Abstract
Microdialysis enables in-depth mechanistic study of the cutaneous circulation in humans. However, whether the insertion or presence of the microdialysis fiber (MDF) affects the skin circulation or its responses is unknown. We tested whether the cutaneous vascular response to whole body heating (WBH) was affected by MDF or by pretreatment with ice (part 1) or local anesthesia (LA; part 2). Eleven subjects participated, 9 in part 1 and 8 in part 2 (5 participated in both). In both parts, four sites on the forearm were selected, providing untreated control, MDF only, ice or LA only, and combined MDF plus ice or LA. A tube-lined suit controlled whole body skin temperature, which was raised to ∼38°C for WBH. Skin sites were instrumented with laser-Doppler flow probes. Data were expressed as cutaneous vascular conductance (CVC). Baseline levels were not different among sites (P > 0.05). In part 1, the internal temperature for the onset of vasodilation was higher (P > 0.05) with MDF with or without ice pretreatment than at untreated control sites (control 36.6 ± 0.1°C, Ice 36.5 ± 0.1, MDF 36.8 ± 0.1°C, and Ice+MDF 36.8 ± 0.1°C). Peak CVC during WBH was decreased (P < 0.05) by MDF (control 73 ± 7 vs. MDF 59 ± 6% of maximal CVC). Ice (73 ± 6% of maximal CVC) or Ice+MDF (69 ± 6% of maximal CVC) did not affect (P > 0.05) peak CVC compared with control. In part 2, the temperature threshold for the onset of vasodilation was increased by MDF with or without LA treatment and by LA alone (P < 0.05; control 36.6 ± 0.1°C, MDF 36.7 ± 0.1°C, LA 36.8 ± 0.1°C, and LA+MDF 36.8 ± 0.1°C). Peak CVC was decreased by MDF (control 69 ± 6% of maximal CVC vs. MDF 58 ± 8% of maximal CVC; P < 0.05). LA only (65 ± 10% of maximal CVC) or MDF in the presence of LA (73 ± 12% of maximal CVC) did not affect (P > 0.05) peak CVC compared with control. Thus LA or MDF increases the temperature threshold for the onset of vasodilation. MDF alone decreases the peak vasodilator response in CVC to WBH; however, this attenuation did not occur if ice or LA is used before MDF placement. Ice or LA alone do not affect the peak response in CVC to WBH. How those treatments prevent or reverse the effect of MDF placement is presently unclear.
Keywords: skin blood flow, active vasodilation, ice, local anesthesia, laser-Doppler flowmetry
microdialysis is a technique that over the past 20 yr has become a common procedure in humans. The first report of the in vivo use of microdialysis in humans concerned studies of the interstitial space (19). In the last decade or so, microdialysis coupled with laser-Doppler flowmetry has provided a powerful system for in-depth mechanistic analysis of how the cutaneous circulation functions in vivo. The microdialysis technique often involves insertion of a “guide,” typically a 25-gauge needle, intradermally for ∼2.5 cm before exiting. The microdialysis fiber is placed through the lumen of this needle; the needle is then removed, leaving the fiber within the dermis (e.g., Refs. 6, 8, 9, 16, 20, 26, 29). Used in this manner, the microdialysis fiber allows the exchange of substances with the interstitial space. One of the primary advantages of this technique is that substances can be applied locally without systemic effects. Several sites on a single human forearm can thereby be individually manipulated as desired. The combination with laser-Doppler flowmetry offers a nearly ideal approach to probing the mechanisms of control in the cutaneous circulation. However, it is possible that the insertion trauma caused by the placement of the microdialysis fiber confounds efforts to establish the mechanisms involved in the cutaneous vascular responses examined. In some instances, ice is used as a temporary anesthetic, which reduces or eliminates the insertion pain. This procedure might be sufficient to lessen the triple response of Lewis (18), that is, flare, local erythema, and wheal, which might affect cutaneous vascular responses (2, 7). The use of ice before needle insertion, as a temporary anesthetic, is common but it is assumed to be sufficiently benign that the process will not affect the vascular responses. However, no work thus far has thoroughly investigated the effects of the preparatory procedures on cutaneous vascular responses.
Local anesthesia (LA) is also effective in preventing the pain of fiber insertion (5, 11, 15, 21). LA is not typically used with microdialysis fiber placement unless sensory nerve blockade is a part of the question, because the application of LA may affect the vascular responses (5, 11, 15, 21). Wong et al. (34) recently provided evidence for an involvement of substance P in the cutaneous vascular response to whole body heat stress. Because sensory nerves are a known source for substance P, this general consideration that LA might affect the response would appear to be well founded. However, that notion has not been directly tested.
