Abstract
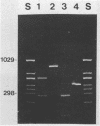
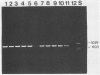
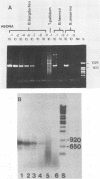
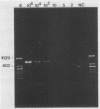
A polymerase chain reaction (PCR) was developed for identification of Borrelia burgdorferi in biological specimens. The diagnostic efficiency was compared with that of in vitro culture. A primer set specifying a 791-bp DNA fragment of the B. burgdorferi B31 flagellin gene was used. Amplified DNA sequences were analyzed by agarose gel electrophoresis, and the identity of amplified DNA was confirmed by restriction enzyme cleavage and Southern blot hybridization with a 32P-labeled probe. By using purified B. burgdorferi DNA, the detection limit of the assay was approximately 0.002 pg of DNA, corresponding to one copy of the B. burgdorferi genome. By using in vitro-cultivated B. burgdorferi without prior DNA purification as the template DNA, 2 to 20 organisms could be detected. A 791-bp DNA fragment was amplified from all of 18 different B. burgdorferi strains tested, as well as from Borrelia hermsii and Borrelia anserina but not from Treponema pallidum. The efficacy of the PCR assay was evaluated on spleen, renal, and urinary bladder tissue specimens from eight experimentally infected gerbils. Specimens from the same organs were cultured in BSK medium in parallel. Of 24 organs, 21 (88%) were PCR positive and 17 (71%) were culture positive. All culture-positive specimens were also PCR positive. Compared with B. burgdorferi cultivation, PCR had at least a comparable diagnostic sensitivity, it was less laborious, and results were available within 1 to 2 days.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asbrink E., Hederstedt B., Hovmark A. The spirochetal etiology of erythema chronicum migrans Afzelius. Acta Derm Venereol. 1984;64(4):291–295. [PubMed] [Google Scholar]
- Asbrink E., Hovmark A., Hederstedt B. The spirochetal etiology of acrodermatitis chronica atrophicans Herxheimer. Acta Derm Venereol. 1984;64(6):506–512. [PubMed] [Google Scholar]
- Asbrink E., Hovmark A. Successful cultivation of spirochetes from skin lesions of patients with erythema chronicum migrans Afzelius and acrodermatitis chronica atrophicans. Acta Pathol Microbiol Immunol Scand B. 1985 Apr;93(2):161–163. doi: 10.1111/j.1699-0463.1985.tb02870.x. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Burgdorfer W., Grunwaldt E., Steere A. C. Antibodies of patients with Lyme disease to components of the Ixodes dammini spirochete. J Clin Invest. 1983 Aug;72(2):504–515. doi: 10.1172/JCI110998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Baril C., Richaud C., Baranton G., Saint Girons I. S. Linear chromosome of Borrelia burgdorferi. Res Microbiol. 1989 Oct;140(8):507–516. doi: 10.1016/0923-2508(89)90083-1. [DOI] [PubMed] [Google Scholar]
- Benach J. L., Bosler E. M., Hanrahan J. P., Coleman J. L., Habicht G. S., Bast T. F., Cameron D. J., Ziegler J. L., Barbour A. G., Burgdorfer W. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983 Mar 31;308(13):740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- Berger B. W., Clemmensen O. J., Ackerman A. B. Lyme disease is a spirochetosis. A review of the disease and evidence for its cause. Am J Dermatopathol. 1983 Apr;5(2):111–124. [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Champion C. I., Miller J. N., Lovett M. A., Blanco D. R. Cloning, sequencing, and expression of two class B endoflagellar genes of Treponema pallidum subsp. pallidum encoding the 34.5- and 31.0-kilodalton proteins. Infect Immun. 1990 Jun;58(6):1697–1704. doi: 10.1128/iai.58.6.1697-1704.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. L., Benach J. L. Isolation of antigenic components from the Lyme disease spirochete: their role in early diagnosis. J Infect Dis. 1987 Apr;155(4):756–765. doi: 10.1093/infdis/155.4.756. [DOI] [PubMed] [Google Scholar]
- Craft J. E., Fischer D. K., Shimamoto G. T., Steere A. C. Antigens of Borrelia burgdorferi recognized during Lyme disease. Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J Clin Invest. 1986 Oct;78(4):934–939. doi: 10.1172/JCI112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft J. E., Grodzicki R. L., Steere A. C. Antibody response in Lyme disease: evaluation of diagnostic tests. J Infect Dis. 1984 May;149(5):789–795. doi: 10.1093/infdis/149.5.789. [DOI] [PubMed] [Google Scholar]
- De Koning J., Bosma R. B., Hoogkamp-Korstanje J. A. Demonstration of spirochaetes in patients with Lyme disease with a modified silver stain. J Med Microbiol. 1987 May;23(3):261–267. doi: 10.1099/00222615-23-3-261. [DOI] [PubMed] [Google Scholar]
- Duray P. H., Johnson R. C. The histopathology of experimentally infected hamsters with the Lyme disease spirochete, Borrelia burgdorferi. Proc Soc Exp Biol Med. 1986 Feb;181(2):263–269. doi: 10.3181/00379727-181-42251. [DOI] [PubMed] [Google Scholar]
