Abstract
Women with systemic lupus erythematosus (SLE) exhibit a high prevalence of hypertension and renal injury. Rosiglitazone (Rosi), a peroxisome proliferator activator receptor gamma (PPARγ) agonist, has renal protective and antihypertensive effects. We tested whether Rosi ameliorates hypertension and renal injury in a female mouse model of SLE (NZBWF1). Thirty-week-old SLE and control (NZW/LacJ) mice (n ≥ 6/group) were fed Rosi (5 mg·kg−1·day−1 in standard chow) or standard chow for 4 wk. SLE mice had increased blood pressure (BP in mmHg) compared with controls (139 ± 4 vs. 111 ± 4, P < 0.05). Rosi treatment lowered BP in SLE mice (127 ± 4, P < 0.05) but not in controls (111 ± 4). Urinary albumin (μg/mg creatinine) was increased in SLE mice compared with controls (12,396 ± 6,525 vs. 50 ± 6) and reduced with Rosi treatment (148 ± 117). Glomerulosclerosis (% of glomeruli with sclerosis) was reduced in Rosi-treated SLE mice (4.2 ± 1.6 vs. 0.4 ± 0.3, P < 0.05). Renal monocyte/macrophage numbers (cell number/1,320 points counted) were reduced in SLE mice treated with Rosi (32.6 ± 11.0 vs. 10.6 ± 3.6, P < 0.05) but unchanged in controls (3.7 ± 1.6 vs. 3.7 ± 2.0). Renal osteopontin expression, a cytokine-regulating macrophage recruitment, was reduced in Rosi-treated SLE mice. Urinary endothelin (in pg/mg creatinine) was increased in SLE mice compared with controls (1.9 ± 0.59 vs. 0.6 ± 0.04, P < 0.05) and reduced in SLE mice treated with Rosi (0.8 ± 0.11, P < 0.05). PPARγ protein expression in the renal cortex was significantly lower in SLE mice compared with controls and was unaffected by Rosi. These data suggest that Rosi may be an important therapeutic option for the treatment of SLE hypertension and renal injury.
Keywords: lupus, inflammation, endothelin, glomerulosclerosis, proliferator activated receptor gamma
systemic lupus erythematosus (SLE) is a chronic autoimmune inflammatory disorder that has a strong predilection for women during their reproductive years. The hallmark of the disease is the production of autoantibodies such as antinuclear antibodies, and more specifically anti-double-stranded DNA (anti-dsDNA) antibodies. Accumulating evidence indicates that the major cause of death among patients with SLE is cardiovascular disease (17, 22, 23, 26). Indeed, studies have reported that women with SLE are at a 50-fold greater risk for developing cardiovascular disease independent of traditional Framingham Heart Study risk factors (35). One of the major factors contributing to the progression of cardiovascular disease is increased arterial pressure. Importantly, SLE is associated with a high incidence of hypertension (1, 41, 56).
The kidneys are prominently affected during SLE in the form of immune complex glomerulonephritis. This occurs in greater than 50% of patients with SLE (18) and is caused, in part, by circulating anti-dsDNA antibody-mediated formation of immune complexes (51). Few advances have been made toward the treatment of SLE and the associated nephritis and hypertension in the past 40 years. Patients with SLE are often treated with blockers of the renin-angiotensin system (RAS) to control blood pressure and reduce proteinuria; however, there is conflicting evidence as to whether angiotensin-converting enzyme inhibition improves renal histopathology during SLE. Although RAS blockade is generally effective for lowering pressure in patients with SLE and can lower the risk of vascular events (36), additional antihypertensive therapies are often required to achieve adequate blood pressure control (54). In addition, there are rare cases in which RAS blockade can cause or exacerbate SLE (4, 42). Therefore, the identification of new therapies for the treatment of SLE and SLE hypertension is important.
Rosiglitazone, a pharmacological agonist for the nuclear receptor peroxisome proliferator activated receptor gamma (PPARγ), is clinically used to regulate glucose levels in insulin-resistant patients. At present, the thiazolidinedione class of drugs (including rosiglitazone) is being considered as a treatment for a wide array of diseases, including cancer (50), Alzheimer's disease (32), atherosclerosis (57), and hypertension (31). Studies indicate that treatment with rosiglitazone can reduce blood pressure (24), improve vascular function (44), and have anti-inflammatory and renal protective effects (47). Given that SLE is a chronic inflammatory disorder with prominent hypertension, inflammation, and renal disease, rosiglitazone could be an attractive therapeutic option. Therefore, in the present study, we tested the hypothesis that treatment with rosiglitazone ameliorates SLE hypertension and renal injury.
To examine the antihypertensive and renal protective effects of rosiglitazone, we used a mouse model (NZBWF1 mice) that closely mimics human SLE. Like humans with SLE, the NZBWF1 mice produce anti-dsDNA antibodies (5), develop immune complex glomerulonephritis (10), and importantly become hypertensive (45). Also similar to humans, the etiology of SLE in this model is thought to be polygenic, and female NZBWF1 mice are more prominently affected than the males.
MATERIALS AND METHODS
Animals.
Thirty-week-old female NZBWF1 (SLE) and NZW/LacJ (control) obtained from Jackson Laboratories (Bar Harbor, ME) were fed either rosiglitazone (Rosi, GlaxoSmithKline, New York, NY) or unsupplemented (Veh) chow for 4 wk. This age was selected because NZBWF1 mice typically begin to develop signs of proteinuria after this time. Consistent with our previous study (46), food intake was not different between SLE and control mice. The targeted dose of rosiglitazone was 5 mg·kg−1·day−1. The achieved dose ranged from 5.5 to 10.9 mg/day and averaged 6.9 ± 0.5 mg·kg−1·day−1 (SLE mice) and 8.9 ± 0.5 mg·kg−1·day−1 (control mice, P < 0.05). The higher dose achieved in control mice reflects the same food intake but lower body weight. Mice were housed in metabolic cages over night for collection of urine samples. Only SLE mice with no signs of urinary albumin at 30 wk of age were included in the study [determined by Albustix, as previously described (45)]. Mice were maintained on 12:12-h light-dark cycle temperature-controlled rooms with access to chow and water ad libitum. Four groups of animals were included: control untreated mice (Ctrl+Veh), control treated with rosiglitazone (Ctrl+Rosi), SLE untreated (SLE+Veh), and SLE treated with rosiglitazone (SLE+Rosi). No deaths occurred over the 4-wk treatment period. Body weight was measured weekly. All of the studies were performed with the approval of the University of Mississippi Medical Center Institutional Animal Care and Use Committee and in accordance with National Institutes of Health guidelines.