Therefore, we designed this study to test whether 1) placement of a microdialysis fiber has an effect on cutaneous vascular responses; 2) using ice as a temporary anesthetic before needle insertion has an effect on subsequent cutaneous vascular responses; 3) application of topical LA affects cutaneous vascular responses; and 4) whether, in conjunction with microdialysis fiber insertion, either ice or LA has any such effects. Cutaneous vascular responses were assessed as the reflex vasodilator responses to whole body heat stress. This was chosen because the response to whole body heating is large and thus likely to show any differences among treatments.
METHODS
Subjects.
The local Institutional Review Board approved this study. All subjects were fully informed of the methods and risks before written informed consent was obtained. All 12 subjects (9 men and 3 women; age 28 ± 4 yr) who participated (9 in part 1 and 8 in part 2; 5 who participated in part 1 did so in part 2) were healthy nonsmokers, nonobese (body mass index: 24 ± 1.9 kg/m2), were not taking any medications, and refrained from alcoholic and caffeinated beverages for at least 12 h before the study. For the female subjects, the phase of the menstrual cycle was recorded because previous studies have reported that the vasodilator responses to whole body heating are affected by female reproductive hormones (4); however, in the context of the questions in the present study, the data from the female subjects displayed no differences from those of the male subjects, so the data were combined.
Instrumentation.
In all studies, subjects had four ventral forearm skin sites chosen for assessment; two of these sites were prepared with microdialysis probes, placed intradermally, and two without. As described previously (6, 16), these custom-built microdialysis probes consisted of 1 cm of microdialysis tubing (regenerated cellulose, inner diameter 200 μm, 18-kDa nominal molecular mass cutoff) attached at each end to polyimide tubing. For both parts, a 25-gauge needle was introduced aseptically for ∼2.5 cm into the dermis before exiting. The microdialysis fiber was introduced into the skin via the lumen of the needle. The needle was then removed, leaving the probe in place. All probes were placed in this manner. The effects of the insertion trauma were allowed to subside over the following 90 min (3, 8). The different probes were placed 3–5 cm apart. In part 1, immediately before implantation of one of the microdialysis fibers, the area of forearm skin was temporarily anesthetized by the application of an ice pack for 5 min; the other microdialysis fiber was inserted into the dermis without pretreatment with ice. The ice treatment was also performed at one of the skin sites not instrumented with a microdialysis fiber; the final site was untreated (neither fiber placement nor ice pretreatment). In part 2, a topical local anesthetic cream was applied for a minimum of 2 h at two skin sites. At one of those sites, a microdialysis fiber was placed. As in part 1, a microdialysis fiber was placed without any pretreatment and a fourth site served as an untreated control site.
Measurements.
All measurements were performed with the subjects resting in the supine posture. Skin blood flow was measured from the ventral aspect of the forearm by laser-Doppler flowmetry (MoorLAB, Moor Instruments, Axminster, UK), and it was expressed as laser-Doppler blood flow (14, 22). Laser-Doppler blood flow measures are exclusive to the skin because they are not contaminated by blood flow to underlying skeletal muscle (24). Local temperature control at the sites of blood flow measurement was achieved with custom-designed metal Peltier cooling-heating probe holders (e.g., Refs. 1, 8, 10, 15, 35). These controlled surface temperature within 0.05°C over an area of 6.3 cm2 with the exception of a small aperture (0.28 cm2) in the center of the holder to enable placement of the laser-Doppler probe. A thermocouple placed between the skin surface and the probe holder enabled local skin temperature assessment and feedback control. In the present study, local skin temperature was maintained at 34°C until the end of the study, at which time it was raised to 42°C for 30 min to achieve maximal cutaneous vasodilation (13, 27). Blood pressure was recorded noninvasively and continuously by the Penaz method (23) from the left middle finger (Finapres, Ohmeda, Madison, WI). Mean arterial pressure was obtained from the electrical integration of the continuous blood pressure signal. Cutaneous vascular conductance (CVC) was calculated as the ratio of laser-Doppler flow to mean arterial pressure (in arbitrary units). Whole body skin temperature was the weighted mean from six thermocouples placed on the body surface (28) and was controlled by the use of water-perfused suits (e.g., Refs. 1, 5, 10, 28). The suit covered the entire body surface apart from the head, hands, feet, and the forearm used for the blood flow measurements. This arrangement allowed independent control of local skin temperature and whole body skin temperature. Oral temperature was recorded by a sublingual thermocouple. The subject was encouraged to keep his or her mouth closed, with the thermocouple as stationary as possible, and to breathe through the nose. All variables were collected at 1-s intervals and stored as 20-s averages for offline analysis.
