Abstract
Reductions in prefrontal oxygenation near maximal exertion may limit exercise performance by impairing executive functions that influence the decision to stop exercising; however, whether deoxygenation also occurs in motor regions that more directly affect central motor drive is unknown. Multichannel near-infrared spectroscopy was used to compare changes in prefrontal, premotor, and motor cortices during exhaustive exercise. Twenty-three subjects performed two sequential, incremental cycle tests (25 W/min ramp) during acute hypoxia [79 Torr inspired Po2 (PiO2)] and normoxia (117 Torr PiO2) in an environmental chamber. Test order was balanced, and subjects were blinded to chamber pressure. In normoxia, bilateral prefrontal oxygenation was maintained during low- and moderate-intensity exercise but dropped 9.0 ± 10.7% (mean ± SD, P < 0.05) before exhaustion (maximal power = 305 ± 52 W). The pattern and magnitude of deoxygenation were similar in prefrontal, premotor, and motor regions (R2 > 0.94). In hypoxia, prefrontal oxygenation was reduced 11.1 ± 14.3% at rest (P < 0.01) and fell another 26.5 ± 19.5% (P < 0.01) at exhaustion (maximal power = 256 ± 38 W, P < 0.01). Correlations between regions were high (R2 > 0.61), but deoxygenation was greater in prefrontal than premotor and motor regions (P < 0.05). Prefrontal, premotor, and motor cortex deoxygenation during high-intensity exercise may contribute to an integrative decision to stop exercise. The accelerated rate of cortical deoxygenation in hypoxia may hasten this effect.
Keywords: altitude, fatigue, near-infrared spectroscopy
reductions in global cerebral oxygenation are associated with impairments in central motor drive (1, 17, 22). This effect is poorly understood, and further progress has been limited, because more specific, regional measurements of cerebral blood flow and oxygenation are difficult to obtain during intense exercise. Single-photon emission computed topography and PET studies in conjunction with MRI have shown regional differences in cerebral oxygenation during submaximal exercise (7, 29, 33), yet the temporal resolution of these tools and their intolerance to head motion preclude studies of high-intensity exercise.
Near-infrared spectroscopy (NIRS) allows noninvasive, continuous monitoring of cortical changes in cerebral oxygenation during incremental exercise to maximal exertion. Cerebral NIRS measurements during exhaustive, whole body exercise have been limited to the prefrontal cortex (19). Results have consistently shown that prefrontal oxygenation is maintained or increased slightly during light- to moderate-intensity exercise but decreases near maximal exercise intensity (2, 26, 27, 30). These findings have led to speculation that reductions in prefrontal oxygenation affect executive functions that influence the decision to stop exercising (1, 27). An alternative, yet untested, hypothesis is that deoxygenation occurs across the cortex and, thus, directly affects motor areas that regulate central motor drive.
Acute hypoxic exposure provides a model that stresses oxygen transport systems and amplifies changes in cerebral oxygenation (26). During exercise, prefrontal cortex oxygenation falls precipitously with increasing exercise intensity and, thus, may exert an earlier and larger influence on the drive to continue exercising than under normoxic conditions (1). Although it appears that the degree of hypoxia is associated with the magnitude of change in prefrontal oxygenation (1, 11), such conclusions are limited by inherent errors in baseline measurements resulting from daily variations and inconsistencies in NIRS probe placement between trials. Continuous NIRS recordings spanning normoxic and hypoxic exposures are therefore needed to better quantify differences in oxygenation between conditions.
The intent of the present study was to address the aforementioned shortcomings by use of multichannel NIRS to obtain uninterrupted measurements of cortical oxygenation during sequential incremental exercise tests in normoxic and hypoxic conditions. Specifically, we tested the null hypothesis that patterns and magnitudes of changes would be similar within prefrontal, premotor, and motor cortices.
METHODS
Subjects.
After approval from the Colorado Multiple Institutional Review Board, 25 active, healthy volunteers (23 men and 2 women) from the Denver, CO, metropolitan area (elevation 1,650 m) provided written, informed consent to participate in a larger study investigating the etiology of acute mountain sickness. Physical examinations, including blood and urine tests, were conducted to verify general health before participation. All study procedures followed ethical guidelines established by the Declaration of Helsinki.
Study design.
