Abstract
Death receptors are members of the tumor necrosis factor receptor superfamily characterized by a cytoplasmic region known as the “death domain” that enables the receptors to initiate cytotoxic signals when engaged by cognate ligands. Binding to the ligand results in receptor aggregation and recruitment of adaptor proteins, which, in turn, initiates a proteolytic cascade by recruiting and activating initiator caspases 8 and 10. Death receptors were once thought to primarily induce cytotoxic signaling cascades. However, recent data indicate that they initiate multiple signaling pathways, unveiling a number of nonapoptosis-related functions, including regulation of cell proliferation and differentiation, chemokine production, inflammatory responses, and tumor-promoting activities. These noncytotoxic cascades are not simply a manifestation of inhibiting proapoptotic pathways but are intrinsically regulated by adaptor protein and receptor internalization processes. Insights into these various death receptor signaling pathways provide new therapeutic strategies targeting these receptors in pathophysiological processes.—Guicciardi, M. E., Gores, G. J. Life and death by death receptors.
Keywords: Fas/CD95/APO1, TNF, TRAIL, Bcl-2 family, caspases, MAP kinases
The tumor necrosis factor (TNF) superfamily comprises a number of plasma membrane receptors with a significant homology in their extracellular domain, characterized by the presence of up to six cysteine-rich domains (CRD), which defines their ligand specificity. The members of this family are type-I transmembrane proteins with a C-terminal intracellular tail, a membrane-spanning region, and an extracellular ligand-binding N-terminal domain. Within this superfamily, a subset of receptors known as the death receptors are characterized by a ∼80 amino acid cytoplasmic sequence termed the death domain (DD), which is essential for apoptosis induction (1,2,3). The most extensively studied death receptors are Fas (CD95/APO-1), TNF-receptor 1 (TNF-R1/p55/CD120a), TNF-related apoptosis-inducing ligand receptor 1 [TRAIL-R1/Death Receptor 4 (DR4)], and receptor 2 (TRAIL-R2/DR5/APO-2/KILLER). Much less is known about two other DD-containing receptors, namely Death Receptors 3 (DR3/APO-3/TRAMP/WSL-1/LARD) and 6 (DR6), which are not potent inducers of apoptosis (4). Activation of Fas, TRAIL-R1, and TRAIL-R2 is often associated with cell death; however, TNF-R1 stimulation usually induces cytokine generation, inflammation, and cell survival, unless the TNF-R1 cytotoxic potential is unmasked by inhibiting these survival pathways.
Death receptors are activated by their cognate ligands, a group of complementary cytokines that belong to the TNF protein family. With the exception of the soluble, lymphocyte-derived cytokine LTα, these proteins (often referred to as death ligands) are mainly expressed as type-II transmembrane proteins, comprising an intracellular N-terminal domain, a transmembrane region, and a C-terminal extracellular tail. In some cases, death ligands can also be released as soluble cytokines by proteolytic cleavage, although the apoptosis-inducing capacity of the soluble forms is generally significantly lower compared to their corresponding membrane-bound forms (5,6,7,8).
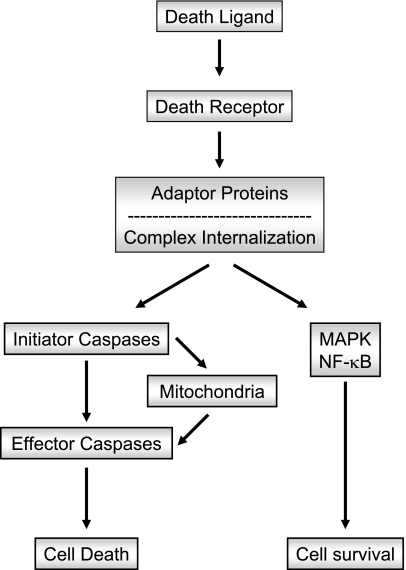
Cytotoxic signal transduction by death receptors proceeds through three general steps: 1) binding to the cognate ligand; 2) recruitment of adaptor/docking proteins, which, in turn, recruit the initiator caspases 8 and 10; and 3) discrete signaling pathways depending on the stoichiometry of the various adaptor proteins and caspases 8 and 10, and cellular internalization events (Fig. 1). Emerging information also implicates numerous noncytotoxic signaling pathways, mainly mediated by the activation of nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK), from the receptor/adaptor protein complexes (Fig. 1). The molecular toggles promoting cytotoxic vs. noncytotoxic signaling is the subject of this review. Each of these steps will be described in detail below.
Figure 1.
Schematic representation of death receptor signaling. Engagement of death receptors by their cognate ligands triggers the recruitment of different adaptor proteins. Distinct pathways originate from the adaptor proteins. A classic proapoptotic pathway is initiated when the adaptor proteins recruit large amounts of the initiator caspases 8 and 10, resulting in their autoactivation. Active caspases 8 and 10 initiate a signaling cascade that results in activation of the effector caspases (caspases 3, 6, and 7) either by directly processing the effector caspases themselves or by engaging the mitochondrial death pathway mediated by the cleavage of the BH3-only protein Bid. The release of proapoptotic proteins from the mitochondria, such as cytochrome c and Smac/DIABLO, ultimately promotes effector caspase activation and apoptosis. Several noncytotoxic signaling pathways can also originate from the association of adaptor proteins with the receptor, which generally results in the activation of MAPK- and/or NF-κB-mediated survival signals.
SHARED DEATH RECEPTOR SIGNALING PROCESSES
Initiation of signal transduction requires the oligomerization of the receptor and the juxtaposition of the intracellular domains (9). Receptor trimerization was initially thought to be triggered by the binding of the ligand in a trimeric form to the receptor’s extracellular domain. This concept has been challenged by the existence of preassembled receptor oligomers on the cell surface (10). The formation of these ligand-independent receptor complexes occurs through the interaction of the membrane-distal, first cysteine-rich domain of the extracellular tail of the receptors, a region termed the preligand assembly domain (PLAD) (11). Although PLAD is not directly involved in ligand binding, deletion of PLAD severely compromises ligand binding to TNFR-1, suggesting that preligand assembly of the receptors is a crucial step for the receptor-ligand interaction (11). Ligand-independent receptor complex formation has also been suggested for TRAIL and Fas receptors (12, 13).
Ligand binding causes a conformational change in the preassembled receptor, which leads to recruitment of different adaptor proteins [i.e., Fas-associated protein with death domain (FADD); TNF receptor-associated protein with death domain, (TRADD)] (Fig. 1). These proteins couple death receptor ligation to the activation of cell death effectors, namely, the initiator caspases (caspase 8 and caspase 10) and the long form of the cellular FLICE/caspase 8-like inhibitory protein (cFLIPL). Adaptor molecules are able to associate with the receptors through homotypic interaction of their DD with the receptor’s DD. They may also contain additional protein-protein interaction modules, such as death effector domains (DEDs) that mediate the recruitment of caspases and cFLIPL through their association with a corresponding DED. The resulting complex is termed the death-inducing signaling complex (DISC) and can generate an apoptotic signaling cascade initiated by the activated caspases. High local concentrations of the initiator caspases lead to their activation, but the mechanism by which this occurs is still debated. One hypothesis, referred to as the induced-proximity model, suggests that clustering of the caspases at the DISC results in their self-processing (14). A second model, the so-called proximity-induced dimerization, proposes that accumulation of initiator caspases at the DISC promotes their dimerization, which, in turn, results in their activation. According to this model, dimerization, and not cleavage, is required for initiator caspase activation, although processing of the caspases may increase the stability of the active form (15). Finally, the recently proposed induced-conformation model suggests that activation of the initiator caspases is achieved through a conformational change in their active site as a consequence of the interaction of the initiator caspase with the adaptor protein complex (16). Regardless of the mechanism of activation, following the autoproteolytic cleavage, the enzyme is released into the cytosol in an active heterotetrameric form containing two large and two small subunits, which, in turn, triggers a proteolytic cascade.
Activation of caspase 8 and 10 at the DISC can be regulated by cFLIP (17). Several c-FLIP variants are generated by alternative mRNA splicing, but only three of them are expressed at the protein level: the most abundant cFLIP long (cFLIPL), a cFLIP short (cFLIPS), and a short variant recently cloned from the Raji B-cell line (cFLIPR) (18). All of the isoforms have two death effector domains and can be recruited to the DISC through DED:DED interactions, but only the long variant also contains a caspase-like domain. Structurally, cFLIPL closely resembles caspase 8 but lacks the catalytic cysteine embedded in the conserved pentapeptide QACRG or QACQG motif and therefore has no cysteine protease activity. The role of cFLIPS in inhibiting death receptor-mediated apoptosis is well established. cFLIPS was shown to block caspase-8 processing and activation at the DISC, probably by competing for binding and recruitment to FADD. As cFLIPR structure closely resembles cFLIPS, it is likely that the two proteins inhibit death receptor-mediated apoptosis through similar mechanisms. On the contrary, the function of cFLIPL at the DISC remains controversial (19). Originally, similarly to cFLIPS, cFLIPL was described as an antiapoptotic molecule that inhibits death receptor-induced apoptosis by interfering with caspase 8 activation at the DISC (20, 21). Indeed, overexpression of cFLIPL results in recruitment of both cFLIPL and caspase 8 to the DISC, followed by defective processing of caspase 8, with the cleaved intermediates remaining bound to the DISC, and no generation of the active heterodimeric form (22). In addition, several reports have implicated cFLIPL in the activation of survival signaling pathways, such as NF-κB- and MAPK-regulated pathways, after death receptor-treatment, due to its ability to recruit adaptor proteins involved in these signaling pathways (23, 24). In contrast, cFLIPL may also have a proapoptotic function by forming caspase 8:cFLIPL heterodimers, which promote activation of caspase 8 at the DISC (25, 26). The hypothesis of a proapoptotic role of cFLIPL is strongly supported by the evidence that cFLIPL-deficient mice show the same phenotype as caspase 8- and FADD-deficient mice, with all three proteins required for embryonic development (20, 27, 28). Moreover, all three proteins are required for T-cell proliferation and survival of activated T and B cells (27, 29). These results suggest that FLIPL has a dual function, as either inhibitor or promoter of caspase activation, with its role being determined by a variety of factors, including its expression level and cellular levels relative to caspase 8 (19).