Drugs.
Sensory nerve blockade was achieved through the use of a topical local anesthetic cream (2.5% lidocaine and 2.5% prilocaine, Fougera, Melville, NY). The cream was applied to an area of 8 cm2 under an occlusive dressing where it was left for a minimum of 2 h (11, 15). The area was tested for sensory loss both before needle insertion and at the completion of the study.
Protocols.
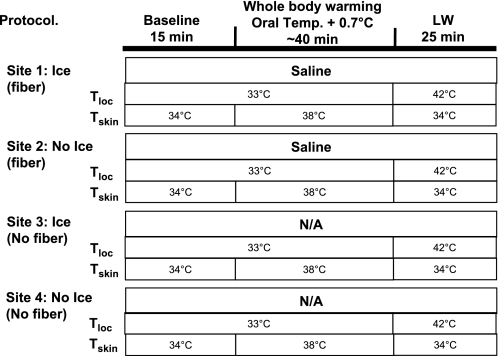
The protocol for part 1 is shown in Fig. 1. Four skin sites on the ventral aspect of the forearm were randomly chosen. Two of these sites were treated with an ice pack for 5 min. One site treated with ice and one site not treated with ice were then instrumented with microdialysis fibers. Hence, there was one untreated site, one site only pretreated with ice, one with only a microdialysis fiber, and a fourth site with both ice pretreatment and a microdialysis fiber.
Fig. 1.
Outline of the protocol for part 1. Four skin sites on the ventral aspect of the forearm were randomly chosen. Two were pretreated with ice for 5 min; one of these was sites was instrumented with a microdialysis probe. A skin site not treated with ice was also instrument with a microdialysis probe. This arrangement yielded untreated control, ice pretreatment only, microdialysis fiber only, and both ice and microdialysis fiber. Ninety minutes were allowed to elapse before data collection. Baseline measurements were then recorded for 10 min, and whole body heat stress was performed for 30–45 min, until oral temperature was raised by 0.7°C. LW, local warming; Tskin, whole body mean skin temperature; Tloc, local skin temperature; Temp, temperature; N/A, not applicable.
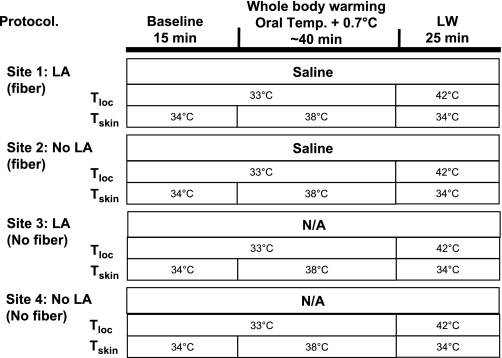
In part 2, again four skin sites on the ventral aspect of the forearm were randomly chosen. The protocol is shown in Fig. 2. Two of these sites were treated with a topical LA for a minimum of 2 h. One site treated with LA and one site not treated with LA were then instrumented with microdialysis fibers, yielding an untreated site, a site pretreated with only LA, one site with a microdialysis fiber but no LA, and a site with both.
Fig. 2.
Outline of the protocol for part 2. Four skin sites on the ventral aspect of the forearm were randomly chosen. Two were pretreated with local anesthesia (LA) for 2 h; one of these sites was instrumented with a microdialysis probe. A skin site not treated with LA was also instrument with a microdialysis probe This yielded an untreated control, LA treated only, microdialysis fiber only, and both LA and microdialysis fiber. Thereafter, the protocol was the same as for part 1. Skin at both LA-treated sites was tested for sensory loss at the beginning and end of the study.
In both parts, a 90-min period was allowed for trauma resolution because this has developed as the “norm” (e.g., Refs. 12, 15, 21, 34) from a study by Anderson et al. (2), who showed that skin perfusion returns to normal following the placement of a microdialysis fiber in about an hour, and Groth et al. (7), who found that histamine levels return to normal levels after 30 min. The experiment began with a 10-min baseline phase. Whole body heating was then performed, with the aim of increasing oral temperature by 0.7°C. This was achieved by pumping 48°C water through the tube-lined suit. After 30–45 min, when oral temperature had increased by 0.7°C, cool water (25°C) was pumped through the suit to return the subject to a thermoneutral temperature. Local skin temperature at the site of measurement was then increased to 42°C to obtain maximal CVC values (14, 28).