Two incremental exercise tests were performed in an environmental chamber under ambient normobaric [normoxic (Norm), 610 Torr barometric pressure (Pb), 118 Torr inspired Po2 (PiO2)] and hypobaric [hypoxic (Hypox), 425 Torr Pb, 79 Torr PiO2] conditions to assess aerobic fitness for the larger study. Both tests were performed during a single chamber session to allow direct comparisons between Norm and Hypox without introduction of error from sensor placement/replacement. Tests were counterbalanced to control for order using a blinding strategy that varied chamber pressure to elicit similar sounds and changes in ear pressure during standardized 15-min ascent and descent periods. After arrival at the target Pb, 15 min were needed to adjust the cycle ergometer (Velotron Dynafit Pro, Racermate, Seattle, WA) and equip subjects with instrumental sensors (see below). Resting data were collected for 2 min before a 5-min warm-up at 50 W. Work rate was then incrementally increased using a 25 W/min ramp protocol to exhaustion. Subjects were blinded to elapsed time, power output, pedal revolutions per minute, and all physiological signals. Cool-down exercise was performed at 50 W for 5 min before chamber pressure was adjusted. After 15 min of ascent/decent and 15 min of rest at the second Pb, the protocol was repeated.
Instrumentation.
A multichannel NIRS instrument (Oxymon III, Artinis) emitting continuous wavelengths of 780- and 850-nm light was used to monitor changes in oxygenated (O2Hb), deoxygenated (HHb), and total (THb) cerebral and muscle heme concentrations. Theoretical and performance details of NIRS have been previously reported (26). Concentration changes in O2Hb and HHb were calculated using the modified Beer-Lambert law and differential pathlength factors of 5.93 for cerebral and 4.95 for muscle tissue (6, 31). THb was calculated as the sum of O2Hb and HHb.
Subjects were instrumented with one of two probe configurations based on hair color and thickness. For subjects with dark and/or thick hair, which is known to obstruct NIR light, configuration 1 was used: probes were placed on the forehead over the left prefrontal cortex (n = 14). Specifically, three transmitters (Tx), spaced 4 mm apart, were paired with a single receiver (Rx), placed 40–45 mm from the middle Tx, to illuminate cortical regions associated with standard Fp1 EEG electrode placement (12). For control, a second set of probes (3 Tx and 1 Rx) was placed on the right vastus lateralis (∼15 cm proximal and 5 cm lateral from the superior border of the patella). The 3 Tx-1 Rx probe configurations allowed for spatially resolved determinations of tissue saturation indexes (TSI = O2Hb/THb) using previously determined absorption and scattering coefficients (14). For subjects with no hair or thin hair, which allows NIRS access to the scalp, configuration 2 was used: 1 Tx-1 Rx optode pairs were placed over four separate regions of the head (n = 11). Two pairs were placed bilaterally over the left and right prefrontal cortex (Fp1 and Fp2 EEG electrode sites) as described above. The other two Tx were placed 40 mm rostral and caudal to a single Rx placed at EEG site C1 to span left premotor and motor cortices. Off-center placement was necessary to illuminate cortical tissue without interference from the sagittal sinus. If necessary, the Tx-Rx set was moved <5 mm to obtain strong pulsatile O2Hb signals, defined as a >0.25 μM systolic- diastolic difference. All probes were held by a flexible, plastic spacer, which was affixed to the skin/scalp with double-sided adhesive tape and attached to a custom-made headset or held in place with elastic bandages to minimize movement artifact and shield ambient light. NIRS signals were recorded throughout the chamber session at 125 Hz and averaged across 5-s windows. Changes in O2Hb, HHb, and THb (μM) were expressed relative to the resting condition in Norm.
Transcranial Doppler (TCD) was used to monitor bilateral middle cerebral artery (MCA) blood flow velocity during exercise. The custom-made NIRS headsets were modified to hold 2-MHz Doppler probes (DWL Multi Dop T2) over the left and right temporal windows to insonate the MCAs. All measurements were optimized at penetration depths between 42 and 48 mm by a single, trained investigator. Continuous traces of the maximal velocity envelope were recorded at 125 Hz and processed offline for determination of beat-by-beat mean velocity (MCA Vmean). Because of potential inconsistencies in bilateral MCA anatomy and, thus, TCD insonation angle, values recorded during exercise were expressed in absolute terms as well as percent change from rest.
Mixed expired O2 and CO2 concentrations from a 3-liter mixing chamber and expired airflow (TrueOne 2400, ParvoMedics, Sandy, UT) were recorded at 200 Hz on a data acquisition system (Powerlab 16/30, ADInstruments, Colorado Springs, CO) that synchronized TCD, end-tidal Po2 and Pco2 (O2Cap, Oxigraf, Mountain View, CA), ECG (Bioamp, ADInstruments), middle finger O2 saturation from pulse oximetry (Sp ; model N-595, Nellcor, Pleasonton, CA), and continuous blood pressure (Nexfin HD) traces. Minute ventilation, O2 uptake (V̇o2), and CO2 output were calculated offline using the Haldane transformation in 15-s intervals.
; model N-595, Nellcor, Pleasonton, CA), and continuous blood pressure (Nexfin HD) traces. Minute ventilation, O2 uptake (V̇o2), and CO2 output were calculated offline using the Haldane transformation in 15-s intervals.
Analyses.