- Ferdows M. S., Barbour A. G. Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5969–5973. doi: 10.1073/pnas.86.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann G. S., Kramer M., Göbel U. B., Wallich R. Nucleotide sequence of a gene encoding the Borrelia burgdorferi flagellin. Nucleic Acids Res. 1989 May 11;17(9):3590–3590. doi: 10.1093/nar/17.9.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K., Bangsborg J. M., Fjordvang H., Pedersen N. S., Hindersson P. Immunochemical characterization of and isolation of the gene for a Borrelia burgdorferi immunodominant 60-kilodalton antigen common to a wide range of bacteria. Infect Immun. 1988 Aug;56(8):2047–2053. doi: 10.1128/iai.56.8.2047-2053.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K., Hindersson P., Pedersen N. S. Measurement of antibodies to the Borrelia burgdorferi flagellum improves serodiagnosis in Lyme disease. J Clin Microbiol. 1988 Feb;26(2):338–346. doi: 10.1128/jcm.26.2.338-346.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K., Pii K., Lebech A. M. Improved immunoglobulin M serodiagnosis in Lyme borreliosis by using a mu-capture enzyme-linked immunosorbent assay with biotinylated Borrelia burgdorferi flagella. J Clin Microbiol. 1991 Jan;29(1):166–173. doi: 10.1128/jcm.29.1.166-173.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindersson P., Knudsen J. D., Axelsen N. H. Cloning and expression of treponema pallidum common antigen (Tp-4) in Escherichia coli K12. J Gen Microbiol. 1987 Mar;133(3):587–596. doi: 10.1099/00221287-133-3-587. [DOI] [PubMed] [Google Scholar]
- Hyde F. W., Johnson R. C., White T. J., Shelburne C. E. Detection of antigens in urine of mice and humans infected with Borrelia burgdorferi, etiologic agent of Lyme disease. J Clin Microbiol. 1989 Jan;27(1):58–61. doi: 10.1128/jcm.27.1.58-61.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson M., Hovind-Hougen K., Svenungsson B., Stiernstedt G. Cultivation and characterization of spirochetes from cerebrospinal fluid of patients with Lyme borreliosis. J Clin Microbiol. 1990 Mar;28(3):473–479. doi: 10.1128/jcm.28.3.473-479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Barbour A. G. Enzyme-linked immunosorbent assays for Lyme disease: reactivity of subunits of Borrelia burgdorferi. J Infect Dis. 1989 Jan;159(1):43–49. doi: 10.1093/infdis/159.1.43. [DOI] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Johnson R. C. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J Infect Dis. 1987 Jul;156(1):183–188. doi: 10.1093/infdis/156.1.183. [DOI] [PubMed] [Google Scholar]
- Malloy D. C., Nauman R. K., Paxton H. Detection of Borrelia burgdorferi using the polymerase chain reaction. J Clin Microbiol. 1990 Jun;28(6):1089–1093. doi: 10.1128/jcm.28.6.1089-1093.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S. L., Young K. K., Barbour A. G. Detection of Borrelia burgdorferi DNA by the polymerase chain reaction. Mol Cell Probes. 1990 Feb;4(1):73–79. doi: 10.1016/0890-8508(90)90041-w. [DOI] [PubMed] [Google Scholar]
- Pallesen L., Hindersson P. Cloning and sequencing of a Treponema pallidum gene encoding a 31.3-kilodalton endoflagellar subunit (FlaB2). Infect Immun. 1989 Jul;57(7):2166–2172. doi: 10.1128/iai.57.7.2166-2172.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N. S., Axelsen N. H., Petersen C. S. Antigenic analysis of Treponema pallidum: cross-reactions between individual antigens of T. pallidum and T. Reiter. Scand J Immunol. 1981;13(2):143–150. doi: 10.1111/j.1365-3083.1981.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Persing D. H., Telford S. R., 3rd, Spielman A., Barthold S. W. Detection of Borrelia burgdorferi infection in Ixodes dammini ticks with the polymerase chain reaction. J Clin Microbiol. 1990 Mar;28(3):566–572. doi: 10.1128/jcm.28.3.566-572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister H. W., Einhäupl K., Preac-Mursic V., Wilske B., Schierz G. The spirochetal etiology of lymphocytic meningoradiculitis of Bannwarth (Bannwarth's syndrome). J Neurol. 1984;231(3):141–144. doi: 10.1007/BF00313682. [DOI] [PubMed] [Google Scholar]
- Rosa P. A., Schwan T. G. A specific and sensitive assay for the Lyme disease spirochete Borrelia burgdorferi using the polymerase chain reaction. J Infect Dis. 1989 Dec;160(6):1018–1029. doi: 10.1093/infdis/160.6.1018. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Shrestha M., Grodzicki R. L., Steere A. C. Diagnosing early Lyme disease. Am J Med. 1985 Feb;78(2):235–240. doi: 10.1016/0002-9343(85)90432-2. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Duray P. H., Butcher E. C. Spirochetal antigens and lymphoid cell surface markers in Lyme synovitis. Comparison with rheumatoid synovium and tonsillar lymphoid tissue. Arthritis Rheum. 1988 Apr;31(4):487–495. doi: 10.1002/art.1780310405. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Wallich R., Moter S. E., Simon M. M., Ebnet K., Heiberger A., Kramer M. D. The Borrelia burgdorferi flagellum-associated 41-kilodalton antigen (flagellin): molecular cloning, expression, and amplification of the gene. Infect Immun. 1990 Jun;58(6):1711–1719. doi: 10.1128/iai.58.6.1711-1719.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linde M. R., Crijns H. J., de Koning J., Hoogkamp-Korstanje J. A., de Graaf J. J., Piers D. A., van der Galiën A., Lie K. I. Range of atrioventricular conduction disturbances in Lyme borreliosis: a report of four cases and review of other published reports. Br Heart J. 1990 Mar;63(3):162–168. doi: 10.1136/hrt.63.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]