Data analysis.
Comparison among sites included the level of CVC achieved at the peak of heating and the level of internal temperature at which CVC began to increase during body heating (threshold for vasodilation). CVC for each site is expressed as a percentage of maximal CVC for that site (%CVCmax). Temperature thresholds at which the onset of vasodilatation occurred during whole body heating were chosen by an investigator who was blinded as to the source of data. Analysis of baseline and maximal CVC values was performed using a repeated-measures one-way ANOVA. Statistical significance was assumed when P < 0.05. Power analysis indicated that with a variance in means of 10 and a common standard deviation of 5 %CVCmax, for both parts 1 and 2, a minimum of seven subjects would be required for a P < 0.05 with 95% power (nQuery Advisor v.3); with nine subjects in part 1 and 8 in part 2, the power for each part was 99.
RESULTS
Part 1.
Baseline CVC values, expressed as %CVCmax, were statistically (P > 0.05) unaffected by the prior application of ice, insertion of a microdialysis fiber, or their combination compared with that at the untreated control site (Fig. 3).
Fig. 3.
Summary of the data from all 9 subjects in part 1 for baseline measurements in cutaneous vascular conductance (CVC). Application of ice, insertion of a microdialysis fiber, or the combination of the pair did not affect baseline blood flow. CVC is expressed as a percentage of maximal CVC (%max).
With body heating, the internal temperature thresholds for the onset of vasodilation at the sites with fibers placed with or without ice pretreatment were significantly (P < 0.05) higher than at the untreated control site (control 36.6 ± 0.1°C vs. Fiber 36.8 ± 0.1°C and Ice+Fiber 36.8 ± 0.1°C; Fig. 4). The onset of cutaneous vasodilation at the Ice+Fiber was also significantly (P < 0.05) higher than the ice only site (36.8 ± 0.1°C vs. 36.5 ± 0.1°C; Fig. 4). Pretreatment with ice had no statistically significant effect on vasodilator threshold, either with or without fiber placement (P > 0.05; Fig. 4).
Fig. 4.
Influence of ice, microdialysis fiber placement, and the combination on the temperature threshold for the onset of vasodilatation during whole body heat stress during part 1. Values are for 9 subjects. Fiber insertion, with or without ice pretreatment, significantly increased the temperature threshold at which vasodilatation occurred. *P < 0.05 relative to control and ice-treated skin sites.
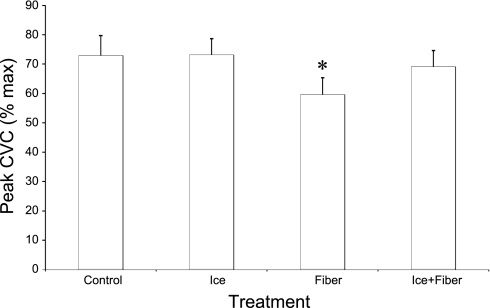
The peak response in CVC during whole body heating was significantly (P < 0.05) decreased by fiber placement on its own (control 73 ± 7% CVCmax vs. fiber 59 ± 6% CVCmax; Fig. 5). However, neither ice pretreatment (73 ± 6% CVCmax) nor fiber placement with ice pretreatment (69 ± 6% CVCmax) significantly affected (P > 0.05) the response in CVC to subsequent whole body heating compared with that at the control site (Fig. 5). These findings are the same if the changes in CVC from baseline to peak heating are used.
Fig. 5.
Mean data from all 9 subjects for the response in CVC to the whole body heating stress in part 1. Oral temperature was raised 0.7°C. Insertion of a microdialysis fiber significantly reduced the response in CVC to the whole body heating. However, placement of a fiber immediately following the application if ice restored the complete response; ice alone had no effect *P < 0.05 compared with the control and ice-treated sites.
Part 2.
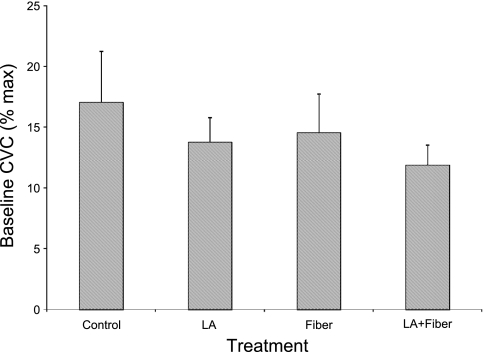
Baseline CVC values were statistically (P > 0.05) unaffected by the application of topical LA, insertion of a microdialysis fiber, or the combination of the two compared with that at the untreated control site (Fig. 6), although there was a trend for a slight decrease (P = 0.07) at both of the sites treated with LA compared with the appropriate untreated site (control 17 ± 4, LA 14 ± 2, Fiber 15 ± 3, and LA+Fiber 12 ± 2% CVCmax).
