Abstract
Laryngeal closure following hypoxic gasps has been documented, but its efficacy in improving autoresuscitation capacity is unknown. We studied SWR/J mice who normally cannot autoresuscitate and the C57/BLJ strain who can. We evaluated the effects of elevated end-inspiratory lung volume immediately following a gasp. We compared upper airway-intact mice with tracheostomized mice in which the vocal cords are bypassed. We used the techniques of repeated autoresuscitate trials to test autoresuscitation capability. Both SWR/J and C57/BLJ mice could maintain elevated lung volume immediately after a gasp (breath holding). Such breath holding increased autoresuscitation ability in C57/BLJ mice but did not in SWR/J mice. In SWR/J mice, the duration of the breath holds was less than that in the C57/BLJ mice. These findings indicate that gasp-associated breath holding improves autoresuscitation capability during repeated autoresuscitation trials. Also, they show that SWR/J mice have a deficiency in central nervous system mechanisms regulating glottic closure during hypoxic gasping.
Keywords: breath holding, control of respiration, SWR/J mice, C57/BLJ mice
past studies have shown that hypoxic gasping is critical for autoresuscitation from hypoxic apnea and that young SWR/J normally cannot autoresuscitate during a critical developmental time period (19–22 days old) (3, 4). Other studies have indicated that hypoxic gasps have an end-inspiratory component that produces vocal cord closure immediately following a gasp (1, 7). This elevates lung volume until the next gasp occurs. Theoretically, this should improve autoresuscitation. However, a prior study of autoresuscitation capability in lambs found that such “breath holding” during gasping failed to show a positive effect of end-inspiratory breath holding on autoresuscitation capability (7).
In the present study, we had two primary hypotheses. First, end-inspiratory breath holding associated with a gasp improves the ability to autoresuscitate in mice. Second, SWR mice that normally cannot autoresuscitate would be different in their ability to breath hold from another inbred strain of mice with good autoresuscitation capability.
METHODS
Twenty-seven SWR/J mice and 44 C57/BLJ mice were studied during the 19- to 21-day critical period. Thirteen SWR/J mice were tracheostomized, and 14 were studied with the upper airway intact. Twenty-four C57/BLJ mice were tracheostomized, and 25 had an intact larynx. Most mice were bred in our own facility. By chance, more C57/BLJ litters were produced in our facility compared with SWR/J mice, and all young C57/BLJ mice were studied. The average ages of both strains were not significantly different. We used a small glass jar pressure plethysmograph for these studies. A port allowed pressure in the jar to be recorded. The lid on the jar had a hole for the mouse's nose and mouth, which projected out of the plethsymograph. An airtight elastic collar was glued to the mouse's head with super glue, and the collar was glued to the plethysmograph opening. The elastic collar allowed the mouth to open during hypoxic gasping. A similar opening in the jar allowed the tracheotomy tube to project out of the phethysmograph.
Both face-out and tracheostomized mice were initially anesthetized with pentobarbital (0.05 mg/g body wt) given intraperitoneally. A midline neck incision was performed, and the trachea exposed. A 1.7-cm-long polyethylene tube was inserted through a small incision in the trachea and was secured with super glue. The inside diameter of the tube was ∼0.8 mm, and that of the trachea was 0.7 mm. The outside diameter of the tube was ∼0.9 mm, and the trachea was stretched slightly wider when the tube was inserted. This incision was closed with super glue. The tracheostomy tube projected out of the jar and was secured by super glue. In face-out mice, the trachea was surgically exposed, and then the skin was closed with the trachea intact. Care was taken in all mice to avoid damage to the recurrent laryngeal nerves. Both face-out and tracheostomized mice were allowed to recover from anesthesia, as determined by return of leg withdrawal to pinch. They then were immediately tested. To induce hypoxia, we used a cone over the plethysmograph lid to expose the mouse to a mixture of 97% nitrogen and 3% CO2, as our laboratory has done in past studies (3, 4). Following the hypoxia-induced hyperpnea, the cone was removed to allow access to air to evaluate autoresuscitation capability. Unlike our prior studies, where air was given at onset of hypoxic seizures, we waited until there was a 1-s time lapse between breaths at the end of hyperpnea before giving access to room air. This was necessary, since precise timing of the onset and termination of seizures could not be made in these partially restrained mice. Consequently, SWR/J mice were able to autoresuscitate for multiple trials due to the fact that one or more gasps occurred before seizure activity. This allowed us to determine the long-term effects of breath holding on autoresuscitation in SWR/J mice. When successful autoresuscitation occurred, as indicated by a respiratory rate of 60 breaths/min, the animal was immediately reexposed to the nitrogen/CO2 gas mixture. This process was repeated until autoresuscitation failed (3, 4). Hypoxic seizures were identified by rapid movements of the extremities. This occurred 3–5 s after the initial gasp (Fig. 1A). This protocol was approved by an institutional review committee for animal experimentation.
