Abstract
On the basis of differences in physiology, e.g., histochemical properties and spindle density, and the structural design of the cat soleus (SO) and medial gastrocnemius (MG) muscles, we hypothesized that 1) fascicle length changes during overground walking would be both muscle and slope dependent, which would have implications for the muscles' force output as well as sensory function, and that 2) muscle-tendon unit (MTU) and fascicle length changes would be different, in which case MTU length could not be used as an indicator of muscle spindle strain. To test these hypotheses, we quantified muscle fascicle length changes and compared them with length changes of the whole MTU in the SO and MG during overground walking at various slopes (0, ± 25, ± 50, +75, and +100%). The SO and MG were surgically instrumented with sonomicrometry crystals and fine-wire electromyogram electrodes to measure changes in muscle fascicle length and muscle activity, respectively. MTU lengths were calculated using recorded ankle and knee joint angles and a geometric model of the hindlimb. The resultant joint moments were calculated using inverse dynamics analysis to infer muscle loading. It was found that although MTU length and velocity profiles of the SO and MG appeared similar, length changes and velocities of muscle fascicles were substantially different between the two muscles. Fascicle length changes of both SO and MG were significantly affected by slope intensity acting eccentrically in downslope walking (−25 to −50%) and concentrically in upslope walking (+25 to +100%). The differences in MTU and fascicle behaviors in both the SO and MG muscles during slope walking were explained by the three distinct features of these muscles: 1) the number of joints spanned, 2) the pennation angle, and 3) the in-series elastic component. It was further suggested that the potential role of length feedback from muscle spindles is both task and muscle dependent.
Keywords: tendon, locomotion, muscle spindle, pennation angle, proprioceptive feedback
since the beginning of the 20th century (see e.g., Refs. 11, 46), the ankle extensors in the cat have frequently been used as an experimental model to study the mechanical and neurophysiological properties of skeletal muscle. As a result, the anatomical, architectural, histochemical, physiological, and mechanical properties of these muscles have been well documented (1, 6, 43, 48), and great differences exist between them, even though they occupy anatomically similar locations. For example, the soleus (SO) muscle crosses only the ankle joint, is a parallel-fibered muscle with a relatively stiff muscle-tendon complex (the estimated ratio of external tendon to muscle belly length is small, ∼1; Ref. 36), and has a high density of muscle spindles (9). In contrast, the medial gastrocnemius (MG) muscle crosses both the ankle and knee joints, is highly pennate with a compliant muscle-tendon complex (the ratio of external tendon to muscle belly length is ∼5; Ref. 36), and has a lower density of muscle spindles (9). It has been shown that, during locomotion, these two ankle extensors contribute differently to the generation of force and mechanical energy (15, 18, 23, 34, 35, 49) and possibly to sensory input to the central nervous system (9, 36). Muscle fascicles are the major source of forces transmitted to the skeleton via various pathways (27) as well as proprioceptive information about muscle length and velocity (42). To date, however, muscle fascicle length changes, recorded in vivo in the cat, have only been reported for the MG during walking on level and minimally sloped (10%) surfaces (21, 25). A comprehensive analysis of in vivo fascicle behavior in the one-joint SO and two-joint MG muscles during cat locomotion is thus lacking. A primary motivation for this study, then, was to obtain this information and gain additional insight into the role that unique features of these muscles play in their force production and sensory function during locomotion.
The behavior of a muscle appears to change in response to alterations in movement demands. For example, fascicles of the lateral gastrocnemius (LG) muscle in the turkey have been shown to work almost isometrically during locomotion on level terrain and concentrically during locomotion on a positive slope (41). This suggests that the turkey ankle extensors during level locomotion are used to economically generate high forces by undergoing small muscle fascicle length changes and to facilitate elastic energy storage and release (5), whereas during upslope walking, they mostly generate mechanical energy. On the basis of what is known about the differences in architecture, mechanical properties, and histochemistry between the cat SO and MG (see above), one would expect differential mechanical responses of these muscles to changes in slope. Thus the first goal of this study was to investigate the effects of slope (0, ±25, ±50, +75, and +100%) on fascicle length changes of SO and MG muscles during overground walking in the cat. We predicted that the fascicle behavior of both muscles would change from predominantly eccentric in downslope walking to predominantly concentric in upslope walking.
Besides motor output, muscles also provide the central nervous system with sensory information regarding muscle length and force through muscle spindles and Golgi tendon organs, respectively. Relating changes in muscle mechanical behavior to potential feedback from peripheral afferents is frequently used to study the neural control of locomotion (19, 38). Muscle-tendon unit (MTU) strain is often measured as an indicator of length-feedback despite the fact that muscle fascicle strain appears to be a more accurate representation of muscle spindle strain (29). (Note: muscle spindles lie in parallel with the extrafusal skeletal muscle fibers.) Therefore, the second goal of this study was to compare MTU and fascicle length changes during slope walking. On the basis of the architectural characteristics of each muscle and their function during walking, we hypothesized that length patterns of MTU and fascicles would be more similar in SO than in MG.
We present the first study in which muscle fascicle lengths of SO and MG, two physiologically and structurally different muscles occupying the same general anatomical location, are examined for such a broad range of walking conditions in the cat. Preliminary results have been presented in abstract form (30).
MATERIALS AND METHODS
Animal Care and Training
All surgical and experimental procedures were in agreement with the “Principles of Laboratory Animal Care” (NIH Publication No. 86-23, Revised 1985) and approved by the Institutional Animal Care and Use Committee of the Georgia Institute of Technology. Adult female cats [Felis domesticus, n = 5, mean body mass = 3.3 kg (SD 0.4)] were selected on the basis of friendliness and attraction to food reward. All cats were housed in one large room with adequate space and access to food and water ad libitum.
Before the experimental measurements were taken, each cat was trained to walk within a Plexiglas-enclosed walkway (2.5 × 0.4 m) on a level surface (0%) as well as on up- and down-sloped surfaces (±25, ±50, +75, and +100%, i.e., ±14, ±27, +37, and +45°) using operant conditioning procedures involving food reward (see e.g., Ref. 37). On average, each cat was trained for 2–4 wk, 5 days/wk, 2 h/day. The walkway surface was covered with a thin layer of nonslip rubberized material to prevent paw slippage during slope walking. The mat over the force plate (see Data Collection) was isolated from the remainder of the walkway.
