Abstract
Previously, we reported that the hypoxic ventilatory response (HVR) in rats was weakest at postnatal day (P) P13, concomitant with neurochemical changes in respiratory nuclei. A major determinant of minute ventilation (V̇e) is reportedly the metabolic rate [O2 consumption (V̇o2) and CO2 production (V̇co2)]. The present study aimed at testing our hypothesis that daily metabolic rates changed in parallel with ventilation during development and that a weak HVR at P13 was attributable mainly to an inadequate metabolic rate in hypoxia. Ventilation and metabolic rates were monitored daily in P0–P21 rats. We found that 1) ventilation and metabolic rates were not always correlated, and V̇e/V̇o2 and V̇e/V̇co2 ratios were not constant during development; 2) metabolic rate and V̇e/V̇o2 and V̇e/V̇co2 ratios at P0–P1 were significantly different from the remaining first postnatal week in normoxia and hypoxia; 3) at P13, metabolic rates and V̇e/V̇o2 and V̇e/V̇co2 ratios abruptly increased in normoxia and were compromised in acute hypoxia, unlike more stable trends during the remaining second and third postnatal weeks; and 4) the respiratory quotient (V̇co2/V̇o2) was quite stable in normoxia and fluctuated slightly in hypoxia from P0 to P21. Thus our data revealed heretofore unsuspected metabolic adjustments at P0–P1 and P13. At P0–P1, ventilation and metabolic rates were uncorrelated, whereas at P13, they were closely correlated under normoxia and hypoxia. The findings further strengthened the existence of a critical period of respiratory development around P13, when multiple physiological and neurochemical adjustments occur simultaneously.
Keywords: carbon dioxide production, critical period, oxygen consumption, respiratory quotient, ventilation
previously, we conducted detailed, day-to-day studies of various brain stem respiratory nuclei of the rat and found that neurotransmitters and receptors underwent distinct developmental changes. Significantly, at or around postnatal day (P) 12, the expression of excitatory neurotransmitter glutamate and its N-methyl-d-aspartate receptors dropped precipitously, whereas the expression of inhibitory neurotransmitter γ-aminobutyric acid (GABA), GABAB receptors, and glycine receptors rose sharply (27, 30, 61). Concomitantly, there was a sudden fall in cytochrome oxidase activity (27, 28, 61), a sensitive indicator of metabolic capacity and neuronal activity (60). We hypothesized that at and around P12 is a sensitive period in the postnatal development of the rat's respiratory control network, when a transient dominance of inhibitory over excitatory neurotransmission renders the animals less capable of overcoming exogenous respiratory stressors. Our subsequent ventilatory studies revealed striking changes in normoxic ventilation as well as hypoxic ventilatory response (HVR) at and around P13, with a peak in respiratory frequency and a transient peak both in tidal volume (Vt) and minute ventilation (V̇e) under normoxia (32). The body temperature also peaked at P13. Significantly, the lowest HVR (decreased frequency response and the lowest Vt response; therefore, the lowest V̇e response) was also at P13 (32). Thus the end of the second postnatal week is likely to be a critical period during which the animals are less responsive to external stressors, such as hypoxia. These findings have special implication for sudden infant death syndrome (SIDS), as the peak incidence of SIDS occurs not at birth but between the second and fourth months after birth, strongly suggesting a critical period of respiratory development (16, 24, 39). Ventilation can be influenced by intrinsic factors, such as body weight, body temperature, level of physical activity, and metabolic rate (42), as well as extrinsic factors, such as ambient temperature and altitude (26, 44). Of these factors, the metabolic rate, which denotes the rate of oxygen consumption (V̇o2) and carbon dioxide production (V̇co2), is the one most directly associated with ventilation, because the gas exchange constitutes an integral part of the ventilatory process. Like ventilation, metabolic rate is dependent on the body size, sex, total body activities, the state of arousal, age, and several other factors, such as ambient temperature and ambient gas composition (20, 42, 43). Both ventilation and metabolic rate are reportedly developmentally regulated, and, in general, V̇co2 is regulated in parallel with V̇o2 (21, 25, 53, 58). However, details of postnatal development of the metabolic rate are poorly understood, because most studies focused on only a few time points. Likewise, postnatal metabolic response to acute hypoxia is also inadequately characterized, because both sample size and time points were limited.
The present study was aimed at monitoring metabolic rates in rats daily throughout the first 3 postnatal weeks. The goal was to test our hypothesis that daily postnatal changes in ventilation observed previously (32) were paralleled by concomitant changes in metabolic rate and that a weak HVR at P13 was attributable largely to an inadequate metabolic rate during hypoxia.
METHODS
Animals.
All experiments and animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health), and all protocols were approved by the Medical College of Wisconsin Animal Care and Use Committee.
A total of 139 Sprague-Dawley rats (Harlan, Indianapolis, IN) from 16 litters were used in this study. The litter size was usually 10–15 pups. Each pup was studied every fifth day to prevent adverse cumulative effect of daily exposure to hypoxia (Ref. 36, and our unpublished observations), first in room air for 6 min and then subjected to 7 min of hypoxia (10% O2 balanced with 90% N2) (see below for analysis). The test days were staggered among the animals such that every single day between P0 (day of birth) and P21 was covered. A total of 18–30 rats from 11 to 16 litters were tested for each of the days from P1 to P21, whereas 13 rats from 7 litters were tested for P0.
Whole body plethysmography under normoxia and hypoxia.
After multiple testings, we found the following combination of practice to be critical: 1) use of a very small plethysmographic chamber that permitted the monitoring of small changes in V̇o2 and V̇co2 in the neonate; 2) use of a highly sensitive pressure transducer custom built for us, again allowing us to detect small changes in respiration in the neonate; 3) warming of the pups for 30 min before testing and continuing the warming with a heating pad on the floor of the plethysmograph [thus the body temperature of pups was maintained at 34–38.5°C (depending on the age), while the ambient temperature of the chamber was maintained at 26–28°C]; 4) careful calibration of the system (gas and volume) before and after each experiment each day; and 5) while the formula for calculating Vt takes into account temperature and humidity, we found that breathing frequency and body volume are equally, if not more, important in determining Vt-induced pressure changes in a small chamber (see below for details).
O2 fraction, CO2 fraction, and ventilation (including respiratory frequency, Vt, and their product V̇e) were determined in awake rat pups placed in an airtight 150-ml plastic syringe (inner diameter 37 mm, length 140 mm) from which the plunger was removed. The inlet of the syringe was connected to a gas cylinder either for calibration or for experiments. The outlet of the syringe was sealed by a rubber cork with ports to measure 1) the fractional contents of O2 and CO2 exiting the chamber (Fe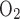 and Fe
and Fe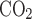 , respectively) using a POET II gas monitor (Criticare Systems, Waukesha, WI) for O2 and a Quantek CO2 analyzer (model 906, Quantek Instruments, Grafton, MA) through a passageway with Drierite before reaching the gas analyzers (the sensitivity was 0.03% for O2 and 0.0003% for CO2); 2) pressure changes with a custom-built pressure transducer referenced to atmosphere via a 15-ft-long, 0.125-in. inner diameter tubing; 3) temperature and relative humidity changes with another transducer connected to a relative humidity-temperature transmitter (model HX93AV-RP1, Omega Engineering, Stamford, CT); and 4) calibration of the system with a 1-ml syringe. The Fe
, respectively) using a POET II gas monitor (Criticare Systems, Waukesha, WI) for O2 and a Quantek CO2 analyzer (model 906, Quantek Instruments, Grafton, MA) through a passageway with Drierite before reaching the gas analyzers (the sensitivity was 0.03% for O2 and 0.0003% for CO2); 2) pressure changes with a custom-built pressure transducer referenced to atmosphere via a 15-ft-long, 0.125-in. inner diameter tubing; 3) temperature and relative humidity changes with another transducer connected to a relative humidity-temperature transmitter (model HX93AV-RP1, Omega Engineering, Stamford, CT); and 4) calibration of the system with a 1-ml syringe. The Fe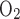 , Fe
, Fe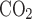 , pressure, relative humidity, and temperature signals were digitized with a data acquisition device (model DI-710, DATAQ Instruments, Akron, OH) and stored on a computer.