Continuous data were collapsed to analyze specific time points of interest corresponding to rest and 25, 50, 75, and 100% of maximal power output (Wmax). Data were analyzed with 2 × 5 repeated-measures ANOVA to evaluate effects of trial (Norm and Hypox) across work rates (rest and 25%, 50%, 75%, and 100% Wmax). Changes at absolute work rates of 100, 150, and 200 W were analyzed similarly. Criterion for significance was set at P < 0.05. Post hoc, pairwise comparisons were made using Holm's sequential method to control for type I error. Pearson's product-moment analyses were used to evaluate relationships between optode pairs throughout exercise. Values are means ± SD.
RESULTS
Subjects.
Twenty-three subjects (29 ± 8 yr of age, 73.7 ± 10.0 kg body wt, 181.7 ± 8.0 cm) completed both trials. Two subjects (with NIRS configuration 1) completed the Norm trial but were excluded from further study because of nausea and/or paresthesia during the subsequent depressurization period. Metabolic and power responses to both conditions were representative of physically fit, age-matched individuals (Table 1). Hypox reduced maximal V̇o2 and Wmax by 16 ± 6% and 21 ± 12%, respectively (P < 0.01). Order of trials did not affect the difference in maximal V̇o2 (P = 0.20) or Wmax (P = 0.95) between conditions, and 8 of 23 subjects were unable to retrospectively identify the order of testing.
Table 1.
Metabolic, respiratory, and cerebral blood flow velocity responses to incremental exercise
| Variable | Normoxia |
Hypoxia | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rest | Wmax |
Rest | Wmax | |||||||
| 25% | 50% | 75% | 100%† | 25% | 50% | 75% | 100%† | |||
| Power, W | 0±0 | 110±15 | 177±27 | 240±40 | 305±52 | 0±0 | 99±9 | 151±19 | 203±28 | 256±38* |
| HR, beats/min | 73±13 | 121±11 | 142±12 | 162±12 | 178±10 | 82±20 | 130±16 | 146±15 | 161±11 | 172±10* |
| O2 saturation, % | 96±2 | 94±2 | 93±2 | 92±2 | 90±3 | 85±4* | 76±4 | 73±5 | 71±6 | 70±7* |
| O2 uptake, l/min | 0.41±0.10 | 1.69±0.29 | 2.24±0.43 | 2.89±0.57 | 3.49±0.76 | 0.45±0.23 | 1.54±0.31 | 2.04±0.47 | 2.35±0.42 | 2.74±0.60* |
| CO2 output, l/min | 0.37±0.10 | 1.53±0.23 | 2.26±0.35 | 3.28±0.53 | 4.49±0.78 | 0.39±0.17 | 1.38±0.31 | 1.91±0.33 | 2.61±0.47 | 3.31±0.71* |
| Minute ventilation, l/min btps | 15.7±5.1 | 48.8±8.2 | 68.6±11.6 | 101.5±20.6 | 159.6±32.6 | 18.6±8.2 | 53.5±11.9 | 73.9±15.7 | 105.9±21.6 | 151.1±32.9 |
| f, breaths/min | 14±5 | 24±4 | 28±6 | 37±8 | 56±11 | 16±6 | 25±6 | 31±8 | 41±9 | 57±14 |
| Vt, liters | 1.1±0.4 | 2.0±0.4 | 2.4±0.5 | 2.6±0.5 | 2.7±0.4 | 1.1±0.4 | 2.0±0.5 | 2.3±0.6 | 2.5±0.6 | 2.5±0.7 |
| Minute ventilation/O2 uptake | 38.1±8.0 | 28.9±2.7 | 30.7±3.9 | 35.0±5.2 | 46.0±8.2 | 43.3±12.3* | 34.8±4.3 | 37.0±6.6 | 45.1±6.2 | 56.0±9.9* |
| Minute ventilation/CO2 output | 41.5±5.0 | 31.7±2.7 | 30.1±3.1 | 30.6±4.0 | 35.3±5.4 | 48.2±10.5* | 39.0±4.9 | 38.9±5.2 | 40.9±5.3 | 46.0±6.1* |
| End-tidal Po2, Torr | 75.8±10.4 | 74.2±8.2 | 77.4±7.1 | 82.9±6.7 | 91.7±6.5 | 47.6±7.2* | 46.7±4.2 | 49.6±4.7 | 54.7±4.6 | 60.0±5.2* |