Surgical Procedures
Under aseptic conditions with isoflurane anesthesia, the right hindlimb was surgically instrumented with sonomicrometry crystals and fine-wire electromyogram (EMG) electrodes. Connecting wires were passed subcutaneously to one of the two head-mounted nine-pin amphenol connectors. For muscle fascicle length measurements, piezoelectric crystals (2 mm; Sonometrics, London, ON, Canada) were implanted in MG and SO muscles. Procedures for implanting these transceivers were based on those described by Biewener et al. (2). Specifically, one pair of piezoelectric crystals was implanted near the origin and insertion (i.e., proximal and distal aponeurosis) of a muscle fascicle in the midregion of each muscle, taking its architecture (i.e., pennation angle) into account. A pair of Teflon-insulated multistranded stainless steel wires (100-μm diameter; Cooner Wire, Chatsworth, CA) was implanted chronically near the sites of muscle fascicle length measurement in the MG and SO muscles for measurement of muscle activity (EMG). EMG electrode placement was verified by stimulation through the implanted wires during surgery. After the cut skin was sutured, the animal was allowed to recover for at least 1 wk before data collection.
Data Collection
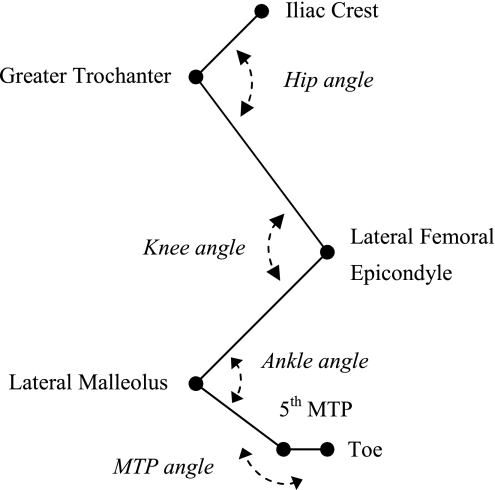
Joint position data, ground reaction forces, and EMG signals were collected according to procedures described previously (19). In short, the right hindlimb was shaved and reflective markers (6-mm diameter) were placed over the iliac crest (IC), greater trochanter, lateral malleolus, fifth metatarsophalangeal joint, and the distal end of the fifth digit (Fig. 1). To minimize the influence of skin movement, knee joint position was estimated by triangulation using hip and ankle coordinates as well as thigh and shank segment lengths. The cats walked within the walkway set at one of the five slope conditions (see Animal Care and Training) at a freely chosen speed. One of the five cats (cat 1) was tested in only three slope conditions (i.e., 0 and ±50%). Segment endpoint position data (120 Hz) and ground reaction forces (360 Hz) were recorded synchronously by a six-camera Vicon 460 motion capture system (Vicon, Oxford, UK). Ground reaction forces were collected using a miniature force plate (0.11 × 0.16 m; Bertec, Columbus, OH) concealed in the walking surface. EMG and sonomicrometry signals were recorded using two separate data acquisition systems. Each channel of EMG was sampled at a rate of 3,000 Hz, and the sonomicrometry signals were recorded at 1,059.3 Hz, with the latter determined by the Sonometrics software. Data collection was started by a trigger pulse to the Sonometrics system. This was immediately followed by a common transistor-transistor logic (TTL) pulse to the Vicon and EMG computers, which was also recorded by an analog channel of the Sonometrics system to indicate the beginning of Vicon-EMG sampling for synchronization of the different systems.
Fig. 1.
Definition of joint angles. The anatomical markers for the endpoints of the segments (i.e., pelvis, thigh, shank, tarsals, and digits) over which the reflective markers were placed are indicated: iliac crest, greater trochanter, 5th metatarsophalangeal joint (MTP), and the most distal part of the fifth digit (toe). Note that knee joint position was estimated by triangulation using hip and ankle coordinates as well as thigh and shank segment lengths.
Data Analysis
Step cycles were accepted only if the cat walked with an uninterrupted gait at a steady pace through the walkway and the right hindlimb made contact with the force plate independently of the surrounding surface. The former was assessed by the velocity of the IC marker parallel to the walking surface. Because the cats had difficulty walking at a steady pace at the 75 and 100% downslope conditions, only upslope walking data are reported for these conditions. In addition, steps in any walking trial in which paw slippage occurred were not used for analysis.
To test the effects of slope and minimize the effects of walking speed, we selected step cycles within a narrow range of stance durations (range ∼200 ms) for each cat, which yielded a total of 201 step cycles (Table 1). The selection of step cycles resulted in similar step cycle parameters for each condition (Table 1). However, for a few of the walking conditions, this criterion could not be met, and step cycles with longer (e.g., cat 1, 50% downslope) stance durations, for example, were included (Table 1). Paw contact (PC) and paw liftoff (PO) were identified using the ground reaction force data. For the SO muscle, a consistent sonomicrometry signal could be obtained in four of the five cats (cats 1, 2, 4, and 5). This also was the case for MG muscle (cats 1, 2, 3, and 4). Consistent EMG signals of both muscles were obtained from all five cats. All data were time-normalized with respect to stance or swing duration. Linear interpolation between measured points was used to compute a value for each 0.5% of the stance phase and a value for each 1% of the swing phase.
Table 1.
Individual step cycle parameters and the number of included step cycles for analysis of the effects of slope condition on MTU length, fascicle length, and joint moments
| Walking Condition, %slope |
|||||||
|---|---|---|---|---|---|---|---|
| −50 | −25 | 0 | +25 | +50 | +75 | +100 | |
| Speed, m/s | |||||||
| Cat 1 | 0.28 (0.02) | 0.40 (0.04) | 0.45 (0.02) | ||||
| Cat 2 | 0.73 (0.08) | 0.76 (0.05) | 0.70 (0.06) | 0.61 (0.05) | 0.60 (0.07) | 0.72 (0.09) | 0.94 (0.10) |
| Cat 3 | 0.69 (0.06) | 0.73 (0.08) | 0.50 (0.05) | 0.55 (0.05) | 0.61 (0.03) | 0.61 (0.08) | 0.61 (0.09) |
| Cat 4 | 0.71 (0.06) | 0.70 (0.11) | 0.69 (0.07) | 0.69 (0.08) | 0.71 (0.06) | 0.74 (0.06) | |
| Cat 5 | 0.56 (0.05) | 0.54 (0.06) | 0.60 (0.08) | 0.71 (0.12) | 0.58 (0.06) | 0.78 (0.07) | 0.66 (0.07) |
| Stance time, ms | |||||||
| Cat 1 | 721 (18) | 560 (64) | 537 (46) | ||||
| Cat 2 | 378 (35) | 357 (16) | 424 (34) | 466 (42) | 508 (54) | 445 (58) | 360 (29) |
| Cat 3 | 447 (46) | 401 (55) | 539 (41) | 485 (41) | 485 (23) | 501 (80) | 543 (76) |
| Cat 4 | 358 (30) | 360 (77) | 380 (35) | 389 (50) | 430 (45) | 428 950) | |
| Cat 5 | 435 (31) | 481 (47) | 431 (52) | 417 (67) | 463 (50) | 388 (20) | 483 (42) |
| Swing time, ms | |||||||
| Cat 1 | 367 (35) | 419 (25) | 283 (19) | ||||
| Cat 2 | 217 (21) | 243 (10) | 250 (13) | 249 (22) | 210 (22) | 194 (26) | 177 (14) |
| Cat 3 | 258 (12) | 291 (32) | 369 (36) | 326 (26) | 290 (32) | 326 (27) | 320 (36) |
| Cat 4 | 278 (21) | 298 (21) | 257 (14) | 271 (26) | 258 (26) | 220 (14) | |
| Cat 5 | 297 (22) | 290 (28) | 309 (24) | 273 (26) | 284 (30) | 221 (26) | 287 (16) |
| No. of step cycles | |||||||
| Cat 1 | 2 | 6 | 10 | ||||
| Cat 2 | 12 | 7 | 9 | 11 | 5 | 7 | 5 |
| Cat 3 | 6 | 11 | 7 | 16 | 6 | 6 | 5 |
| Cat 4 | 3 | 5 | 5 | 7 | 5 | 5 | |
| Cat 5 | 5 | 4 | 9 | 5 | 8 | 4 | 5 |
Values are means (SD). It should be noted that for the soleus (SO) muscle, a reliable sonomicrometry signal could be obtained in only 4 of the 5 cats (poor signal in cat 3). For medial gastrocnemius (MG) muscle, reliable sonomicrometry signals were obtained in all cats except cat 5. MTU, muscle-tendon unit.