, pressure, relative humidity, and temperature signals were digitized with a data acquisition device (model DI-710, DATAQ Instruments, Akron, OH) and stored on a computer.
Gas calibration was taken before and after each animal's experiment. Before each calibration, a minimum of 5 min was taken to equilibrate the gases to appropriate concentrations in the outflow. Concentrations of 19% O2-1% CO2-80% N2 and 9% O2-1% CO2-90% N2 were used for calibration system to monitor Fe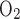 and Fe
and Fe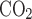 under normoxia or hypoxia, respectively. These gas concentrations were determined empirically by testing the exhaled gases of P0–P21 rats breathing either room air (former) or 10% O2 (latter). During recording, the syringe chamber was continuously flushed with a specific gas mixture (either room air or 10% O2) at a flow rate of 150 ml/min, and the pressure in the chamber was kept at around atmospheric by finely adjusting air inflow via a regulator, a needle valve, and a flowmeter installed in the inflow airway to match 150 ml/min vacuum power on the excurrent side provided by the POET II gas monitor. Thus the pressure in the chamber was kept at around atmospheric during the experiment.
under normoxia or hypoxia, respectively. These gas concentrations were determined empirically by testing the exhaled gases of P0–P21 rats breathing either room air (former) or 10% O2 (latter). During recording, the syringe chamber was continuously flushed with a specific gas mixture (either room air or 10% O2) at a flow rate of 150 ml/min, and the pressure in the chamber was kept at around atmospheric by finely adjusting air inflow via a regulator, a needle valve, and a flowmeter installed in the inflow airway to match 150 ml/min vacuum power on the excurrent side provided by the POET II gas monitor. Thus the pressure in the chamber was kept at around atmospheric during the experiment.
For recording of ventilation, the system was calibrated by applying 0.1 ml of air into the plethysmographic chamber 10 times before and after each animal's recording. These applications were done via a 1-ml syringe driven by a weight to produce stable 0.1-ml injections at a speed of ∼200 min−1, comparable to the mean frequency of respiration between P0 and P21. We found that the ratio of body volume to body weight (ml/g) in rat pups is 1.081. Thus, when we performed the calibration, we put a combination of blocks in the chamber with the mass equal to that of the animal tested. In the absence of a live animal, the calibration was more accurate and repeatable. Because calibration was done at a presumed frequency of 200 (= the mean of frequencies found in rats from P0 to P21), yet the actual frequency obtained from each animal may be lower or higher than 200, the calibration factor had to be adjusted to avoid under- or overestimation. We, therefore, created a table that expressed the relationship between simulated breathing frequency, simulated body volume, and voltage change (signifying Vt) induced by 0.1-ml injection of air into the plethysmograph. The table included a range of body volume from 7 to 50 ml (P0–P21) with 3- to 5-ml intervals, and breathing frequencies from 60 to 320 breaths/min (including and extending beyond the range observed in P0 to P21 rats) with 10–20/min intervals. The table allowed us to see the expected voltage change at the breathing frequency of the animal and adjust our Vt values accordingly (by multiplying our Vt values with a ratio of “voltage change at a calibrated frequency of 200” and the “voltage change at the observed frequency”). Rectal temperature of each animal was measured before and after each recording with a 30-gauge microprocessor thermometer (model HH21, Omega Engineering). At each age between P0 and P21, each animal was studied for 6 min while breathing room air (normoxia) and then subjected to 7 min of 10% O2 + 90% N2 (hypoxia). The plethysmograph was set on a heating pad, and the temperature of the chamber was maintained at 26–28°C. Internal air temperature was continuously monitored and did not change more than 0.5°C during any single experiment. Starting at ∼30 min before each experiment, each animal was warmed with a heating pad to maintain its normal rectal temperature (at or above 34°C depending on the age) before being placed in the chamber. Body contact of the rat pups with the warmed floor of the plethysmograph helped to maintain the pups' temperature at or above 34°C [varied between 34 and 38.5°C from P0 to P21 during normoxia and hypoxia, comparable to those shown in our laboratory's previous study (32)]. This was confirmed by the measurement of body temperature before and after each session.
Data collection and statistical analyses.
O2 fraction, CO2 fraction, and ventilation in normoxia and hypoxia were continuously monitored in animals during each session. The sampling rate was 206/s. V̇o2 and V̇co2 were calculated by applying the Fick principle (17) and Zwemer et al.'s equations (62), using the difference in gas content between inflow air and outflow air:
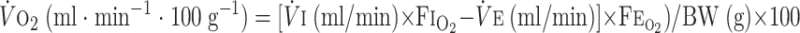 |
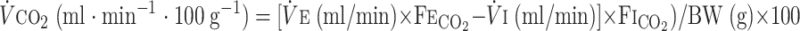 |
Where Fi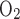 , Fe
, Fe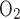 , Fi
, Fi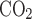 , and Fe
, and Fe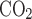 are the fractions of inflow and outflow of O2 or CO2, respectively; V̇e is outflow rate (150 ml/min in this study); V̇i is inflow rate, equal to V̇e × (1 − Fe
are the fractions of inflow and outflow of O2 or CO2, respectively; V̇e is outflow rate (150 ml/min in this study); V̇i is inflow rate, equal to V̇e × (1 − Fe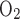 − Fe
− Fe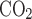 )/(1 − Fi
)/(1 − Fi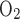 − fi
− fi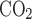 ); and BW is body weight.
); and BW is body weight.
Ventilation data, Vt, respiratory frequency, and V̇e, were calculated by using the formulae of Drorbaugh and Fenn (15). Thus the ratios V̇e/V̇o2 and V̇e/V̇co2, and respiratory quotient (RQ; V̇co2/V̇o2) were calculated.
The values were grouped into 30-s bins during the fifth and sixth minute in normoxia and sixth and seventh minute in hypoxia, when breathing and gas concentrations were more stable (Fig. 1, A and B), and expressed as the mean of the last 2 min either in 6-min normoxia or 7-min hypoxia ± SE. Values that were >2 SDs above or below the mean were deleted. These outliers (∼5%) appeared to result from greater body movements and/or leakage in the plethysmograph and, therefore, did not represent true values. Even after the deletion, there were still 17–30 rats from 11–16 litters for each postnatal day from P1 to P21, and 12 rats from 7 litters for P0 for analyses. We found that it took close to 3 min (with a time constant of 56.7 s) for the plethysmographic chamber to equilibrate to 10% O2 after the inflow gas was switched from room air to hypoxic gas. Under hypoxic exposure, as shown in Fig. 1B, it took close to 3 min for excurrent gas concentrations to reach a plateau. Thus, when we measured the hypoxic response at the sixth and seventh minute in the present study, it was comparable to about the third and fourth minute of hypoxic response in our laboratory's previous study (32), which utilized a different set-up that equilibrated gases in ∼0.5 min.
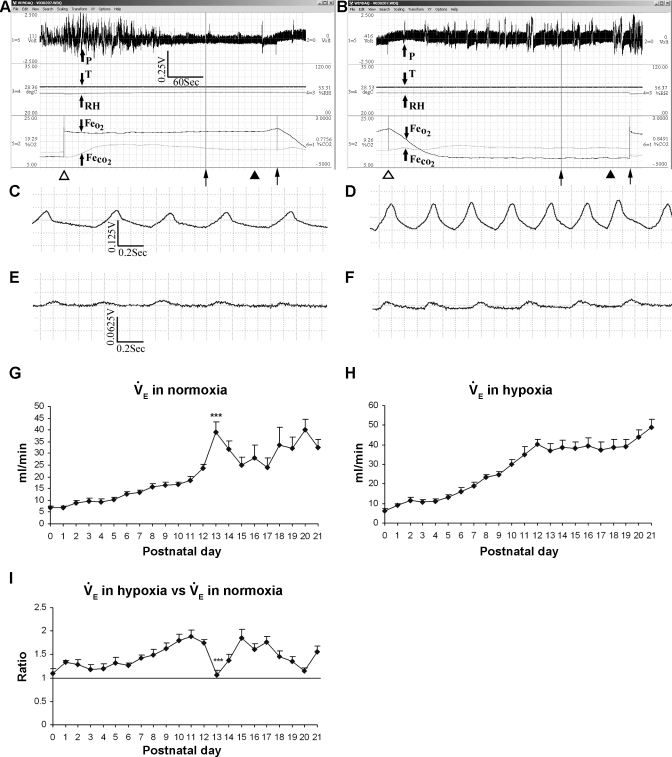
Fig. 1.