Fig. 6.
Baseline CVC from part 2. Values are for 8 subjects. Application of topical local anesthesia, insertion of a microdialysis fiber, or the combination of the pair did not affect baseline blood flow. CVC is expressed as a percentage of maximal CVC.
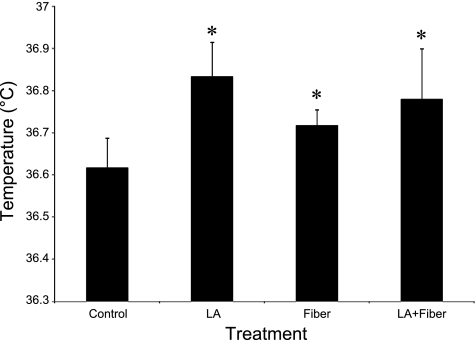
As found in part 1, the temperature threshold at which the onset of cutaneous vasodilation occurs during whole body heating was increased by the placement of the microdialysis fiber. Fiber placement with or without LA treatment, or LA treatment alone, caused temperature thresholds to be significantly (P < 0.05) higher than at the untreated control site (control 36.6 ± 0.1°C, fiber 36.7 ± 0.1°C, LA 36.8 ± 0.1°C, and LA+Fiber 36.8 ± 0.1°C; Fig. 7). Nevertheless, there was no significant difference (P > 0.05) in the onset of vasodilation at the LA+Fiber site compared with that of the LA only site (Fig. 7). Similarly, there was no statistical difference between thresholds at the Fiber and LA+Fiber sites (P > 0.05; Fig. 7).
Fig. 7.
Internal temperature thresholds for the onset of vasodilation in part 2. Values are for 8 subjects. All treatments raised the temperature threshold compared with the untreated control. There were no statistical differences among the three treatments. *P < 0.05 that LA, Fiber and LA+Fiber sites are significantly different compared with the control site.
As in part 1, the peak response in CVC to whole body heating was significantly (P < 0.05) decreased by fiber placement (control 69 ± 6% CVCmax vs. fiber 58 ± 8% CVCmax; Fig. 8). LA only (65 ± 10% CVCmax) or fiber placement in the presence of LA (73 ± 12% CVCmax) did not significantly affect (P > 0.05) the peak response in CVC to whole body heating compared with that at the control site (Fig. 8). These results are the same if the changes in CVC from baseline to peak heating are analyzed.
Fig. 8.
Peak CVC during body heating from all 8 subjects for part 2. Oral temperature was raised 0.7°C. Insertion of a microdialysis fiber significantly reduced the response in CVC to the whole body heating. However, placement of a fiber following treatment with topical local anesthesia restored the complete response; local anesthesia alone had no significant effect. *P < 0.05 is significant different compared with the control and the LA+Fiber sites.
DISCUSSION
The major findings of this investigation are that microdialysis fiber placement offsets the temperature threshold for the onset of cutaneous vasodilation during heat stress to a higher temperature and that this occurs with or without the anesthetic effects of ice pretreatment or the application of topical LA before fiber placement. Also, LA alone (but not ice pretreatment alone) increases the temperature threshold for the onset of active vasodilation. Fiber placement alone decreased the ultimate vasodilator response in CVC to whole body heating; however, this attenuation did not occur if ice or LA is used before fiber placement. Ice and LA alone did not affect the ultimate response in CVC to whole body heating.
As expected from earlier studies (2, 7), resting CVC was unaffected in all instances; there were no statistically identifiable differences in the baseline levels of blood flow among the four treatments in either part 1 or part 2. This outcome is consistent with previous work, in which the initial increase in histamine levels from the trauma due to the microdialysis needle insertion subsides in about 30 min (7). Our laboratory (e.g., Refs. 12, 15, 25) and others (e.g., Refs. 17, 30, 31, 33) routinely wait 1–2 h following the placement of microdialysis fibers before taking measurements, and baseline blood flows that are not perceptibly different from skin that has not had such treatment are observed. As for the efforts to eliminate pain from fiber insertion, ice was originally used because we believed that its effects would be relatively benign and temporary while still decreasing or eliminating the pain of needle insertion (6). Indeed, we found that such pretreatment with ice had no measurable effects on either the vasodilator threshold or the degree of vasodilation with subsequent body heating. Although one might expect LA to reduce baseline and/or peak CVC due to its known vasoconstrictor effects (3), only a statistically insignificant trend for a decline in baseline CVC at the LA+Fiber site in part 2 was seen (Fig. 6) with no apparent effect on peak CVC (Fig. 8).