| End-tidal Pco2, Torr | 33.8±4.5 | 37.5±4.6 | 38.1±4.5 | 36.2±5.3 | 28.7±5.4 | 29.0±4.9* | 30.9±4.3 | 30.3±4.8 | 27.8±4.2 | 23.3±4.1* |
| MCA Vmean, cm/s | ||||||||||
| Left | 53.8±10.0 | 62.0±12.6 | 68.0±15.1 | 70.3±16.0 | 65.1±14.6 | 56.6±11.4 | 68.9±16.2 | 75.4±14.8 | 75.2±15.3 | 70.4±14.7* |
| Right | 56.2±10.2 | 65.9±11.3 | 72.0±14.0 | 74.4±16.0 | 69.9±15.5 | 60.0±11.4 | 74.0±10.5 | 79.3±12.9 | 79.3±14.6 | 76.7±14.4* |
| MCA Vmean, %change | ||||||||||
| Left | 0±0 | 15.4±13.1 | 26.0±16.1 | 31.0±20.1 | 21.2±21.4 | 0±0 | 25.9±21.1 | 33.5±24.7 | 35.1±24.3 | 26.9±21.0 |
| Right | 0±0 | 18.2±12.0 | 28.9±13.6 | 32.7±17.2 | 24.8±18.3 | 0±0 | 25.9±21.1 | 34.8±25.0 | 34.2±22.0 | 30.4±24.9 |
| Mean BP, %change | 0±0 | 16.1±8.9 | 25.6±8.9 | 32.2±10.0 | 38.1±12.8 | 0±0 | 16.1±9.4 | 22.9±9.7 | 30.4±12.8 | 36.8±17.2 |
Values are means ± SD (n = 23). HR, heart rate; f, respiratory rate; MCA Vmean, middle cerebral artery mean blood flow velocity; BP, blood pressure. O2 saturation in the middle finger was measured by pulse oximetry. Repeated-measures ANOVA (2 trials × 5 work rates) were performed on each variable. Post hoc paired t-tests were performed on rest and 100% maximal work rate (Wmax) values only.
P < 0.05 vs. normoxia.
All 100% Wmax values were different from rest (P < 0.05).
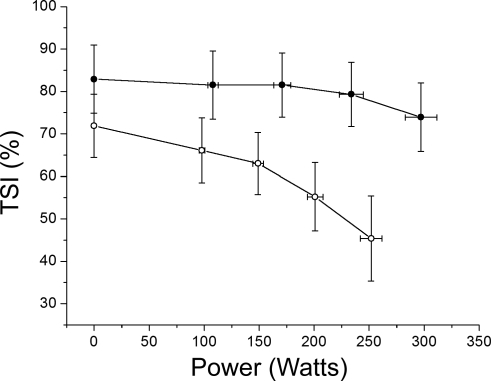
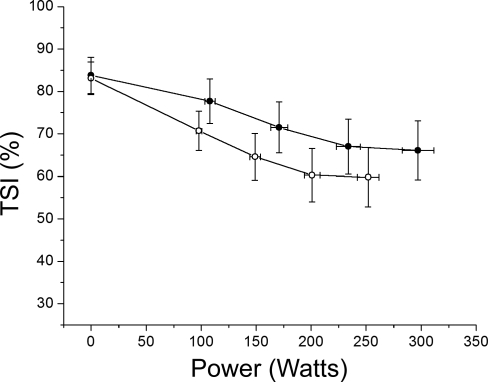
Differences in tissue oxygenation during Norm and Hypox.
During Norm, resting cerebral TSI (82.9 ± 27.9%) was maintained up to 75% Wmax but fell by 9.0 ± 10.7% thereafter (P = 0.03) because of large increases in HHb relative to O2Hb (Fig. 1). In comparison, muscle TSI (83.1 ± 14.8%) decreased 17.5 ± 12.3% as work rate increased (P < 0.01; Fig. 2). Resting cerebral TSI (71.9 ± 25.9%) was lower during Hypox than Norm (P = 0.01) and was not influenced by trial order (P = 0.11). Cerebral TSI fell throughout exercise, exhibiting 26.5 ± 19.5% decrease from rest (P < 0.01). Larger changes in cerebral TSI during Hypox (P < 0.01) resulted from greater reductions in O2Hb (P = 0.02, Norm vs. Hypox). In comparison, muscle TSI was not affected by Hypox at rest but decreased 23.5 ± 15.7% during exercise (P = 0.02, Norm vs. Hypox).
Fig. 1.
Cerebral tissue saturation index (TSI) during exercise (n = 12). Data points correspond to rest and 25, 50, 75, and 100% maximal power output (Wmax) in normoxia (Norm, •) and hypoxia (Hypox, ○). Cerebral TSI is maintained in Norm until 75% Wmax and declines thereafter (P < 0.05). In Hypox, resting TSI is reduced (P < 0.05) and continues to fall as exercise intensity increases (P < 0.01).
Fig. 2.
Muscle TSI during exercise (n = 12). Data points correspond to rest and 25, 50, 75, and 100% Wmax in Norm (•) and Hypox (○). Muscle TSI decreases until 75% Wmax (P < 0.05) and plateaus thereafter. In Hypox, resting TSI is unaffected (P > 0.75) and follows a pattern similar to that in Norm during exercise.
