Abstract
The purpose of this study was to determine whether exercise prescriptions differing in volume or intensity also differ in their ability to retain insulin sensitivity during an ensuing period of training cessation. Sedentary, overweight/obese subjects were assigned to one of three 8-mo exercise programs: 1) low volume/moderate intensity [equivalent of ∼12 miles/wk, 1,200 kcal/wk at 40–55% peak O2 consumption (V̇o2peak), 200 min exercise/wk], 2) low volume/vigorous intensity (∼12 miles/wk, 1,200 kcal/wk at 65–80% V̇o2peak, 125 min/wk), and 3) high volume/vigorous intensity (∼20 miles/wk, 2,000 kcal/wk at 65–80% V̇o2peak, 200 min/wk). Insulin sensitivity (intravenous glucose tolerance test, SI) was measured when subjects were sedentary and at 16–24 h and 15 days after the final training bout. SI increased with training compared with the sedentary condition (P ≤ 0.05) at 16–24 h with all of the exercise prescriptions. SI decreased to sedentary, pretraining values after 15 days of training cessation in the low-volume/vigorous-intensity group. In contrast, at 15 days SI was significantly elevated compared with sedentary (P ≤ 0.05) in the prescriptions utilizing 200 min/wk (low volume/moderate intensity, high volume/vigorous intensity). In the high-volume/vigorous-intensity group, indexes of muscle mitochondrial density followed a pattern paralleling insulin action by being elevated at 15 days compared with pretraining; this trend was not evident in the low-volume/moderate-intensity group. These findings suggest that in overweight/obese subjects a relatively chronic persistence of enhanced insulin action may be obtained with endurance-oriented exercise training; this persistence, however, is dependent on the characteristics of the exercise training performed.
Keywords: obesity, physical activity, cardiovascular disease, metabolic syndrome
physical activity can improve insulin action in individuals with insulin-resistant conditions such as the metabolic syndrome, cardiovascular disease, obesity, and type 2 diabetes (2, 6, 28). This desirable response is, unfortunately, typically short-lived because insulin sensitivity can decline significantly after as little as 38 h after the final exercise training bout (14, 25, 32). This transient response should be considered when designing physical activity programs for the general populace, because both the magnitude of the improvement in insulin action and the ability to retain this improvement when not exercising should be optimized. However, despite such potentially important considerations, the long-term maintenance characteristics after exercise training is stopped are notably understudied. The purpose of the present investigation was to determine whether there are differences between exercise prescriptions commonly used to treat and prevent disease in the ability to retain the physical activity-induced improvement in insulin action during a 2-wk period of training cessation. Possible mechanistic hypotheses for such adaptations were also explored to the extent that data were available. Persistence of an exercise-mediated improvement in insulin action would provide evidence for additional health benefits of endurance-oriented exercise training beyond the well-characterized short-term postexercise effects.
MATERIALS AND METHODS
Subjects.
These data are part of the Studies of Targeted Risk Reduction Intervention Through Defined Exercise (STRRIDE) project described in detail elsewhere (22). Inclusion criterion were sedentary, overweight/obese [body mass index (BMI) >25 to ≤35 kg/m2], and dyslipidemia (either an LDL cholesterol concentration of 130–190 mg/dl or an HDL cholesterol concentration below 40 mg/dl for men or 45 mg/dl for women). Women participants were postmenopausal. Exclusion criteria included medications that could alter carbohydrate metabolism, the presence of diabetes, hypertension, or heart disease, and orthopedic conditions prohibiting exercise. The experimental protocol was approved by the Institutional Review Boards at Duke and East Carolina Universities. Subjects provided written informed consent.
Study design.
Subjects were stratified for race, age, and sex and randomly assigned to one of three exercise training groups. After 8 mo of supervised exercise, subjects stopped training and insulin action was assessed 16–24 h after the final training bout and after 15 days of training cessation. The number of subjects per group (n) were 46 for the low-volume/moderate-intensity group, 49 for the low-volume/vigorous-intensity group, and 58 for the high-volume/vigorous-intensity group. Data from these subjects were included in a report (20) comparing insulin action at only the sedentary vs. 16–24 h postexercise testing points. Data analysis in that report (20) was conducted before the completion of STRRIDE recruitment; overlap between the present and previous (20) data at the pretraining and 16–24 h time points is n = 37 for the low-volume/moderate-intensity group, n = 28 for the low-volume/vigorous-intensity group, and n = 36 for the high-volume/vigorous-intensity group.
Exercise training.