The amount of active caspase 8, and perhaps caspase 10, released into the cytosol likely determines the apoptosis signaling pathways initiated downstream of the DISC. Large amounts of active caspase 8 start a cascade of caspase activation by directly processing and activating the so-called effector caspases (e.g., caspase 3, caspase 6, and caspase 7), which then proceed to cleave and degrade several crucial cellular proteins. Alternatively, small amounts of caspase 8 depend on cleavage of the proapoptotic BH3-only protein Bid to induce cell death. This truncated carboxy-terminal fragment (tBid) translocates to the mitochondria, causing oligomerization of the proapoptotic, multidomain Bcl-2 proteins Bax or Bak, which, in turn, mediate the mitochondrial pathway of cell death (30,31,32,33).
In recent years, it has become evident that the nature of the signal transduction events originating from the death receptors is determined by the formation of spatially and temporally distinct receptor/adaptor complexes. Within these complexes, the receptor-interacting protein 1 (RIP1), a DD-containing serine/threonine kinase, plays a crucial role in switching between death and survival signaling. RIP1 binds to all death receptors, as well as to DD-containing adaptors like FADD and TRADD, via interaction with its own DD (34). Following post-translational modifications, RIP1 can initiate a death cascade or a survival signal. For example, a polyubiquitinated form of RIP1 is required for TNF-mediated NF-κB activation (35), and RIP1 phosphorylation is necessary for ERK activation (36), although not for NF-κB or p38 MAPK activation (37). On the contrary, deubiquitination of RIP1 results in enhanced formation of RIP1/FADD/caspase 8 complexes, and consequent caspase 8 activation and apoptosis (38). During death receptor-mediated apoptosis, RIP1 is cleaved and inactivated by caspase 8, providing a mechanism to silence RIP1-activated survival signaling (39). Finally, RIP1 may also act as a central initiator of Fas-mediated necrosis in cells lacking caspase 8 (40, 41).
Although the above molecules and pathways are common to all death receptors, their assembly and execution is unique to each death receptor and will now be reviewed in detail.
FAS AND FAS LIGAND
Fas is ubiquitously expressed but is particularly abundant in liver, heart, kidney, pancreas, brain, thymus, lymphoid tissues, and activated mature T lymphocytes. It is largely found bound to the plasma membrane, although soluble forms of the receptor lacking the transmembrane domain can result from alternative splicing (42). In vitro, these soluble forms of the receptor appear to have a negative regulatory effect on the membrane-bound Fas, likely due to a competitive binding to Fas ligand (FasL) (43). FasL is mainly expressed in preassociated homotrimeric structures on the plasma membrane of activated T cells (mFasL), but cleavage by a metalloprotease between Ser-126 and Leu127 in its extracellular domain can generate a soluble form whose biological activity remains controversial (sFasL) (5).
Cytotoxic signaling pathways
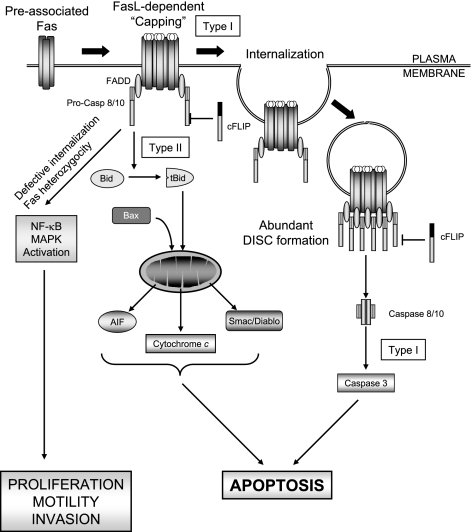
The complex early events in Fas signaling resulting in the activation of effector caspases have recently been elucidated (Fig. 2). Fas ligand stimulation induces the formation of SDS- and β-mercaptoethanol-stable microaggregates of preassociated receptors, which involves palmitoylation of the membrane proximal cysteine 199. Palmitoylation is the targeting signal for Fas localization to lipid rafts, and facilitates the subsequent internalization of the receptor and caspase 8 activation (44, 45). Only low levels of DISC are formed at this step. Subsequently, the receptor complexes organize into higher-order aggregates (46), which recruit FADD and move into lipid rafts to form microscopically visible signaling protein oligomerization structures (SPOTS) (47, 48). Effective homophilic interaction between FADD and the receptor is critical for the formation of SPOTS, as FADD-deficient cells or cells expressing a mutated Fas incapable of binding FADD are unable to form SPOTS. The function of SPOTS is to recruit procaspase 8 at sufficiently high levels to achieve full enzymatic activation. In a caspase 8-dependent manner, the receptor further aggregates to form large lipid raft platforms, a process referred to as receptor clustering or “capping” (48,49,50). The receptors are then internalized through clathrin-mediated endocytosis and delivered to the early endosomal compartment, where most of the DISC formation occurs. Both internalization of the receptor and localization to the endosomal compartment are required steps for efficient DISC assembly, amplification of the signal, and execution of apoptosis (51). It has recently been suggested that the components of the receptor-associated signaling complex comprising the DED-containing proteins FADD, c-FLIP, and procaspase 8, may be released from the receptor to form a secondary cytosolic complex, or complex II (52). The function of this newly identified complex II is likely to enhance caspase 8 activation. The formation of complex II does not depend on receptor internalization, as Fas is not coimmunoprecipitated in the complex. Therefore, internalization of the receptor and formation of the cytosolic complex II appear to be independent pathways both aimed to amplify caspase 8 activation.
Figure 2.
Fas/CD95-mediated signaling pathways. Model of Fas/CD95-mediated cytotoxic and noncytotoxic pathways. See text for details.
The signal downstream of DISC formation, leading to the activation of the effector caspases 3 and 7, proceeds via two different pathways (53, 54) (Fig. 2). In the so-called type I cells, relatively large amounts of DISC are rapidly assembled and internalized, and caspase 8 is mainly activated at the DISC. Caspase 8 then directly cleaves and activates caspase 3. In these cells, Fas is associated with membrane rafts, which favors its internalization (48, 55). Colocalization of Fas with lipid rafts is a crucial step, as disruption of lipid raft structure by cholesterol depletion inhibits Fas-mediated apoptosis in type I cells (56). Overexpression of the antiapoptotic proteins Bcl-2 or Bcl-XL does not prevent activation of caspase 8 or caspase 3, nor does it inhibit apoptosis, suggesting mitochondria are not required for caspase activation. In contrast, DISC formation in the so-called type II cells is delayed and less effective. After stimulation, Fas does not fully colocalize with lipid rafts and is not efficiently internalized (50). Activation of caspases, including caspase 8, requires engagement of mitochondria, as both caspase activation and apoptosis can be blocked by overexpression of Bcl-2 or Bcl-XL. Notably, Fas triggers mitochondria dysfunction in both type I and type II cells, but only in type II cells does overexpression of Bcl-XL or Bcl-2 block apoptosis. Thus, in type II cells, mitochondria are essential for execution of the apoptotic program, whereas in type I cells mitochondrial dysfunction likely functions as an amplifier of the apoptotic signal (57). Mitochondrial dysfunction during Fas signaling is triggered by caspase 8-mediated cleavage of Bid to generate tBid which, in turn, triggers the mitochondrial pathway of cell death (30). Although the definition of type I and type II cells originally referred to the Fas signaling pathway, a large body of work now suggests that the same distinction may apply to other death receptors as well.