Anti-dsDNA.
Plasma anti-dsDNA antibodies were measured by a commercial ELISA (Alpha Diagnostic International, San Antonio, TX). Mice were considered anti-dsDNA positive at values ≥1 SD from controls. Only NZBWF1 mice with positive anti-dsDNA antibodies were considered to have SLE. Data are expressed in nanogram per milliliter.
Blood glucose, plasma insulin, and triglycerides.
At the end of the experimental protocol, glucose was measured in blood (100–200 μl) obtained by retro-orbital puncture using the Accu-Chek Advantage glucometer (Roche), as previously described (46). Remaining blood samples were centrifuged, and plasma samples were used to measure insulin as previously described (46) and triglycerides using a commercially available kit (Pointe Scientific, Canton, MI). Data are expressed as follows: glucose (mg/dl), insulin (ng/ml), and triglycerides (mg/dl).
Blood pressure measurements.
Mean arterial pressure (MAP) was recorded using indwelling carotid artery catheters in conscious mice at the end of the 4-wk treatment period, as previously described in our laboratory (46). Data are presented as the mean pressure measured over two consecutive days.
Western blot analysis of renal cortical PPARγ expression.
Kidneys were removed, and the renal cortex was dissected to make whole tissue protein homogenates to use in a Western blot, as previously described (48). Briefly, 20 μg of protein were electrophoretically separated. PPARγ expression was determined using a rabbit anti-PPARγ primary antibody (1:1,000 SC-7196, Santa Cruz Biotechnology, Santa Cruz, CA). The secondary antibody (1:8,000 donkey anti-rabbit horseradish peroxidase, NA9340) was obtained from Amersham Biosciences (Pittsburgh, PA). GAPDH was used as a loading control. Data are expressed as arbitrary units PPARγ/GAPDH.
Assessment of renal injury.
ALBUMINURIA.
Urinary albumin was measured using a commercial ELISA (Alpha Diagnostic International) and was normalized to urinary creatinine (CR01 Oxford Biomedical Research, Oxford, MI). The data are expressed as micrograms of albumin per milligram creatinine.
RENAL MACROPHAGE INFILTRATION.
At the time of death, one whole kidney was removed and fixed in formalin for histological analysis. The remaining kidney was used for protein and/or RNA analyses. Renal sections (4 μm) were stained for the presence of monocyte/macrophage using the F4/80 antibody (1:75 dilution, from AbD Serotec, Raleigh, NC) and analyzed using a point counting method with a 40× objective lens. Fifteen fields with 88 points/field were analyzed for each histological section, and the results are expressed as the number of cells positive for F4/80 per tissue section (1,320 total points counted per animal).
MORPHOLOGY.
Glomerulosclerosis was determined in Periodic Acid Schiff-stained sections. At least 150 glomeruli per section were evaluated by an observer blinded to the experimental groups. The degree of sclerosis was graded as follows: Sclerotic area up to 25%, sclerotic area 26 to 50%, sclerotic area 51 to 75%, sclerotic area 76 to 100%, and global sclerosis. The results are expressed as a percentage of glomeruli exhibiting each degree of sclerosis.
Real-time PCR.
RNA was isolated from kidney cortex of control and SLE mice using the RNeasy Protect Minikit (Qiagen), as previously described (45). The reverse transcription reaction was performed with 0.5 μg of RNA. Detailed methods, including primer sequences for mouse osteopontin, monocyte chemotactic protein-1 (MCP-1), and 18s ribosomal RNA can be found in the online supplement. Samples were run in duplicate using the Bio-Rad iCycler (Bio-Rad Laboratories, Hercules, CA). The results were standardized to 18S mRNA expression and presented as fold change from Ctrl+Veh.
Urinary endothelin-1 (ET-1) levels.
Urine proteins were extracted with methanol and 20% acetic acid, as previously described in plasma (34). ET-1 concentrations were measured using a Quantiglo ET-1 ELISA (R&D Systems, Minneapolis, MN). Data are expressed as picograms urinary ET-1 per milligram creatinine.
Statistical analyses.
Data are presented as means ± SE. Statistical analyses were performed using GraphPad Prism 5 Software (San Diego, CA). A two-factor ANOVA was used to test for drug or group interactions in data sets that passed the normality test followed by Student-Newman-Keuls to determine individual differences among experimental groups. When the normality test failed, a nonparametric ANOVA on ranks was used with a Dunn's post hoc test. Significance was accepted at P < 0.05.
RESULTS
Physical and metabolic characteristics.
Body weight was significantly higher in SLE mice than control mice (Table 1) as previously described (46). SLE mice treated with rosiglitazone weighed more than vehicle-treated SLE mice at the completion of the study. Body weight was not different in rosiglitazone and vehicle-treated control mice. Fasted blood glucose and plasma triglycerides were not significantly different in SLE mice compared with controls, and mice treated with rosiglitazone did not have lower fasted glucose. As we previously reported, plasma insulin levels were higher in SLE mice (46), but these were not significantly reduced with rosiglitazone treatment. Plasma anti-dsDNA antibodies were significantly greater in SLE mice compared with control mice. Treatment with rosiglitazone did not change the level of anti-dsDNA in either SLE or control mice.
Table 1.