The 8-mo exercise training programs were as follows (volume/intensity): 1) low volume/moderate intensity [caloric equivalent of ∼12 miles/wk, 1,200 kcal/wk at 40–55% peak O2 consumption (V̇o2peak)]; 2) low volume/vigorous intensity (∼12 miles/wk, 1,200 kcal/wk at 65–80% V̇o2peak), and 3) high volume/vigorous intensity (∼20 miles/wk, 2,000 kcal/wk at 65–80% V̇o2peak). The training regimens were chosen to compare different exercise volume (1,200 vs. 2,000 kcal/wk) in groups exposed to the same training intensity (65–80% V̇o2peak) and different intensities (40–55% vs. 65–80% V̇o2peak) in groups with the same exercise volume (1,200 kcal/wk). The exercise volumes (1,200 and 2,000 kcal/wk) were consistent with recommendations of the Surgeon General's report (6) and the Harvard Alumni Study (24). The 65–80% V̇o2peak level was chosen because this is the traditional exercise intensity prescribed for cardiovascular fitness benefits (1). The lower training intensity (40–55% V̇o2peak) approximated brisk walking or moderate-intensity exercise as advised in health guidelines (1, 27, 28). While exercise volume is expressed in terms of walking or jogging, the exercise modes included stationary cycling, treadmill, and elliptical trainers in order to enhance variety and adherence. All exercise sessions were verified by direct supervision and/or use of a heart rate monitor (Polar Electro, Woodbury, NY). To minimize musculoskeletal injury, there was an initial ramp period of 2–3 mo followed by 6 mo of the appropriate exercise prescription. Subjects were counseled to maintain body mass and that the goal of the intervention was not weight loss.
After the 8 mo of training, subjects were asked to cease all exercise and perform only normal daily activities for the subsequent 15 days; they were offered the incentive that they could reenter the exercise training program for 3 mo at no cost after adhering to the withdrawal period. Subjects were questioned at 5 days and 15 days after the last exercise session to verify that they had not performed physical activity other than normal activities of daily living. No subjects indicated that they had performed structured exercise.
Anthropometrics.
Body mass was measured to the nearest tenth of a kilogram on a digital electronic scale at the time that cardiorespiratory fitness (V̇o2peak) was determined (before and after training and after 15 days of training cessation). Height was measured to the nearest 0.5 cm with a stadiometer, and BMI was calculated as mass/height2.
Insulin action.
Insulin action was determined with a 3-h intravenous glucose tolerance test (IVGTT) (3). After fasting blood was obtained, glucose (50%) was injected through a catheter placed in an antecubital vein at a dose of 0.3 g/kg body mass. Insulin at a dose of 0.025 U/kg body mass was injected at minute 20. Blood samples were obtained at minutes 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 25, 30, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160, and 180 and centrifuged, and plasma was frozen at −80°C for subsequent measurements. Insulin was measured by immunoassay (Access Immunoassay System, Beckman Coulter, Fullerton, CA) and glucose with an oxidation reaction (YSI model 2300 Stat Plus, Yellow Springs Instruments, Yellow Springs, OH). An insulin sensitivity index (SI) was calculated with the minimal model of Bergman et al. (3). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as fasting insulin (μU/ml) × glucose (mmol/l)/22.5 (4).
Cardiorespiratory fitness.
Cardiorespiratory fitness was measured with an incremental, maximal treadmill test as previously described (12). Briefly, an initial test was performed to screen for underlying cardiovascular disease and to acclimate the subject. If no evidence for cardiovascular disease was detected, a second test was conducted to determine the pretraining V̇o2peak. Additional V̇o2peak tests were performed after the ramp period and periodically during training in order to modify the exercise workload to maintain the weekly caloric expenditure prescription. V̇o2peak was also determined during the final week of training (after training time point) and 15–18 days after the final training bout. The V̇o2peak test at 15–18 days was performed after the IVGTT to alleviate any acute effect of this exercise bout on measurements of insulin action. For consistency, testing points for V̇o2peak and body mass are denoted as before training, 16–24 h after training, and 15 days after training, although actual testing times differ slightly.
Skeletal muscle analyses.
To explore possible mechanisms for changes in insulin action, we examined available muscle biopsy samples in subjects from each exercise prescription. Muscle was obtained from the vastus lateralis by a percutaneous needle biopsy before each IVGTT. Part of the sample was mounted in an optimum cutting temperature medium (OCT)-tragacanth gum mixture, frozen in isopentane cooled over liquid nitrogen, and stored in liquid nitrogen for subsequent histochemical analyses of muscle fiber type (myosin ATPase staining) (15), intramuscular triglyceride (IMTG) content (Oil Red O) (15), muscle glycogen content (periodic acid Schiff method) (29), capillary density (11), and an index of mitochondrial density (NADH tetrazolium reductase staining) (18). Muscle citrate synthase (CS) activity was also obtained as an additional index of mitochondrial content and determined with a fluorometric end point assay (11).
Sections stained for fiber type, IMTG, NADH, and glycogen content were viewed under light microscopy. In a previous study in our laboratory (15) the image taken with ×10 magnification produced results similar to ×40. Because the ×10 magnification encompassed a larger area, we felt it produced a better representative index of the variables being measured. When the desired field of view was obtained, software (Spot Advanced 3.2.4, Diagnostic Instruments, Sterling Heights, MI) was used to generate a live image. The image was digitally captured, stamped with a calibration mark, and saved as a 32-bit tagged image format file. Images taken from serial sections were used to determine fiber type-specific lipid content as described previously (15). Sigma Scan Pro 5.0 (SPSS Science, Chicago, IL) software was used to perform image analysis.