Regional differences in cortical oxygenation during exercise.
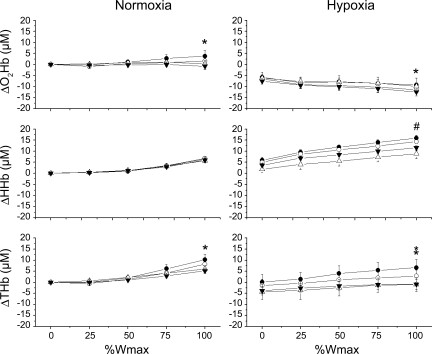
In Norm, bilateral changes in prefrontal oxygenation (O2Hb, HHb, and THb) were highly correlated throughout exercise (R2 = 0.94–1.00, P < 0.01; Fig. 3), although at 100% Wmax, prefrontal oxygen delivery (increased O2Hb and THb) was slightly greater in the right than left prefrontal region (P < 0.05). Correlations were also strong between left prefrontal, premotor, and motor regions with respect to HHb and THb (R2 = 0.94–1.00, P < 0.01), but not O2Hb (R2 = 0.01–0.55), suggesting slight variations in oxygen delivery, particularly within the motor cortex, where reductions in O2Hb were inversely correlated with prefrontal regions (R = −0.43, P < 0.01). These differences were not significant at 100% Wmax.
Fig. 3.
Regional changes in cerebral oxygenation during incremental exercise (n = 11). Continuous recordings obtained across conditions are shown as change from Norm at rest. O2Hb, oxyhemoglobin; HHb, deoxyhemoglobin; THb, total hemoglobin. Patterns of deoxygenation are similar in right prefrontal (○), left prefrontal (•), left premotor (▵), and left motor (▴) cortices. Extent of deoxygenation is greater in Hypox (P < 0.05). Note slight regional differences at 100% Wmax. *P < 0.05, right prefrontal > left prefrontal. #P < 0.05, left prefrontal > left premotor and motor.
Hypox decreased resting cerebral oxygenation (ΔO2Hb = −6.5 ± 6.2 μM and ΔHHb = 4.1 ± 3.8 μM, P < 0.01) similarly across regions (P > 0.50) without affecting cerebral blood volume (ΔTHb = −2.4 ± 9.6 μM, P > 0.30). During exercise, patterns of cerebral oxygenation were well correlated between regions (R2 = 0.61–1.00, P < 0.01), yet small differences at 100% Wmax were noted. Perfusion (increased O2Hb and THb) was greater in the right than left prefrontal region (P < 0.01). Deoxygenation (increased HHb) was greater in the prefrontal cortex than premotor and motor regions (P < 0.01).
Changes in cerebral blood flow velocity.
Resting MCA Vmean was similar between hemispheres and was not affected by acute Hypox (Table 1). Throughout exercise, MCA Vmean increased slightly during the early stages of exercise but fell from 75 to 100% Wmax in Norm and Hypox (P < 0.05). Small bilateral differences were not significant (P > 0.20).
DISCUSSION
The major finding of this study was cerebral deoxygenation at maximal exercise across prefrontal, premotor, and motor cortices. Results support previous findings in prefrontal regions (2, 11, 26, 27, 30), yet they provide new evidence to suggest that deoxygenation within the motor cortex may influence central motor drive during exhaustive aerobic exercise. Acute hypoxia increased the rate and magnitude of cortical deoxygenation and, thus, may have hastened the effect on central motor drive, explaining lower Wmax.
Regional changes in cortical oxygenation during maximal exercise.
Overall patterns of cortical oxygenation during incremental exercise were similar in prefrontal, premotor, and motor regions (Fig. 3). Bilaterally, O2Hb and THb were greater in the right than left prefrontal region at maximal exercise intensity in Norm and Hypox. These findings seem to suggest greater right-sided vasodilation during exercise, yet given the similarity in bilateral patterns of HHb (Fig. 2), the discrepancy is more likely explained by variation in arterial and venous contributions of illuminated regions. Specifically, because venous contributions account for >70% of NIRS signals (4), changes in HHb are more representative of regional changes in oxygenation (8). Thus uniform changes in HHb demonstrate widespread cortical deoxygenation during exhaustive aerobic exercise.
It has been suggested that cerebral deoxygenation during high-intensity exercise is caused by hypocapnia-induced vasoconstriction of pial arteries, thereby reducing oxygen delivery (20). This mechanism cannot adequately explain the changes in oxygenation, since cortical blood volume (THb) rises throughout exercise and is poorly correlated with changes in MCA Vmean (27). Increased THb near maximal may be the result of parasympathetically mediated cortical vasodilation in response to relative hypoperfusion (13) and/or larger resistance to venous outflow (25).
Regional influence of cerebral oxygenation on performance.