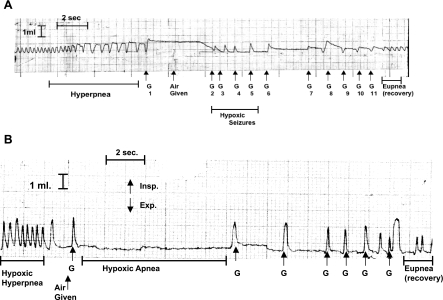
Fig. 1.
A: tracing of respiration in a C57/BLJ mouse during an autoresuscitation (AR) trial. Gasps (G) 1, 6, 7, and 8 were counted in our protocol. Those during seizures, G2-5, were not. Note that some degree of end-inspiratory breath holding is present during hypoxic hyperpnea. After a 1.5-s time lapse between breaths, N2/CO2 is discontinued and is replaced by air. Our protocol was giving air after 1 s, but there was some degree of variability in attempting to reach this precise target. Note the prolonged breath hold after the first gasp and its absence in gasps during hypoxic seizures. The final gasps before recovery (G10 and 11) have decreased peak inspiratory volume, possibly due to decreased neural output from the medulary center(s) that regulates gasping. B: tracing of respiration in a tracheostomized C57/BLJ mouse during an AR trial. Note the absence of breath holding associated with gasps. The first 4 gasps were considered in our protocol. Insp, inspiratory; Exp, expiratory.
Expiratory time was determined as time from the peak of the gasp inspiratory volume until the volume returned to the pregasp baseline. The expiratory time of the first three gasps was measured and averaged. In doing this, the rapid gasps during hypoxic seizures were excluded, since breath holding was absent in these gasps, indicating a different neuro-regulating mechanism. Expiratory time was measured from the polygraph charts using a ruler. To reduce the number of these time-consuming measurements, expiratory time was determined in the initial four gasps in the first and last three successful trials in both mouse strains. To determine the progressive changes in breath holding, we measured expiratory time in the first four successful autoresuscitation trials and the last four trials. The trial that was midway between the first and last trial was also studied. The unpaired t-test and ANOVA were used for statistical analysis.
RESULTS
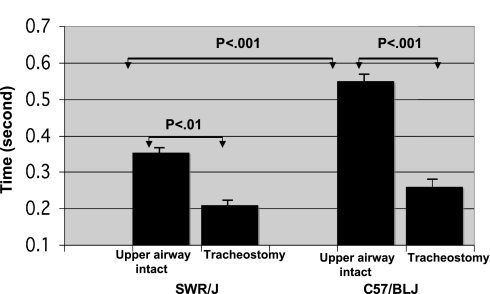
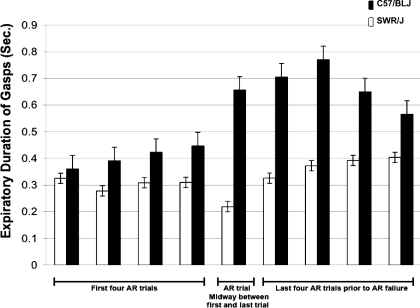
Airway-intact mice of both strains displayed postgasp breath holding during autoresuscitation. This was not present in tracheostomized mice (Fig. 1). The average expiratory time for tracheotomized mice was significantly less than that of the upper airway-intact mice, and duration of the gasp-associated breath hold was increased in C57/BLJ mice compared with SWR/J mice (Fig. 2). With an increasing number of successful autoresuscitation trials, breath-hold duration increased in both SWR/J and C57/BLJ mice (P < 0.01 ANOVA) (Fig. 3). However, breath holds were longer in C57/BLJ compared with SWR/J mice, and this became more pronounced in the preterminal trials (P < 0.01 ANOVA) (Fig. 3).