Vascular density, expressed as endothelial cells per muscle fiber and endothelial cells per square millimeter, was determined by examining the total number of endothelial cells relative to the number of muscle fibers via light microscopy. Endothelial cells were identified in histological sections by immunohistological techniques with an endothelial cell-specific monoclonal antibody (11) as described below. Sections were cut throughout the tissue block for analysis, ensuring a homogeneous sample. A minimum of six different ×200 fields were counted, with the mean number of fibers for each sample being 100. Slides were allowed to come to room temperature and placed in ice-cold acetone for 2 min and phosphate-buffered saline (PBS) for 5 min. Blocking solution (10% horse serum in PBS) was applied for 1 h at room temperature. The primary antibody CD-31 (mouse anti-human CD31, catalog no. BBA7, R&D Systems) was applied for 30 min at room temperature, followed by incubation with a biotinylated anti-mouse IgG (mouse IgG alkaline phosphatase Vectastain ABC kit, Vector Laboratories, Burlingame, CA). Immune complexes were localized with the chromogenic alkaline phosphatase substrate Vector Red (Vector Laboratories), and levamisole was added to block endogenous alkaline phosphatase activity. The sections were counterstained with hematoxylin, dehydrated, and mounted with Cytoseal XYL (Richard Allen Scientific). The stained slide of human muscle was placed on the Olympus 1X70 microscope and transferred onto a computer screen at a magnification of ×100 through an Optronics Engineering DEI-750 camera (Goleta, CA) to the Adobe Premiere 4.2.1 program (San Jose, CA). The image was captured and saved to the Adobe Photoshop LE program and then opened into the NIH Image 1.6/ppc program, and the individual muscle fiber was outlined and total area calculated.
Statistics.
Data were compared with a repeated-measures ANOVA and contrast-contrast comparisons. Relative changes were compared with between-groups ANOVA. Statistical significance was denoted at the P ≤ 0.05 level. All data are presented as means ± SE.
RESULTS
Subjects.
Characteristics for subjects that completed the detraining component of the study (pretraining, 16–24 h, and 15 days of training cessation) with insulin action data are presented in Table 1. There were no differences between groups in age, stature, or BMI. It should be noted that the numbers per group may differ from previously published studies from the STRRIDE cohort (12, 20) because the individuals included in the present analyses were those who had IVGTT data at all three study points.
Table 1.
Subject characteristics
| Variable | Low Volume/Moderate Intensity (n = 46) | Low Volume/Vigorous Intensity (n = 49) | High Volume/Vigorous Intensity (n = 58) |
|---|---|---|---|
| Age, yr | 53.0±0.8 | 52.0±1.0 | 51.1±0.8 |
| Height, m | 1.71±0.02 | 1.71±0.01 | 1.72±0.01 |
| BMI, kg/m2 | 29.4±0.4 | 30.0±0.4 | 29.6±0.3 |
| Race, n (%) | |||
| White | 38 (83%) | 38 (78%) | 50 (86%) |
| AA | 8 (17%) | 8 (16%) | 4 (7%) |
| Other | 0 (%) | 3 (6%) | 4 (7%) |
| Sex, n (%) | |||
| Female | 21 (46%) | 21 (43%) | 22 (38%) |
| Male | 25 (54%) | 28 (57%) | 36 (62%) |
Parameter values are expressed as means ± SE for n subjects. BMI, body mass index; AA, African American. There were no significant differences between groups in these parameters (P < 0.05).
Exercise training.
Characteristics of the exercise programs are presented in Table 2. As expected, actual training volume (kcal of energy expenditure on exercise) per week was highest in the high-volume/vigorous-intensity group. To achieve the prescribed volumes (kcal/wk), the low-volume/moderate-intensity and high-volume/vigorous-intensity groups devoted significantly (P < 0.05) more time to training per week and per exercise session than the low-volume/vigorous-intensity group. The frequency (sessions per week) needed to attain the assigned exercise dosage was significantly (P < 0.05) lower in the low-volume/vigorous-intensity group.
Table 2.
Characteristics of exercise training programs
| Variable | Low Volume/Moderate Intensity | Low Volume/Vigorous Intensity | High Volume/Vigorous Intensity |
|---|---|---|---|
| Prescribed intensity, %V̇o2peak | 40–55% | 65–80% | 65–80% |
| Prescribed volume, miles/wk | 12 | 12 | 20 |
| Adherence, % | 89.0±2.0 | 90.0±1.7 | 84.4±2.1 |
| Actual volume, miles/wk | 10.6±0.2 | 10.8±0.2 | 16.8±0.4† |
| Actual time, min/wk | 196.5±5.3* | 125.0±4.2 | 202.2±5.7* |
| Frequency, sessions/wk | 3.5±0.1* | 2.9±0.1 | 3.5±0.1* |
| Time per session, min | 58.3±2.4* | 44.0±1.5 | 60.1±3.0* |
Data are expressed as means ± SE. Prescribed volume, approximate no. of miles per week calorically equivalent to prescribed 14 kcal·kg−1·wk−1 for the low-volume groups and 23 kcal·kg−1·wk−1 for the high-volume group; V̇o2peak, peak O2 consumption; actual volume, prescribed volume multiplied by rate of adherence for each subject. Adherence is expressed as % of optimal by dividing actual time spent exercising by time needed to meet defined exercise prescription.