Body weight, blood glucose, plasma insulin, triglycerides, and anti-dsDNA antibody levels in untreated control mice, control mice treated with rosiglitazone, untreated SLE mice, and SLE mice treated with rosiglitazone
| Ctrl+Veh | Ctrl+Rosi | SLE+Veh | SLE+Rosi | |
|---|---|---|---|---|
| Body weight, g | 34±0 | 35±0 | 40±1* | 44±1*† |
| Anti-dsDNA, ng/ml | 100±14 | 94±12 | 671±155* | 694±217* |
| Glucose, mg/dl | 82±5 | 79±4 | 90±4 | 93±4 |
| Insulin, ng/ml | 0.2±0.1 | 0.2±0.1 | 0.8±0.3* | 0.5±0.1 |
| Triglycerides, mg/dl | 59±6 | 50±5 | 74±12 | 72±6 |
Ctrl+Veh, control mice; Ctrl+Rosi, control mice treated with rosiglitazone; SLE+Veh, untreated SLE mice; and SLE+Rosi, SLE mice treated with rosiglitazone.
P < 0.05 vs. Ctrl+Veh, Ctrl+Rosi.
P < 0.05 vs. SLE+Veh.
MAP.
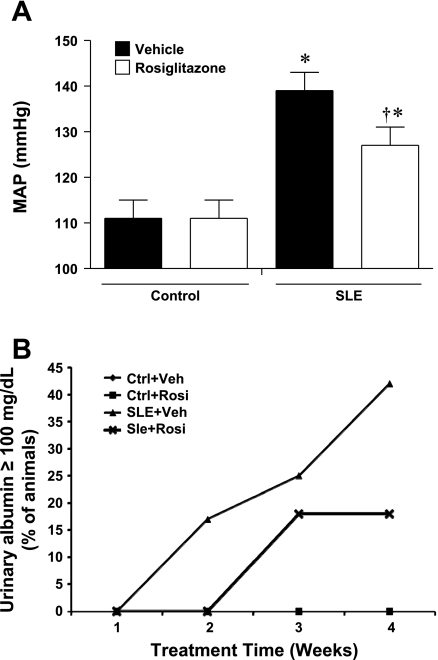
Consistent with our previous studies (45, 46), MAP was significantly higher in vehicle-treated SLE mice compared with vehicle-treated control mice (Fig. 1A). The SLE mice treated with rosiglitazone for 4 wk had significantly lower blood pressure compared with SLE+Veh. Rosiglitazone treatment did not alter blood pressure in control animals. We measured pressure in a separate group of 30-wk-old SLE mice with no albuminuria and found them to be hypertensive compared with controls (135 ± 2 vs. 121 ± 3 mmHg, P < 0.05) and have increased anti-dsDNA antibodies (340 ± 163 vs. 17 ± 3, P < 0.05). These data suggest that there may be beneficial therapeutic effects of rosiglitazone in SLE hypertension rather than only prophylactic benefits. Consistent with our previous findings (45), SLE mice had higher urinary albumin compared with control mice (12,396 ± 6,525 vs. 50 ± 6 μg/mg creatinine, SLE+Veh vs. Ctrl+Veh, respectively). Urinary albumin was decreased in SLE mice treated with rosiglitazone for 4 wk (148 ± 117 μg/mg creatinine). These differences did not reach a statistical difference due to the variable levels of urinary albumin within the SLE group. For example, albumin ranged only from 25 to 75 (median 41) and 18 to 75 (median 43) in control mice and control mice treated with rosiglitazone. To the contrary, urinary albumin ranged from 11 to 45,682 (median 457) and 20 to 970 (median 43) in SLE mice and SLE mice treated with rosiglitazone. The percentage of animals that developed albuminuria as measured by albustix is shown in Fig. 1B (42% SLE+Veh, 18% SLE+Rosi). Subsequent studies were performed using experimental groups with a similar makeup of animals with albuminuria.
Fig. 1.
A: systemic lupus erythematosus (SLE) mice treated with rosiglitazone have lower mean arterial pressure (MAP) compared with SLE mice fed standard chow for 4 wk (34 wk of age). Rosiglitazone treatment did not affect blood pressure in control mice. *P < 0.05 vs. Ctrl+Veh, Ctrl+Rosi. †P < 0.05 vs. SLE+Veh (n ≥ 7). B: weekly percentage of mice with positive urinary albumin as measured by albustix (n ≥ 10).
Renal monocyte/macrophage infiltration.
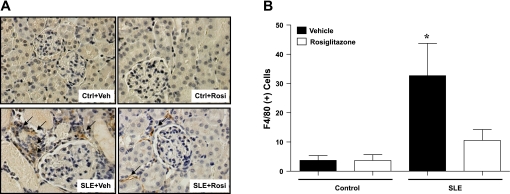
Renal monocyte/macrophage infiltration was significantly higher in SLE mice compared with control animals (Fig. 2), and treatment with rosiglitazone reduced the number of F4/80-positive cells in the kidneys of SLE mice but not in control mice. The majority of inflammatory cells were observed in the renal cortex. The numbers of inflammatory cells in the medulla were not different between SLE or control mice after vehicle or rosiglitazone treatment. (Ctrl+Veh: 0, Ctrl+Rosi: 0, SLE+Veh: 2 ± 0.6, SLE+Rosi: 1.3 ± 0.8).
Fig. 2.
A: renal cortical sections stained with the F4/80 antibody to identify monocyte and macrophage infiltration (arrows pointing to brown stain areas). The sections are counter-stained with hematoxylin-and-eosin (×40). B: summary data showing that SLE mice have increased monocyte/macrophage number compared with control animals and that treatment with rosiglitazone significantly reduces the monocyte/macrophage number in the kidney of SLE mice. *P < 0.05 vs. Ctrl+Veh, ANOVA on ranks (n ≥ 6).
Glomerulosclerosis.
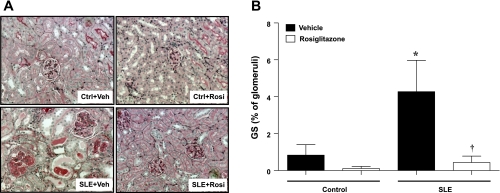
The percentage of glomeruli that exhibited a sclerotic area up to 25% is shown in Fig. 3. Glomerulosclerosis was greater in SLE mice compared with control mice. SLE mice treated with rosiglitazone had significantly lower levels of sclerosis. Treatment with rosiglitazone did not affect glomerulosclerosis in control mice. For complete histological analyses of glomerulosclerosis, please refer to the online supplement (Supplemental Fig. 1).