Noncytotoxic signaling pathways
In many cell types and tissues, Fas is not cytotoxic but rather promotes cell proliferation, migration, and cytokine generations (58, 59). Only recently have these nonapoptotic signaling cascades been appreciated. For example, Fas activation of extracellular signal-regulated kinase (ERK) signaling is essential for neuronal regeneration (60) and stimulates proliferation of quiescent hepatic stellate cells after liver injury (61). Fas is also known to promote liver regeneration following partial hepatectomy (62). Hepatic resection causes the activation of antiapoptotic signaling pathways, such as Akt, STAT3, and NF-κB (63,64,65), as well as a change in expression profiles of Bcl-2 family members that increases resistance to apoptosis (66). Therefore, Fas treatment of apoptosis-resistant hepatocytes may result in a switch of Fas-mediated signals from apoptotic to proliferative. In addition, Fas enhances proliferation of T-cell receptor (TCR)-stimulated T cells and thymocytes via a caspase-dependent, FADD-mediated pathway (67, 68). Because of its pivotal role in the killing of tumor cells by tumor-infiltrating lymphocytes, Fas has been long viewed as a tumor suppressor. Inactivating mutations in the Fas DD are, indeed, very frequent in human tumors, and are associated with increased resistance to Fas-mediated apoptosis. Moreover, germline and somatic mutations of the Fas gene are associated with a high risk to develop both lymphoid and solid tumors in humans and mice (69, 70). However, recent studies indicate that in tumor cells with disabling Fas mutations, FasL stimulation triggers the activation of nonapoptotic pathways, such as NF-κB and MAP kinase pathways, which result in the induction of tumorigenic or prosurvival genes (51, 71) (Fig. 2). Gene chip analyses have identified a large number of potential candidate genes that are transcriptionally up-regulated by FasL in these cell lines, among them urokinase plasminogen activator (uPA) and the serine/threonine protein kinase SNARK, both promoting FasL-induced motility and invasiveness (58). Moreover, Fas has been shown to facilitate basal invasion of apoptosis-resistant glioblastoma in vivo via activation of phosphatidylinositol 3-kinase (PI3K). Binding of FasL to the receptor results in recruitment of the Src family member Yes and the p85 PI3K subunit, leading to PI3K activation. Activated PI3K, in turn, inhibits glycogen synthase kinase 3-β (GSK3β) and induces expression of matrix metalloproteinases (MMPs), resulting in increased invasiveness (72). Interestingly, although a large number of tumors have congenital or somatic mutations in the Fas death domain, they very rarely display loss of heterozygocity, suggesting that maintaining one wild-type receptor may confer an oncogenic advantage (73). This advantage is likely due to the fact that Fas-induced apoptosis requires two functional Fas alleles in order to ensure efficient DISC formation (74). In contrast, the signaling threshold to activate NF-κB is significantly lower and can be achieved even in the presence of only one functional Fas allele (74). Indeed, when internalization of the receptor is impaired and DISC formation is reduced (i.e., in caspase 8-defective tumor cells), the receptor aggregates activate prosurvival signals such as NF-κB- and ERK-mediated pathways, demonstrating that compartmentalization of Fas signal is critical to determine the cellular outcome after Fas stimulation in many cells (51) (Fig. 2). Recent studies have identified a conserved extracellular glycosphingolipid-binding motif (GBM) on Fas as one of the regulatory elements in the selection of the receptor internalization route. Loss-of-function mutations of GBM result in defective DISC formation and cause switching from clathrin-mediated receptor endocytosis to a clathrin-independent internalization process, which potentiates Fas nondeath signals (75). The mechanisms by which the receptor activates these prosurvival pathways, however, have not been fully elucidated.
TRAIL RECEPTORS AND TRAIL
TNF-related apoptosis-inducing ligand (TRAIL)/APO2L triggers apoptosis on binding to one of its two cognate death receptors, TRAIL-R1 (DR4) and TRAIL-R2 (DR5) (76, 77). TRAIL-R1 is expressed in most human tissues, including spleen, thymus, liver, peripheral blood leukocytes, activated T cells, small intestine, and some tumor cell lines (78). TRAIL-R2 expression has a ubiquitous distribution both in normal tissue and tumor cell lines, but it is particularly abundant in spleen, peripheral blood leukocytes, and activated lymphocytes. Studies in TRAIL receptor-deficient mice (mice express only one TRAIL receptor) support the role of TRAIL-R as an inflammation and metastasis suppressor in multiple tissues in vivo (79, 80). Indeed, TRAIL-R-knockout mice display increased susceptibility to metastasis from lymphoma and diethylnitrosamine (DEN)-induced liver tumors (81, 82). TRAIL can also bind to two other receptors, the so-called decoy receptors TRAIL-R3 (DcR1 or TRID or LIT) and TRAIL-R4 (DcR2/TRUNDD) (83, 84). The decoy receptors resemble TRAIL-R1 and TRAIL-R2 in their extracellular and transmembrane regions; however, TRAIL-R3 lacks the entire intracellular domain, including the death domain, and is a glycosylphosphatidyl-inositol (GPI)-linked plasma membrane receptor, and TRAIL-R4 possesses a truncated, dysfunctional death domain. Therefore, binding of TRAIL to these receptors fails to trigger apoptosis and can antagonize the apoptotic signal through TRAIL-R1 and TRAIL-R2. Indeed, transient overexpression of TRAIL-R3 or TRAIL-R4 prevents TRAIL-induced apoptosis in several normal and cancer cells (12, 84, 85). Recent studies suggest TRAIL-R3 and TRAIL-R4 inhibit TRAIL-induced apoptosis by distinct mechanisms. TRAIL-R3 localizes within the lipid rafts, where it prevents the formation of TRAIL-R2-associated DISC formation (86). On the contrary, TRAIL-R4 is recruited to the TRAIL-R2 DISC through binding to TRAIL, inhibiting proper recruitment, processing, and activation of initiator caspases within the DISC (86). TRAIL-R4 has also been suggested to inhibit TRAIL-mediated apoptosis via activation of NF-κB (84). TRAIL also binds to a fifth receptor, the soluble osteoprotegerin receptor (OPG). However, TRAIL binding affinity for this receptor is weak at physiological temperatures (87).
TRAIL is constitutively present in many tissues at the level of mRNA, but it is expressed mainly by cells of the immune system, especially natural killer (NK) cells, natural killer T (NKT) cells, and macrophages. This ligand is synthesized as a type II transmembrane protein that can also be proteolytically cleaved by a cysteine protease to generate a soluble form (88). It is biologically active as a homotrimer, with the cysteine residues in position 230 coordinating a zinc ion, essential for proper folding, trimer association, and activity of the cytokine itself (89, 90). TRAIL plays a crucial role in maintaining T-cell homeostasis, as well as in killing of tumor and virally transformed cells by NK and NKT cells (91,92,93). Over the years, TRAIL has generated considerable interest among clinicians for its preferential toxicity toward transformed cells and tumor xenografts, with generally little or no toxicity to normal tissues, which makes it an ideal candidate for cancer therapy (94,95,96,97,98). Despite intensive studies, the reason for this differential sensitivity has not yet been fully clarified. Preclinical studies have established that using a nontagged, soluble zinc-replete form of TRAIL is effective, nontoxic on various organs, including the liver, and, therefore, suitable for investigations on human subjects (96, 99, 100). As a result, recombinant TRAIL, as well as agonistic antibodies against TRAIL receptors, are currently in Phase I/II clinical trials for treatment of solid tumors and hematological malignancies (101).
Cytotoxic signaling pathways
TRAIL binds to TRAIL-R1 and TRAIL-R2 with nanomolar affinity, and both receptors are often expressed simultaneously on the same cell. Nonetheless, generally TRAIL-R2 seems to play a more important role than TRAIL-R1 in triggering apoptosis in cells expressing both receptors (102). Why humans have evolved to express two TRAIL receptors and the potential differences in their signaling cascades has yet to be explained. To date, they are assumed to signal in a similar manner, although there are likely some distinctions. Engagement of TRAIL-R1 and TRAIL-R2 results in recruitment of FADD, caspase 8 and caspase 10 to their respective DISCs, and initiation of a caspase cascade, similarly to what occurs when Fas is activated (103) (Fig. 3). However, unlike Fas, which have been shown to depend on receptor endocytosis to induce apoptosis, TRAIL-R1 and TRAIL-R2 do not require internalization for the formation of the DISC and the transmission of an apoptotic signal in type I cells (104). Indeed, despite ligand-engaged TRAIL-R1 and TRAIL-R2 being rapidly internalized via both clathrin-dependent and clathrin-independent pathways, inhibition of endocytosis does not prevent recruitment of FADD and caspase 8, or apoptosis. On the contrary, internalization of the receptor seems to be essential for TRAIL-induced apoptosis in hepatocytes, a classic example of type II cells (unpublished results). Therefore, it appears that type I and type II cells have opposite requirements for receptor internalization during TRAIL- and Fas-induced cell death. Hepatocytes also heavily rely on lysosomal permeabilization to mediate TRAIL killing, and perhaps trafficking of receptor-bound DISC-containing vesicles to the lysosomes may be required to initiate the lysosomal pathway. On the other hand, Fas-mediated hepatocyte apoptosis does not involve lysosomal permeabilization, which, in turn, may explain the lack of receptor internalization during Fas-mediated apoptosis in these type II cells. A post-translational modification, O-glycosylation of the TRAIL receptors, increases TRAIL-sensitivity by promoting ligand-induced receptor clustering and consequent caspase 8 activation. Elevated expression of O-glycosyltransferases, the enzymes responsible for O-glycosylation of the receptors, correlates with sensitivity to TRAIL-mediated apoptosis in a large number of tumor cell lines, and represent a potential new biomarker to screen cancer patients for TRAIL-based clinical trials (105).
Figure 3.
TRAIL-R1/2-mediated signaling pathways. Model of TRAIL-R1/2 signaling and the formation of signaling complexes triggered by engagement of TRAIL to TRAIL receptors. See text for details.