Significantly different (P < 0.05) from the low-volume/vigorous-intensity group;
significantly different (P < 0.05) from the low-volume groups.
Insulin action.
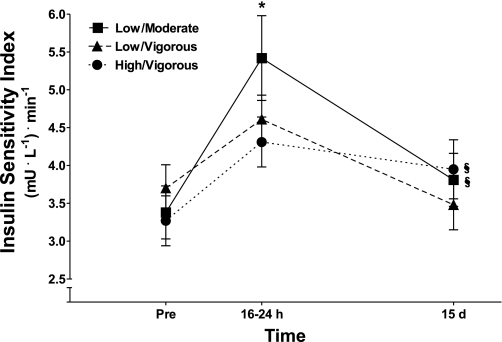
After 8 mo of exercise training, mean SI increased with all of the exercise prescriptions as determined 16–24 h after the final exercise training bout and compared with the sedentary pretraining condition (Fig. 1). After 15 days of training cessation, SI in the low-volume/vigorous-intensity exercise group declined to a value that was not significantly different from the sedentary pretraining level (Fig. 1). In contrast, in both the low-volume/moderate-intensity and high-volume/vigorous-intensity groups insulin action (SI) at 15 days remained at a level that was significantly elevated compared with the sedentary pretraining condition (P ≤ 0.05) (Fig. 1). Insulin action was reduced at 15 days compared with 16–24 h after training in the low-volume/moderate-intensity, low-volume/vigorous-intensity (P ≤ 0.001), and high-volume/vigorous-intensity (P = 0.08) groups (Fig. 1).
Fig. 1.
Insulin sensitivity index (SI) before exercise training (Pre) and after 16–24 h and 15 days of training cessation. All values are means ± SE. *Significant difference (P ≤ 0.05) from all other times points within an exercise prescription (difference between 16–24 h and 15 days in the high-volume/vigorous-intensity group, P = 0.08); §significant difference (P ≤ 0.05) from Pre (sedentary) in the low-volume/moderate-intensity and high-volume/vigorous-intensity groups.
Other components of insulin action such as fasting insulin and HOMA-IR demonstrated an exercise training and subsequent detraining main effect with no differences between the exercise prescriptions. As presented in Table 3, fasting plasma insulin significantly decreased at 16–24 h after the final training bout compared with before training and subsequently returned to sedentary, before training levels after 15 days of training withdrawal. A similar pattern was evident for HOMA-IR (Table 3).
Table 3.
Variables that exhibited no statistically significant differential effect between the three exercise prescriptions with exercise training and training cessation
| Variable | Before Training | 16–24 h After Training | 15 Days After Training |
|---|---|---|---|
| Fasting insulin, μU/ml | 9.3±0.5 | 7.8±0.4* | 9.0±0.4 |
| Fasting glucose, mg/dl | 93.5±0.7 | 94.0±0.8 | 93.7±0.9 |
| HOMA-IR | 2.1±0.1 | 1.9±0.1* | 2.1±0.1 |
| Body mass, kg | 87.2±1.1 | 86.1±1.1* | 85.8±1.1† |
| TTE, s | 783.3±16.2 | 927.0±18.3* | 870.6±18.0† |
Data are expressed as means ± SE. HOMA-IR, homeostasis model of insulin resistance; TTE, time to exhaustion during maximal exercise stress test.
Significantly different (P < 0.05) from other time points;
significantly different (P < 0.05) from before training.
Body mass.
Body mass declined significantly (P < 0.0001) by a mean of −1.1 kg from before (sedentary) to 16–24 h after training and then decreased further (a total of −1.3 kg vs. the sedentary condition) at 15 days of training cessation. There were no differences between the exercise prescriptions in the body mass response (Table 3).
Cardiorespiratory fitness.
V̇o2peak (l/min) increased with training (Fig. 2). In the low-volume/moderate-intensity group V̇o2peak was retained at trained levels after 15 days of training cessation. In contrast, absolute V̇o2peak declined significantly at 15 days of training cessation compared with trained values in the low-volume/vigorous-intensity (−5%) and high-volume/vigorous-intensity (−5%) groups; 15 day values, however, remained significantly elevated compared with before training (Fig. 2) in all of the exercise prescriptions. Exercise time to exhaustion (TTE) increased with training and remained elevated after 15 days of no training compared with sedentary values but declined significantly from the 16–24 h value (Table 3); there were no differences between the exercise training groups in TTE.
Fig. 2.
Peak O2 consumption (V̇o2peak) before exercise training (Pre) and after 16–24 h and 15 days of training cessation. All values are means ± SE. *Significant difference (P ≤ 0.05) vs. pretraining (sedentary) in all exercise groups; ‡significant difference (P ≤ 0.05) from after training (16–24 h) in the low-volume/vigorous-intensity and high-volume/vigorous-intensity groups.