Representative monitoring of ventilation and metabolic rate as well as postnatal development of minute ventilation (V̇e) in normoxia and hypoxia. A: representative plethysmographic tracing of pressure (arrow, P), temperature (T, in °C), relative humidity (RH, %), excurrent fractional concentration of O2 (Fe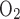 , %), and excurrent fractional concentration of CO2 (Fe
, %), and excurrent fractional concentration of CO2 (Fe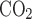 , %) during normoxia in a P18 rat. Time and voltage scales are indicated. ▵, Beginning of a 6-min monitoring during normoxia. Two thin arrows at the bottom indicate the last 2 min of a 6-min period when breathing and gas concentrations were more stable and the normoxic data were analyzed. ▴, Point where the plethysmographic pressure tracing was magnified in 1C. B: representative monitory of the same rat as in A and C but in 10% O2, and the data were obtained during the 6th and 7th min after the introduction of 10% O2. The CO2 concentration (V̇co2) at the beginning of the 6th min in hypoxia was 0.8491% (V̇co2: 3.56 ml·min−1·100 g−1), whereas that at the beginning of the 5th min of normoxia was 0.7756% (V̇co2: 3.24 ml·min−1·100 g−1). C and D: raw plethysmographic tracings obtained at the time indicated by filled triangles in A and B during normoxia and hypoxia, respectively. Breathing frequency was ∼125 breaths/min in normoxia but ∼200 breaths/min in hypoxia, and voltage change was ∼ 0.067 V in normoxia but 0.125 V in hypoxia. E and F: raw plethysmographic tracings obtained from a P0 animal during normoxia and hypoxia, respectively. Breathing frequency was ∼140 breaths/min in normoxia but ∼172 breaths/min in hypoxia, and voltage change was ∼0.015 V in normoxia but 0.02 V in hypoxia. G: postnatal development of V̇e during normoxia. V̇e gradually increased with age from P0 to P12, peaking at P13 (P < 0.001), followed by a decline at P14 and P15, a plateau at P16-P17, and a subsequent rise until P21. H: V̇e in hypoxia increased gradually with age from P0 to P12, plateaued from P13 to P19, an then increased again at P20–P21. I: V̇e in hypoxia vs. that in normoxia. One-sample t-test revealed that in the majority of age groups, the ratio was significantly different from 1 (P < 0.05), except for P0, P3, P4, and P13. P13 is the only time point that shows a statistically significant difference between any adjacent 2 age groups (P < 0.001). For statistical comparisons between successive age groups, please see methods. ***P < 0.001.
, %) during normoxia in a P18 rat. Time and voltage scales are indicated. ▵, Beginning of a 6-min monitoring during normoxia. Two thin arrows at the bottom indicate the last 2 min of a 6-min period when breathing and gas concentrations were more stable and the normoxic data were analyzed. ▴, Point where the plethysmographic pressure tracing was magnified in 1C. B: representative monitory of the same rat as in A and C but in 10% O2, and the data were obtained during the 6th and 7th min after the introduction of 10% O2. The CO2 concentration (V̇co2) at the beginning of the 6th min in hypoxia was 0.8491% (V̇co2: 3.56 ml·min−1·100 g−1), whereas that at the beginning of the 5th min of normoxia was 0.7756% (V̇co2: 3.24 ml·min−1·100 g−1). C and D: raw plethysmographic tracings obtained at the time indicated by filled triangles in A and B during normoxia and hypoxia, respectively. Breathing frequency was ∼125 breaths/min in normoxia but ∼200 breaths/min in hypoxia, and voltage change was ∼ 0.067 V in normoxia but 0.125 V in hypoxia. E and F: raw plethysmographic tracings obtained from a P0 animal during normoxia and hypoxia, respectively. Breathing frequency was ∼140 breaths/min in normoxia but ∼172 breaths/min in hypoxia, and voltage change was ∼0.015 V in normoxia but 0.02 V in hypoxia. G: postnatal development of V̇e during normoxia. V̇e gradually increased with age from P0 to P12, peaking at P13 (P < 0.001), followed by a decline at P14 and P15, a plateau at P16-P17, and a subsequent rise until P21. H: V̇e in hypoxia increased gradually with age from P0 to P12, plateaued from P13 to P19, an then increased again at P20–P21. I: V̇e in hypoxia vs. that in normoxia. One-sample t-test revealed that in the majority of age groups, the ratio was significantly different from 1 (P < 0.05), except for P0, P3, P4, and P13. P13 is the only time point that shows a statistically significant difference between any adjacent 2 age groups (P < 0.001). For statistical comparisons between successive age groups, please see methods. ***P < 0.001.
For statistical analyses, each of the outcomes was analyzed separately. For each outcome, a mixed-effect model with fixed categorical day effect, and random litter and animal effects was fitted using PROC MIXED in SAS (Cary, NC). The random effects accounted for the correlations of the multiple measurements within animal, as well as multiple animals within a litter. Twenty-one contrasts comparing consecutive day differences with the average of all consecutive day differences were estimated from the model. Standard multiple comparison procedures would adjust significance levels for all 210 pairwise comparisons between days, resulting in overly conservative results for the much lower number of comparisons of interest. Thus simulation-based adjustment for multiple testing implemented in the %SimTests macro (59) was used to ensure a 5% type I error rate over the 21 day-to-day contrasts.
RESULTS
The daily ventilatory data (frequency and Vt) in normoxia and hypoxia, as well as body temperature and body weight of rats from P0 to P21 in this study were comparable to those reported previously (32), and will not be detailed here.
Postnatal developmental trend of V̇e during normoxia and acute hypoxia.
Figure 1A shows representative plethysmographic tracing of pressure (P), temperature (T), relative humidity (RH), FeO2, and Fe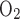 (%) during normoxia in a P18 rat. Data were analyzed during the last 2 min of a 6-min period, when breathing and gas concentrations were more stable. Likewise, data were taken from the last 2 min of a 7-min period in hypoxia (Fig. 1B). The CO2 concentration at the beginning of the sixth minute in hypoxia was ∼0.8,491% (V̇co2: 3.56 ml·min−1·100 g−1), whereas that at the beginning of the fifth minute of normoxia was ∼0.7,756% (V̇co2: 3.24 ml·min−1·100 g−1). In this representative animal, breathing frequency was ∼125 breaths/min in normoxia (Fig. 1C) but ∼200 breaths/min in hypoxia (Fig. 1D), and voltage change was ∼0.067 V in normoxia but 0.125 V in hypoxia. By comparison, breathing frequency of a representative P0 animal was ∼140 breaths/min in normoxia (Fig. 1E) but ∼172 breaths/min in hypoxia (Fig. 1F), and voltage change was ∼0.015 V in normoxia but 0.02 V in hypoxia.
(%) during normoxia in a P18 rat. Data were analyzed during the last 2 min of a 6-min period, when breathing and gas concentrations were more stable. Likewise, data were taken from the last 2 min of a 7-min period in hypoxia (Fig. 1B). The CO2 concentration at the beginning of the sixth minute in hypoxia was ∼0.8,491% (V̇co2: 3.56 ml·min−1·100 g−1), whereas that at the beginning of the fifth minute of normoxia was ∼0.7,756% (V̇co2: 3.24 ml·min−1·100 g−1). In this representative animal, breathing frequency was ∼125 breaths/min in normoxia (Fig. 1C) but ∼200 breaths/min in hypoxia (Fig. 1D), and voltage change was ∼0.067 V in normoxia but 0.125 V in hypoxia. By comparison, breathing frequency of a representative P0 animal was ∼140 breaths/min in normoxia (Fig. 1E) but ∼172 breaths/min in hypoxia (Fig. 1F), and voltage change was ∼0.015 V in normoxia but 0.02 V in hypoxia.