Muscle-tendon unit length.
For MG and SO muscles, MTU lengths during the selected step cycles were calculated using the ankle and knee joint angles and the geometric model presented by Goslow et al. (17). Joint angles (Fig. 1) were determined from the filtered segment endpoint position data (low-pass, fourth-order, zero-lag Butterworth filter; 98.5% of the power spectrum of the signal was used to determine the cutoff frequency, which was typically between 5 and 10 Hz) and a five-segment two-dimensional model of the cat hindlimb described in detail elsewhere (31). After MTU lengths were calculated, MTU velocities were computed using the method of finite differences. Positive velocity values indicated MTU stretch; negative values indicated shortening. MTU length was expressed relative to the resting length, which was calculated by averaging the maximum and minimum lengths in swing during level walking (16).
Muscle fascicle length.
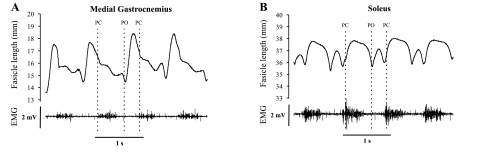
The distance between the sonomicrometry crystals was obtained by measuring the transit time of the ultrasound (∼1.5 MHz) pulses between the two crystals that emit and receive them. This transit time was then converted to a linear length measure using the speed of sound in vertebrate skeletal muscle (i.e., 1,540 m/s, see Ref. 3). Exemplar recordings (cat 2) of fascicle length in MG and SO during walking on a level surface together with their EMG signals are shown in Fig. 2. These length measures were corrected for the offset error (0.82 mm) introduced by the faster speed of sound through the epoxy lens of the crystals (4).
Fig. 2.
Representative fascicle length patterns and electromyogram (EMG) signals of medial gastrocnemius (MG; A) and soleus (SO; B) muscles recorded synchronously for 4 step cycles during walking on a level surface (cat 2). PC, paw contact; PO, paw liftoff.
Muscle dissection was performed postmortem (see below) to determine the fraction of total fascicle length corresponding to the distance between the crystals. In all muscles of all cats, the crystals were inserted near the fascicle origin and insertion, except in cat 1 for which the distance between crystals was only 65% in SO and 80% in MG of the full fascicle length. After collection and low-pass filtering of fascicle length data (same filtering procedures as for segment endpoint position data), fascicle velocities were computed using the method of finite differences. Positive velocity values indicated fascicle stretch; negative values indicated shortening. Fascicle length was expressed relative to the resting length, which was calculated by averaging the maximum and minimum lengths in swing during level walking (16).
Joint moments.
Joint moments at the ankle and knee were calculated using Newton-Euler equations of motion and the methods of inverse dynamics as reported previously (15, 37). The hindlimb was modeled as a five-segment (Fig. 1) planar, rigid body system. Segment mass, center of mass location, and the moment of inertia for each segment were calculated using regression equations derived by Hoy and Zernicke (26). Flexor and extensor joint moments were used to characterize loading of the flexor and extensor muscles, respectively. Extensor moments were assigned positive values, and their peaks in the stance phase were identified.
Muscle activity.
Before analysis, the EMG signals were band-pass filtered (30–1,000 Hz, 3 dB) and full wave rectified. The mean and peak EMG magnitude in the step cycle and EMG burst duration before and after PC were determined. To assess the onset and offset of EMG for each muscle, we calculated the mean and standard deviation (SD) of EMG values during muscle silent periods (i.e., most of the swing phase, see Fig. 2). The muscle was considered “on” if its EMG values exceeded 2 SDs of the silent mean EMG for at least 50 ms. Correspondingly, the times of EMG onset and offset were determined when EMG values became greater and smaller, respectively, than the 2 SD threshold. EMG means and peaks were normalized to their maximum values observed across all conditions within each cat (usually upslope walking). Because of a low activity level of MG muscle during downslope walking (typically lower than the 2 SDs of the mean muscle silent period), MG EMG parameters could not be consistently determined for those conditions. Because of intermittent electrical interference between the sonomicrometry and the EMG signals, a separate set of step cycles was used for EMG analysis. To test the effects of slope on muscle activity, we used a total of 251 step cycles for SO and 133 step cycles for MG. Step cycle parameters (i.e., walking speed, stance time) were similar to those in trials presented in Table 1.
Statistics
Statistical analysis focused on the stance phase, because this is when MG and SO muscles are predominantly active during walking (19, 49). To evaluate the effects of slope condition on fascicle and MTU length, joint moments, and EMG characteristics, we performed several one-way ANOVAs. If significant, the above variables were tested for differences between walking conditions by using t-tests with Bonferroni correction. The values of the variables obtained in every step cycle of each cat except cat 1 were used together in this analysis. The data of cat 1 were excluded because not all slope conditions were tested and the walking speed was significantly lower than in the other animals. P values <0.05 were considered statistically significant. All statistical tests were performed using SPSS (version 11.5; SPSS, Chicago, IL).