Skeletal muscle.
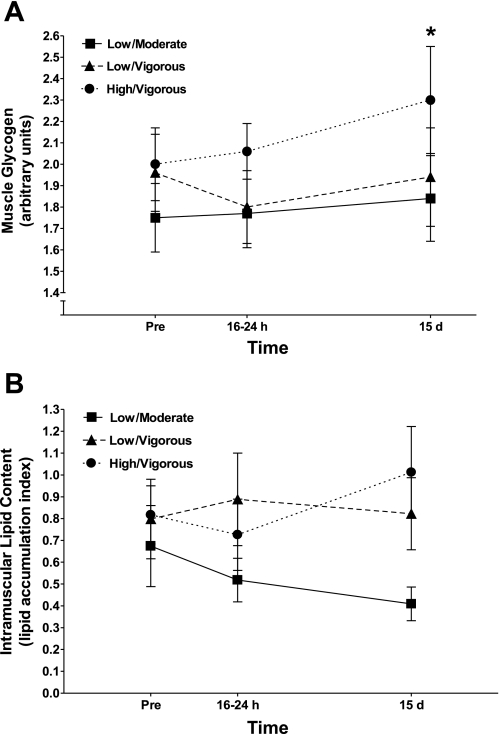
Some subjects did not have tissue for all time points; the number of subjects per comparison is indicated in each presentation. Muscle glycogen content (Fig. 3A) was elevated at 15 days compared with 16–24 h after training (n = 27, main effect). IMTG content expressed as a lipid accumulation index (n = 24) was not altered at either 16–24 h or 15 days after exercise training compared with the sedentary condition (Fig. 3B). Similar to total content, type I- and type II-specific IMTG content did not change with exercise training (data not shown). Type I muscle fibers were found to have a significantly higher IMTG content compared with type II fibers (P < 0.0001).
Fig. 3.
Histochemical determinations of muscle glycogen content (A) and intramuscular triglyceride content (B) with physical activity and exercise cessation in each of the 3 exercise prescriptions. *Significant (P ≤ 0.05) main effect for glycogen content compared with 16–24 h after training.
Measures indicative of mitochondrial density/content are presented in Fig. 4. A histochemical index of mitochondrial density (NADH staining) (Fig. 4A) was elevated at 16–24 h compared with before training (n = 29, main effect). In the high-volume/vigorous-intensity group, histochemically determined mitochondrial density was significantly elevated at 15 days compared with pretraining (Fig. 4A) (n = 10 subjects), similar to that of insulin action (Fig. 1). Results were similar for CS activity. As presented in Fig. 4B, there was a main effect (n = 100) for CS activity to increase with exercise training (16–24 h). CS activity remained significantly (P < 0.05) elevated in the high-volume/vigorous-intensity group (n = 42) after 15 days of training cessation compared with pretraining.
Fig. 4.
Changes in histochemical (NADH staining; A) and biochemical (citrate synthase activity; B) indexes of mitochondrial content/density with exercise training and training cessation in each of the 3 exercise prescriptions. ‡Significant (P ≤ 0.05) main effect for mitochondrial density vs. pretraining (sedentary); §significant difference (P ≤ 0.05) from Pre (sedentary) in the high-volume/vigorous-intensity group.
Vascular density, expressed as endothelial cells per muscle fiber and endothelial cells per square millimeter (n = 146) exhibited a main effect with exercise training. The number of endothelial cells per muscle fiber increased (P < 0.001) with exercise training (1.60 ± 0.03 vs. 1.85 ± 0.04 for before vs. 16–24 h after training, respectively) and remained elevated (P < 0.001) compared with the sedentary condition at 15 days of training cessation (1.81 ± 0.05), with no difference between prescriptions. Capillary density exhibited a similar pattern of change, with a significant main effect (P < 0.001) with exercise training (338.0 ± 6.3 vs. 393.1 ± 8.3 capillaries/mm2), and remained elevated (P < 0.001) at 15 days of training withdrawal (377.9 ± 10.5 capillaries/mm2) compared with the sedentary condition. Muscle fiber type, as determined with myosin ATPase staining (type I, type II), and muscle fiber cross-sectional area were not altered with exercise training or training cessation (data not shown).
Comparison of relative changes.