We observed a 9.0 ± 10.7% reduction in prefrontal cortex tissue saturation (increased HHb relative to O2Hb) before the cessation of exercise in Norm. Since regional blood flow and oxygenation are directly correlated with neuronal activity, the reduction in prefrontal oxygenation may have, at least in part, influenced the ability to maintain motor output. This hypothesis is supported by the fact that prefrontal oxygenation is directly correlated with maximal handgrip strength and is a strong predictor of motor performance (22). Prefrontal function is mainly responsible for higher-order cognitive functions but may integrate goal-directed behavior, task-related memory, sensory information, and motivation to plan motor movements (15, 21). In this scenario, it is important to stress that prefrontal oxygenation would be only one of many afferent signals influencing complex regulation of motor drive during high-intensity exercise (24).
Our data are the first to show that deoxygenation also occurs in premotor and motor regions during high-intensity aerobic exercise. We therefore raise the possibility that deoxygenation in motor areas may directly impair motor neuron excitability by altering ion channel and/or neurotransmitter function (10). Alternatively, deoxygenation in motor areas may have had a mediating effect on interneurons connected to basal ganglia, which integrate sensory signals and regulate motor drive (9). In either case, data suggest that motor drive may be influenced by multiple regions of cerebral deoxygenation during high-intensity exercise but do not exclude a role for other physiological (e.g., accumulation of muscle metabolites) and psychological states associated with exhaustion.
Effect of acute hypoxia on maximal aerobic performance.
Uninterrupted NIRS recordings were obtained throughout each experiment to assess differences between Norm and Hypox without error from probe placement/replacement. Acute Hypox decreased resting cerebral tissue saturation (−11 ± 14%, P < 0.01) in direct proportion with Sp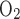 (−11 ± 3%, P < 0.01). This level of cerebral hypoxia was similar to that at maximal intensity in Norm (P = 0.71). This new observation supports the argument that normoxic exercise could not have been limited exclusively by cerebral deoxygenation (1, 26, 27); otherwise, subjects would have been unable to begin exercising in Hypox.
(−11 ± 3%, P < 0.01). This level of cerebral hypoxia was similar to that at maximal intensity in Norm (P = 0.71). This new observation supports the argument that normoxic exercise could not have been limited exclusively by cerebral deoxygenation (1, 26, 27); otherwise, subjects would have been unable to begin exercising in Hypox.
During exercise, a faster rate of deoxygenation led to a larger relative (compared with rest) and absolute (compared with Norm) degree of cerebral hypoxia at maximal effort. Greater deoxygenation in Hypox was primarily caused by reduced availability of oxygen (reduced O2Hb), resulting from reduced Sp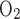 , but was exacerbated by greater oxygen extraction (increased HHb). Severe hypoxia increases the relative influence of cerebral hypoxia on motor drive and perception of effort (1, 22, 23) and, thus, may have hastened the decision to stop exercising, explaining lower Wmax. Whether the extent of cerebral deoxygenation at maximal intensity in severely hypoxic conditions approaches a critical absolute limit remains to be resolved but could be determined using a similar design if exercise with progressively greater levels of hypoxia is stopped at comparable levels of cerebral oxygenation.
, but was exacerbated by greater oxygen extraction (increased HHb). Severe hypoxia increases the relative influence of cerebral hypoxia on motor drive and perception of effort (1, 22, 23) and, thus, may have hastened the decision to stop exercising, explaining lower Wmax. Whether the extent of cerebral deoxygenation at maximal intensity in severely hypoxic conditions approaches a critical absolute limit remains to be resolved but could be determined using a similar design if exercise with progressively greater levels of hypoxia is stopped at comparable levels of cerebral oxygenation.
Overall, the increase in cerebral blood volume (THb) during incremental exercise was lower in Hypox. This may reflect diversion of blood from cortical regions to protect deeper structures, such as the basal ganglia and thalamus (3, 5), from severe hypoxia. Although patterns of deoxygenation were similar between prefrontal, premotor, and motor regions, the extent of deoxygenation (HHb) was largest in prefrontal areas (P < 0.05). Hypoxic impairment of prefrontal processing (32) may thus have played a more dominant role in the decision to stop exercise during Hypox.
Differences between cerebral and muscle tissue oxygenation: potential for steal?
In Norm, cerebral and muscle tissue saturations exhibited varied responses to exercise. Near maximal intensity, reductions in end-tidal Pco2, MCA Vmean, and cerebral oxygenation were concurrent with a plateau in muscle oxygenation (Table 1, Figs. 1 and 2). It is possible that hypocapnia-induced cerebral vasoconstriction allowed for a greater percentage of cardiac output to be diverted toward working muscle, effectively maintaining the balance between leg oxygen delivery and demand from 75 and 100% Wmax. A similar steal-like mechanism may have also occurred during Hypox, since resting reductions in Sp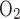 and cerebral oxygenation were associated with preservation of muscle oxygenation (18). To test a cerebral steal hypothesis, future studies that maintain arterial Pco2, and thus cerebral blood flow, during high-intensity exercise are needed.
and cerebral oxygenation were associated with preservation of muscle oxygenation (18). To test a cerebral steal hypothesis, future studies that maintain arterial Pco2, and thus cerebral blood flow, during high-intensity exercise are needed.