Noncytotoxic signaling pathways
In addition to inducing cell death, TRAIL promotes activation of prosurvival proteins such as NF-κB, protein kinase B (PBK)/Akt, and MAP kinases through distinct, independent pathways. In particular, TRAIL-R1, TRAIL-R2, and TRAIL-R4 have been shown to activate NF-κB via a TRAF-2 (TNF-associated factor 2)-NIK (NF-κB-inducing kinase)-IKK complex (IκB kinase complex)-dependent signaling cascade, whereas TRAIL-R1 induces JNK (c-Jun NH2-terminal kinase)/SAPK (stress-activated protein kinase) activation via a TRAF-2-MEKK1 (mitogen-activated protein/ERK kinase kinase 1)-MKK4 (mitogen-activated protein/ERK kinase 4)-dependent pathway (106). This suggests a bifurcation in the signaling pathway at the level of TRAF-2 similar to that described for TNF-R1 (Fig. 4). Interestingly, activation of NF-κB is not sufficient to block TRAIL-induced apoptosis in all cell types (107, 108). To explain this apparent paradox, it has been proposed that TRAIL-induced activation of NF-κB and MAP kinase pathways is mediated by the delayed formation of a secondary intracellular signaling complex that follows the assembly of the primary apoptosis-inducing DISC (Fig. 3). Ligand engagement of TRAIL-R2 quickly leads to recruitment of FADD and caspase 8 to the receptor, forming the membrane-bound, primary complex (DISC) that signals apoptosis. Subsequently, the primary complex dissociates and multiple proteins, including RIP1, TRAF2, IKKγ, and TRADD, organize into a secondary complex that lacks the ligand and the receptor, but contains FADD and caspase 8. This secondary complex is responsible for activation of NF-κB and MAP kinase pathways, such as JNK and p38 (109). However, sucrose gradient techniques have not been employed to conclusively prove this proposition. Furthermore, in the absence of c-IAP1/2 (cellular inhibitor of apoptosis 1/2), death receptors such as TNF-R1 (vide infra) can signal cell death despite NF-κB activation (110). The role of c-IAP1/2 in TRAIL receptor signaling has not been examined but may explain why NF-κB activation may not prevent apoptosis in many cell lines. TRAIL has also been shown to stimulate the activation of ERK, which is generally associated with antiapoptotic functions. Consistently, ERK activation inhibits TRAIL-mediated apoptosis by suppressing caspase 8 activation and Bid cleavage (111, 112), and, conversely, inhibition of ERK phosphorylation sensitizes cells to TRAIL-induced apoptosis (113, 114).
Figure 4.
TNF-R1-mediated signaling pathways. Model of TNF-R1 signaling and the formation of signaling complexes triggered by engagement of TNF-α to TNF-R1. See text for details.
The activation of noncytotoxic pathways has great clinical relevance in light of the possible use of TRAIL as an anticancer drug. For example, in apoptosis-resistant cells, TRAIL may actually promote oncogenic features, such as tumor metastasis and invasion (115, 116), due to NF-κB-dependent production of proinflammatory cytokines (117). TRAIL-induced NF-κB activation has been reported in several tumor cells, including cholangiocarcinoma, hepatocellular carcinoma and colon carcinoma cell lines (108, 115, 118). Moreover, recent studies indicate TRAIL expression is enhanced in primary sclerosing cholangitis, an inflammatory disease of the bile duct, which predisposes the patient to the development of cholangiocarcinoma (115), suggesting that TRAIL may also be involved in NF-κB-driven neoplasia development and progression. Finally, TRAIL has also been reported to potently promote angiogenesis by increasing migration, invasion, proliferation, and differentiation of human endothelial cells. This function is mediated by rapid activation of ERK but appears to be independent of NF-κB (119). These observations, together with the evidence that TRAIL is often overexpressed in tumor cells (119,120,121,122,123,124), strongly point to a role for TRAIL in cancer biology. Thus, TRAIL is strongly involved in the development of the malignant phenotype, with a role in almost all oncogenic features, from proliferation to invasion and metastasis, to tumor angiogenesis.
TNF-R1 AND TNF-α
The TNF/TNF-receptor signaling system consists of two distinct receptors, TNF-R1 and TNF-R2 (p75/CD120b), and three ligands, the membrane-bound TNF-α (mTNF-α), the soluble TNF-α (sTNF-α), and the soluble lymphocyte-derived cytokine (LTα/TNF-β) (125). However, only TNF-R1 is considered a canonical death receptor, as TNR-R2 does not possess an intracellular death domain. TNF-R1 is ubiquitously expressed at constitutively low levels and controlled by a noninducible promoter. Although TNF-R1 and TNF-R2 interact with both forms of TNF-α, as well as with LTα, TNF-R1 appears to be largely responsible for TNF signaling in most cell types. Studies in TNF-R1-deficient mice indicate TNF-R1 is essential for TNF-induced apoptosis of pathogen-infected cells (126, 127).
Tumor necrosis factor-α (TNF-α) plays a key role in inflammation and immunity, as well as in proliferation and differentiation of many different target cells (128). TNF-α is mainly produced by macrophages, monocytes, and T cells in response to infection and inflammatory conditions, but also by other cell types, such as B cells, fibroblasts, and hepatocytes. Both soluble and membrane-bound TNF-α are biologically active, and while the soluble form acts as an effector molecule at a distance from the producer cell, the membrane-bound form likely has a specific role in localized TNF-α responses.
Noncytotoxic signaling pathways
Unlike Fas and TRAIL receptors 1/2, TNF-R1 is primarily involved in mediating inflammation and not cell death. Engagement of TNF-R1 leads to the formation of two subsequent signaling complexes. The initial complexes regulate the expression of antiapoptotic proteins that prevent cell death, whereas the later complexes trigger cell death (Fig. 4) (129). Within minutes, engagement of TNF-R1 by TNF-α induces recruitment of RIP1, TRAF2, and c-IAP1/2 to the receptor to form the membrane-bound complex I (129, 130). The presence of TRADD in this complex is controversial (129, 131). Assembly of complex I requires translocation of TNF-R1 to lipid rafts, where several proteins in the complex undergo post-translational modifications (132). In particular, RIP1 is polyubiquitinated at Lys377 by cIAP-1 and cIAP-2, which act as E3 ligase, a step required for activation of NF-κB (35, 133). These lysine 63-linked polyubiquitin chains of RIP1 are essential for the recruitment of TAK1 and IKK complexes to the TNR receptor complex (35). Two distinct pathways originate from the association of RIP1 and TRAF-2 to the receptor, whose balance determines the ultimate fate of the cell in response to TNF-α (134). The first one promotes survival and signals through binding of the protein kinase TAK1 via interaction of the TAK1 complex, consisting of the TAK1 kinase and its associated proteins TAK1-binding protein 1/2/3 (TAB1, TAB2, and TAB3) with RIP1 lysine 63-linked polyubiquitin chains, resulting in TAK1 kinase phosphorylation and activation (35). TAK1 proceeds to activate the catalytic IKK complex, comprising the three proteins IKKα (IKK1), IKKβ (IKK2), and IKKγ (NEMO), leading to phosphorylation of the NF-κB inhibitory protein IκBα. Phospho-IκBα is then degraded via the ubiquitin-proteasome pathway, allowing NF-κB to translocate to the nucleus and initiate transcription of antiapoptotic target genes, such as cFLIP, cIAP-1, cIAP-2, TRAF1, and TRAF2 (135). Thus, post-translational modifications of RIP1 and the nature of the polyubiquitination chains dictate its survival signaling cascade.
Cytotoxic signaling pathways
The second pathway originating from the association of RIP1 and TRAF-2 with the receptor leads to activation of JNK via the consequential activation of MEKK-1 and JNKK (JNK kinase). JNK phosphorylates and activates c-Jun, a member of the transcription factor complex termed activator protein-1 (AP-1). Transient JNK prodeath stimuli are normally inhibited by NF-κB-regulated genes, such as xiap, which encodes the endogenous inhibitor of apoptosis, XIAP, and promotes cell survival (136). However, JNK-sustained activation mediates phosphorylation and activation of the E3 ubiquitin ligase Itch, which specifically ubiquitinates c-FLIP and promotes its proteasomal degradation, resulting in enhanced caspase 8 activation and apoptosis (137).
Following the transient formation of complex I, the receptor is internalized by endocytosis (138). It was first suggested that RIP1, TRAF2, and perhaps TRADD dissociated from TNF-R1 during endocytosis, allowing the DD of RIP1 to interact with the DD of FADD, which, in turn, recruited caspase 8 and 10. The newly formed cytosolic complex (named complex II) containing TRADD, TRAF2, RIP1, FADD, and caspase 8/10, promoted the activation of caspase 8/10, initiating the apoptotic cascade (129). The composition of this cytosolic complex, however, has been questioned after analysis of TNF-R1-containing endocytic vesicles (named TNF receptosomes) isolated by magnetic sorting. Using this sophisticated technique, Schneider-Brachert et al. (131) suggest FADD and caspase 8/10 are recruited during endocytosis of the receptor to the TNF receptosomes to form a cytosolic TNF-R1-associated DISC (Fig. 4). Regardless of its composition, this cytosolic complex is temporally and spatially distinct from complex I. In TNF-sensitive cells, caspase 8 and 10 are abundantly recruited to the cytosolic complex. On the contrary, in TNF-resistant cells, activation of initiator caspases in the TNF receptosomes is reduced by complex I-driven, NF-κB-dependent cFLIP expression. Indeed, inhibition of NF-κB, or impairment of RNA or protein synthesis, sensitizes cells to TNF-α-induced apoptosis (139). Therefore, the temporal delay in the assembly of the two complexes provides a checkpoint to control cell death. The assembly of complex II is critically dependent on receptor endocytosis, which is regulated by a cytoplasmic region between aa 205 and aa 214 named TNF-R1 internalization domain (TRID) (131). In the absence of a functional TRID, stimulation with TNF-α still triggers a low level of caspase 3 activation, although the receptor is retained at the cell surface and no functional DISC is formed. However, to explain cell death from plasma membrane-associated complexes, it has been suggested that, over time, persistence of the receptor at the plasma membrane increases its interaction with factor associated with neutral sphingomyelinase (FAN), resulting in overactivation of neutral sphingomyelinase (N-SMase) (140), which, in turn, is able to activate caspase 3 (141).