The mean relative change induced by exercise training or training cessation for each group was obtained by calculating the individual relative change for a subject and using this value to derive a mean relative change for each exercise prescription. Data are presented in this manner (%) to account for the range of relative changes and to subsequently determine whether similar patterns of change were evident in SI and other variables offering possible mechanistic implications. The mean relative changes over the sedentary condition at 15 days of training cessation in SI were +35.2 ± 1.3% for low volume/moderate intensity, +6.0 ± 0.9% for low volume/vigorous intensity, and +27.4 ± 0.7% for high volume/vigorous intensity. Comparing log-adjusted values, the mean increase above the sedentary condition at 15 days was significantly (P < 0.05) greater in the low-volume/moderate-intensity and high-volume/vigorous-intensity groups compared with the low-volume/vigorous-intensity group. The high-volume/vigorous-intensity training group exhibited the greatest retention of V̇o2peak over pretraining (sedentary) levels at 15 days of training cessation; values were +5.6 ± 0.1, +3.9 ± 0.2, and +10.6 ± 0.2% for the low-volume/moderate-intensity, low-volume/vigorous-intensity, and high-volume/vigorous-intensity groups, respectively. Indicators of mitochondrial density (NADH staining and CS activity) demonstrated a pattern similar to V̇o2peak, with the increment over the sedentary condition being the greatest in the high-volume/vigorous-intensity group (Fig. 4). Other variables such as body mass, muscle glycogen or IMTG content, capillary density, muscle fiber type, and fiber cross-sectional area did not exhibit differential patterns of change between the exercise groups. Thus, while V̇o2peak and mitochondrial content remained elevated at 15 days in the high-volume/vigorous-intensity group, a consistent pattern of change similar to insulin action (increase over the sedentary condition in both low-volume/moderate-intensity and high-volume/vigorous-intensity groups; Fig. 1) was not evident in the variables examined.
DISCUSSION
Endurance-oriented exercise training improves insulin action in insulin-resistant individuals (6, 16, 30). The intent of the present study was to determine which, if any, clinically utilized exercise prescriptions retain the training-induced improvement in insulin action during an ensuring period of training cessation in an at-risk population of overweight/obese individuals. The main finding of the present investigation was that insulin action (SI) remained elevated above pretraining sedentary levels after 15 days of training cessation in two of the three exercise prescriptions examined (low volume/moderate intensity and high volume/vigorous intensity) (Fig. 1). The persistence of training-induced improvements in insulin action does not appear to be due to subjects remaining physically active during the 15-day training withdrawal period because other studies examining 10–14 days of training cessation reported a 0 to −7% decline in maximal O2 consumption (V̇o2max) (8, 9, 17). The declines in V̇o2peak from the trained level in the low-volume/moderate-intensity and high-volume/vigorous-intensity groups in the present study were at the upper end of this range (−5%) (Fig. 1). Also supportive were observed declines in TTE during the maximal test for all three exercise protocols (Table 3). Together, these observations suggest that adaptation(s) linked with the low-volume/moderate-intensity and high-volume/vigorous-intensity but not the low-volume/vigorous-intensity prescriptions resulted in an improvement in insulin action that remained evident at 15 days after the final exercise training bout (Fig. 1).
Others have also observed a more long-lived influence of exercise training on insulin action even when training is stopped. In master athletes, 10 days of inactivity reduced insulin action to values that were intermediate between young and older untrained men rather than reverting completely to the older, sedentary control group; this observation suggests that there is some retention of a training-related improvement in insulin action with 10 days of no physical activity (32). Dela et al. (10) reported a training-induced elevation in insulin action that persisted after 6 days of inactivity in overweight subjects. The present data thus support other observations of an improvement in insulin action that persists beyond an early compensation phase of 2–4 days following a prolonged period of endurance-oriented exercise training (10, 32). In relation to magnitude of the response, at 15 days the mean elevation in SI over the sedentary condition in the low/moderate and high/vigorous groups was ∼30% (results). This contrasts with the approximate doubling over the sedentary condition we have reported in similar subjects with identical methods (7, 20, 21) when an insulin sensitivity index is obtained within 48 h of the final training bout (Fig. 1). Although the increment in insulin sensitivity at 15 days of detraining clearly is not similar to that evident within 24–48 h after the final training bout, the observed retention at 15 days could be important clinically and indicative of a persistent effect of exercise training on insulin action.
In the present study, common components of the exercise prescriptions where insulin action was retained (Fig. 1) were weekly exercise duration and frequency. Weekly training duration averaged ∼200 min/wk in both the low-volume/moderate-intensity and high-volume/vigorous-intensity prescriptions; this 200 min/wk was obtained with three or four exercise sessions per week of ∼60-min duration. This is in contrast to the low-volume/moderate-intensity prescription, which consisted of exercising 3 days per week for a total of 125 min or ∼45 min per session (Table 2). This finding suggests that exercise duration may be a critical factor to consider when designing exercise prescriptions to optimize the persistence of the training-induced improvement in insulin action. To our knowledge, there is no other information concerning retention characteristics of insulin action with various exercise prescriptions. The present data thus provide the novel finding that the training-induced improvement in insulin action can be at least partially retained, but that this persistence is dependent on the nature of the original exercise exposure. Although weekly exercise duration and frequency may be very important parameters that determine the longevity of insulin action effects, it is also possible that the beneficial effects of moderate-intensity exercise at low volume and vigorous-intensity exercise at high volume result in similar long-term effects, but through different mechanisms outside of weekly duration and frequency. It should be noted that when moderate-intensity and vigorous-intensity exercise were compared at the same volume (low-volume/moderate-intensity vs. low-volume/vigorous-intensity prescriptions; Table 3), the moderate-intensity exercise was clearly superior (Fig. 1), implying that there is some aspect of moderate-intensity exercise training that has more beneficial effects on insulin action than vigorous exercise.