RESULTS
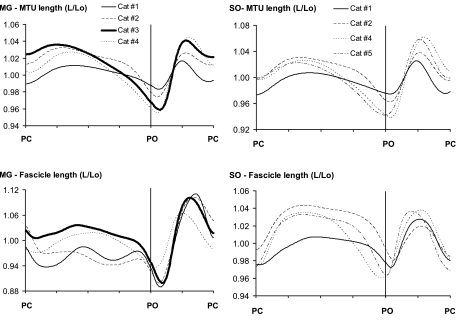
The MTU and fascicle length trajectories of SO and MG muscles were similar across four cats as seen from length data of each cat shown for walking on a level surface (Fig. 3). Note that the length patterns of cat 1, although similar qualitatively, were shifted down in early stance and up in midstance. As a result, the peak stretch and shortening velocities of MTUs and fascicles of this cat were profoundly different from those of the other cats. This also was the case for the other slope conditions. For this reason and because cat 1 walked slower and was not tested at all slope conditions (see Statistics), the data from this cat were not included in the means and statistical results presented. Nevertheless, variations in slope angle altered the different length parameters in a qualitatively similar way as in the other cats.
Fig. 3.
Mean normalized muscle-tendon unit (MTU) and fascicle length of MG and SO muscles as a function of normalized cycle time (in arbitrary units) during walking on a level surface for each of 4 cats for each muscle. An increase in length corresponds to MTU and fascicle stretch; a decrease corresponds to shortening. L, length, Lo, resting length, determined as the mean of the maximum and minimum lengths in swing during level walking.
Effects of Positive Slopes on MG and SO Muscle Behavior
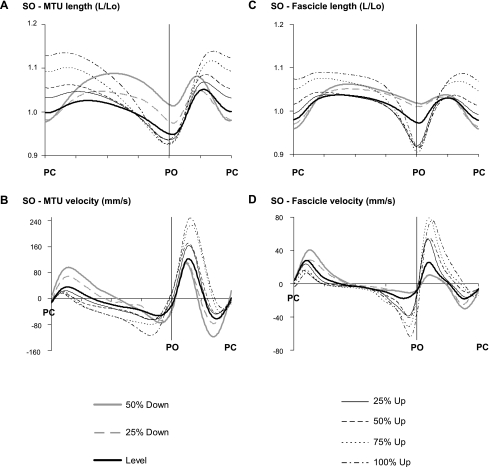
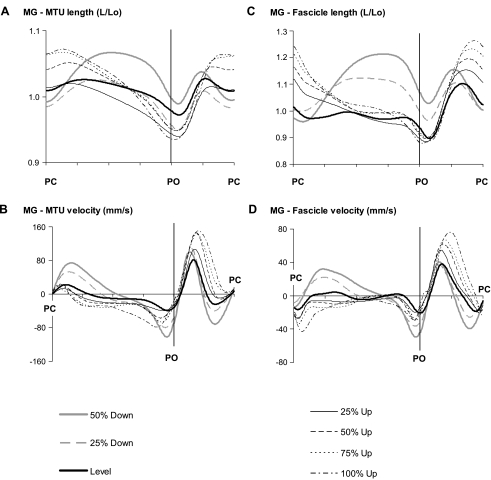
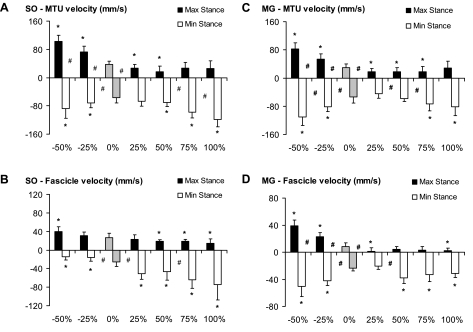
MTU length (Figs. 4A and 5A) at paw contact was greater with steeper positive slopes compared with level walking in both the MG and SO muscles. MTU stretch and peak stretch velocity during stance in upslope walking were significantly smaller than in level walking, but no significant differences in peak velocities between upslope conditions were found (Fig. 6). The extent of MTU shortening during stance increased significantly with intensity in upslope walking (0 < 25 < 50 < 75 < 100%). This also was reflected in higher peak MTU shortening velocities. In contrast to the MTU results, length and velocity patterns of SO fascicles (Fig. 4, C and D) were substantially different from those in the MG (Fig. 5, C and D). The general pattern of length changes in SO fascicles was similar to that observed at the MTU level. For example, fascicle shortening and peak shortening velocity (end of stance) were significantly higher for the upslope conditions compared with level walking. However, peak fascicle velocity was significantly lower than peak MTU velocity (Fig. 6). As a consequence, discrepancies between MTU and fascicle length changes were found for the SO. (Note: in midstance, the fascicles operated almost isometrically, whereas shortening was observed at the MTU level.)
Fig. 4.
Length and velocity of SO MTU and muscle fascicles as a function of normalized cycle time (in arbitrary units) for all slope conditions. A: MTU length. B: MTU velocity. C: fascicle length. D: fascicle velocity. Means across 3 cats (cats 2, 4, and 5) are shown. Positive velocity values correspond to MTU and fascicle stretch; negative values correspond to shortening.
Fig. 5.
Length and velocity of MG MTU and muscle fascicles as a function of normalized cycle time (in arbitrary units) for all slope conditions. A: MTU length. B: MTU velocity. C: fascicle length. D: fascicle velocity. Means across 3 cats (cats 2, 3, and 4) are shown. Positive velocity values correspond to MTU and fascicle stretch; negative values correspond to shortening.
Fig. 6.
Peak lengthening (+) and shortening (−) velocities during stance for all slope conditions. A: SO MTU velocity. B: SO fascicle velocity. C: MG MTU velocity. D: MG fascicle velocity. Mean and SD including the step cycles of 3 cats are shown (i.e., cats 2, 4, and 5 for SO; cats 2, 3, and 4 for MG). *P < 0.05, significant difference from 0% (level walking, shaded bars). #P < 0.05, significant difference between 2 bordering conditions.
Greater differences between MTU and fascicle length patterns were observed in MG muscle (Fig. 5). During early stance in level and upslope walking, when MTU lengthening was found (Fig. 5A), the MG fascicles were shortening. In level walking, this was followed by a short lengthening phase, but the upslope conditions consisted mainly of shortening, an observation supported by the close-to-zero peak lengthening velocities (Fig. 6D). For the steeper slopes (50–100%), peak shortening velocity of fascicles was significantly higher than with level walking.