Postnatal development of V̇e during normoxia showed a gradual increase with age from P0 to P12, peaking at P13 (P < 0.001), followed by a decline at P14 and P15, a plateau at P16–P17, and a subsequent rise until P21 (Fig. 1G). V̇e in hypoxia increased gradually with age from P0 to P12, plateaued from P13 to P19, and then increased again at P20–p21 (Fig. 1H). When V̇e in hypoxia was compared with that in normoxia, the ratio fluctuated at a level slightly above 1 from P0 to P6 (except for P0, P3, and P4), gradually increased from P7 to P12, but precipitously fell at P13 to P1 (P < 0.001), followed by an increase at P14–P15, declining thereafter from P16 to P20, and increased again at P21 (Fig. 1I). P13 is the only time point that showed a statistically significant difference between any adjacent two age groups. One-sample t-test revealed that in the majority of age groups, the ratio of V̇e in hypoxia vs. that in normoxia was significantly different from 1 (P < 0.05), except for P0, P3, P4, and P13.
The ventilation data in the present study had a trend very similar to that reported previously (32). Minor differences in the absolute values were related to the following: 1) a difference in the chamber size (smaller chamber size was necessary in the present study to reveal small changes in metabolic rates in normoxia and hypoxia, especially in neonatal animals; 2) measurements were taken from the last 2 min of a 6- or 7-min analysis in the present study to allow for equilibration of the chamber to 10% O2 in the present set-up, comparable to about the third and fourth minute of hypoxic response in our laboratory's previous study, i.e., immediately after the peak response to acute hypoxia (32), whereas they represented the average of 5 min in the previous study, where equilibration was achieved within the first 0.5 min; and 3) the smaller chamber size prevented major movements of animals, allowing for more stabilized normoxic data and greater hypoxic response.
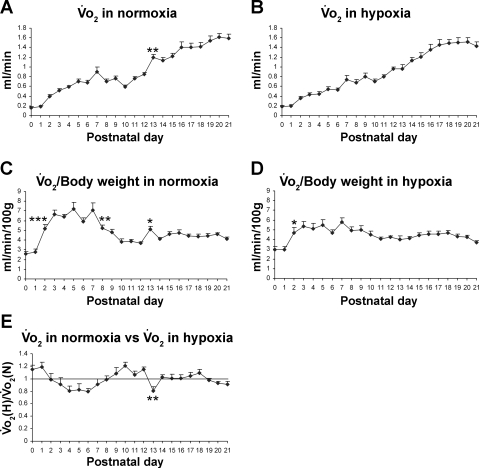
Postnatal developmental trend of V̇o2 in normoxia.
The postnatal developmental trend of V̇o2 in normoxia was a gradual increase with age. However, the rise in successive age groups was significant only at P13 (P < 0.01; Fig. 2A). When normalized to body weight (per 100 g), V̇o2 exhibited the lowest level at P0 and P1, a significant increase at P2 (P < 0.001), followed by a plateau until P7. The values attained during the plateau were the highest in the first 3 postnatal wk (5.2–7.2 ml·min−1·100 g−1). V̇o2 then declined significantly at P8 (P < 0.01), with a further decline at P10 followed by a relative plateau until P21 (3.8–4.7 ml·min−1·100 g−1), except for a sudden rise in V̇o2 at P13 (P < 0.05; Fig. 2C).
Fig. 2.
Postnatal developmental trend of O2 consumption (V̇o2) in normoxia and hypoxia. A: developmental trend of V̇o2 in normoxia. V̇o2 levels increased gradually with age, but significant increases are evident at postnatal day (P) 13. B: developmental trend of V̇o2 in hypoxia. V̇o2 levels progressively increased with age. C: V̇o2 values normalized to body weight (100 g) in normoxia. V̇o2 was the lowest at P0–P1, increased significantly at P2, followed by a relative plateau until P7. It then decreased markedly at P8 and again at P10, followed by a plateau until P21. However, a significant peak occurred at P13. D: V̇o2 normalized to body weight (100 g) in hypoxia. V̇o2 was the lowest at birth, increased significantly at P2, followed by fluctuations and a plateau until P21. E: V̇o2 in hypoxia [V̇o2(H)] vs. that in normoxia [V̇o2(N)]. One-sample t-test demonstrated that the ratio was significantly different from 1 (P < 0.05) at P0, P4, P6, P10, P12, and P13. P13 is the only time point that shows a statistically significant difference between any adjacent 2 age groups (P < 0.01). For statistical comparisons between successive age groups, please see methods. *P < 0.05. **P < 0.01. ***P < 0.001.
Postnatal developmental trend of V̇o2 in acute hypoxia.
V̇o2 in acute hypoxia also increased progressively with age (Fig. 2B). When normalized to body weight, V̇o2 was also the lowest at P0–P1 but increased significantly at P2 (P < 0.05) followed by a relative plateau until P21 (Fig. 2D). When the V̇o2 in hypoxia was compared with that in normoxia, the ratio was higher than the normoxic baseline at P0 and P1 but fell below the baseline from P3 to P7. During P9–P12, the ratio was generally above the baseline (between 1.07 and 1.21). At P13, it fell significantly below the baseline (0.84; P < 0.01), followed by a rise again to the baseline the next day, and plateauing thereafter (Fig. 2E). One-sample t-test demonstrated that the ratio of V̇o2 in hypoxia vs. that in normoxia was significantly different from 1 (P < 0.05) at P0, P4, P6, P10, P12, and P13.
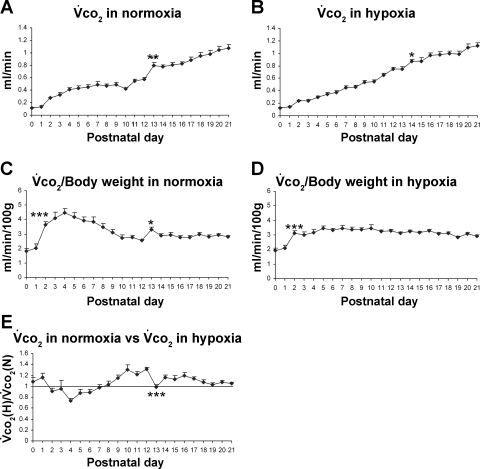
Postnatal developmental trend of V̇co2 in normoxia.
V̇co2 in normoxia paralleled that of V̇o2 during development in that V̇co2 increased progressively with age. However, as in the case of V̇o2, the rise in successive age groups was significant only at P13 (P < 0.01; Fig. 3A). When normalized to body weight, V̇co2 was lowest at P0–P1, increased significantly at P2 (P < 0.001), and plateaued for the rest of the first postnatal week (3.7–4.5 ml·min−1·100 g−1). The values were reduced to between 2.6 and 3.5 ml·min−1·100 g−1 during the second and third postnatal weeks, with a notable peak at P13 (P < 0.05; Fig. 3C).
Fig. 3.
Postnatal developmental trend of V̇co2 in normoxia and hypoxia. A: developmental trend of V̇co2 in normoxia. V̇co2 levels increased gradually with age, but significant successive age group increase is evident at P13 only. B: developmental trend of V̇co2 in hypoxia. Generally, V̇co2 levels progressively increased with age, but a significant rise occurred at P14, compared with P13. C: V̇co2 values normalized to body weight (100 g) in normoxia. V̇co2 was the lowest at P0–P1, increased significantly at P2, followed by a relative plateau for the remaining first postnatal week. The values were reduced and maintained at a relative plateau for the 2nd and 3rd postnatal weeks, except for a small but statistically significant peak at P13. D: V̇co2 normalized to body weight (100 g) in hypoxia. V̇co2 was the lowest at birth, increased significantly at P2, followed by fluctuations and a plateau until P21. E. V̇co2 in hypoxia [V̇co2(H)] vs. that in normoxia [V̇co2(N)]. One-sample t-test showed that the ratio was significantly different from 1 (P < 0.05) at P1, P4, P9–P12, and P14–P17. P13 is the only time point that shows a statistically significant difference between any adjacent 2 age groups (P < 0.001). For statistical comparisons between successive age groups, please see methods. *P < 0.05. **P < 0.01. ***P < 0.001.
Postnatal developmental trend of V̇co2 in hypoxia.