The present study also attempted to provide insight into possible cellular mechanisms by which the longer-duration prescriptions conferred a persistence of insulin action. Our approach was to utilize comparisons of recovery kinetics between insulin action and candidates for regulation of cellular insulin action, with the reasoning that if patterns were similar then a mediator would be considered a good mechanistic candidate. For instance, glycogen supercompensation can occur during the early stages of training withdrawal and induce insulin resistance (13, 19); the accumulation of IMTG is also associated with insulin resistance at least in sedentary individuals (15, 16). However, when we compared the recovery kinetics across groups for insulin action (Fig. 1), muscle glycogen content, and IMTG content (Fig. 3), no pattern similarities were evident. A noncongruent pattern of change was also evident for capillary density, muscle fiber type, and muscle fiber area with insulin action, suggesting no mechanistic link with these variables (results).
Indicators of mitochondrial density were measured, because mitochondrial content may be an important factor governing insulin action (5, 23, 33) and would be anticipated to change with training and training cessation (5a). Mitochondrial density reproduced the pattern of change for SI in the high-volume/vigorous-intensity prescription in that it remained above sedentary values at day 15 of training cessation (Figs. 1, 4). V̇o2peak was also more highly retained in the high-volume/vigorous-intensity group with training cessation (results). From these observations it could be hypothesized that the high-volume/vigorous-intensity prescription evoked adaptations that resulted in a more substantial retention of factors associated with V̇o2peak and muscle mitochondrial content; these adaptations could in turn have contributed to the persistence of the training-induced improvement in SI. However, in the low-volume/moderate-intensity group, in whom SI was also elevated (Fig. 1), mitochondrial content and V̇o2peak were not similarly maintained (Figs. 2, 4), suggesting that different mechanisms may be responsible for retaining insulin action with a moderate-intensity training regimen. However, the present findings could also be interpreted as indicating that the true cellular mediators responsible for regulating insulin action in human subjects with a prolonged exercise training program simply were not measured because no indexes consistently mimicked the relative increments in SI over the sedentary condition. The mechanisms that govern insulin action are multifactorial and complex (14, 16, 17); further studies are needed to explain why the exercise prescriptions studied provided persistence of the training-induced improvement in insulin action.
The goal of the STRRIDE project was to provide information to aid in determining effective exercise prescriptions for treating and preventing chronic disease. The present study and others (20) suggest that exercise prescriptions similar to the low-volume/moderate-intensity and high-volume/vigorous-intensity (Table 2) programs will confer gains, as well as the persistence of enhanced insulin action, even over 2 wk after cessation of the exercise exposure, which may be clinically significant. It is our hope that this information contributes to a larger body of evidence that can aid in defining specific exercise prescriptions that improve and maintain health benefits yet can be realistically performed by sedentary overweight individuals.
In summary, the present findings suggest there is some persistence of the training-induced improvement in insulin action after 15 days of training cessation in middle-aged, overweight to obese individuals. This effect, however, is dependent on the intensity and weekly amount of exercise employed during the training period. The cellular mechanisms responsible for the persistence of insulin sensitivity following exercise cessation were not obvious in the present study, and there may be more than one mechanism involved dependent on the nature of the exercise exposure. These data provide a basis for exercise training prescriptions in sedentary populations at risk for the development of diabetes and cardiovascular disease.
GRANTS
This study was supported by Research Grants RO1-57354 from the National Heart, Lung, and Blood Institute and RO1-56112 from the National Institute of Diabetes and Digestive and Kidney Diseases.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription (5th ed.). Baltimore, MD: Williams and Wilkins, 1995.
- 2.Anonymous. Physical activity and cardiovascular health. NIH Consens Statement 13: 1–33, 1995. [PubMed] [Google Scholar]
- 3.Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev 6: 45–86, 1985. [DOI] [PubMed] [Google Scholar]
- 4.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity. Diabetes Care 23: 57–63, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Bruce CR, Anderson MJ, Carey AL, Newman DG, Bonen A, Kriketos AD, Cooney GJ, Hawley JA. Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status. J Clin Endocrinol Metab 88: 5444–5451, 2003. [DOI] [PubMed] [Google Scholar]
- 5a.Bruce CR, Thrush AB, Mertz VA, Bezaire V, Chabowski A, Heigenhauser GJ, Dyck DJ. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am J Physiol Endocrinol Metab 291: E99–E107, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Physical Activity and Health: Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services, 1996, S/N 017-023-00196-5.