Fig. 2.
Duration of gasp-associated breath holding in upper airway-intact vs. tracheostomized SWR/J and C57/BLJ mice. Duration of expiratory time is longer in upper airway-intact mice than in tracheostomized mice. Note that expiratory time prolongation is greater in C57/BLJ mice compared with SWR/J mice.
Fig. 3.
Progressive changes in breath holding duration (expiratory time) in SWR/J and C57/BLJ mice with repeated hypoxic exposures. Since the C57/BLJ mice underwent an increased number of trials, their overall exposure to hypoxia was increased compared with that of SWR/J mice. Data for the initial 4 trials, the middle AR trial, and the terminal 4 trials are shown. Note the increased duration of gasp-associated breath holding in C57/BLJ mice as opposed to SWR/J mice. Also note the increasing duration of breath holds with the increasing number of trials.
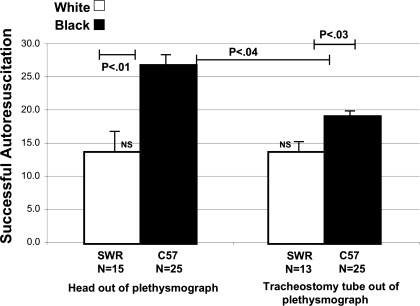
The number of successful autoresuscitation trials was greater for C57/BLJ than SWR/J mice (Fig. 4). The number of successful trials in face-out C57/BLJ was greater than that of tracheostomized mice. In contrast, there was no difference in successful trials in tracheostomized vs. upper airway-intact SWR/J mice (P > 0.05).
Fig. 4.
Number (N) of successful AR trials in upper airway-intact and tracheostomized SWR/J and C57/BLJ mice. Note that both tracheostomized and airway-intact SWR mice had reduced trials compared with C57/BLJ mice. Also note that the upper airway-intact C57/BLJ mice had more successful trials than tracheostomized mice. However, this was not the case in SWR/J mice. NS, nonsignificant.
Additional findings of interest were that gasps during hypoxic seizures were not associated with breath holding (Fig. 1A). Also, the degree of laryngeal closure appeared to vary from complete closure in the initial gasps to partial or delayed closure, as indicated by progressively decreasing inspiratory volume during the breath holds, leading up to completion of autoresuscitations (Fig. 1A).
DISCUSSION
In a past study, we found that pentobarbital (0.06 mg/g body wt) increased the ability of SWR/J mice to autoresuscitate (5). Therefore, the pentobarbital anesthesia needed for surgery in the present study may have contributed to the increased ability of SWR/J mice to repeatedly autoresuscitate. However, in contrast to that prior study, the mice in the present study were allowed to partially recover from anesthesia before testing. In any event, both mouse strains received pentobarbital in the present study, and SWR/J mice had reduced ability to autoresuscitate compared with C57/BLJ mice, as predicted. Prior studies in rabbits of the laryngeal constricting muscle motoneurons during gasping indicated activation of laryngeal constricting muscles immediately following a gasp (1). Also, recordings of tidal volume in lambs, using a face mask, established that breath holding is associated with hypoxic gasping (7).