The onset of vasodilation at the sites with fibers placed was offset to higher temperatures compared with control sites. A possible explanation for this observation is that the needle insertion may damage a few nerve endings, thereby reducing the ability of those areas of skin to respond to the whole body heat stress. LA also increased the temperature at which the onset of vasodilation occurred, possibly due to LA causing reduced nerve function and leading to decreased sensitivity to activation of the vasodilator system during whole body heat stress. Previous studies of cutaneous vascular responses to local cooling (11, 15) and local heating (21) found the initiation of the local vasoconstrictor and vasodilator responses to be altered following treatment with LA. The finding that the combination of ice or LA with fiber placement reverses the increased threshold is not in keeping with this speculation about damage to nerve fibers by fiber placement. The finding that fiber placement increases the threshold for activation of the active vasodilator system to a higher temperature might also be due to fiber placement disrupting local transmitters, such as substance P (34) or others (4, 17, 20, 25, 32, 33). It could also be that the elevated levels of histamine observed in the first 30 min following fiber placement (7) lead to its depletion. This scenario could affect the sensitivity of the active vasodilator system because histamine has been suggested to be involved in the active vasodilator response (32). Although the cause for the displacement of threshold is not made clear by these studies, the data are important in the use of vasodilator thresholds as an analytic tool (e.g., Ref. 4). It is important that control sites should have microdialysis probes if the test sites do.
Application of ice before fiber insertion partially restored the peak vasodilator response, such that there was not a statistical difference in peak CVC between the untreated control and ice plus fiber sites. This could be due to the ice acting as a temporary anesthetic and reducing an acute inflammatory response. As previously mentioned, Groth et al. (7) found that the trauma from microdialysis fiber insertion was associated with elevated levels of histamine. The concentration of histamine would steadily decrease and plateau about 30 min after fiber placement. We waited 1.5 h following microdialysis fiber placement and did not see any effects on baseline. With ice or LA pretreatment before fiber placement, a full CVC response to whole body heating is observed. Perhaps the anesthetic properties reduce the inflammatory response, in which case histamine might not be depleted.
The lack of effect of LA on neurogenic vasodilator function, although surprising, is in keeping with our laboratory's earlier observations of intact vasoconstrictor function following such LA treatment (11, 15). Our laboratory's earlier speculation was that a greater depth of autonomic than sensory fibers may account for this observation. Deeper application of the anesthetic, for example by microdialysis, would be an interesting test of this possibility.
The combination of LA and needle insertion was consistently associated with an unchanged peak vasodilator response to whole body heat stress (in comparison to responses at the untreated control site). Interestingly, application of LA alone did not reduce the ultimate vasodilator response to whole body heat stress. Cracowski et al. (5) recently showed that 40 min application of LA cream significantly reduced perceived needle insertion pain and after 2 h did not affect postocclusive or thermal hyperemia. Here, having applied topical LA for 2 h, there was a complete absence of any sensation to the needle insertion. This loss of sensory function was maintained throughout the investigation. The presence of the sensory blockade from LA throughout the study (unlike that from ice) suggests this not to be a generally useful adjunct to microdialysis. However, it produced a potentially important finding. Wong et al. (34) showed that desensitization of neurokinin type 1 (NK1) receptors decreased the response in CVC to whole body heating. This was an intriguing and provocative finding, suggesting that substance P is involved in the active vasodilator system. However, the evidence was gained in an unconventional manner. Rather than antagonism of NK1 receptors, they were desensitized by pretreating the skin with an infusion of substance P via microdialysis, exploiting a finding from their previous work (33). Although sensory nerves are a major source of substance P in the skin, our finding that treatment with topical LA did not affect the peak response to whole body heating at the LA only site suggests that substance P of sensory nerve origin is not involved in the cutaneous vasodilator response to whole body heating.
A thorough examination of the effect of the trauma of the insertion of the microdialysis fiber on the cutaneous vascular responses had not previously been performed. This study highlights the importance of ensuring that all sites within a study are treated in a similar manner. That simple procedures such as the application of an ice pack to temporarily anesthetize the skin before needle insertion are valuable, not only to reduce subject discomfort, but the findings of this investigation would suggest that is useful in preventing effects that can cause small, albeit statistically significant changes in the cutaneous vascular responses to stimuli.