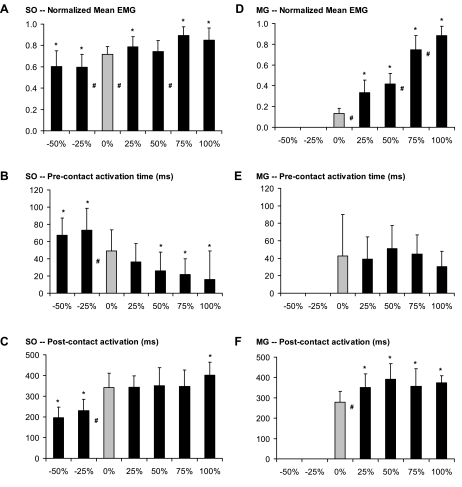
The mean and peak normalized EMG in both muscles increased significantly during upslope walking compared with level walking, but modulation of EMG magnitude was more pronounced in MG (the mean changed from 0.13 to 0.88 in MG vs. 0.72 to 0.89 in SO; Fig. 7, A and D). In both muscles, the EMG burst started just before paw contact. Although this precontact activation time was independent of slope in MG, the SO precontact activation time decreased significantly in upslope walking. In contrast, the postcontact activation time was increased in MG during upslope walking, but no significant differences were found between the different upslope conditions. Finally, in SO, significantly longer postcontact activation time was found only for the steepest slope (100%).
Fig. 7.
Normalized mean values and timing of EMG bursts in SO and MG muscles for all slope conditions. A: SO normalized mean EMG. B: SO precontact activation time. C: SO postcontact activation time. D: MG normalized mean EMG. E: MG precontact activation time. F: MG postcontact activation time. Peak EMG data resemble those of mean EMG and are therefore not shown. Precontact activation time is the time between EMG onset and PC; postcontact activation time is the time from PC to burst cessation. Mean and SD including the step cycles of 4 cats are shown (cats 2, 3, 4, and 5). *P < 0.05, significant difference from 0% (level walking). #P < 0.05, significant difference between 2 bordering conditions.
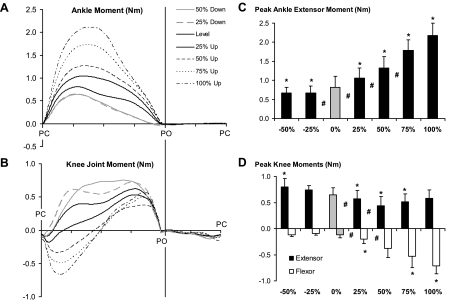
Because the hindlimb does not support body weight during swing, the largest joint moments were observed during stance. The magnitude of the ankle extensor moment (Fig. 8, A and C) increased progressively from walking on a level surface to walking on a positive slope of 100%. At the knee (Fig. 8B), a flexor moment was calculated in early stance, changing to an extensor moment later in stance. Peak flexor moment increased from level walking to the 100% upslope condition, but the peak knee extensor moment was essentially the same in upslope and level walking (Fig. 8D).
Fig. 8.
Mean joint moment patterns of ankle (A) and knee joints (B) as a function of normalized cycle time (in arbitrary units) for all slope conditions as well as the respective mean (±SD) peak moments during stance (C and D). Means include the data of 4 cats (cats 2, 3, 4, and 5). Positive values correspond to extensor moments; negative values correspond to flexor moments. *P < 0.05, significant difference from 0% (level walking). #P < 0.05, significant difference between 2 bordering conditions.
These results suggest that changing the walking surface from level to a positive slope alters the contractile behavior of each muscle in a qualitatively similar fashion. MG muscle action clearly changed from predominantly isometric to concentric when the slope increased. A shift toward concentric muscle action was also found for SO muscle: less lengthening in early stance, earlier start of fascicle shortening in midstance, and significantly greater shortening at the end of stance. Even though EMG and joint moments are indirect estimates of loading in individual muscles, they suggest that walking on a positive slope increases the load during stance in SO (increased EMG, higher ankle extensor moment) and MG (increased EMG, higher ankle extensor and knee flexor moments). The EMG data indicate that the load in MG muscle is altered more profoundly than the load in SO as positive slope intensity increases.
Effects of Negative Slopes on MG and SO Muscle Behavior
Walking downslope significantly enhanced MTU lengthening and peak stretch velocity in the first half of the stance phase compared with walking on a level surface for both SO (Figs. 4 and 6) and MG (Figs. 5 and 6). In both muscles, MTU shortening started later in stance, but peak shortening velocities were significantly higher than in level walking. In contrast to level walking, the length patterns in MG muscle fascicles during downslope walking appeared similar to those at the MTU level. For example, high fascicle lengthening and stretch velocities were found in early stance. MTU and fascicle length changes in SO followed similar trends, except at the end of stance. In this phase, the MTU showed greater shortening and higher velocities during downslope compared with level walking. Fascicle shortening, however, was minimal and peak shortening velocity was significantly lower.
Because of low amplitude (see materials and methods), no EMG data were processed for the MG during downslope walking. It should be noted for purposes of further discussion, however, that MG was still active during all downslope conditions studied, an observation supported in the literature (19, 47). The mean and peak normalized EMG in SO were significantly lower in downslope compared with level walking. Similar to the results for the positive slopes, these changes were small (i.e., from 0.72 to 0.59; Fig. 7A). Also, the timing of EMG bursts was altered significantly during downslope walking; precontact activation time increased (Fig. 7B), whereas postcontact activation time decreased (Fig. 7C).
The ankle extensor moment (Fig. 8A) was lower in downslope walking than in level walking. However, no significant differences in peak ankle moment between downslope conditions were found (Fig. 8C). Similar to level walking, the knee flexor moment during downslope walking was small and present only at the very beginning of stance. The knee extensor moment was higher for most of the stance phase of downslope walking than the moment calculated for level walking (Fig. 8, B and D).
These results suggest that walking downslope involves eccentric activity in SO as well as MG during the first half of stance. Note that in the MG, 1) very low EMG activity was present in downslope walking, and 2) predominantly isometric behavior was found during level walking. Furthermore, walking downslope appears to decrease the muscle load during stance in SO (decreased EMG, lower ankle extensor moment) as well as in MG (very small EMG values, lower ankle extensor moment).
DISCUSSION
The first goal of this study was to investigate the effects of slope (0, ±25, ±50, +75, and +100%) on fascicle length changes of two distinctly different hindlimb muscles in overground walking in the cat (i.e., SO and MG). We hypothesized that fascicle length changes would be both muscle and slope dependent and predicted that fascicle behavior in both muscles would change from predominantly eccentric in downslope to predominantly concentric in upslope walking. For this objective, we quantified fascicle length changes, patterns of muscle activity, and joint moments in the above-mentioned conditions. We found our predictions to be true. Our second goal was to compare MTU and fascicle length changes under the same experimental conditions to determine whether they were similar, in which case MTU length could be used as an indicator of muscle spindle strain. As hypothesized, greater discrepancies between MTU and muscle fascicle length changes were observed in MG than in SO. In our discussion of these results we have highlighted three features distinguishing these two muscles (i.e., the number of joints spanned, the pennation angle, and the in-series elastic component) that could explain most of the observations.