We found that expiratory time was substantially shortened in tracheostomized mice. In this study, we used a technique of giving air earlier than we have done in the past. This allowed us to fully test autoresuscitation capability in SWR/J mice, which normally cannot autoresuscitate on the first hypoxic trial. Unlike the prior study in lambs (7), we were able to show in C57BL/J mice an increased efficacy of autoresuscitation, as indicated by an increased number of successful autoresuscitation trials. The prior study was of only one successful autoresuscitation, and this may be why no positive effect was seen (6). The beneficial effect of breath holding was seen only in C57/BLJ mice. That autoresuscitation was not improved in breath-holding SWR mice was unexpected. Our laboratory's past work has indicated that SWR/J mice have reduced stores of cardiac glycogen (2). This would cause early heart failure during hypoxia due to the reduced capacity for anaerobic metabolism in heart muscle. Thus early heart failure during hypoxia could be responsible, in part, for the failure of SWR/J mice to resuscitate on the first autoresuscitation trial. It is possible that the breath-holding mechanism does not compensate for such cardiac failure. Also, we have recently found evidence that SWR/J mice have deficient epinephrine release during hypoxia compared with C57/BLJ mice (unpublished observations). This also might contribute to autoresuscitation failure. Potentially, the ability to breath hold would have no effect on epinephrine release. Also, it is noteworthy that SWR/J mice had fewer hypoxic trials than C57/BLJ mice and had shorter breath holds. Therefore, as well as deficient glycogen and adrenal function, the shortened breath holds in SWR/J mice may have contributed to their reduced autoresuscitation capability during multiple hypoxic trials.
Respiratory rate during hypoxic hyperpnea slows before the onset of hypoxic apnea, and we allowed access to room air after a 1-s pause between hyperpneic breaths. We made an arbitrary decision to call the last breath during hyperpnea a gasp. This is because the distinctive pattern of breath holding associated with gasps was universally present in the breaths after the 1-s pause. Traditionally, hypoxic gasping is defined as the breaths that follow hypoxic apnea. We previously viewed hypoxic apnea as being irrelevant to autoresuscitation, as no gasps occur during this phase. However, the present findings indicate that breath holding during hypoxic apnea could contribute to recovery in certain clinical situations. For instance, when hypoxic apnea and gasping occurs during infantile breath-holding spells, the initial breath hold during hypoxic apnea could contribute to a more rapid autoresuscitation. The gasps associated with hypoxic seizures were not accompanied by breath holding and suggest that, although they may contribute to successful autoresuscitation, they have a different central nervous system regulatory mechanism than other gasps.
In a prior study, arterial blood gases were measured during autoresuscitation (7). It was found that breath holding had no significant effect on elevating arterial Po2, although there was a trend in that direction. Failure to note a significant increase may have been due to the small number of animals studied. It may also be that the positive airway pressure during a breath hold may delay the appearance of pulmonary edema as autoresuscitation trials progress. Such edema often accompanies severe hypoxia, and, in the present case, it would have compromised the positive effect of gasps on increasing arterial oxygen (6).
GRANTS
This research was funded by National Institute of Child Health and Human Development Grant HD-10993.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Davis PF, Macefield G, Nail BS. Laryngeal motoneurone activity in the rabbit during asphyxic gasping. Respir Physiol 70: 327–342, 1987. [DOI] [PubMed] [Google Scholar]
- 2.Despande P, Khurana A, Hensen P, Wilkins D, Thach BT. Failure of the autoresuscitation mechanism in weaning mice: significance of cardiac glycogen and heart rate regulation. J Appl Physiol 87: 203–210, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Jacobi MS, Gershan WM, Thach BT. Mechanism of failure of recovery from hypoxic apnea by gasping in 17- to 23-day-old mice. J Appl Physiol 71: 1098–1105, 1991. [DOI] [PubMed] [Google Scholar]
- 4.Jacobi MS, Thach BT. Effect of maturation on spontaneous recovery from hypoxic apnea by gasping. J Appl Physiol 66: 2384–2390, 1989. [DOI] [PubMed] [Google Scholar]
- 5.Jacobi MS, Gershan WM, Thach BT. Effect of pentobarbital on spontaneous recovery from hypoxic apnea in mice. Respir Physiol 84: 337–349, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Sarada S, Himadri P, Chitaranjan M, Geetali P, Sai Ram M, Ilavazhagan G. Role of oxidative stress and NFkB in hypoxia-induced pulmonary edema. Exp Biol Med (Maywood) 233: 1088–1098, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Thuot F, Lemaire D, Dorian D, Letourneau P, Praud JP. Active glottal closure during anoxic gasping in lambs. Respir Physiol 128: 208–218, 2001. [DOI] [PubMed] [Google Scholar]