Fig. 3.
A: periodic acid Schiff staining of renal sections in SLE and control mice fed standard chow, or standard chow containing rosiglitazone. B: summary data showing that SLE mice have an increased percentage of sclerosed glomeruli (sclerotic area up to 25%) compared with control animals and that treatment with rosiglitazone significantly reduces the glomerulosclerosis. *P < 0.05 vs. Ctrl+Veh, Ctrl+Rosi, †P < 0.05 vs. SLE+Veh (n ≥ 6).
Renal cortical osteopontin mRNA expression.
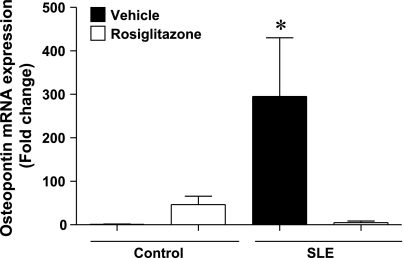
Because SLE mice have increased inflammatory cells in the kidney that were reduced in the rosiglitazone-treated mice, we assessed whether rosiglitazone would affect the expression of two cytokines involved in macrophage recruitment. Renal cortical mRNA expression of osteopontin was 295 ± 135-fold greater in SLE mice compared with control mice (Ctrl+Veh, P < 0.05) (Fig. 4). Osteopontin expression in SLE mice treated with rosiglitazone was not different from controls. We also measured renal cortical MCP-1 mRNA expression. The renal cortex from SLE mice had increased levels of MCP-1 that were prevented by treatment with rosiglitazone; however, this did not reach a statistically significant difference. The data are shown in the online supplement (Supplemental Fig. 2).
Fig. 4.
The expression of renal cortical osteopontin is increased in SLE mice compared with control animals. Treatment with rosiglitazone significantly reduces osteopontin expression. *P < 0.05 vs. Ctrl+Veh. ANOVA on ranks (n ≥ 7).
Effect of rosiglitazone on urinary ET-1 levels.
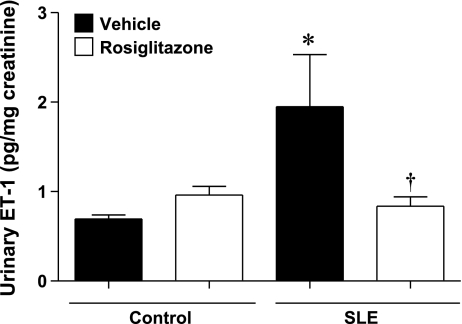
Previous studies demonstrated that ET-1 levels are increased in kidneys from NZBWF1 mice, and circulating ET-1 levels correlate directly with SLE disease activity in humans (33). On the basis of this evidence and data demonstrating that treatment with thiazolidinediones prevents ET-1-mediated hypertension (28) and renal injury (49), we measured urinary ET-1 levels in SLE and control mice after 4-wk treatment with rosiglitazone (Fig. 5). Urinary ET-1 was increased in SLE mice compared with control animals. SLE mice treated with rosiglitazone had reduced urinary ET-1 levels. Treatment with rosiglitazone did not alter urinary ET-1 levels in control mice.
Fig. 5.
Urinary ET-1 is increased in SLE mice compared with control animals. SLE mice treated with rosiglitazone have significantly lower urinary ET-1 production compared with SLE mice fed standard chow. *P < 0.05 vs. Ctrl+Veh, Ctrl+Rosi, †P < 0.05 vs. SLE+Veh (n ≥ 6).
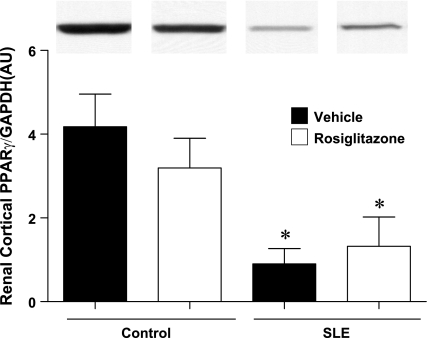
Renal cortical PPARγ protein expression.
PPARγ protein expression was significantly lower in mice with SLE compared with control mice. Treatment with rosiglitazone did not affect renal cortical PPARγ expression in either control or SLE mice (Fig. 6).
Fig. 6.
SLE mice have decreased renal cortical PPARγ protein expression compared with control mice. Rosiglitazone treatment did not affect PPARγ expression in either group. *P < 0.05 vs. Ctrl+Veh and Ctrl+Rosi (n = 4).
DISCUSSION
The present study tested the hypothesis that treatment with rosiglitazone would ameliorate SLE hypertension, renal injury, and inflammation. There are several major findings in this study: 1) treatment with rosiglitazone lowers blood pressure in a mouse model of SLE hypertension, independently of effects on peripheral glucose, insulin, or triglycerides; 2) treatment with rosiglitazone has renal protective effects in SLE mice demonstrated by the reduced glomerulosclerosis and urinary albumin concentration; 3) SLE mice treated with rosiglitazone exhibit reduced inflammation in the renal cortex demonstrated by decreased cytokine expression (osteopontin, MCP-1) and fewer inflammatory cells (monocyte/macrophage); 4) SLE mice treated with rosiglitazone have reduced urinary ET-1 levels; and 5) a mouse model with SLE has reduced PPARγ protein expression in the renal cortex compared with control mice.
Hypertension and SLE.
SLE is a chronic autoimmune inflammatory disorder with accelerated cardiovascular and renal disease and a high prevalence of hypertension. Inflammation has been strongly implicated as an important factor in the development of hypertension, and therefore, it is conceivable that chronic inflammation during SLE may contribute to the hypertension in these patients. We previously demonstrated that an established mouse model of SLE (NZBWF1) has increased blood pressure in addition to albuminuria and renal macrophage infiltration (45, 46). Data from the current study show that SLE mice treated with rosiglitazone have lower blood pressure compared with untreated SLE mice. These data are consistent with the blood pressure-lowering effects of rosiglitazone previously described in humans (24) and in other experimental models of hypertension, including angiotensin and endothelin-mediated hypertension (14, 19, 28, 44). The current study was designed to test whether rosiglitazone can prevent the progression of renal injury and lower blood pressure during SLE. However, because the SLE mice are hypertensive at the start of the treatment regimen, our data also highlight the importance of conducting studies to examine the therapeutic effect of rosiglitazone to lower blood pressure and protect the kidneys in patients with active disease.