Features of SO and MG Affecting Differential Behavior of MTU and Fascicles
Number of joints spanned.
In all slope conditions, the SO muscle experienced larger MTU length changes and peak velocities than MG. This can be explained by the fact that the SO is a one-joint muscle whereas the MG spans both the ankle and knee joints, demonstrating the coordinated pattern of movements during walking (i.e., both joints either simultaneously flex or simultaneously extend, Ref. 19). Because the SO and MG have the same moment arm about the ankle (7), MTU length changes as a function of ankle angle are equal in both muscles. Thus the smaller MTU length changes in the MG compared with those in the SO originate from the knee joint motion.
Pennation angle.
The differences in length changes between MG and SO were found to be more pronounced at the muscle fascicle level. For example, although the fascicles of SO muscle were lengthening in early stance during all slope conditions, MG fascicle lengthening was found only during downslope walking. As described in the inroduction, there are several differences between SO and MG contributing to this finding. One of them is that SO has a more parallel fiber arrangement (angle of pennation ∼6°) compared with the highly pennated MG (∼21°) (43). In situ experiments have shown that pennation effects reduce the ratio of fascicle velocity to muscle belly velocity in maximally activated rat MG (52). This means that part of the muscle length change originates from a change in fascicle pennation angle rather than a change in fascicle length. The finding that MG muscle fascicles can shorten while the MTU is lengthening also may be partly mediated by this phenomenon. However, for the conditions that involved active lengthening of MTU and fascicles in both muscles (i.e., −50%), the difference in MTU-fascicle velocity was smaller in the pennate MG than in the parallel-fibered SO.
In-series elastic component.
The fact that the difference between MTU and fascicle velocities was smaller in the MG than in the SO suggests that muscle-specific changes in fascicle length may be caused to a greater extent by differences in length changes of the elastic tissues that lay in series with the muscle fascicles (i.e., aponeurosis and tendon). The in-series structures can take up a considerable part of the MTU length changes due to their relatively high compliance, especially when at the toe region of the stress-strain curve (40). This behavior is dependent on the compliance of the series elastic tissues and on the force exerted on them. Note that SO has long fascicles and relatively short tendons compared with MG muscle (10). These characteristics of SO are likely to result in more comparable length changes in MTU and muscle fascicles as opposed to MG, even if equal forces are produced by these muscles. However, previous studies have reported different forces exerted at the distal tendons of SO and MG during locomotion (e.g., 15, 20, 28, 49, 50). The EMG data presented in this study (Fig. 7) also show differences between SO and MG in the modulation of activity and its timing with changes in slope, suggesting that changes in MG force were greater than changes in SO force (see also Refs. 20, 28). Thus, with similar MTU length changes, greater differences in fascicle length behavior between these muscles may be expected. Our fascicle length results support this conclusion.
However, some of the obtained results are not as easily explained by the above three features of SO and MG. For example, peak shortening velocity in SO muscle fascicles at the end of stance always occurred at a point later in time than the peak shortening velocity of the MTU. During the upslope conditions, this resulted in a slightly higher shortening velocity of the fascicles in SO than in the MTU (Fig. 4), which was most pronounced at 100% slope. Since muscle force is declining at the end of stance (28), this cannot be explained by lengthening of the in-series elastic tissues. It should be noted that earlier in stance, shortening velocities in MTU were higher than in the fascicles. This suggests that the tendon was recoiling at speeds not achievable by the muscle fascicles. Later, at the very end of stance, the fibers were “catching up.” A similar muscle fascicle-tendon interaction has been observed during a quick MTU release experiment (44). Comparing the shortening velocities of the muscle fascicles with data from the literature (e.g., Ref. 45) shows good agreement (i.e., the peak shortening velocities found in our study are well below the maximal shortening velocity values reported). Also, in a maximally excited and isolated SO muscle, the shortening velocities of the MTU were consistently higher than those of the fascicles (45).
Another less intuitive finding was the higher lengthening velocities of the MTU compared with the fascicle velocities during swing, when the muscles are mainly inactive. In particular for MG, this may be partly explained by length amplification effects of decreasing pennation angle during muscle lengthening (52). Because the difference in MTU and fascicle velocity during this phase of the step cycle was higher for SO than for MG, this was most likely mediated by other effects. Except for downslope walking, the muscles were at their lowest length in the beginning of swing. It is likely that at this shorter length and low active state of the muscle (Figs. 2 and 7), the in-series connective tissues are slack or operating near the toe region of the stress-strain curve (36). During the uptake of slack in the in-series tissues, the MTU can be lengthened by external forces without length changes in the muscle fascicles.
Muscle Fascicle Length as an Indicator for Muscle Spindle Strain in MG and SO
In studying the neural control of locomotion, mechanical variables are frequently used to judge potential feedback from peripheral afferents. MTU length is typically used to assess the role of length feedback from muscle spindles, assuming a given fusimotor drive and presynaptic inhibition (19, 38). Since muscle spindles are located among the extrafusal fibers, muscle fascicle length is more closely related to muscle spindle strain than is MTU length and, thus, seems to be better suited as the mechanical variable to estimate strain of the muscle spindles and, indirectly, activity of Ia and II afferents (29). Although many agree with this view (13, 14, 21, 25, 39), it remains open to debate (see recent comment in Ref. 51). In contrast to our results, Elek et al. (12) reported small discrepancies between MTU and fascicle length changes. Accordingly, only subtle effects of the in-series compliance on muscle spindle output were observed. However, spindle firing was measured with MTU length changes and muscle activation imposed on an isolated MG or LG, which was meant to mimic the in vivo muscle conditions of a cat step cycle during walking on a level surface. A possible explanation for their different conclusion is that the MTU length changes they imposed deviated from MTU length trajectories observed in this and several previous reports (17, 19, 25). MTU length was simulated to decrease after paw contact and to remain at a constant length in the remainder of stance (see Fig. 2 in Ref. 12). The present results as well as the above-mentioned previous studies all indicate that MTU length of both MG and LG increases in early stance. Note that this is the portion of the stance phase in which the effects of in-series compliance on the MTU-fascicle relationship are most pronounced.
If muscle fascicle length and velocity are related to muscle spindle strain, the present results suggest that the potential role of length feedback is both task and muscle dependent. Note that a large contribution of length feedback to muscle output is found during active fascicle lengthening, whereas only a limited contribution of length feedback is expected during shortening contractions (32). Thus Ia and II afferent activity from MG and SO in stance during downslope walking is likely to be higher than during level and upslope walking. With regard to muscle dependency, the potential contribution of length feedback from SO in modulating muscle activity in stance during level and upslope walking is expected to be higher than that from MG muscle, in which the fascicles are shortening during early stance. This fits well with the observation that SO muscle has a greater density of muscle spindles than gastrocnemius (9).