Limitations.
The four regions monitored during this study represent a large cross section of cortical tissue. Illuminated volumes spanned areas associated with cognitive and motor performance but were unable to discriminate between specific functional areas that are active during gross motor movements (16, 28). Additional channels of NIRS (Tx-Rx pairs) would permit higher-resolution cortical mapping of neuronal activity during exhaustive exercise. Such functional NIRS (NIRS) assessments would significantly advance our understanding of cerebral contributions to whole body fatigue but are not without limitations.
Penetration depths of NIRS are limited to superficial layers (2–3 mm deep) of the cerebrum, which are sufficient to illuminate cortical gray matter. However, deeper structures associated with motor, respiratory, cardiovascular, and temperature regulation, such as the basal ganglia, brain stem, and cerebellum, remain inaccessible by NIRS. Additionally, NIRS accurately monitors relative changes in O2Hb and HHb, but mathematical assumptions needed to obtain absolute concentration values (i.e., absorption and scattering coefficients) introduce error in tissue saturation estimates. During pilot work, even slight variance in probe placement was noted to introduce significant discrepancies in cerebral TSI values. This was likely due to heterogeneity of arterial and venous composition of illuminated volumes, as previously discussed, and accounts for large variance between subjects. In general, TSI measurements were greater than would be expected in mixed venous blood (14). For this reason, we designed the protocol to collect all necessary data from each individual during a single session in which NIRS probes were left in place. We also restricted our interpretations to relative changes in NIRS values across conditions and exercise. We encourage others using NIRS to follow similar precautions in designing and interpreting results to directly address limitations of the technology.
Conclusions.
Cortical deoxygenation during high-intensity exercise is not restricted to prefrontal regions of the brain. Deoxygenation in premotor and motor cortices may contribute to fatigue and/or decisions to stop exercising. Acute hypoxia exacerbates cortical deoxygenation and, thus, may hasten these effects. Future functional NIRS studies are needed to expand our understanding of the role of cerebral activity in exhaustive whole body exercise.
GRANTS
This project was funded in part by National Heart, Lung, and Blood Institute Grant HL-070362.
Acknowledgments
The authors are grateful to Ruth Johnson and Martin Heine for assistance in project management and Janet Uhde for medical expertise during subject recruitment and screening.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Amann M, Romer LM, Subudhi AW, Pegelow DF, Dempsey JA. Severity of arterial hypoxaemia affects the relative contributions of peripheral muscle fatigue to exercise performance in healthy humans. J Physiol 581: 389–403, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhambhani Y, Malik R, Mookerjee S. Cerebral oxygenation declines at exercise intensities above the respiratory compensation threshold. Respir Physiol Neurobiol 156: 196–202, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Binks AP, Cunningham VJ, Adams L, Banzett RB. Gray matter blood flow change is unevenly distributed during moderate isocapnic hypoxia in humans. J Appl Physiol 104: 212–217, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Boushel R, Langberg H, Olesen J, Gonzales-Alonzo J, Bulow J, Kjaer M. Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand J Med Sci Sports 11: 213–222, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Buck A, Schirlo C, Jasinksy V, Weber B, Burger C, von Schulthess GK, Koller EA, Pavlicek V. Changes of cerebral blood flow during short-term exposure to normobaric hypoxia. J Cereb Blood Flow Metab 18: 906–910, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Duncan A, Meek JH, Clemence M, Elwell CE, Tyszczuk L, Cope M, Delpy DT. Optical pathlength measurements on adult head, calf and forearm and the head of the newborn infant using phase resolved optical spectroscopy. Phys Med Biol 40: 295–304, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Fink GR, Adams L, Watson JD, Innes JA, Wuyam B, Kobayashi I, Corfield DR, Murphy K, Jones T, Frackowiak RS, et al. Hyperpnoea during and immediately after exercise in man: evidence of motor cortical involvement. J Physiol 489: 663–675, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grassi B, Pogliaghi S, Rampichini S, Quaresima V, Ferrari M, Marconi C, Cerretelli P. Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. J Appl Physiol 95: 149–158, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Grillner S, Wallen P, Saitoh K, Kozlov A, Robertson B. Neural bases of goal-directed locomotion in vertebrates—an overview. Brain Res Rev 57: 2–12, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Haddad GG, Jiang C. O2 deprivation in the central nervous system: on mechanisms of neuronal response, differential sensitivity and injury. Prog Neurobiol 40: 277–318, 1993. [DOI] [PubMed] [Google Scholar]