The renal protective effects and reduction of endothelin in this study raise the possibility that improved kidney function may contribute to the lower blood pressure. However, a complete study examining renal hemodynamic effects of rosiglitazone during SLE will be necessary to definitively answer this question. In addition, the fact that there is a greater decrease in markers of renal inflammation and injury than there is in blood pressure suggests that there may be extrarenal mechanisms contributing to SLE hypertension and/or that the reduced blood pressure is mediated, in part, by extrarenal actions of rosiglitazone. This should not be surprising given that thiazolidinediones can exert their beneficial effects through PPARγ-dependent effects, PPARγ-independent effects, or both. For example, independently of PPARγ, rosiglitazone can directly cause vasodilation, through calcium channel-blocking actions of thiazolidinediones (8, 53, 63). This vasodilatory action of thiazolidinediones has been speculated to promote sodium and water reabsorption in the kidneys (52) and may partly explain why blood pressure was not completely normalized. Rosiglitazone also likely increases sodium and water reabsorption via a PPARγ-dependent mechanism in the collecting duct. Elegant studies have demonstrated that the sodium and water retention during rosiglitazone treatment is ameliorated in mice with collecting duct-specific deletion of the PPARγ gene (25, 64).
In this study, the decreased pressure appears to be independent of its metabolic effects since blood glucose, plasma insulin, and triglycerides levels were not different in rosiglitazone-treated mice. This is not entirely unexpected given that previous studies have reported reductions in blood pressure with rosiglitazone in the absence of changes in metabolic parameters (3, 6).
Renal inflammation and injury.
Renal inflammation is linked to the development of hypertension in experimental models through the promotion of renal injury (7, 20). Indeed, several studies report that anti-inflammatory or immunosuppressive therapies, such as corticosteroids, mycophenalate mofetil, or cyclophosphamide can reduce urinary albumin in humans or animal models with SLE (11, 12, 21, 30). However, the potential for anti-inflammatory therapies in SLE hypertension has not been previously examined. The current study focused on the renal cortex because the glomeruli are most prominently affected by immune complex deposition in patients with SLE. Although we observed only a limited number of macrophage in the renal medulla from SLE mice, it is still important to recognize the importance of the renal medulla to blood pressure regulation. We show here that renal macrophage infiltration is increased in the kidneys from SLE mice (predominantly in the renal cortex) and treatment with rosiglitazone significantly reduced renal monocyte/macrophage number. Our data suggest that the lower number of inflammatory cells may be mediated by the reduced expression of two inflammatory cytokines involved in the recruitment of monocytes/macrophages, osteopontin, and MCP-1. On the basis of these data, we speculate that the attenuation of renal inflammation in rosiglitazone-treated mice may contribute to the reduced renal injury. Increased urinary albumin and renal injury are common in humans with SLE and is well established in the NZBWF1 model of SLE (9, 10). The data here show that urinary albumin and glomerulosclerosis are both decreased in SLE mice treated with rosiglitazone.
It is unlikely that the renal protective effects of rosiglitazone in this study are simply the result of decreased blood pressure. Previous studies in both the NZBWF1 and MRL/lpr mouse models of SLE show that while ACE inhibitors, angiotensin receptor blockers, calcium channel blockers, and sympathetic blockers all lower blood pressure to similar levels, only the blockade of the renin-angiotensin system reduces renal damage measured by albuminuria (27, 40). This is consistent with studies in humans with SLE showing that calcium channel blockers and ACE inhibitors equally lower blood pressure, yet only ACE inhibition reduces proteinuria (29). It may not be surprising that renal injury associated with SLE is not pressure dependent since it is well known to be mediated by immune complex deposition in the glomeruli and inflammation. Therefore, consistent with previous reports, treatment with rosiglitazone imparts strong renal-protective effects (47). Whether there is a causal link between reduced renal injury and lower blood pressure in this model remains to be determined.
Alternatively to the renal protective effects of rosiglitazone, one mechanism that could contribute to the reduced blood pressure is decreased sympathetic activity. Studies in both animal models of hypertension and in humans with insulin resistance suggest that treatment with thiazolidinediones reduces urinary catecholamines, skin sympathetic nerve activity, and cardiac sympathetic nervous system function (55, 59, 61). The contribution of sympathetic activation to SLE hypertension has not been previously examined. There is also evidence that rosiglitazone protects against renin-angiotensin system-mediated hypertension (15, 19, 44). The role for the renin-angiotensin system in SLE hypertension has not been extensively studied; however, both the NZBWF1 and MRL/lpr models of SLE reportedly have low plasma renin activity (43). Finally, it is conceivable that immune system-related mechanisms not affected by rosiglitazone treatment contribute to SLE hypertension. This possibility is supported by our data showing that rosiglitazone treatment does not alter levels of the anti-dsDNA autoantibodies that are critical for immune complex formation and SLE disease progression.
PPARγ and SLE.
A possible role for PPARγ in the progression of SLE or SLE hypertension has not been extensively examined. Previously published work from Gilkeson and colleagues showed that the lupus-prone MRL/lpr mice exhibit a reduction in renal mesangial cell PPARγ expression (13), making it tempting to postulate that a reduction in PPARγ expression and activity could, in part, contribute to SLE hypertension and renal injury. While this study was under consideration for publication, Rifkin and colleagues reported that treatment of the MRL/lpr model of SLE with rosiglitazone reduced glomerular area and cellularity (2). The authors demonstrate, using MRL/lpr-adiponectin knockout mice, that the protective actions of rosiglitazone occur through activation of adiponectin. The authors did not report on blood pressure in this study. The link between adiponectin and PPARγ is worthy of pursuing further given our recent report that the NZBWF1 model has several characteristics of the metabolic syndrome (46).