Experimental considerations.
During the 0.7°C increase in oral temperature not all skin sites reached a plateau; this might be a potential source of error in the interpretation that peak CVC values are different between treatments. That is, the lower peak response may relate to the delay in the onset of vasodilation, and, if greater thermal stress or a longer period of body heating were used to allow stabilization of flow, the present findings might not be observed. It is also important to note that the use of oral temperature as a guide to the degree of heat stress has some potential sources of error; although breathing only through the nose is encouraged, breathing through the mouth can cause large changes in temperature values, as can changes in position of the probe in the mouth. This should not be a problem in interpretation in the present study because data from sites treated differently were taken simultaneously and, therefore, at the same central temperature. There is a potential issue reporting the changes in CVC as percentages of maximal CVC achieved by local heating, because it is not known whether microdialysis fiber placement affects the maximal cutaneous vasodilator response. This would be most likely to reduce maximal CVC through a physical effect on the microcirculation. Although such an effect is not supported by the lack of a significant effect on baseline CVC, nevertheless the possibility underscores the necessity of treating control and experimental sites equally, including the placement of microdialysis fibers. Finally, these results apply to reflex responses to body heating, but they do not necessarily anticipate the effects of ice pretreatment, local anesthesia, or microdialysis fiber insertion on other (e.g., vasoconstrictor) reflex responses or on the effects of local skin warming or cooling. This would ultimately be interesting considering the involvement of autonomic and sensory nerves and of local factors in those responses (1, 8–12, 15, 16, 20, 21, 25, 26, 30, 35).
In summary, we found that microdialysis fiber placement raises the temperature threshold for the onset of cutaneous vasodilation during heat stress. This shift occurs with or without the anesthetic affects of ice treatment or application of topical LA before fiber placement. Furthermore, LA alone increases the temperature threshold for the onset of active vasodilation. Fiber placement alone decreases the peak cutaneous vasodilator response to whole body heating. Interestingly, this attenuation did not occur if ice or LA was used before fiber placement. Ice and LA alone do not affect the peak cutaneous vascular responses to whole body heating.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant R01 HL-059166.
Acknowledgments
We thank the subjects for their participation.
Present address of G. J. Hodges: School of Kinesiology, The University of Western Ontario, London, Ontario, Canada N6A 5B8 (ghodges@uwo.ca).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alvarez GE, Zhao K, Kosiba WA, Johnson JM. Relative roles of local and reflex components in cutaneous vasoconstriction during skin cooling in humans. J Appl Physiol 100: 2083–2088, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Anderson C, Andersson T, Wardell K. Changes in skin circulation after insertion of a microdialysis probe visualized by laser Doppler perfusion imaging. J Invest Dermatol 102: 807–811, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Ashinoff R, Geronemus RG. Effect of the topical anesthetic EMLA on the efficacy of pulsed dye laser treatment of port-wine stains. J Dermatol Surg Oncol 16: 1008–1011, 1990. [DOI] [PubMed] [Google Scholar]
- 4.Charkoudian N, Johnson JM. Reflex control of cutaneous vasoconstrictor system is reset by exogenous female reproductive hormones. J Appl Physiol 87: 381–385, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Cracowski JL, Lorenzo S, Minson CT. Effects of local anaesthesia on subdermal needle insertion pain and subsequent tests of microvascular function in human. Eur J Pharmacol 559: 150–154, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Crandall CG, Etzel RA, Johnson JM. Evidence of functional β-adrenoceptors in the cutaneous vasculature. Am J Physiol Heart Circ Physiol 273: H1038–H1043, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Groth L, Jørgensen A, Serup J. Cutaneous microdialysis in the rat: insertion trauma and effect of anaesthesia studied by laser Doppler perfusion imaging and histamine. Skin Pharmacol Appl Skin Physiol 111: 125–132, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Hodges GJ, Kosiba WA, Zhao K, Alvarez GE, Johnson JM. The role of baseline in the cutaneous vasoconstrictor responses during combined local and whole body cooling in humans. Am J Physiol Heart Circ Physiol 293: H3187–H3192, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Hodges GJ, Kosiba WA, Zhao K, Johnson JM. The involvement of norepinephrine, neuropeptide Y, and nitric oxide in the cutaneous vasodilator response to local heating in humans. J Appl Physiol 105: 233–240, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodges GJ, Kosiba WA, Zhao K, Johnson JM. The involvement of heating rate and vasoconstrictor nerves in the cutaneous vasodilator response to skin warming. Am J Physiol Heart Circ Physiol 296: H51–H56, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodges GJ, Traeger JA 3rd, Tang T, Kosiba WA, Zhao K, Johnson JM. Role of sensory nerves in the cutaneous vasoconstrictor response to local cooling in humans. Am J Physiol Heart Circ Physiol 293: H784–H789, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Hodges GJ, Zhao K, Kosiba WA, Johnson JM. The involvement of nitric oxide in the cutaneous vasoconstrictor response to local cooling in humans. J Physiol 574: 849–857, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson JM The cutaneous circulation. In: Laser-Doppler Blood Flowmetry, edited by Shepherd AP and Öberg PA. New York: Springer, 1990, p. 121–139.