In addition to blocking the NF-κB transcriptional activity, TNF signaling can also be switched from prosurvival to pro-cell death in transformed cells by simultaneous use of a Smac mimetic, a small molecule designed to resemble the N terminus of Smac that interacts with and inhibits XIAP, cIAP-1, and cIAP-2 (110, 142). Interestingly, although both the protein synthesis inhibitor cycloheximide and the Smac mimetic promote caspase 8 activation, the pathways involved appear to be different. Indeed, cycloheximide stimulates TNF-α-induced caspase 8 activation mainly by blocking NF-κB-mediated neosynthesis of cFLIP (Fig. 5A), whereas the Smac mimetic binds to cIAP-1 and cIAP-2 and activates their E3 ubiquitin ligase activity, resulting in their autoubiquitination and proteasomal degradation. This leads to deubiquitination and release of RIP1 from the receptor-associated complex I, which is then able to recruit FADD and provide a platform for caspase 8 activation (143) (Fig. 5B). Indeed, RIP1 ubiquitination status appears to determine its function, switching from a prosurvival molecule in a receptor-bound, polyubiquitinated form to a proapoptotic, caspase 8-activating molecule when deubiquitinated (38). Consistently, it has recently been shown that cIAP-1and cIAP-2 directly ubiquitinate RIP1 and induce constitutive RIP1 polyubiquitination in cancer cells, therefore sustaining cancer cell proliferation (144).
Figure 5.
Protein synthesis inhibitors and Smac mimetics sensitize cells to TNF-mediated apoptosis via two different pathways of caspase 8 activation. Schematic representation of molecular mechanisms of sensitization to TNF-mediated apoptosis by cycloheximide (A) and Smac mimetics (B). Cycloheximide blocks NF-κB-mediated transcription of cFLIP, promoting caspase 8 activation and apoptosis. Smac mimetics trigger proteasomal degradation of cIAP-1 and cIAP-2, preventing cIAP-1/2-mediated ubiquitination of RIP1. Deubiquitinated RIP1 is released from the receptor complex into the cytosol, where it recruits FADD and caspase 8, enhancing caspase 8 activation.
CONCLUSIONS
Death receptors play a major role in maintaining tissue homeostasis through regulation of cell death and survival, as demonstrated by the apparent redundancy of their signaling pathways. The intensive study of death receptors over the past decade has shed a new light on the physiological and pathological functions of these molecules and have widened the array of identified responses elicited by these receptors beyond cell death. Death receptor signaling is now recognized as a dynamic process that integrates different events, which can generate diverse outcomes. Detailed analyses of the assembly of the death receptor complexes have now demonstrated that molecular events, such as receptor translocation and subcellular localization, and post-translational modifications of the receptor complex components, play a more critical role in determining cell fate. As we continue to study these fascinating molecules, it is likely that more interesting receptor biology will be discovered.
Acknowledgments
This work was supported by grants from the U.S. National Institutes of Health (DK63947 to G.J.G.) and the Mayo Foundation, Rochester, Minnesota, USA.
References
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Locksley R M, Killeen N, Lenardo M J. The TNF and TNF receptor superfamily: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Wajant H. Death receptors. Essays Biochem. 2003;39:53–71. doi: 10.1042/bse0390053. [DOI] [PubMed] [Google Scholar]
- Schneider P, Holler N, Bodmer J L, Hahne M, Frei K, Fontana A, Tschopp J. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med. 1998;187:1205–1213. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shudo K, Kinoshita K, Imamura R, Fan H, Hasumoto K, Tanaka M, Nagata S, Suda T. The membrane-bound but not the soluble form of human Fas ligand is responsible for its inflammatory activity. Eur J Immunol. 2001;31:2504–2511. doi: 10.1002/1521-4141(200108)31:8<2504::aid-immu2504>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Wajant H, Moosmayer D, Wuest T, Bartke T, Gerlach E, Schonherr U, Peters N, Scheurich P, Pfizenmaier K. Differential activation of TRAIL-R1 and -2 by soluble and membrane TRAIL allows selective surface antigen-directed activation of TRAIL-R2 by a soluble TRAIL derivative. Oncogene. 2001;20:4101–4106. doi: 10.1038/sj.onc.1204558. [DOI] [PubMed] [Google Scholar]
- Grell M, Wajant H, Zimmermann G, Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci U S A. 1998;95:570–575. doi: 10.1073/pnas.95.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldin M P, Mett I L, Varfolomeev E E, Chumakov I, Shemer-Avni Y, Camonis J H, Wallach D. Self-association of the “death domains” of the p55 tumor necrosis factor (TNF) receptor and Fas/APO1 prompts signaling for TNF and Fas/APO1 effects. J Biol Chem. 1995;270:387–391. doi: 10.1074/jbc.270.1.387. [DOI] [PubMed] [Google Scholar]
- Chan K F, Siegel M R, Lenardo J M. Signaling by the TNF receptor superfamily and T cell homeostasis. Immunity. 2000;13:419–422. doi: 10.1016/s1074-7613(00)00041-8. [DOI] [PubMed] [Google Scholar]
- Chan F K, Chun H J, Zheng L, Siegel R M, Bui K L, Lenardo M J. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 2000;288:2351–2354. doi: 10.1126/science.288.5475.2351. [DOI] [PubMed] [Google Scholar]
- Clancy L, Mruk K, Archer K, Woelfel M, Mongkolsapaya J, Screaton G, Lenardo M J, Chan F K. Preligand assembly domain-mediated ligand-independent association between TRAIL receptor 4 (TR4) and TR2 regulates TRAIL-induced apoptosis. Proc Natl Acad Sci U S A. 2005;102:18099–18104. doi: 10.1073/pnas.0507329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R M, Frederiksen J K, Zacharias D A, Chan F K, Johnson M, Lynch D, Tsien R Y, Lenardo M J. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science. 2000;288:2354–2357. doi: 10.1126/science.288.5475.2354. [DOI] [PubMed] [Google Scholar]
- Salvesen G S, Dixit V M. Caspase activation: the induced-proximity model. Proc Natl Acad Sci U S A. 1999;96:10964–10967. doi: 10.1073/pnas.96.20.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. Caspase activation: revisiting the induced proximity model. Cell. 2004;117:855–858. doi: 10.1016/j.cell.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Chao Y, Shiozaki E N, Srinivasula S M, Rigotti D J, Fairman R, Shi Y. Engineering a dimeric caspase-9: a re-evaluation of the induced proximity model for caspase activation. [Online] PLoS Biol. 2005;3:e183. doi: 10.1371/journal.pbio.0030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J, Irmler M, Thome M. Inhibition of Fas death signals by FLIPs. Curr Opin Immunol. 1998;10:552–558. doi: 10.1016/s0952-7915(98)80223-9. [DOI] [PubMed] [Google Scholar]
- Golks A, Brenner D, Fritsch C, Krammer P H, Lavrik I N. c-FLIPR, a new regulator of death receptor-induced apoptosis. J Biol Chem. 2005;280:14507–14513. doi: 10.1074/jbc.M414425200. [DOI] [PubMed] [Google Scholar]
- Chang D W, Xing Z, Pan Y, Algeciras-Schimnich A, Barnhart B C, Yaish-Ohad S, Peter M E, Yang X. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 2002;21:3704–3714. doi: 10.1093/emboj/cdf356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasper D M, Vaillancourt J P, Hadano S, Houtzager V M, Seiden I, Keen S L, Tawa P, Xanthoudakis S, Nasir J, Martindale D, Koop B F, Peterson E P, Thornberry N A, Huang J, MacPherson D P, Black S C, Hornung F, Lenardo M J, Hayden M R, Roy S, Nicholson D W. Cell death attenuation by ‘Usurpin’, a mammalian DED-caspase homologue that precludes caspase-8 recruitment and activation by the CD-95 (Fas, APO-1) receptor complex. Cell Death Differ. 1998;5:271–288. doi: 10.1038/sj.cdd.4400370. [DOI] [PubMed] [Google Scholar]
- Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer J L, Schroter M, Burns K, Mattmann C, Rimoldi D, French L E, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Krueger A, Baumann S, Krammer P H, Kirchhoff S. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol Cell Biol. 2001;21:8247–8254. doi: 10.1128/MCB.21.24.8247-8254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W H, Johnson H, Shu H B. Activation of NF-κB by FADD, Casper, and Caspase-8. J Biol Chem. 2000;275:10838–10844. doi: 10.1074/jbc.275.15.10838. [DOI] [PubMed] [Google Scholar]
- Kataoka T, Budd R C, Holler N, Thome M, Martinon F, Irmler M, Burns K, Hahne M, Kennedy N, Kovacsovics M, Tschopp J. The caspase-8 inhibitor FLIP promotes activation of NF-κB and Erk signaling pathways. Curr Biol. 2000;10:640–648. doi: 10.1016/s0960-9822(00)00512-1. [DOI] [PubMed] [Google Scholar]
- Micheau O, Thome M, Schneider P, Holler N, Tschopp J, Nicholson D W, Briand C, Grutter M G. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem. 2002;277:45162–45171. doi: 10.1074/jbc.M206882200. [DOI] [PubMed] [Google Scholar]