The data in the current study show that renal cortex PPARγ protein expression is significantly lower in the NZBWF1 model of SLE. Although protein expression does not directly reflect activity, the decreased PPARγ expression in the NZBWF1 model is at least consistent with a potential role for decreased PPARγ in the progression of SLE hypertension and renal injury. Our data showing that PPARγ protein was not increased after rosiglitazone are consistent with a previous study showing that another thiazolidinedione (pioglitazone) did not increase PPARγ mRNA in the renal cortex of obesity-prone rats (16). However, contrary to our results, pioglitazone treatment did increase expression in control rats. This discrepancy may be related to species, sex, disease model, and methodological differences that are apparent in these studies. Taken together, these data suggest that extrarenal targets may also contribute to the anti-hypertensive actions of the thiazolidinediones.
At present, there is scant evidence related to renal PPARγ expression in SLE in humans. One study (abstract only available) (60) suggests that renal PPARγ expression is increased in patients with SLE. To our knowledge, this is the only published study in humans with SLE and highlights the need to more completely examine the role of PPARγ in SLE hypertension and renal disease. Despite the limited available data related to PPARγ and SLE, investigators have called for experiments to test possible therapeutic benefits of PPARγ agonists for SLE (39, 58). Our data clearly show that mice treated with rosiglitazone, a PPARγ agonist, have lower blood pressure and reduced renal inflammation and injury.
Endothelin, PPARγ, and SLE.
One mechanism by which treatment with rosiglitazone might protect the kidneys and reduce inflammation during SLE is through its effects to lower renal endothelin (ET-1). A potential pathogenic role for ET-1 has been proposed in the development of vascular injury (28) and lupus nephritis in humans, and plasma ET-1 concentrations directly correlate with SLE activity (62). In addition, previous studies using the NZBWF1 model of SLE show that renal ET-1 expression is increased and that treatment with an ET-1 receptor antagonist (ETA) reduces renal injury, proteinuria, and blood pressure (37). Consistent with these studies, we show that urinary ET-1 is increased in SLE mice. However, neither the source of the ET-1 (medullary or cortical) nor the physiological role of the increased ET-1 can be directly ascertained in this study. Renal ET-1 levels are altered by sodium intake; however, we believe that this is not the cause for increased urinary ET-1 since food intake is not different between SLE and control mice. Nevertheless, that SLE mice treated with rosiglitazone had reduced urinary ET-1 levels compared with vehicle-treated SLE mice is consistent with a mechanism by which rosiglitazone protects the kidney in SLE. In agreement with our work, others report that PPARγ agonists reduce urinary ET-1 and albumin excretion in patients with type 2 diabetes mellitus (38) and protects against ET-1-dependent hypertension and renal injury in experimental animal models.
Perspectives and Significance
The current study shows that a female mouse model of SLE treated with rosiglitazone has reduced blood pressure compared with vehicle-treated SLE mice. These data suggest rosiglitazone treatment as a promising therapeutic strategy for SLE hypertension and renal injury and indicate that the clinical use of rosiglitazone in patients with SLE needs to be examined. On the basis of these data, it will be important to design studies comparing the clinical efficacy of thiazolidinediones with blockers of the renin angiotensin system that are now commonly used in patients with SLE.
GRANTS
The work in this study was supported by the National Heart Lung and Blood Institute awards R01-HL085907 and K02-HL092284 (M. J. Ryan), as well as P01-HL51971.
Supplementary Material
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Al-Herz A, Ensworth S, Shojania K, Esdaile JM. Cardiovascular risk factor screening in systemic lupus erythematosus. J Rheumatol 30: 493–496, 2003. [PubMed] [Google Scholar]
- 2.Aprahamian T, Bonegio RG, Richez C, Yasuda K, Chiang LK, Sato K, Walsh K, Rifkin IR. The peroxisome proliferator-activated receptor gamma agonist rosiglitazone ameliorates murine lupus by induction of adiponectin. J Immunol 182: 340–346, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakris GL, Ruilope LM, McMorn SO, Weston WM, Heise MA, Freed MI, Porter LE. Rosiglitazone reduces microalbuminuria and blood pressure independently of glycemia in type 2 diabetes patients with microalbuminuria. J Hypertens 24: 2047–2055, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Bertin P, Kamdem J, Bonnet C, Arnaud M, Treves R. Captopril-induced lupus. Clin Exp Rheumatol 11: 695, 1993. [PubMed] [Google Scholar]
- 5.Blank M, Shoenfeld Y. Experimental models of systemic lupus erythematosus: anti-dsDNA in murine lupus. Rheumatology 44: 1086–1089, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Bolten CW, Payne MA, McDonald WG, Blanner PM, Chott RC, Ghosh S, Arhancet GB, Staten NR, Gulve EA, Sullivan PM, Hromockyj AE, Colca JR. Thiazolidinediones inhibit the progression of established hypertension in the Dahl salt-sensitive rat. Diab Vasc Dis Res 4: 117–123, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Bravo Y, Quiroz Y, Ferrebuz A, Vaziri ND, Rodriguez-Iturbe B. Mycophenolate mofetil administration reduces renal inflammation, oxidative stress, and arterial pressure in rats with lead-induced hypertension. Am J Physiol Renal Physiol 293: F616–F623, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan TA, Meehan WP, Jeng YY, Yang D, Chan TM, Nadler JL, Scott S, Rude RK, Hsueh WA. Blood pressure lowering by pioglitazone. Evidence for a direct vascular effect. J Clin Invest 96: 354–360, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnet FM, Holmes MC. The natural history of the NZB/NZW F1 hybrid mouse: a laboratory model of systemic lupus erythematosus. Australas Ann Med 14: 185–191, 1965. [DOI] [PubMed] [Google Scholar]