- 14.Johnson JM, O'Leary DS, Taylor WF, Kosiba W. Effect of local warming on forearm reactive hyperaemia. Clin Physiol 6: 337–346, 1986. [DOI] [PubMed] [Google Scholar]
- 15.Johnson JM, Yen TC, Zhao K, Kosiba WA. Sympathetic, sensory, and nonneuronal contributions to the cutaneous vasoconstrictor response to local cooling. Am J Physiol Heart Circ Physiol 288: H1573–H1579, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Kellogg DL, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol 86: 1185–1190, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Kellogg DL, Zhao JL, Wu Y. Neuronal nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. J Physiol 586: 847–857, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis T The Blood Vessels of the Human Skin and Their Responses. London: Shaw & Sons, 1927.
- 19.Lönnroth P, Jansson PA, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol Endocrinol Metab 253: E228–E231, 1987. [DOI] [PubMed] [Google Scholar]
- 20.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91: 1619–1626, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol 9: 1644–1649, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Öberg PA Laser-Doppler flowmetry. Crit Rev Biomed Eng 18: 125–163, 1990. [PubMed] [Google Scholar]
- 23.Parati G, Casadei R, Groppelli A, Di Rienzo M, Mancia G. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension 13: 647–655, 1989. [DOI] [PubMed] [Google Scholar]
- 24.Saumet JL, Kellogg DL Jr, Taylor WF, Johnson JM. Cutaneous laser-Doppler flowmetry: influence of underlying muscle blood flow. J Appl Physiol 65: 478–481, 1988. [DOI] [PubMed] [Google Scholar]
- 25.Stephens DP, Saad AR, Bennett LA, Kosiba WA, Johnson JM. Neuropeptide Y antagonism reduces reflex cutaneous vasoconstriction in humans. Am J Physiol Heart Circ Physiol 287: H1404–H1409, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Stewart JM, Medow MS, Minson CT, Taneja I. Cutaneous neuronal nitric oxide is specifically decreased in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 293: H2161–H2167, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol 66: 1586–1592, 1989. [DOI] [PubMed] [Google Scholar]
- 28.Taylor WF, Johnson JM, O'Leary D, Park MK. Effect of high local temperature on reflex cutaneous vasodilation. J Appl Physiol 57: 191–196, 1984. [DOI] [PubMed] [Google Scholar]
- 29.Thompson CS, Holowatz LA, Kenney WL. Cutaneous vasoconstrictor responses to norepinephrine are attenuated in older humans. Am J Physiol Regul Integr Comp Physiol 288: R1108–R1113, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Thompson CS, Kenney WL. Altered neurotransmitter control of reflex vasoconstriction in aged human skin. J Physiol 558: 697–704, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilkins BW, Chung LH, Tublitz NJ, Wong BJ, Minson CT. Mechanisms of vasoactive intestinal peptide-mediated vasodilation in human skin. J Appl Physiol 97: 1291–1298, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Wong BJ, Minson CT. Neurokinin-1 receptor desensitization attenuates cutaneous active vasodilatation in humans. J Physiol 577: 1043–1051, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong BJ, Tublitz NJ, Minson CT. Neurokinin-1 receptor desensitization to consecutive microdialysis infusions of substance P in human skin. J Physiol 568: 1047–1056, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong BJ, Wilkins BW, Minson CT. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J Physiol 560: 941–948, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamazaki F, Sone R, Zhao K, Alvarez GE, Kosiba WA, Johnson JM. Rate dependency and role of nitric oxide in the vascular response to direct cooling in human skin. J Appl Physiol 100: 42–50, 2006. [DOI] [PubMed] [Google Scholar]