- Dohrman A, Russell J Q, Cuenin S, Fortner K, Tschopp J, Budd R C. Cellular FLIP long form augments caspase activity and death of T cells through heterodimerization with and activation of caspase-8. J Immunol. 2005;175:311–318. doi: 10.4049/jimmunol.175.1.311. [DOI] [PubMed] [Google Scholar]
- Yeh W C, Itie A, Elia A J, Ng M, Shu H B, Wakeham A, Mirtsos C, Suzuki N, Bonnard M, Goeddel D V, Mak T W. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12:633–642. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- Yeh W C, Pompa J L, McCurrach M E, Shu H B, Elia A J, Shahinian A, Ng M, Wakeham A, Khoo W, Mitchell K, El-Deiry W S, Lowe S W, Goeddel D V, Mak T W. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- Peter M E, Krammer P H. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu C J, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- Milhas D, Cuvillier O, Therville N, Clave P, Thomsen M, Levade T, Benoist H, Segui B. Caspase-10 triggers Bid cleavage and caspase cascade activation in FasL-induced apoptosis. J Biol Chem. 2005;280:19836–19842. doi: 10.1074/jbc.M414358200. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S J, Wei M C, Saito M, Weiler S, Oh K J, Schlesinger P H. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- Meylan E, Tschopp J. The RIP kinases: crucial integrators of cellular stress. Trends Biochem Sci. 2005;30:151–159. doi: 10.1016/j.tibs.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Ea C K, Deng L, Xia Z P, Pineda G, Chen Z J. Activation of IKK by TNFα requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22:245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Devin A, Lin Y, Liu Z G. The role of the death-domain kinase RIP in tumour-necrosis-factor-induced activation of mitogen-activated protein kinases. EMBO Rep. 2003;4:623–627. doi: 10.1038/sj.embor.embor854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T H, Shank J, Cusson N, Kelliher M A. The kinase activity of Rip1 is not required for tumor necrosis factor-alpha-induced IκB kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. J Biol Chem. 2004;279:33185–33191. doi: 10.1074/jbc.M404206200. [DOI] [PubMed] [Google Scholar]
- O'Donnell M A, Legarda-Addison D, Skountzos P, Yeh W C, Ting A T. Ubiquitination of RIP1 regulates an NF-κB-independent cell-death switch in TNF signaling. Curr Biol. 2007;17:418–424. doi: 10.1016/j.cub.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Devin A, Rodriguez Y, Liu Z G. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell’s decision to live or die. Cell Death Differ. 2007;14:400–410. doi: 10.1038/sj.cdd.4402085. [DOI] [PubMed] [Google Scholar]
- Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer J L, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- Cascino I, Fiucci G, Papoff G, Ruberti G. Three functional soluble forms of the human apoptosis-inducing Fas molecule are produced by alternative splicing. J Immunol. 1995;154:2706–2713. [PubMed] [Google Scholar]
- Cheng J, Zhou T, Liu C, Shapiro J P, Brauer M J, Kiefer M C, Barr P J, Mountz J D. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science. 1994;263:1759–1762. doi: 10.1126/science.7510905. [DOI] [PubMed] [Google Scholar]
- Chakrabandhu K, Herincs Z, Huault S, Dost B, Peng L, Conchonaud F, Marguet D, He H T, Hueber A O. Palmitoylation is required for efficient Fas cell death signaling. EMBO J. 2007;26:209–220. doi: 10.1038/sj.emboj.7601456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig C, Tchikov V, Schutze S, Peter M E. Palmitoylation of CD95 facilitates formation of SDS-stable receptor aggregates that initiate apoptosis signaling. EMBO J. 2007;26:221–231. doi: 10.1038/sj.emboj.7601460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkler F, Behrle E, Dennehy K M, Wicovsky A, Peters N, Warnke C, Pfizenmaier K, Wajant H. The extracellular domains of FasL and Fas are sufficient for the formation of supramolecular FasL-Fas clusters of high stability. J Cell Biol. 2005;168:1087–1098. doi: 10.1083/jcb.200501048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R M, Muppidi J R, Sarker M, Lobito A, Jen M, Martin D, Straus S E, Lenardo M J. SPOTS: signaling protein oligomeric transduction structures are early mediators of death receptor-induced apoptosis at the plasma membrane. J Cell Biol. 2004;167:735–744. doi: 10.1083/jcb.200406101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legembre P, Daburon S, Moreau P, Moreau J F, Taupin J L. Modulation of Fas-mediated apoptosis by lipid rafts in T lymphocytes. J Immunol. 2006;176:716–720. doi: 10.4049/jimmunol.176.2.716. [DOI] [PubMed] [Google Scholar]
- Algeciras-Schimnich A, Shen L, Barnhart B C, Murmann A E, Burkhardt J K, Peter M E. Molecular ordering of the initial signaling events of CD95. Mol Cell Biol. 2002;22:207–220. doi: 10.1128/MCB.22.1.207-220.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eramo A, Sargiacomo M, Ricci-Vitiani L, Todaro M, Stassi G, Messina C G, Parolini I, Lotti F, Sette G, Peschle C, De Maria R. CD95 death-inducing signaling complex formation and internalization occur in lipid rafts of type I and type II cells. Eur J Immunol. 2004;34:1930–1940. doi: 10.1002/eji.200324786. [DOI] [PubMed] [Google Scholar]
- Lee K H, Feig C, Tchikov V, Schickel R, Hallas C, Schutze S, Peter M E, Chan A C. The role of receptor internalization in CD95 signaling. EMBO J. 2006;25:1009–1023. doi: 10.1038/sj.emboj.7601016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrik I N, Mock T, Golks A, Hoffmann J C, Baumann S, Krammer P H. CD95 stimulation results in the formation of a novel death effector domain protein-containing complex. J Biol Chem. 2008;283:26401–26408. doi: 10.1074/jbc.M800823200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K M, Krammer P H, Peter M E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart B C, Alappat E C, Peter M E. The CD95 type I/type II model. Semin Immunol. 2003;15:185–193. doi: 10.1016/s1044-5323(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Algeciras-Schimnich A, Peter M E. Actin dependent CD95 internalization is specific for Type I cells. FEBS Lett. 2003;546:185–188. doi: 10.1016/s0014-5793(03)00558-1. [DOI] [PubMed] [Google Scholar]
- Hueber A O, Bernard A M, Herincs Z, Couzinet A, He H T. An essential role for membrane rafts in the initiation of Fas/CD95-triggered cell death in mouse thymocytes. EMBO Rep. 2002;3:190–196. doi: 10.1093/embo-reports/kvf022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi C, Schmitz I, Zha J, Korsmeyer S J, Krammer P H, Peter M E. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem. 1999;274:22532–22538. doi: 10.1074/jbc.274.32.22532. [DOI] [PubMed] [Google Scholar]
- Barnhart B C, Legembre P, Pietras E, Bubici C, Franzoso G, Peter M E. CD95 ligand induces motility and invasiveness of apoptosis-resistant tumor cells. EMBO J. 2004;23:3175–3185. doi: 10.1038/sj.emboj.7600325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajant H, Pfizenmaier K, Scheurich P. Non-apoptotic Fas signaling. Cytokine Growth Factor Rev. 2003;14:53–66. doi: 10.1016/s1359-6101(02)00072-2. [DOI] [PubMed] [Google Scholar]
- Desbarats J, Birge R B, Mimouni-Rongy M, Weinstein D E, Palerme J S, Newell M K. Fas engagement induces neurite growth through ERK activation and p35 upregulation. Nat Cell Biol. 2003;5:118–125. doi: 10.1038/ncb916. [DOI] [PubMed] [Google Scholar]
- Reinehr R, Sommerfeld A, Haussinger D. CD95 ligand is a proliferative and antiapoptotic signal in quiescent hepatic stellate cells. Gastroenterology. 2008;134:1494–1506. doi: 10.1053/j.gastro.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Desbarats J, Newell M K. Fas engagement accelerates liver regeneration after partial hepatectomy. Nat Med. 2000;6:920–923. doi: 10.1038/78688. [DOI] [PubMed] [Google Scholar]
- Hong F, Nguyen V A, Shen X, Kunos G, Gao B. Rapid activation of protein kinase B/Akt has a key role in antiapoptotic signaling during liver regeneration. Biochem Biophys Res Commun. 2000;279:974–979. doi: 10.1006/bbrc.2000.4044. [DOI] [PubMed] [Google Scholar]
- Jackson L N, Larson S D, Silva S R, Rychahou P G, Chen L A, Qiu S, Rajaraman S, Evers B M. PI3K/Akt activation is critical for early hepatic regeneration after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1401–G1410. doi: 10.1152/ajpgi.00062.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub R. Liver regeneration 4: transcriptional control of liver regeneration. FASEB J. 1996;10:413–427. [PubMed] [Google Scholar]
- Tzung S P, Fausto N, Hockenbery D M. Expression of Bcl-2 family during liver regeneration and identification of Bcl-x as a delayed early response gene. Am J Pathol. 1997;150:1985–1995. [PMC free article] [PubMed] [Google Scholar]