The V̇co2 in acute hypoxia gradually increased with age, except for P14 when the V̇co2 significantly increased (P < 0.05), compared with its adjacent younger age group (P13; Fig. 3B). When normalized to body weight, V̇co2 exhibited the lowest level at P0 and P1 (1.9–2.1 ml·min−1·100 g−1), a significant rise at P2 (P < 0.001), followed by a relative plateau until P21 (2.85–3.5 ml·min−1·100 g−1; Fig. 3D). When V̇co2 in hypoxia was compared with that in normoxia, the trend was similar to that of V̇o2 in that the ratio was above 1 at P0 and P1 but reduced to 1 or below 1 for the remaining first postnatal week (0.74–0.97). During the second and third postnatal weeks, the ratio was generally above the baseline (1.03–1.3) except for a significant decline at P13 (to 0.99; P < 0.001) and P18–P21 (Fig. 3E). The value reached by P21 was close to the baseline. One-sample t-test showed that the ratio of V̇co2 in hypoxia vs. that in normoxia was significantly different from 1 (P < 0.05) at P1, P4, P9–12, and P14–P17.
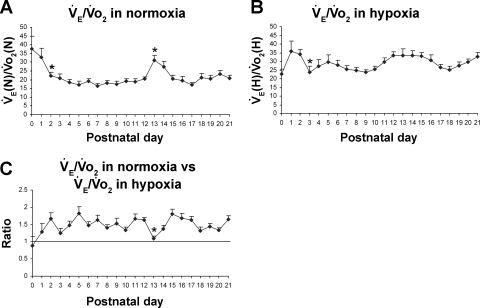
Postnatal changes in V̇e/V̇o2 ratios in normoxia and hypoxia.
At birth (P0), the V̇e/V̇o2 ratio was at its highest under normoxia but fell significantly at P2 (P < 0.05) followed by a relative plateau for the remaining first week. The ratio plateaued between 17 and 23 during the second and third postnatal weeks, except for a sudden and significant rise at P13 to 31 (P < 0.05) and at P14 to 27.3 (Fig. 4A). Under hypoxia, the V̇e/V̇o2 ratio fluctuated between 23 and 36 from P0 to P21, with a significant reduction in V̇e/V̇o2 at P3 (P < 0.05) (Fig. 4B). When V̇e/V̇o2 in hypoxia was compared with that in normoxia, the ratio (V̇e/V̇o2 in hypoxia vs. that in normoxia) was below 1 at P0 (0.88), but it rose to above 1 at P1 and thereafter (Fig. 4C). The ratio fluctuated between 1.25 and 1.82 for most of the remaining postnatal days until P21. However, it fell precipitously at P13 to 1.09 (P < 0.05) before returning at P15 to previous levels (Fig. 4C). Thus, compared with normoxic level, V̇e/V̇o2 in hypoxia was lowest at P0 during the first postnatal week and at P13 during the remaining period studied. One-sample t-test indicated that in the majority of age groups, the ratio of V̇e/V̇o2 in hypoxia vs. that in normoxia was significantly different from 1 (P < 0.05), except for P0, P1, P3, and P13.
Fig. 4.
Postnatal changes in V̇e/V̇o2 ratios in normoxia and hypoxia. A: V̇e/V̇o2 ratios in normoxia [V̇e(N)/V̇o2 (N)]. The ratio was highest at P0 and decreased significantly at P2, followed by a relative plateau until P12. It then increased abruptly and significantly at P13, decreased at P14 and P15, and plateaued thereafter until P21. B: V̇e/V̇o2 in hypoxia [V̇e(H)/V̇o2(H)]. V̇e/V̇o2 response fluctuated from P0 through P21, with the lowest level at P0 and a significant decrease at P3. C: V̇e/V̇o2 in hypoxia vs. that in normoxia. One-sample t-test indicated that in the majority of age groups, the ratio was significantly different from 1 (P < 0.05), except for P0, P1, P3, and P13. P13 is the only time point that shows a statistically significant difference between any adjacent 2 age groups (P < 0.05). For statistical comparisons between successive age groups, please see methods. *P < 0.05.
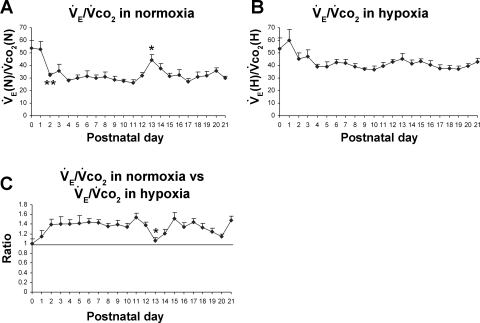
Postnatal changes in V̇e/V̇co2 ratios in normoxia and hypoxia.
Under normoxia, V̇e/V̇co2 ratio was highest at P0–P1 but plunged at P2 (P < 0.01) and plateaued (26.4–37.6) for most of the remaining days until P21; however, there was a distinct, sudden rise at P13 to 44.3 (P < 0.05) followed by a fall at P14–P15 (Fig. 5A). Under hypoxia, the V̇e/V̇co2 ratio was the highest at P0 and P1, but it fell at P2, followed by fluctuations between 36.6 and 47.4 from P3 to P21 without significant differences between adjacent age groups (Fig. 5B). When V̇e/V̇co2 under hypoxia was compared with that in normoxia, the ratio (V̇e/V̇co2 in hypoxia vs. that in normoxia) hovered between 1.2 and 1.5 for most of the first 3 postnatal wk, except for two time points (P0 and P13) when it was at 1 (P < 0.05 in comparing P13 to P12; Fig. 5C). One-sample t-test showed that in the majority of age groups, the ratio of V̇e/V̇co2 in hypoxia vs. that in normoxia was significantly different from 1 (P < 0.05), except for P0, P1, and P13.
Fig. 5.
Postnatal changes in V̇e/V̇co2 ratios in normoxia and hypoxia. A: V̇e/V̇co2 ratios in normoxia [V̇e(n)/V̇co2(N)]. The ratio was highest at P0–P1, then reduced significantly at P2 followed by a plateau until P12. It then rose significantly at P13, followed by a gradual decline at P14-P15 and a plateau until P21. B: V̇e/V̇co2 in hypoxia [V̇e(h)/V̇co2(H)]. This ratio was highest at P0 and P1, but fell insignificantly at P2, followed by a relative plateau until P21. C: V̇e/V̇co2 in hypoxia vs. that in normoxia. One-sample t-test showed that in the majority of age groups, the ratio was significantly different from 1 (P < 0.05), except for P0, P1, and P13. P13 is the only time point that shows a statistically significant difference between any adjacent 2 age groups (P < 0.05). For statistical comparisons between successive age groups, please see methods. *P < 0.05. **P < 0.01.
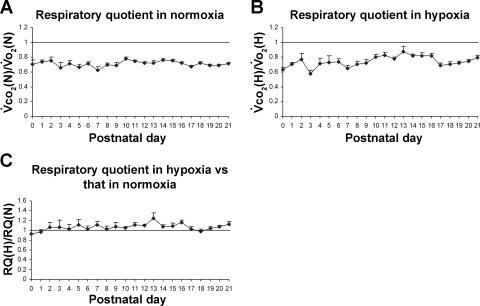
Postnatal development of RQ in normoxia and hypoxia.
Under normoxia, RQ (V̇co2/V̇o2) remained between 0.63 and 0.78 from P0 to P21, without significant differences among and between age groups (Fig. 6A). During acute hypoxia, RQ fluctuated between 0.58 (at P3) and 0.83 (at P11), with a peak closer to 1 at P13 (0.88) (Fig. 6B). When RQ under hypoxia was compared with that in normoxia, the ratio was about 1 at P0–P10, then slightly above 1 at P11–P13, with a small peak at P13, followed by fluctuations close to or slightly above 1 (Fig. 6C). However, there were no significant differences among and between the age groups. One-sample t-test revealed that the ratio of RQ in hypoxia vs. that in normoxia was significantly different from 1 (P < 0.05) at P11–P13, P16, P20, and P21.
Fig. 6.