- 10.Burnett R, Ravel G, Descotes J. Clinical and histopathological progression of lesions in lupus-prone (NZB × NZW) F1 mice. Exp Toxicol Pathol 56: 37–44, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Casey TP Immunosuppression by cyclophosphamide in NZB × NZW mice with lupus nephritis. Blood 32: 436–444, 1968. [PubMed] [Google Scholar]
- 12.Chan TM, Li FK, Tang CS, Wong RW, Fang GX, Ji YL, Lau CS, Wong AK, Tong MK, Chan KW, Lai KN. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong Kong-Guangzhou Nephrology Study Group. N Engl J Med 343: 1156–1162, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Crosby MB, Zhang J, Nowling TM, Svenson JL, Nicol CJ, Gonzalez FJ, Gilkeson GS. Inflammatory modulation of PPAR gamma expression and activity. Clin Immunol 118: 276–283, 2006. [DOI] [PubMed] [Google Scholar]
- 14.De CC, Amiri F, Iglarz M, Cohn JS, Touyz RM, Schiffrin EL. Synergistic vascular protective effects of combined low doses of PPARα and PPARγ activators in angiotensin II-induced hypertension in rats. Br J Pharmacol 151: 45–53, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diep QN, El Mabrouk M, Cohn JS, Endemann D, Amiri F, Virdis A, Neves MF, Schiffrin EL. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: role of peroxisome proliferator-activated receptor-gamma. Circulation 105: 2296–2302, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Dobrian AD, Schriver SD, Khraibi AA, Prewitt RL. Pioglitazone prevents hypertension and reduces oxidative stress in diet-induced obesity. Hypertension 43: 48–56, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Domsic R, Maksimowicz-McKinnon K, Manzi S. Prevention of cardiovascular disease in patients with rheumatic diseases. Best Pract Res Clin Rheumatol 20: 741–756, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Donadio JV, Hart GM, Bergstralh EJ, Holley KE. Prognostic determinants in lupus nephritis: a long-term clinicopathologic study. Lupus 4: 109–115, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Efrati S, Berman S, Ilgiyeav E, Averbukh Z, Weissgarten J. PPAR-γ activation inhibits angiotensin II synthesis, apoptosis, and proliferation of mesangial cells from spontaneously hypertensive rats. Nephron Exp Nephrol 106: e107–e112, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. TNF-α inhibition reduces renal injury in DOCA-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol 294: R76–R83, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faedda R, Palomba D, Satta A, Pirisi M, Tanda F, Bartoli E. Immunosuppressive treatment of the glomerulonephritis of systemic lupus. Clin Nephrol 44: 367–375, 1995. [PubMed] [Google Scholar]
- 22.Frostegard J SLE, atherosclerosis and cardiovascular disease. J Intern Med 257: 485–495, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Frostegard J Systemic lupus erythematosus and cardiovascular disease. Lupus 17: 364–367, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Giles TD, Sander AG. Effects of thiazolidinediones on blood pressure. Curr Hypertens Rep 9: 332–337, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, Magnuson MA, Redha R, Zhang Y, Breyer MD. Thiazolidinediones expand body fluid volume through PPARγ stimulation of ENaC-mediated renal salt absorption. Nat Med 11: 861–866, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Hahn BH, McMahon M. Atherosclerosis and systemic lupus erythematosus: the role of altered lipids and of autoantibodies. Lupus 17: 368–370, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Herlitz H, Svalander C, Tarkowski A, Westberg G. Effect of captopril on murine systemic lupus erythematosus disease. J Hypertens Suppl 6: S684–S686, 1988. [DOI] [PubMed] [Google Scholar]
- 28.Iglarz M, Touyz RM, Amiri F, Lavoie MF, Diep QN, Schiffrin EL. Effect of peroxisome proliferator-activated receptor-α and -γ activators on vascular remodeling in endothelin-dependent hypertension. Arterioscler Thromb Vasc Biol 23: 45–51, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Ikeda T, Nakayama D, Gomi T, Sakurai J, Yamazaki T, Yuhara M. Captopril, an angiotensin I-converting enzyme inhibitor, decreases proteinuria in hypertensive patients with renal diseases. Nephron 52: 72–75, 1989. [DOI] [PubMed] [Google Scholar]
- 30.Jonsson CA, Svensson L, Carlsten H. Beneficial effect of the inosine monophosphate dehydrogenase inhibitor mycophenolate mofetil on survival and severity of glomerulonephritis in systemic lupus erythematosus (SLE)-prone MRLlpr/lpr mice. Clin Exp Immunol 116: 534–541, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komajda M, Curtis P, Hanefeld M, Beck-Nielsen H, Pocock SJ, Zambanini A, Jones NP, Gomis R, Home PD. Effect of the addition of rosiglitazone to metformin or sulfonylureas versus metformin/sulfonylurea combination therapy on ambulatory blood pressure in people with type 2 diabetes: a randomized controlled trial (the RECORD study) [Online]. Cardiovasc Diabetol 7: 10, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kummer MP, Heneka MT. PPARs in Alzheimer's disease (Online). PPAR Res 2008: 403896, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuryliszyn-Moskal A, Klimiuk PA, Ciolkiewicz M, Sierakowski S. Clinical significance of selected endothelial activation markers in patients with systemic lupus erythematosus. J Rheumatol 35: 1307–1313, 2008. [PubMed] [Google Scholar]
- 34.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension 46: 82–86, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA Jr, Jansen-McWilliams L, D'Agostino RB, Kuller LH. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol 145: 408–415, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Mikdashi JA, Kittner Handwerger BS. The impact of antihypertensive therapy on the prevalence of cerebrovascular events in systemic lupus erythematosus. American College of Rheumatology 2004 meeting, San Antonio, TX, October 16–21, 2004, Abstract 380.