- 11.Imray CH, Myers SD, Pattinson KT, Bradwell AR, Chan CW, Harris S, Collins P, Wright AD. Effect of exercise on cerebral perfusion in humans at high altitude. J Appl Physiol 99: 699–706, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Jasper H The ten twenty electrode system of the international federation. Electroencephalogr Clin Neurophysiol 10: 371–375, 1958. [PubMed] [Google Scholar]
- 13.Kano M, Moskowitz MA, Yokota M. Parasympathetic denervation of rat pial vessels significantly increases infarction volume following middle cerebral artery occlusion. J Cereb Blood Flow Metab 11: 628–637, 1991. [DOI] [PubMed] [Google Scholar]
- 14.Matcher SJ, Kirkpatrick P, Nahid K, Cope M, Delpy DT. Absolute quantification methods in tissue near infrared spectroscopy. Proc SPIE 2389: 486–495, 1995. [Google Scholar]
- 15.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Miyai I, Tanabe HC, Sase I, Eda H, Oda I, Konishi I, Tsunazawa Y, Suzuki T, Yanagida T, Kubota K. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage 14: 1186–1192, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Nybo L, Rasmussen P. Inadequate cerebral oxygen delivery and central fatigue during strenuous exercise. Exerc Sport Sci Rev 35: 110–118, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Peltonen JE, Kowalchuk JM, Paterson DH, DeLorey DS, duManoir GR, Petrella RJ, Shoemaker JK. Cerebral and muscle tissue oxygenation in acute hypoxic ventilatory response test. Respir Physiol Neurobiol 155: 71–81, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Perrey S Non-invasive NIR spectroscopy of human brain function during exercise. Methods 45: 289–299, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Querido JS, Sheel AW. Regulation of cerebral blood flow during exercise. Sports Med 37: 765–782, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci 5: 184–194, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen P, Dawson EA, Nybo L, van Lieshout JJ, Secher NH, Gjedde A. Capillary-oxygenation-level-dependent near-infrared spectrometry in frontal lobe of humans. J Cereb Blood Flow Metab 27: 1082–1093, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Romer LM, Haverkamp HC, Amann M, Lovering AT, Pegelow DF, Dempsey JA. Effect of acute severe hypoxia on peripheral fatigue and endurance capacity in healthy humans. Am J Physiol Regul Integr Comp Physiol 292: R598–R606, 2007. [DOI] [PubMed] [Google Scholar]
- 24.St. Clair Gibson A, Noakes TD. Evidence for complex system integration and dynamic neural regulation of skeletal muscle recruitment during exercise in humans. Br J Sports Med 38: 797–806, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stolz E, Fox BC, Hoffmann O, Gerriets T, Blaes F, Kraus J, Kaps M. Cranial venous outflow under lower body positive and negative pressure conditions and head-up and -down tilts. J Neuroimaging. In press. [DOI] [PubMed]
- 26.Subudhi AW, Dimmen AC, Roach RC. Effects of acute hypoxia on cerebral and muscle oxygenation during incremental exercise. J Appl Physiol 103: 177–183, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Subudhi AW, Lorenz MC, Fulco CS, Roach RC. Cerebrovascular responses to incremental exercise during hypobaric hypoxia: effect of oxygenation on maximal performance. Am J Physiol Heart Circ Physiol 294: H164–H171, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki M, Miyai I, Ono T, Oda I, Konishi I, Kochiyama T, Kubota K. Prefrontal and premotor cortices are involved in adapting walking and running speed on the treadmill: an optical imaging study. Neuroimage 23: 1020–1026, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Tashiro M, Itoh M, Fujimoto T, Masud MM, Watanuki S, Yanai K. Application of positron emission tomography to neuroimaging in sports sciences. Methods 45: 300–306, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Thomas R, Stephane P. Prefrontal cortex oxygenation and neuromuscular responses to exhaustive exercise. Eur J Appl Physiol 102: 153–163, 2008. [DOI] [PubMed] [Google Scholar]
- 31.van der Zee P, Cope M, Arridge SR, Essenpreis M, Potter LA, Edwards AD, Wyatt JS, McCormick DC, Roth SC, Reynolds EO, et al. Experimentally measured optical pathlengths for the adult head, calf and forearm and the head of the newborn infant as a function of inter optode spacing. Adv Exp Med Biol 316: 143–153, 1992. [DOI] [PubMed] [Google Scholar]
- 32.Virues-Ortega J, Buela-Casal G, Garrido E, Alcazar B. Neuropsychological functioning associated with high-altitude exposure. Neuropsychol Rev 14: 197–224, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Williamson JW, Nobrega AC, McColl R, Mathews D, Winchester P, Friberg L, Mitchell JH. Activation of the insular cortex during dynamic exercise in humans. J Physiol 503: 277–283, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]