769–F776, 2000. [DOI] [PubMed] [Google Scholar]
- 186.White SM, Imig JD, Kim TT, Hauschild BC, Inscho EW. Calcium signaling pathways utilized by P2X receptors in freshly isolated preglomerular MVSMC. Am J Physiol Renal Physiol 280: F1054–F1061, 2001. [DOI] [PubMed] [Google Scholar]
- 187.Widgren BR, Herlitz H, Aurell M, Berglund G, Wikstrand J, Andersson OK. Increased systemic and renal vascular sensitivity to angiotensin II in normotensive men with positive family histories of hypertension. Am J Hypertens 5: 167–174, 1992. [DOI] [PubMed] [Google Scholar]
- 188.Wilcox CS, Welch WJ. TGF and nitric oxide: effects of salt intake and salt-sensitive hypertension. Kidney Int Suppl 55: S9–S13, 1996. [PubMed] [Google Scholar]
- 189.Xu X, Yang J, Rogus J, Chen C, Schork N, Xu X. Mapping of a blood pressure quantitative trait locus to chromosome 15q in a Chinese population. Hum Mol Genet 8: 2551–2555, 1999. [DOI] [PubMed] [Google Scholar]
- 190.Ying WZ, Sanders PW. Enhanced expression of EGF receptor in a model of salt-sensitive hypertension. Am J Physiol Renal Physiol 289: F314–F321, 2005. [DOI] [PubMed] [Google Scholar]
- 191.Ying Z, Jin L, Dorrance AM, Webb RC. Increased expression of mRNA for regulator of G protein signaling domain-containing Rho guanine nucleotide exchange factors in aorta from stroke-prone spontaneously hypertensive rats. Am J Hypertens 17: 981–985, 2004. [DOI] [PubMed] [Google Scholar]
- 192.Yiu SS, Zhao X, Inscho EW, Imig JD. 12-Hydroxyeicosatetraenoic acid participates in angiotensin II afferent arteriolar vasoconstriction by activating L-type calcium channels. J Lipid Res 44: 2391–2399, 2003. [DOI] [PubMed] [Google Scholar]
- 193.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res 87: 992–998, 2000. [DOI] [PubMed] [Google Scholar]
- 194.Zhang J, Lee MY, Cavalli M, Chen L, Berra-Romani R, Balke CW, Bianchi G, Ferrari P, Hamlyn JM, Iwamoto T, Lingrel JB, Matteson DR, Wier WG, Blaustein MP. Sodium pump alpha2 subunits control myogenic tone and blood pressure in mice. J Physiol 569: 243–56, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhao L, Funk CD. Lipoxygenase pathways in atherogenesis. Trends Cardiovasc Med 14: 191–195, 2004. [DOI] [PubMed] [Google Scholar]
- 196.Zou AP, Li N, Cowley AW Jr. Production and actions of superoxide in the renal medulla. Hypertension 37: 547–553, 2001. [DOI] [PubMed] [Google Scholar]
- 197.Zou MH, Ullrich V. Peroxynitrite formed by simultaneous generation of nitric oxide and superoxide selectively inhibits bovine aortic prostacyclin synthase. FEBS Lett 382: 101–104, 1996. [DOI] [PubMed] [Google Scholar]