- 37.Nakamura T, Ebihara I, Tomino Y, Koide H. Effect of a specific endothelin A receptor antagonist on murine lupus nephritis. Kidney Int 47: 481–489, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura T, Ushiyama C, Shimada N, Hayashi K, Ebihara I, Koide H. Comparative effects of pioglitazone, glibenclamide, and voglibose on urinary endothelin-1 and albumin excretion in diabetes patients. J Diabetes Complications 14: 250–254, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Oates JC, Reilly CM, Crosby MB, Gilkeson GS. Peroxisome proliferator-activated receptor gamma agonists: potential use for treating chronic inflammatory diseases. Arthritis Rheum 46: 598–605, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Perez De LG, De WC, Cohen CD, Nieto E, Molina A, Banas B, Luckow B, Vicente AB, Mampaso F, Schlondorff D. Angiotensin inhibition reduces glomerular damage and renal chemokine expression in MRL/lpr mice. J Pharmacol Exp Ther 307: 275–281, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Petri M, Perez-Gutthann S, Spence D, Hochberg MC. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am J Med 93: 513–519, 1992. [DOI] [PubMed] [Google Scholar]
- 42.Ratliff NB, III. Captopril induced lupus. J Rheumatol 29: 1807–1808, 2002. [PubMed] [Google Scholar]
- 43.Rudofsky UH, Dilwith RL, Roths JB, Lawrence DA, Kelley VE, Magro AM. Differences in the occurrence of hypertension among (NZB X NZW)F1, MRL-lpr, and BXSB mice with lupus nephritis. Am J Pathol 116: 107–114, 1984. [PMC free article] [PubMed] [Google Scholar]
- 44.Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. PPARγ agonist rosiglitazone improves vascular function and lowers blood pressure in hypertensive transgenic mice. Hypertension 43: 661–666, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Ryan MJ, McLemore, GR Jr. Hypertension and impaired vascular function in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 292: R736–R742, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Ryan MJ, McLemore, GR Jr, Hendrix ST. Insulin resistance and obesity in a mouse model of systemic lupus erythematosus. Hypertension 48: 988–993, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Sarafidis PA, Bakris GL. Protection of the kidney by thiazolidinediones: an assessment from bench to bedside. Kidney Int 70: 1223–1233, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Sartori-Valinotti JC, Iliescu R, Yanes LL, Dorsett-Martin W, Reckelhoff JF. Sex differences in the pressor response to angiotensin II when the endogenous renin-angiotensin system is blocked. Hypertension 51: 1170–1176, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Sasser JM, Sullivan JC, Hobbs JL, Yamamoto T, Pollock DM, Carmines PK, Pollock JS. Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol 18: 143–154, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sertznig P, Seifert M, Tilgen W, Reichrath J. Present concepts and future outlook: function of peroxisome proliferator-activated receptors (PPARs) for pathogenesis, progression, and therapy of cancer. J Cell Physiol 212: 1–12, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Sherer Y, Gorstein A, Fritzler MJ, Shoenfeld Y. Autoantibody explosion in systemic lupus erythematosus: more than 100 different antibodies found in SLE patients. Semin Arthritis Rheum 34: 501–537, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Song J, Knepper MA, Hu X, Verbalis JG, Ecelbarger CA. Rosiglitazone activates renal sodium- and water-reabsorptive pathways and lowers blood pressure in normal rats. J Pharmacol Exp Ther 308: 426–433, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Song J, Walsh MF, Igwe R, Ram JL, Barazi M, Dominguez LJ, Sowers JR. Troglitazone reduces contraction by inhibition of vascular smooth muscle cell Ca2+ currents and not endothelial nitric oxide production. Diabetes 46: 659–664, 1997. [DOI] [PubMed] [Google Scholar]
- 54.Teplitsky V, Shoenfeld Y, Tanay A. The renin-angiotensin system in lupus: physiology, genes and practice, in animals and humans. Lupus 15: 319–325, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Uchida A, Nakata T, Hatta T, Kiyama M, Kawa T, Morimoto S, Miki S, Moriguchi J, Nakamura K, Fujita H, Itoh H, Sasaki S, Takeda K, Nakagawa M. Reduction of insulin resistance attenuates the development of hypertension in sucrose-fed SHR. Life Sci 61: 455–464, 1997. [DOI] [PubMed] [Google Scholar]
- 56.Urowitz MB, Gladman DD, Ibanez D, Berliner Y. Modification of hypertension and hypercholesterolaemia in patients with systemic lupus erythematosus: a quality improvement study. Ann Rheum Dis 65: 115–117, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Viljoen A New approaches in the diagnosis of atherosclerosis and treatment of cardiovascular disease. Recent Patents Cardiovasc Drug Discov 3: 84–91, 2008. [DOI] [PubMed] [Google Scholar]
- 58.Wardle EN PPAR: Receptors that regulate inflammation. Saudi J Kidney Dis Transpl 15: 1–6, 2004. [PubMed] [Google Scholar]
- 59.Watanabe K, Komatsu J, Kurata M, Inaba S, Ikeda S, Sueda S, Suzuki J, Kohara K, Hamada M. Improvement of insulin resistance by troglitazone ameliorates cardiac sympathetic nervous dysfunction in patients with essential hypertension. J Hypertens 22: 1761–1768, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Xiong Z, Huang H, Li J, Wang H. [PPAR-gamma expression in the kidney of actively proliferating glomerulonephritis] [Abstract]. Beijing Da Xue Xue Bao 35: 499–502, 2003. [PubMed] [Google Scholar]
- 61.Yosefy C, Magen E, Kiselevich A, Priluk R, London D, Volchek L, Viskoper RJ Jr. Rosiglitazone improves, while Glibenclamide worsens blood pressure control in treated hypertensive diabetic and dyslipidemic subjects via modulation of insulin resistance and sympathetic activity. J Cardiovasc Pharmacol 44: 215–222, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Yoshio T, Masuyama J, Mimori A, Takeda A, Minota S, Kano S. Endothelin-1 release from cultured endothelial cells induced by sera from patients with systemic lupus erythematosus. Ann Rheum Dis 54: 361–365, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang F, Sowers JR, Ram JL, Standley PR, Peuler JD. Effects of pioglitazone on calcium channels in vascular smooth muscle. Hypertension 24: 170–175, 1994. [DOI] [PubMed] [Google Scholar]
- 64.Zhang H, Zhang A, Kohan DE, Nelson RD, Gonzalez FJ, Yang T. Collecting duct-specific deletion of peroxisome proliferator-activated receptor gamma blocks thiazolidinedione-induced fluid retention. Proc Natl Acad Sci USA 102: 9406–9411, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.