- Kennedy N J, Kataoka T, Tschopp J, Budd R C. Caspase activation is required for T cell proliferation. J Exp Med. 1999;190:1891–1896. doi: 10.1084/jem.190.12.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Harris A W, Bath M L, Smith K G, Strasser A. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J. 1998;17:706–718. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschen M, Warskulat U, Beckmann M W. Defining CD95 as a tumor suppressor gene. J Mol Med. 2000;78:312–325. doi: 10.1007/s001090000112. [DOI] [PubMed] [Google Scholar]
- Straus S E, Jaffe E S, Puck J M, Dale J K, Elkon K B, Rosen-Wolff A, Peters A M, Sneller M C, Hallahan C W, Wang J, Fischer R E, Jackson C M, Lin A Y, Baumler C, Siegert E, Marx A, Vaishnaw A K, Grodzicky T, Fleisher T A, Lenardo M J. The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood. 2001;98:194–200. doi: 10.1182/blood.v98.1.194. [DOI] [PubMed] [Google Scholar]
- Legembre P, Barnhart B C, Peter M E. The relevance of NF-κB for CD95 signaling in tumor cells. Cell Cycle. 2004;3:1235–1239. doi: 10.4161/cc.3.10.1194. [DOI] [PubMed] [Google Scholar]
- Kleber S, Sancho-Martinez I, Wiestler B, Beisel A, Gieffers C, Hill O, Thiemann M, Mueller W, Sykora J, Kuhn A, Schreglmann N, Letellier E, Zuliani C, Klussmann S, Teodorczyk M, Grone H J, Ganten T M, Sultmann H, Tuttenberg J, von Deimling A, Regnier-Vigouroux A, Herold-Mende C, Martin-Villalba A. Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell. 2008;13:235–248. doi: 10.1016/j.ccr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Peter M E, Legembre P, Barnhart B C. Does CD95 have tumor promoting activities? Biochim Biophys Acta. 2005;1755:25–36. doi: 10.1016/j.bbcan.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Legembre P, Barnhart B C, Zheng L, Vijayan S, Straus S E, Puck J, Dale J K, Lenardo M, Peter M E. Induction of apoptosis and activation of NF-κB by CD95 require different signalling thresholds. EMBO Rep. 2004;5:1084–1089. doi: 10.1038/sj.embor.7400280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabandhu K, Huault S, Garmy N, Fantini J, Stebe E, Mailfert S, Marguet D, Hueber A O. The extracellular glycosphingolipid-binding motif of Fas defines its internalization route, mode and outcome of signals upon activation by ligand. Cell Death Differ. 2008;15:1824–1837. doi: 10.1038/cdd.2008.115. [DOI] [PubMed] [Google Scholar]
- Pan G, Ni J, Wei Y F, Yu G, Gentz R, Dixit V M. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- Pan G, O'Rourke K, Chinnaiyan A M, Gentz R, Ebner R, Ni J, Dixit V M. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- Golstein P. Cell death: TRAIL and its receptors. Curr Biol. 1997;7:R750–R753. doi: 10.1016/s0960-9822(06)90000-1. [DOI] [PubMed] [Google Scholar]
- Grosse-Wilde A, Voloshanenko O, Bailey S L, Longton G M, Schaefer U, Csernok A I, Schutz G, Greiner E F, Kemp C J, Walczak H. TRAIL-R deficiency in mice enhances lymph node metastasis without affecting primary tumor development. J Clin Invest. 2008;118:100–110. doi: 10.1172/JCI33061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse-Wilde A, Kemp C J. Metastasis suppressor function of tumor necrosis factor-related apoptosis-inducing ligand-R in mice: implications for TRAIL-based therapy in humans? Cancer Res. 2008;68:6035–6037. doi: 10.1158/0008-5472.CAN-08-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnberg N, Klein-Szanto A J, El-Deiry W S. TRAIL-R deficiency in mice promotes susceptibility to chronic inflammation and tumorigenesis. J Clin Invest. 2008;118:111–123. doi: 10.1172/JCI29900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer U, Voloshanenko O, Willen D, Walczak H. TRAIL: a multifunctional cytokine. Front Biosci. 2007;12:3813–3824. doi: 10.2741/2354. [DOI] [PubMed] [Google Scholar]
- Degli-Esposti M A, Smolak P J, Walczak H, Waugh J, Huang C P, DuBose R F, Goodwin R G, Smith C A. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med. 1997;186:1165–1170. doi: 10.1084/jem.186.7.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degli-Esposti M A, Dougall W C, Smolak P J, Waugh J Y, Smith C A, Goodwin R G. The novel receptor TRAIL-R4 induces NF-κB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997;7:813–820. doi: 10.1016/s1074-7613(00)80399-4. [DOI] [PubMed] [Google Scholar]
- Riccioni R, Pasquini L, Mariani G, Saulle E, Rossini A, Diverio D, Pelosi E, Vitale A, Chierichini A, Cedrone M, Foa R, Lo Coco F, Peschle C, Testa U. TRAIL decoy receptors mediate resistance of acute myeloid leukemia cells to TRAIL. Haematologica. 2005;90:612–624. [PubMed] [Google Scholar]
- Merino D, Lalaoui N, Morizot A, Schneider P, Solary E, Micheau O. Differential inhibition of TRAIL-mediated DR5-DISC formation by decoy receptors 1 and 2. Mol Cell Biol. 2006;26:7046–7055. doi: 10.1128/MCB.00520-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truneh A, Sharma S, Silverman C, Khandekar S, Reddy M P, Deen K C, McLaughlin M M, Srinivasula S M, Livi G P, Marshall L A, Alnemri E S, Williams W V, Doyle M L. Temperature-sensitive differential affinity of TRAIL for its receptors. DR5 is the highest affinity receptor. J Biol Chem. 2000;275:23319–23325. doi: 10.1074/jbc.M910438199. [DOI] [PubMed] [Google Scholar]
- Mariani S M, Krammer P H. Differential regulation of TRAIL and CD95 ligand in transformed cells of the T and B lymphocyte lineage. Eur J Immunol. 1998;28:973–982. doi: 10.1002/(SICI)1521-4141(199803)28:03<973::AID-IMMU973>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Bodmer J L, Meier P, Tschopp J, Schneider P. Cysteine 230 is essential for the structure and activity of the cytotoxic ligand TRAIL. J Biol Chem. 2000;275:20632–20637. doi: 10.1074/jbc.M909721199. [DOI] [PubMed] [Google Scholar]
- Trabzuni D, Famulski K S, Ahmad M. Functional analysis of tumour necrosis factor-alpha-related apoptosis-inducing ligand (TRAIL): cysteine-230 plays a critical role in the homotrimerization and biological activity of this novel tumoricidal cytokine. Biochem J. 2000;350:505–510. [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y, Screpanti V, Yagita H, Grandien A, Ljunggren H G, Smyth M J, Chambers B J. NK cell TRAIL eliminates immature dendritic cells in vivo and limits dendritic cell vaccination efficacy. J Immunol. 2004;172:123–129. doi: 10.4049/jimmunol.172.1.123. [DOI] [PubMed] [Google Scholar]
- Janssen E M, Droin N M, Lemmens E E, Pinkoski M J, Bensinger S J, Ehst B D, Griffith T S, Green D R, Schoenberger S P. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- Smyth M J, Takeda K, Hayakawa Y, Peschon J J, van den Brink M R, Yagita H. Nature’s TRAIL—on a path to cancer immunotherapy. Immunity. 2003;18:1–6. doi: 10.1016/s1074-7613(02)00502-2. [DOI] [PubMed] [Google Scholar]
- Wiley S R, Schooley K, Smolak P J, Din W S, Huang C P, Nicholl J K, Sutherland G R, Smith T D, Rauch C, Smith C A, Goodwin R G. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- Pitti R M, Marsters S A, Ruppert S, Donahue C J, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Pai R C, Fong S, Leung S, Lawrence D A, Marsters S A, Blackie C, Chang L, McMurtrey A E, Hebert A, DeForge L, Koumenis I L, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall R H. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence D, Shahrokh Z, Marsters S, Achilles K, Shih D, Mounho B, Hillan K, Totpal K, DeForge L, Schow P, Hooley J, Sherwood S, Pai R, Leung S, Khan L, Gliniak B, Bussiere J, Smith C A, Strom S S, Kelley S, Fox J A, Thomas D, Ashkenazi A. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med. 2001;7:383–385. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- Walczak H, Miller R E, Ariail K, Gliniak B, Griffith T S, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin R G, Rauch C T, Schuh J C, Lynch D H. Tumoricidal activity of tumor necrosis factor-related apoptosis- inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- Gores G J, Kaufmann S H. Is TRAIL hepatotoxic? Hepatology. 2001;34:3–6. doi: 10.1053/jhep.2001.25173. [DOI] [PubMed] [Google Scholar]
- Ganten T M, Koschny R, Sykora J, Schulze-Bergkamen H, Buchler P, Haas T L, Schader M B, Untergasser A, Stremmel W, Walczak H. Preclinical differentiation between apparently safe and potentially hepatotoxic applications of TRAIL either alone or in combination with chemotherapeutic drugs. Clin Cancer Res. 2006;12:2640–2646. doi: 10.1158/1078-0432.CCR-05-2635. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Herbst R S. To kill a tumor cell: the potential of proapoptotic receptor agonists. J Clin Invest. 2008;118:1979–1990. doi: 10.1172/JCI34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley R F, Totpal K, Lindstrom S H, Mathieu M, Billeci K, Deforge L, Pai R, Hymowitz S G, Ashkenazi A. Receptor-selective mutants of apoptosis-inducing ligand 2/tumor necrosis factor-related apoptosis-inducing ligand reveal a greater contribution of death receptor (DR) 5 than DR4 to apoptosis signaling. J Biol Chem. 2005;280:2205–2212. doi: 10.1074/jbc.M410660200. [DOI] [PubMed] [Google Scholar]