Postnatal development of respiratory quotient (RQ) in normoxia and hypoxia. A: RQ in normoxia [V̇co2 (N)/V̇o2(N)]. The values remained quite stable from 0.63 to 0.78 from P0 to P21. B: RQ in hypoxia [V̇co2(H)/V̇o2(H)]. This ratio fluctuated mainly between 0.58 (at P0) and 0.83 (at P11) for most the 3 postnatal weeks, with a distinct peak close to 1 (0.88) at P13. C: RQ in hypoxia [RQ(H)] vs. that in normoxia [RQ(N)]. One-sample t-test revealed that the ratio was significantly different from 1 (P < 0.05) at P11–P13, P16, P20, and P21. For statistical comparisons between successive age groups, please see methods.
DISCUSSION
The present study is a comprehensive investigation of daily changes in the metabolic rate of rats throughout the first 3 postnatal wk under normoxia and hypoxia. It has uncovered striking features in metabolic development not heretofore recognized. Specifically, metabolic rates at P0–P1 and at P13 were distinctly different from those of the rest of the first and second postnatal weeks, respectively, and V̇e/V̇o2 and V̇e/V̇co2 ratios are not maintained at a constant value during the first 3 wk of postnatal development as previously thought (21). On the other hand, our findings are consistent with our hypothesis that insufficient metabolic rate is one of the major mechanisms underlying inadequate hypoxic ventilatory response around P13, a critical period of respiratory development. These properties may be closely linked to unique ventilatory characteristics observed in normoxia and acute hypoxia at the same time (Ref. 32; the present study) as well as to other known and unknown changes in bodily functions. Our data have revealed four temporally distinct patterns that are discussed below.
P0–P1.
Most developmental studies of ventilation and metabolism in the rat did not examine the first 2 days after birth; rather, animals within the remaining first postnatal week were grouped as “newborn” or “neonates” (22, 45, 48). What the present study revealed was that the metabolic rate at P0–P1 was very different from that of the remaining first postnatal week. V̇o2 and V̇co2 in normoxia were at their lowest, but V̇e/V̇o2 and V̇e/V̇co2 ratios were at their highest at P0–P1 compared with the rest of the first 3 postnatal weeks. Greater or lesser V̇e/V̇o2 and V̇e/V̇co2 ratios than normal generally imply hyperventilation or hypoventilation, respectively (19, 37, 52) and may indicate ventilatory perturbations (14, 48). Thus the normal state at P0–P1 is a combination of a relatively high rate of ventilation and a relatively low rate of metabolism, the exact opposite to that during the rest of the first week. The higher rate of ventilation serves to compensate for a poor efficiency in gas exchange due to limited alveolar surface area (10, 11). The low metabolic rate can be attributable to a weak or absent thermoregulatory response and moderate tissue growth at that time (41). Acute hypoxic challenge at P0–P1 induced relatively high V̇o2 and V̇co2 responses and a relatively low ventilatory response. The relatively high metabolic response in hypoxia results mainly from relatively low metabolic rate under normoxia and from a weak or absent hypometabolic response mechanism that probably commences at P2. On the other hand, a relatively low ventilatory response to acute hypoxia is attributable mainly to low chemosensitivity at birth. The sudden increase in arterial partial pressure of oxygen from ∼25–30 Torr in utero to ∼60 Torr at birth (9) transiently suppresses peripheral chemosensitive organs, primarily the carotid bodies (7, 42), resulting in a low response to the hypoxic challenge. Thus V̇e/V̇o2 and V̇e/V̇co2 ratios in hypoxia are at their lowest at P0–P1 compared with the rest of the first 3 postnatal wk.
P2–P7.
The metabolic rate in the remaining first postnatal week was exactly the opposite of those at P0–P1. Under normoxia, it was a combination of a relatively high rate of metabolism and reduced though stable V̇e/V̇o2 and V̇e/V̇co2 ratios. A relatively high rate of metabolism is necessary to meet the requirements of tissue growth and to compensate for greater heat diffusion and heat loss from a greater surface area-to-body mass ratio and the lack of a thick coat of fur (40, 41, 51). Concurrently, the lungs are developing and improving on gas exchange (8, 10, 11). The reduction in V̇e/V̇o2 and V̇e/V̇co2 ratios at P2 and stabilization thereafter (except for P13–P14, see below) indicate greater efficiency of gas convection (4, 5), and suggests that the respiratory control network is now able to modulate ventilation to match lung development and body's metabolic needs. Our values are comparable to or within the midrange of those reported by other investigators (6, 20, 22, 35, 45, 48, 50). When confronted with acute hypoxia, however, the metabolic rate was at a very low level compared with that in normoxia at P2–P7, most likely reflecting a self-protective mechanism against hypoxia and is consistent with the maxim that “the higher the normoxic V̇o2 (as evident during this time), the greater its decrease during hypoxia” (43). The protective hypometabolic response, combined with relatively high ventilatory rate (32), high V̇e/V̇o2 and V̇e/V̇co2 ratios (the present study), and maturing central respiratory network and peripheral chemosensors (carotid bodies) (2, 54) are able to meet the hypoxic challenge at this time. Our hypoxic data are comparable to some reports (22, 48, 50) but differ from others (35). Neonatal hypometabolic response to hypoxia also includes decreasing body temperature and other O2-dependent functions, such as maintaining muscle tone, cell excitability, tissue development, and organ growth (43). By conserving O2 delivery to central organs, neonates are better able to protect themselves against the hypoxic challenge. On the other hand, increases in ventilation promote the delivery of O2 to the mitochondria (20) for the generation of ATP.
Second and third postnatal weeks excluding P13.
After an initial period of adjustment (P8–P10), rats have acquired a more mature pattern of metabolism and ventilation (with the exception of P13, see below), with stable V̇o2/body weight and V̇co2/body weight ratios as well as relatively stable V̇e/V̇o2 and V̇e/V̇co2 ratios both in normoxia and acute hypoxia. Our values are comparable to most reports (14, 45, 48, 51), whereas they differ slightly from others (19, 22), although the hypoxic paradigms are sometimes different and relatively few time points were monitored in most studies. Thus, as the animal matures through the second and third postnatal weeks, the ventilatory machinery is generally able to meet the metabolic needs of growth under normoxia and the metabolic requirements under acute hypoxia. However, the end of the second postnatal week presents an entirely different scenario not previously recognized (see below).
P13.
Our most striking finding was that the metabolic rate and ventilation at P13 in both normoxia and hypoxia were distinctly different from those during the second and third postnatal weeks. The sudden enhancement of the metabolic rate and V̇e/V̇o2 and V̇e/V̇co2 ratios in normoxia were matched by significant increases in respiratory frequency, Vt, V̇e, as well as an abrupt rise in body temperature, all occurring at P13 (Ref. 32; present study). The reasons for such surges are not clear at this time, but several factors deserve our consideration: 1) sudden hormonal adjustments involving the hypothalamo-pituitary-adrenal axis; 2) changes in sympathetic activity; 3) thyroid hormone surges; 4) alterations in the mechanism of thermogenesis; 5) growth spurts in tissue or organ; and 6) changes in sleep patterns. These factors may exist in isolation or in combination, and their effects may be cumulative, subtractive, or interactive. The hypothalamic paraventricular nucleus (PVN) is important in the regulation of energy metabolism (57). Corticotropin-releasing hormone (CRH) (33, 34) and urocortin (12, 13) in the PVN can increase sympathetic activity, V̇o2, and the expression of uncoupling proteins (UCP; an indicator of thermogenesis). Between P7 and P14, CRH mRNA level undergoes significant changes in the rat (1). The PVN neurons that release CRH reportedly project to the locus coeruleus (49) and rostral ventrolateral medulla (38), both of which are important for sympathetic activity. The sympathetic nervous system is postulated to be the primary efferent pathway for regulating energy expenditure, because it innervates thermogenic target tissues, such as brown adipose tissue, leading to increased UCP activity and heat production (57). Thyroid hormone is a major agent involved in the enhancement of the metabolic rate (23), for it facilitates catecholamine-induced thermogenesis in brown adipose tissue (46, 56). A sharp rise in body temperature at P13 (32) may trigger hyperventilation to help dissipate the heat. Between P12 and P16, there is a reduction in active sleep (considered to be an immature form of rapid eye movement sleep) and an increase in quiet sleep (thought to represent an immature form of slow-wave sleep) in the rat (18), further affecting respiratory pattern.