- 7.Cox JH, Cortright RN, Dohm GL, Houmard JA. Effect of aging on response to exercise training in humans: skeletal muscle GLUT-4 and insulin sensitivity. J Appl Physiol 86: 2019–2025, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Coyle EF, Martin WH, Sinacore DR, Joyner MJ, Hagberg JM, Holloszy JO. Time course of loss of adaptations after stopping prolonged intense endurance training. J Appl Physiol 57: 1857–1864, 1984. [DOI] [PubMed] [Google Scholar]
- 9.Cullinane EM, Sady SP, Badeboncoeur L, Burke M, Thompson PD. Cardiac size and VO2max do not decrease after short-term exercise cessation. Med Sci Sports Exerc 18: 420–424, 1986. [PubMed] [Google Scholar]
- 10.Dela F, Larsen JJ, Mikines KJ, Ploug T, Peterson LN, Galbo H. Insulin-stimulated glucose clearance in patients with NIDDM: effects of one-legged physical training. Diabetes 44: 1010–1020, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Duscha BD, Annex BH, Keteyian SJ, Green HJ, Sullivan MJ, Samsa GP, Brawner CA, Schachat FH, Kraus WE. Differences in skeletal muscle between men and women with chronic heart failure. J Appl Physiol 90: 280–286, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Duscha BD, Slentz CA, Johnson JL, Houmard JA, Bensimhon DR, Knetzger KJ, Kraus WE. Effects of exercise training amount and intensity on peak oxygen consumption in middle-age men and women at risk for cardiovascular disease. Chest 128: 2788–2793, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Goforth HW, Arnall DA, Bennett BL, Law PG. Persistence of supercompensated muscle glycogen in trained subjects after carbohydrate loading. J Appl Physiol 82: 342–347, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med 49: 235–261, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Gray RE, Tanner CJ, Pories WJ, MacDonald KG, Houmard JA. Effect of weight loss on muscle lipid content in morbidly obese subjects. Am J Physiol Endocrinol Metab 284: E726–E732, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Hawley JA Exercise as a therapeutic intervention for the prevention and treatment of insulin resistance. Diabetes Metab Res Rev 20: 383–393, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Heath GW, Gavin JR, Hinderliter JM, Hagberg JM, Bloomfield SA, Holloszy JO. Effects of exercise and lack of exercise on glucose tolerance and insulin sensitivity. J Appl Physiol 55: 512–517, 1983. [DOI] [PubMed] [Google Scholar]
- 18.Holloszy JO, Kohrt WM, Hansen PA. The regulation of carbohydrate and fat metabolism during and after exercise. Front Biosci 3: 1011–1027, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Host HH, Hansen PA, Nolte LA, Chen MM, Holloszy JO. Glycogen supercompensation masks the effect of a training-induced increase in GLUT-4 on muscle glucose transport. J Appl Physiol 85: 133–138, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE. Effect of the volume of intensity of exercise training on insulin sensitivity. J Appl Physiol 96: 101–106, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Houmard JA, Tyndall GL, Midyette JB, Hickey MS, Dolan PL, Gavigan KE, Weidner ML, Dohm GL. Effect of reduced training and training cessation on insulin action and muscle GLUT-4. J Appl Physiol 81: 1162–1168, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Kraus WE, Torgan CE, Duscha BD, Norris J, Brown SA, Cobb FR, Bales CS, Annex BH, Samsa GP, Houmard JA, Slentz CA. Studies of a targeted risk reduction intervention through defined exercise (STRRIDE). Med Sci Sports Exerc 33: 1774–1784, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Kriketos AD, Pan DA, Lillioja S, Cooney GJ, Baur LA, Milner MR, Sutton JR, Jenkins AB, Bogardus C, Storlien LH. Interrelationships between muscle morphology, insulin action, and adiposity. Am J Physiol Regul Integr Comp Physiol 270: R1332–R1339, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Lee IM, Hsieh CC, Paffenbarger RS. Exercise intensity and longevity in men: the Harvard Alumni Study. JAMA 328: 538–545, 1995. [Google Scholar]
- 25.Mikines KJ, Sonne B, Tronier B, Galbo H. Effects of acute exercise and detraining on insulin action in trained men. J Appl Physiol 66: 704–711, 1989. [DOI] [PubMed] [Google Scholar]
- 26.Novikoff AF, Schin W, Drucker J. Mitochondrial localization of oxidation enzymes: staining result with two tetrazolium salts. J Histochem Cytochem 9: 47–61, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paffenbarger RS, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical activity level and other lifestyle characteristics with mortality among men. N Engl J Med 328: 538–545, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Pate RR, Pratt M, Blair SN, Haskell WL, Macera AA, Bouchard C, Buchner D, Ettinger W, Health GW, King A, Kriska A, Leon AS, Marcus BH, Morris J, Paffenbarger RS, Patrick K, Pollock ML, Rippe JM, Sallis J, Wilmore JH. Physical activity and public health: a recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA 273: 402–407, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Pearse AG Histochemistry: Theoretical and Applied. Boston, MA: Little, Brown, 1968.
- 30.Pedersen BK, Saltin B. Evidence for prescribing exercise as a therapy in chronic disease. Scand J Med Sci Sports 16, Suppl 1: 3–63, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Rogers MA, King DS, Hagberg JM, Ehsani AA, Holloszy JO. Effect of 10 days of physical inactivity on glucose tolerance in master athletes. J Appl Physiol 68: 1833–1837, 1990. [DOI] [PubMed] [Google Scholar]
- 33.Simoneau JA, Colberg SR, Thaete FL, Kelley DE. Skeletal muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. FASEB J 9: 273–278, 1995. [PubMed] [Google Scholar]