Limitations of MTU and Muscle Fascicle Length Measurements
MTU length was not directly measured but was estimated from two-dimensional kinematic data of the ankle and knee joints and the geometric model presented by Goslow et al. (17). In that report, the input parameters for MTU length calculations (i.e., segment lengths, distances between joint centers of rotation and muscle attachment points) were reported for three weight groups of cats. We used the original data set from 50 cats (Goslow GE Jr., personal communication) to calculate the linear regression equations for estimation of the input parameters (a technique previously reported, Ref. 50). A major potential source of error in this method is that muscles are modeled as straight lines. This assumption is likely to affect MTU length estimates of MG, because it wraps around the knee joint when the joint is close to full extension. We estimated this error by calculating MTU length during level walking, allowing for wrapping MG around the knee arc with different radii (3, 5, and 10 mm). The errors due to the straight line assumption were within ∼2% of the MTU length. Furthermore, the use of two-dimensional kinematics, and thus assuming that locomotion is planar, will result in length underestimations (50). Nevertheless, the errors of our MTU length estimations seem relatively small, which is also confirmed by the agreement of our data with MTU lengths measured directly by implanted length transducer (25).
There are several sources of error when using sonomicrometry to measure muscle fascicle length. These have been discussed extensively in several previous reports (3, 21, 33) and are therefore described only briefly in this report. The main assumption with this method is that sound travels at a constant speed through the tissue during the active and passive phases of the step cycle. Because only very small speed changes were found during a tetanic muscle contraction compared with passive muscle (1.3% in vitro, Ref. 22; up to 0.4% in situ, Ref. 8), a minimal error was introduced. When implanting the crystals, care was taken to align the line connecting the crystals with the fiber direction. However, errors in fascicle length due to misalignment of the crystals relative to the fascicle axis are relatively small. This error is proportional to the cosine of the angle between the crystals' line and fascicle axis: <1% for misalignment of up to 8° and <5% for misalignment of up to 18° (calculated for MG muscle fascicles). We further implanted the crystals in such a way that fascicle lengths in the midsagittal plane were monitored. Effects of fiber curvature due to shape changes of the muscle are minimal in this plane. Although regional differences in fascicle length changes may occur within a muscle (24), fascicle lengths in the midbelly region were assumed to provide a representative sample of the whole muscle. Finally, effects of crystal movements not related to fascicle length changes due to a poor immobilization in the muscle are expected to be negligible due to the fact that all measurements were performed at least a week after the implantation surgery, which was sufficient for the crystals to be tightly embedded within the muscle tissue (Biewener AA, personal communication). This conclusion was confirmed by low variability of fascicle length measurements within each cat.
GRANTS
This research was supported by National Institutes of Health Grants HD032571 and NS048844 as well as the Center for Human Movement Studies at the Georgia Institute of Technology.
Acknowledgments
We thank Dr. Andrew Biewener for overtly showing his surgical and experimental procedures to measure muscle fascicle length using sonomicrometry and Dr. Alan Sokoloff for allowing the use of the Sonometrics system. In addition, we thank Dr. Guayhaur Shue for technical support and Denise Larkins for help with data analysis.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ariano MA, Armstron RB, Edgerton VR. Hindlimb muscle fiber populations of 5 mammals. J Histochem Cytochem 21: 51–55, 1973. [DOI] [PubMed] [Google Scholar]
- 2.Biewener AA, Corning WR, Tobalske BW. In vivo pectoralis muscle force-length behavior during level flight in pigeons (Columba livia). J Exp Biol 201: 3293–3307, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Biewener AA, Konieczynski DD, Baudinette RV. In vivo muscle force-length behavior during steady-speed hopping in tammar wallabies. J Exp Biol 201: 1681–1694, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Biewener AA, McGowan C, Card GM, Baudinette RV. Dynamics of leg muscle function in tammar wallabies (M. eugenii) during level versus incline hopping. J Exp Biol 207: 211–223, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Biewener AA, Roberts TJ. Muscle and tendon contributions to force, work, and elastic energy savings: a comparative perspective. Exerc Sport Sci Rev 28: 99–107, 2000. [PubMed] [Google Scholar]
- 6.Burke RE Motor units: anatomy, physiology, and functional organization. In: Handbook of Physiology. The Nervous System. Motor Control. Bethesda, MD: Am. Physiol. Soc., 1981, p. 345–422.
- 7.Burkholder TJ, Nichols TR. Three-dimensional model of the feline hindlimb. J Morphol 261: 118–129, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caputi AA, Hoffer JA, Pose IE. Velocity of ultrasound in active and passive cat medial gastrocnemius muscle. J Biomech 25: 1067–1074, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Chin NK, Cope M, Pang M. Number and distribution of spindle capsules in seven hindlimb muscles of the cat. In: Symposium on Muscle Receptors, edited by Barker D. Hong Kong: Hong Kong University Press, 1962, p. 241–248.
- 10.Cui L, Perreault EJ, Maas H, Sandercock TG. Modeling short-range stiffness of feline lower hindlimb muscles. J Biomech 41: 1945–1952, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denny-Brown DE The histological features of striped muscle in relation to its functional activity. Proc R Soc Lond B Biol Sci 104: 371–411, 1929. [Google Scholar]
- 12.Elek J, Prochazka A, Hulliger M, Vincent S. In-series compliance of gastrocnemius muscle in cat step cycle: do spindles signal origin-to-insertion length? J Physiol 429: 237–258, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eng JJ, Hoffer JA. Regional variability of stretch reflex amplitude in the cat medial gastrocnemius muscle during a postural task. J Neurophysiol 78: 1150–1154, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Fellows SJ, Rack PMH. Changes in the length of the human biceps brachii muscle during elbow movements. J Physiol 383: 405–412, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowler EG, Gregor RJ, Hodgson JA, Roy RR. Relationship between ankle muscle and joint kinetics during the stance phase of locomotion in the cat. J Biomech 26: 465–483, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Gabaldon AM, Nelson FE, Roberts TJ. Mechanical function of two ankle extensors in wild turkeys: shifts from energy production to energy absorption during incline versus decline running. J Exp Biol 207: 2277–2288, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Goslow GE, Reinking RM, Stuart DG. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. J Morphol 141: 1–41, 1973. [DOI] [PubMed] [Google Scholar]
- 18.Gregor RJ, Roy RR, Whiting WC, Lovely RG, Hodgson JA, Edgerton VR. Mechanical output of the cat soleus during treadmill locomotion: in vivo vs in situ characteristics. J Biomech 21: 721–732, 1988. [DOI] [PubMed] [Google Scholar]
- 19.Gregor RJ, Smith DW, Prilutsky BI. Mechanics of slope walking in the cat: quantification of muscle load, length change and ankle extensor EMG patterns. J Neurophysiol 95: 1397–1409, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Gregor RJ, Smith JL, Smith DW, Oliver A, Prilutsky BI. Hindlimb kinetics and neural control during slope walking in the cat: unexpected findings. J Appl Biomech 17: 277–286, 2001. [Google Scholar]
- 21.Griffiths RI Shortening of muscle fibres during stretch of the active cat medial gastrocnemius muscle: the role of tendon compliance. J Physiol 436: 219–236, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatta I, Sugi H, Tamura Y. Stiffness changes in frog skeletal muscle during contraction recorded using ultrasonic waves. J Physiol 403: 193–209, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herzog W, Leonard TR, Guimaraes AC. Forces in gastrocnemius, soleus, and plantaris tendons of the freely moving cat. J Biomech 26: 945–953, 1993. [DOI] [PubMed] [Google Scholar]
- 24.Hoffer JA, Caputi AA, Pose IE. Activity of muscle proprioceptors in cat posture and locomotion: relation to EMG, tendon force, and the movement of fibres and aponeurotic segments. In: Muscle Afferents and Spinal Control of Movement, edited by Jami L, Pierrot-Deseilligny E, and Zytnicki D. New York: Pergamon, 1992.