The response to acute hypoxia at P13 was especially noteworthy compared with responses at other times during the second and third postnatal weeks. Both the metabolic rate (V̇o2 and V̇co2) and V̇e/V̇o2 and V̇e/V̇co2 ratios in hypoxia did not keep pace with the heightened values under normoxia. When challenged with hypoxia, the animals responded at the same metabolic rate as the day before and after P13, but this did not compensate for the fact that its normoxic rate has been drastically increased. Thus, compared with the days before and after P13, the animal was operating at a relative low, compromised metabolic rate under hypoxia at P13. If hypoxia became more severe and prolonged, the animal's metabolic rate and energy production will not be sufficient to meet the increased metabolic demand at P13, and the survival of the animal may be at stake. This combined pattern of relatively low metabolism, low V̇e/V̇o2 and V̇e/V̇co2 ratios, as well as low ventilation (Ref. 32; present study) is unique only to P13 for the entire first 3 postnatal wk. Unlike the case of P2–P7, when the neonates need to reduce their metabolic rate while they are hyperventilating in order to survive under hypoxic conditions (43), pups at P13 exhibit a low metabolic rate in hypoxia relative to the distinctly high metabolic rate in normoxia, and hypopnea in hypoxia relative to increased ventilation in normoxia when they are at a more mature developmental stage, and only for that single day. It can be argued that hypoventilation and hypometabolism in hypoxia at P13 is an appropriate response. However, it is difficult to reconcile that a “protective” mechanism exists for only 1 day. It is more likely that multiple physiological and neurochemical changes occur during this narrow window (see below) to which the animals are not able to adjust appropriately, resulting in a response to hypoxia at a level not different from those before or after P13 but inadequate for P13. Measurement of blood gases may be needed to further elucidate this issue. Low metabolism at P13, however, is consistent with Mortola's maxim (43) that “the higher the normoxic V̇o2, the greater its decrease during hypoxia.” The weak hypoxic ventilatory response at P13 is due mainly to a decrease in tidal volume, a response that is different from those in the rest of the first 3 postnatal wk (32). By P14, the metabolic response to hypoxia has returned to normal, but the HVR remained low (32); thus the V̇e/V̇o2 and V̇e/V̇co2 ratios in hypoxia also stayed low as those at P13.
RQ.
As expected, the RQ did not exhibit any significant change during postnatal development from P0 to P21, either in normoxia or hypoxia, although some fluctuations were present, especially in the first week of life. These findings (RQ values of 0.63–0.75 during the first 3 postnatal wk) are consistent with published reports (6, 55). However, at P13, RQ did show a small although statistically insignificant peak during hypoxia. This suggests that the sympathetic system is even more activated on this day to combat hypoxia, and greater utilization of glucose than fat as energy source by the sympathetic system would increase the RQ (47).
The end of the second postnatal week: a potential critical period.
The present study provides further evidence that the end of the second postnatal week is a critical period in the development of the respiratory system in the rat. Sudden changes during this period include a drastic imbalance in the expressions of excitatory (reduced) and inhibitory (enhanced) neurotransmitters and receptors at P12 (reviewed in Ref. 61), a switch in GABAA receptor subunit expressions around P12 (29, 31), a sudden drop in cytochrome oxidase activity in several brain stem respiratory nuclei at P12 (reviewed in Ref. 61), a spike in body temperature, and a significant increase in V̇e due to an increase in both frequency and Vt at P13 (32), as well as a significant increase in the metabolic rate at P13 (present study). These changes all occur under normal conditions. When confronted with acute hypoxia, both the ventilatory response (32) and the relative metabolic rate (present study) are at their weakest. Thus this period can be regarded as a “critical period” of respiratory development, when the system is undergoing a great deal of normal adjustments and when the animal is less capable of responding to respiratory stressors, such as hypoxia. Other physiological and behavioral changes have been discussed previously (32). The implication of such a critical period in seemingly normal infants who have succumbed to SIDS deserves future investigation. Of special note is the reported correspondence in brain development between human postnatal months 2–4 (the peak incidence of SIDS) and P11–P14 (around our critical period) in rats (3).
GRANTS
This study was supported by National Institute of Child Health and Human Development Grant HD-048954 and by a grant from the Children's Hospital and Health System Foundation, Milwaukee, Wisconsin.
Acknowledgments
We thank Dr. A. Szabo and S. Jackson for their expert consultation and performance of the statistical analyses, we thank Dr. H. Forster for critically reading the manuscript, and we greatly appreciate the late Dr. R. Franciosi for his support through the years.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aird F, Halasz I, Redei E. Ontogeny of hypothalamic corticotropin-releasing factor and anterior pituitary pro-opiomelanocortin expression in male and female offspring of alcohol-exposed and adrenalectomized dams. Alcohol Clin Exp Res 21: 1560–1566, 1997. [PubMed] [Google Scholar]
- 2.Aitken ML, Franklin JL, Pierson DJ, Schoene RB. Influence of body size and gender on control of ventilation. J Appl Physiol 60: 1894–1899, 1986. [DOI] [PubMed] [Google Scholar]
- 3.Ballanyi K Neuromodulation of the perinatal respiratory network. Curr Neuropharm 2: 221–243, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Bech C, Johansen K. Ventilation and gas exchange in the mute swan, Cygnus olor. Respir Physiol 39: 285–295, 1980. [DOI] [PubMed] [Google Scholar]
- 5.Bennett AF, Hicks JW. Postprandial exercise: prioritization or additivity of the metabolic responses? J Exp Biol 204: 2127–2132, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Bertin R, De Marco F, Mouroux I, Portet R. Postnatal development of nonshivering thermogenesis in rats: effects of rearing temperature. J Dev Physiol 19: 9–15, 1993. [PubMed] [Google Scholar]
- 7.Blanco CE, Dawes GS, Hanson MA, McCooke HB. The response to hypoxia of arterial chemoreceptors in fetal sheep and new-born lambs. J Physiol 351: 25–37, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanco LN Mechanisms for the generation of gas-exchange surface area in rat lung. Am J Physiol Lung Cell Mol Physiol 269: L698–L708, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Brouillette RT, Waxman DH. Evaluation of the newborn's blood gas status. National Academy of Clinical Biochemistry. Clin Chem 43: 215–221, 1997. [PubMed] [Google Scholar]
- 10.Burri PH The postnatal growth of the rat lung. 3. Morphology. Anat Rec 180: 77–98, 1974. [DOI] [PubMed] [Google Scholar]
- 11.Burri PH, Dbaly J, Weibel ER. The postnatal growth of the rat lung. I. Morphometry. Anat Rec 178: 711–730, 1974. [DOI] [PubMed] [Google Scholar]
- 12.Currie PJ, Coscina DV, Bishop C, Coiro CD, Koob GF, Rivier J, Vale W. Hypothalamic paraventricular nucleus injections of urocortin alter food intake and respiratory quotient. Brain Res 916: 222–228, 2001. [DOI] [PubMed] [Google Scholar]
- 13.De Fanti BA, Martínez JA. Central urocortin activation of sympathetic-regulated energy metabolism in Wistar rats. Brain Res 930: 37–41, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Dotta A, Mortola JP. Postnatal development of the denervated lung in normoxia, hypoxia, or hyperoxia. J Appl Physiol 73: 1461–1466, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics 16: 81–87, 1955. [PubMed] [Google Scholar]
- 16.Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate 65: 194–197, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Fishman AP, McClement J, Himmelstein A, Cournand A. Effects of acute anoxia on the circulation and respiration in patients with chronic pulmonary disease studied during the steady state. J Clin Invest 31: 770–781, 1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank MG, Heller HC. Development of REM and slow wave sleep in the rat. Am J Physiol Regul Integr Comp Physiol 272: R1792–R1799, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Frappell PB, Mortola JP. Hamsters vs. rats: metabolic and ventilatory response to development in chronic hypoxia. J Appl Physiol 77: 2748–2752, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Frappell P, Lanthier C, Baudinette RV, Mortola JP. Metabolism and ventilation in acute hypoxia: a comparative analysis in small mammalian species. Am J Physiol Regul Integr Comp Physiol 262: R1040–R1046, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Fukuda Y Maintenance of ventilatory control by CO2 in the rat during growth and aging. Pflügers Arch 419: 38–42, 1991. [DOI] [PubMed] [Google Scholar]
- 22.Gozal D, Reeves SR, Row BW, Neville JJ, Guo SZ, Lipton AJ. Respiratory effects of gestational intermittent hypoxia in the developing rat. Am J Respir Crit Care Med 167: 1540–1547, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Guyton AC Energetics and metabolic rate. In: Textbook of Medical Physiology (7th ed.). Philadelphia, PA: Saunders, 1986, p. 841–848.