- 25.Hoffer JA, Caputi AA, Pose IE, Griffiths RI. Roles of muscle activity and load on the relationship between muscle spindle length and whole muscle length in the freely walking cat. Prog Brain Res 80: 75–85, 1989. [DOI] [PubMed] [Google Scholar]
- 26.Hoy MG, Zernicke RF. Modulation of limb dynamics in the swing phase of locomotion. J Biomech 18: 49–60, 1985. [DOI] [PubMed] [Google Scholar]
- 27.Huijing PA Muscular force transmission necessitates a multilevel integrative approach to the analysis of function of skeletal muscle. Exerc Sport Sci Rev 31: 167–175, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Kaya M, Leonard T, Herzog W. Coordination of medial gastrocnemius and soleus forces during cat locomotion. J Exp Biol 206: 3645–3655, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Maas H, Lichtwark GA. Is muscle-tendon unit length a valid indicator for muscle spindle output? J Physiol 587: 13–14, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maas H, Prilutsky BI, Gregor RJ. In vivo fascicle length of cat medial gastrocnemius and soleus muscles during slope walking. XXth Congress of the International Society of Biomechanics, Cleveland, OH, 2005, p. 90.
- 31.Maas H, Prilutsky BI, Nichols TR, Gregor RJ. The effects of self-reinnervation of cat medial and lateral gastrocnemius muscles on hindlimb kinematics in slope walking. Exp Brain Res 181: 377–393, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nichols TR, Houk JC. Improvement in linearity and regulation of stiffness that results from actions of stretch reflex. J Neurophysiol 39: 119–142, 1976. [DOI] [PubMed] [Google Scholar]
- 33.Olson JM, Marsh RL. Activation patterns and length changes in hindlimb muscles of the bullfrog Rana catesbeiana during jumping. J Exp Biol 201: 2763–2777, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Prilutsky BI, Herzog W, Allinger TL. Force-sharing between cat soleus and gastrocnemius muscles during walking: explanations based on electrical activity, properties, and kinematics. J Biomech 27: 1223–1235, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Prilutsky BI, Herzog W, Allinger TL. Mechanical power and work of cat soleus, gastrocnemius and plantaris muscles during locomotion: possible functional significance of muscle design and force patterns. J Exp Biol 199: 801–814, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Prilutsky BI, Herzog W, Leonard TR, Allinger TL. Role of the muscle belly and tendon of soleus, gastrocnemius, and plantaris in mechanical energy absorption and generation during cat locomotion. J Biomech 29: 417–434, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Prilutsky BI, Sirota MG, Gregor RJ, Beloozerova IN. Quantification of motor cortex activity and full-body biomechanics during unconstrained locomotion. J Neurophysiol 94: 2959–2969, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Prochazka A, Gorassini M. Models of ensemble firing of muscle spindle afferents recorded during normal locomotion in cats. J Physiol 507: 277–291, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rack PMH, Ross HF, Thilmann AF, Walters DKW. Reflex responses at the human ankle - The importance of tendon compliance. J Physiol 344: 503–524, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rack PMH, Westbury DR. Elastic properties of the cat soleus tendon and their functional importance. J Physiol 347: 479–495, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts TJ, Marsh RL, Weyand PG, Taylor CR. Muscular force in running turkeys: the economy of minimizing work. Science 275: 1113–1115, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Rossignol S, Dubuc RJ, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev 86: 89–154, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Sacks RD, Roy RR. Architecture of the hind limb muscles of cats: functional significance. J Morphol 173: 185–195, 1982. [DOI] [PubMed] [Google Scholar]
- 44.Sandercock TG Nonlinear summation of force in cat soleus muscle results primarily from stretch of the common-elastic elements. J Appl Physiol 89: 2206–2214, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Scott SH, Brown IE, Loeb GE. Mechanics of feline soleus. I. Effect of fascicle length and velocity on force output. J Muscle Res Cell Motil 17: 207–219, 1996. [DOI] [PubMed] [Google Scholar]
- 46.Sherrington CS The Integrative Action of the Nervous System. New Haven, CT: Yale University Press, 1906, p. 433.
- 47.Smith JL, Carlson-Kuhta P, Trank TV. Forms of forward quadrupedal locomotion. III. A comparison of posture, hindlimb kinematics, and motor patterns for downslope and level walking. J Neurophysiol 79: 1702–1716, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Spector SA, Gardiner PF, Zernicke RF, Roy RR, Edgerton VR. Muscle architecture and force-velocity characteristics of cat soleus and medial gastrocnemius: implications for motor control. J Neurophysiol 44: 951–960, 1980. [DOI] [PubMed] [Google Scholar]
- 49.Walmsley B, Hodgson JA, Burke RE. Forces produced by medial gastrocnemius and soleus muscles during locomotion in freely moving cats. J Neurophysiol 41: 1203–1216, 1978. [DOI] [PubMed] [Google Scholar]
- 50.Whiting WC, Gregor RJ, Roy RR, Edgerton VR. A technique for estimating mechanical work of individual muscles in the cat during treadmill locomotion. J Biomech 17: 685–694, 1984. [DOI] [PubMed] [Google Scholar]
- 51.Windhorst U Muscle spindles are multi-functional. Brain Res Bull 75: 507–508, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Zuurbier CJ, Huijing PA. Influence of muscle geometry on shortening speed of fiber, aponeurosis and muscle. J Biomech 25: 1017–1026, 1992. [DOI] [PubMed] [Google Scholar]