- 24.Kinney HC, Filiano JJ, White WF. Medullary serotonergic network deficiency in the sudden infant death syndrome: review of a 15-year study of a single dataset. Neuropathol Exp Neurol 60: 228–247, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Kleiber M Respiratory exchange and metabolic rate. In: Handbook of Physiology. Respiration. Washington, DC, Am. Physiol. Soc., 1965, sect. 3, vol II, chapt. 35, p. 927–937.
- 26.Lagneaux D Ventilatory responses to brief hypoxic stimuli after simulated altitude exposure in rat. Respir Physiol 97: 157–173, 1994. [DOI] [PubMed] [Google Scholar]
- 27.Liu Q, Wong-Riley MTT. Postnatal expression of neurotransmitters, receptors, and cytochrome oxidase in the rat pre-Botzinger complex. J Appl Physiol 92: 923–934, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Liu Q, Wong-Riley MTT. Postnatal changes in cytochrome oxidase expressions in brain stem nuclei of rats: implications for sensitive periods. J Appl Physiol 95: 2285–2291, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Liu Q, Wong-Riley MTT. Developmental changes in the expression of GABAA receptor subunits α1, α2, and α3 in the rat pre-Bötzinger complex. J Appl Physiol 96: 1825–1831, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Liu Q, Wong-Riley MTT. Postnatal developmental expressions of neurotransmitters and receptors in various brain stem nuclei of rats. J Appl Physiol 98: 1442–1457, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Liu Q, Wong-Riley MTT. Developmental changes in the expression of GABA(A) receptor subunits alpha1, alpha2, and alpha3 in brain stem nuclei of rats. Brain Res 1098: 129–138, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Liu Q, Lowry TF, Wong-Riley MT. Postnatal changes in ventilation during normoxia and acute hypoxia in the rat: implication for a sensitive period. J Physiol 577: 957–970, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu XY, Barsh GS, Akil H, Watson SJ. Interaction between alpha-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. J Neurosci 23: 7863–7872, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masaki T, Yoshimichi G, Chiba S, Yasuda T, Noguchi H, Kakuma T, Sakata T, Yoshimatsu H. Corticotropin-releasing hormone-mediated pathway of leptin to regulate feeding, adiposity, and uncoupling protein expression in mice. Endocrinology 144: 3547–3554, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Matsuoka T, Mortola JP. Effects of hypoxia and hypercapnia on the Hering-Breuer reflex of the conscious newborn rat. J Appl Physiol 78: 5–11, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Matsuoka T, Yoda T, Ushikubo S, Matsuzawa S, Sasano T, Komiyama A. Repeated acute hypoxia temporarily attenuates the ventilatory respiratory response to hypoxia in conscious newborn rats. Pediatr Res 46: 120–125, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Mautz WJ, Bufalino C. Breathing pattern and metabolic rate responses of rats exposed to ozone. Respir Physiol 76: 69–77, 1989. [DOI] [PubMed] [Google Scholar]
- 38.Milner TA, Reis DJ, Pickel VM, Aicher SA, Giuliano R. Ultrastructural localization and afferent sources of corticotropin-releasing factor in the rat rostral ventrolateral medulla: implications for central cardiovascular regulation. J Comp Neurol 333: 151–167, 1993. [DOI] [PubMed] [Google Scholar]
- 39.Moon RY, Horne RS, Hauck FR. Sudden infant death syndrome. Lancet 370: 1578–1587, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Mortola JP Breathing pattern in newborns. J Appl Physiol 56: 1533–1540, 1984. [DOI] [PubMed] [Google Scholar]
- 41.Mortola JP Dynamics of breathing in newborn mammals. Physiol Rev 67: 187–243, 1987. [DOI] [PubMed] [Google Scholar]
- 42.Mortola JP Respiratory Physiology of Newborn Mammals. Baltimore, MD: The Johns Hopkins University Press, 2001.
- 43.Mortola JP Implications of hypoxic hypometabolism during mammalian ontogenesis. Respir Physiol Neurobiol 141: 345–356, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Mortola JP Influence of temperature on metabolism and breathing during mammalian ontogenesis. Respir Physiol Neurobiol 149: 155–164, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Mortola JP, Morgan CA, Virgona V. Respiratory adaptation to chronic hypoxia in newborn rats. J Appl Physiol 61: 1329–1336, 1986. [DOI] [PubMed] [Google Scholar]
- 46.Mory G, Ricquier D, Hémon P. Effects of chronic treatments upon the brown adipose tissue of rats. II. Comparison between the effects of catecholamine injections and cold adaptation. J Physiol (Paris) 76: 859–864, 1981. [PubMed] [Google Scholar]
- 47.Nonogaki K New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia 43: 533–495, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Peyronnet J, Roux JC, Geloen A, Tang LQ, Pequignot JM, Lagercrantz H, Dalmaz Y. Prenatal hypoxia impairs the postnatal development of neural and functional chemoafferent pathway in rat. J Physiol 524: 525–537, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reyes BA, Valentino RJ, Xu G, Van Bockstaele EJ. Hypothalamic projections to locus coeruleus neurons in rat brain. Eur J Neurosci 22: 93–106, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Saetta M, Mortola JP. Interaction of hypoxic and hypercapnic stimuli on breathing pattern in the newborn rat. J Appl Physiol 62: 506–512, 1987. [DOI] [PubMed] [Google Scholar]
- 51.Sant'Anna GM, Mortola JP. Thermal and respiratory control in young rats with altered caloric intake during postnatal development. Respir Physiol Neurobiol 133: 215–227, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Sant'Anna GM, Mortola JP. Thermal and respiratory control in young rats exposed to cold during postnatal development. Comp Biochem Physiol A Mol Integr Physiol 134: 449–459, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Simakajornboon N, Kuptanon T. Maturational changes in neuromodulation of central pathways underlying hypoxic ventilatory response. Respir Physiol Neurobiol 149: 273–286, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Tattersall GJ, Blank JL, Wood SC. Ventilatory and metabolic responses to hypoxia in the smallest simian primate, the pygmy marmoset. J Appl Physiol 92: 202–210, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Thomas AJ, Erokwu BO, Yamamoto BK, Ernsberger P, Bishara O, Strohl KP. Alterations in respiratory behavior, brain neurochemistry and receptor density induced by pharmacologic suppression of sleep in the neonatal period. Brain Res Dev Brain Res 120: 181–189, 2000. [DOI] [PubMed] [Google Scholar]
- 56.Triandafillou J, Gwilliam C, Himms-Hagen J. Role of thyroid hormone in cold-induced changes in rat brown adipose tissue mitochondria. Can J Biochem 60: 530–537, 1982. [DOI] [PubMed] [Google Scholar]
- 57.Wang C, Bomberg E, Billington C, Levine A, Kotz CM. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus reduces energy intake. Am J Physiol Regul Integr Comp Physiol 293: R1003–R1012, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Waters KA, Gozal D. Responses to hypoxia during early development. Respir Physiol Neurobiol 136: 115–129, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Westfall PH Multiple testing of general contrasts using logical constraints and correlations. J Am Stat Assoc 92: 299–306, 1997. [Google Scholar]
- 60.Wong-Riley MTT Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci 12: 94–101, 1989. [DOI] [PubMed] [Google Scholar]
- 61.Wong-Riley MTT, Liu Q. Neurochemical development of brain stem nuclei involved in the control of respiration. Respir Physiol Neurobiol 149: 83–98, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Zwemer CF, Song MY, Carello KA, D'Alecy LG. Strain differences in response to acute hypoxia: CD-1 versus C57BL/6J mice. J Appl Physiol 102: 286–293, 2007. [DOI] [PubMed] [Google Scholar]