- Kuang A A, Diehl G E, Zhang J, Winoto A. FADD is required for DR4- and DR5-mediated apoptosis: lack of trail-induced apoptosis in FADD-deficient mouse embryonic fibroblasts. J Biol Chem. 2000;275:25065–25068. doi: 10.1074/jbc.C000284200. [DOI] [PubMed] [Google Scholar]
- Kohlhaas S L, Craxton A, Sun X M, Pinkoski M J, Cohen G M. Receptor-mediated endocytosis is not required for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. J Biol Chem. 2007;282:12831–12841. doi: 10.1074/jbc.M700438200. [DOI] [PubMed] [Google Scholar]
- Wagner K W, Punnoose E A, Januario T, Lawrence D A, Pitti R M, Lancaster K, Lee D, von Goetz M, Yee S F, Totpal K, Huw L, Katta V, Cavet G, Hymowitz S G, Amler L, Ashkenazi A. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070–1077. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- Falschlehner C, Emmerich C H, Gerlach B, Walczak H. TRAIL signalling: decisions between life and death. Int J Biochem Cell Biol. 2007;39:1462–1475. doi: 10.1016/j.biocel.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Hu W H, Johnson H, Shu H B. Tumor necrosis factor-related apoptosis-inducing ligand receptors signal NF-κB and JNK activation and apoptosis through distinct pathways. J Biol Chem. 1999;274:30603–30610. doi: 10.1074/jbc.274.43.30603. [DOI] [PubMed] [Google Scholar]
- Kim Y S, Schwabe R F, Qian T, Lemasters J J, Brenner D A. TRAIL-mediated apoptosis requires NF-κB inhibition and the mitochondrial permeability transition in human hepatoma cells. Hepatology. 2002;36:1498–1508. doi: 10.1053/jhep.2002.36942. [DOI] [PubMed] [Google Scholar]
- Varfolomeev E, Maecker H, Sharp D, Lawrence D, Renz M, Vucic D, Ashkenazi A. Molecular determinants of kinase pathway activation by Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand. J Biol Chem. 2005;280:40599–40608. doi: 10.1074/jbc.M509560200. [DOI] [PubMed] [Google Scholar]
- Petersen S L, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, Harran P, Wang X. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom T S, Poukkula M, Holmstrom T H, Heiskanen K M, Eriksson J E. Mitogen-activated protein kinase/extracellular signal-regulated kinase signaling in activated T cells abrogates TRAIL-induced apoptosis upstream of the mitochondrial amplification loop and caspase-8. J Immunol. 2002;169:2851–2860. doi: 10.4049/jimmunol.169.6.2851. [DOI] [PubMed] [Google Scholar]
- Tran S E, Holmstrom T H, Ahonen M, Kahari V M, Eriksson J E. MAPK/ERK overrides the apoptotic signaling from Fas, TNF, and TRAIL receptors. J Biol Chem. 2001;276:16484–16490. doi: 10.1074/jbc.M010384200. [DOI] [PubMed] [Google Scholar]
- Lee T J, Lee J T, Park J W, Kwon T K. Acquired TRAIL resistance in human breast cancer cells are caused by the sustained cFLIP(L) and XIAP protein levels and ERK activation. Biochem Biophys Res Commun. 2006;351:1024–1030. doi: 10.1016/j.bbrc.2006.10.163. [DOI] [PubMed] [Google Scholar]
- Guicciardi M E, Bronk S F, Werneburg N W, Gores G J. cFLIPL prevents TRAIL-induced apoptosis of hepatocellular carcinoma cells by inhibiting the lysosomal pathway of apoptosis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1337–G1346. doi: 10.1152/ajpgi.00497.2006. [DOI] [PubMed] [Google Scholar]
- Ishimura N, Isomoto H, Bronk S F, Gores G J. Trail induces cell migration and invasion in apoptosis-resistant cholangiocarcinoma cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G129–G136. doi: 10.1152/ajpgi.00242.2005. [DOI] [PubMed] [Google Scholar]
- Trauzold A, Siegmund D, Schniewind B, Sipos B, Egberts J, Zorenkov D, Emme D, Roder C, Kalthoff H, Wajant H. TRAIL promotes metastasis of human pancreatic ductal adenocarcinoma. Oncogene. 2006;25:7434–7439. doi: 10.1038/sj.onc.1209719. [DOI] [PubMed] [Google Scholar]
- Leverkus M, Sprick M R, Wachter T, Denk A, Brocker E B, Walczak H, Neumann M. TRAIL-induced apoptosis and gene induction in HaCaT keratinocytes: differential contribution of TRAIL receptors 1 and 2. J Invest Dermatol. 2003;121:149–155. doi: 10.1046/j.1523-1747.2003.12332.x. [DOI] [PubMed] [Google Scholar]
- Inoue H, Shiraki K, Yamanaka T, Ohmori S, Sakai T, Deguchi M, Okano H, Murata K, Sugimoto K, Nakano T. Functional expression of tumor necrosis factor-related apoptosis-inducing ligand in human colonic adenocarcinoma cells. Lab Invest. 2002;82:1111–1119. doi: 10.1097/01.lab.0000027838.69455.39. [DOI] [PubMed] [Google Scholar]
- Secchiero P, Gonelli A, Carnevale E, Corallini F, Rizzardi C, Zacchigna S, Melato M, Zauli G. Evidence for a proangiogenic activity of TNF-related apoptosis-inducing ligand. Neoplasia. 2004;6:364–373. doi: 10.1593/neo.03421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Koike N, Adachi S. Expression of TNF-related apoptosis-inducing ligand (TRAIL) and its receptors in gastric carcinoma and tumor-infiltrating lymphocytes: a possible mechanism of immune evasion of the tumor. J Cancer Res Clin Oncol. 2002;128:73–79. doi: 10.1007/s004320100292. [DOI] [PubMed] [Google Scholar]
- Zhao S, Asgary Z, Wang Y, Goodwin R, Andreeff M, Younes A. Functional expression of TRAIL by lymphoid and myeloid tumour cells. Br J Haematol. 1999;106:827–832. doi: 10.1046/j.1365-2141.1999.01630.x. [DOI] [PubMed] [Google Scholar]
- Rieger J, Ohgaki H, Kleihues P, Weller M. Human astrocytic brain tumors express AP02L/TRAIL. Acta Neuropathol. 1999;97:1–4. doi: 10.1007/s004010050948. [DOI] [PubMed] [Google Scholar]
- Frank S, Kohler U, Schackert G, Schackert H K. Expression of TRAIL and its receptors in human brain tumors. Biochem Biophys Res Commun. 1999;257:454–459. doi: 10.1006/bbrc.1999.0493. [DOI] [PubMed] [Google Scholar]
- Lancaster J M, Sayer R, Blanchette C, Calingaert B, Whitaker R, Schildkraut J, Marks J, Berchuck A. High expression of tumor necrosis factor-related apoptosis-inducing ligand is associated with favorable ovarian cancer survival. Clin Cancer Res. 2003;9:762–766. [PubMed] [Google Scholar]
- Wallach D, Varfolomeev E E, Malinin N L, Goltsev Y V, Kovalenko A V, Boldin M P. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- Pfeffer K, Matsuyama T, Kundig T M, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi P S, Kronke M, Mak T W. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- Zhao Y X, Lajoie G, Zhang H, Chiu B, Payne U, Inman R D. Tumor necrosis factor receptor p55-deficient mice respond to acute Yersinia enterocolitica infection with less apoptosis and more effective host resistance. Infect Immun. 2000;68:1243–1251. doi: 10.1128/iai.68.3.1243-1251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- Harper N, Hughes M, MacFarlane M, Cohen G M. Fas-associated death domain protein and caspase-8 are not recruited to the tumor necrosis factor receptor 1 signaling complex during tumor necrosis factor-induced apoptosis. J Biol Chem. 2003;278:25534–25541. doi: 10.1074/jbc.M303399200. [DOI] [PubMed] [Google Scholar]
- Schneider-Brachert W, Tchikov V, Neumeyer J, Jakob M, Winoto-Morbach S, Held-Feindt J, Heinrich M, Merkel O, Ehrenschwender M, Adam D, Mentlein R, Kabelitz D, Schutze S. Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity. 2004;21:415–428. doi: 10.1016/j.immuni.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Legler D F, Micheau O, Doucey M A, Tschopp J, Bron C. Recruitment of TNF receptor 1 to lipid rafts is essential for TNFα-mediated NF-κB activation. Immunity. 2003;18:655–664. doi: 10.1016/s1074-7613(03)00092-x. [DOI] [PubMed] [Google Scholar]
- Li H, Kobayashi M, Blonska M, You Y, Lin X. Ubiquitination of RIP is required for tumor necrosis factor alpha-induced NF-κB activation. J Biol Chem. 2006;281:13636–13643. doi: 10.1074/jbc.M600620200. [DOI] [PubMed] [Google Scholar]
- Wajant H, Scheurich P. Tumor necrosis factor receptor-associated factor (TRAF) 2 and its role in TNF signaling. Int J Biochem Cell Biol. 2001;33:19–32. doi: 10.1016/s1357-2725(00)00064-9. [DOI] [PubMed] [Google Scholar]
- Wang C Y, Mayo M W, Korneluk R G, Goeddel D V, Baldwin A S. NF-κB antiapoptosis: induction of TRAF 1 and TRAF 2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- Tang G, Minemoto Y, Dibling B, Purcell N H, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-κB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- Chang L, Kamata H, Solinas G, Luo J L, Maeda S, Venuprasad K, Liu Y C, Karin M. The E3 ubiquitin ligase itch couples JNK activation to TNFα-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Schneider-Brachert W, Tchikov V, Merkel O, Jakob M, Hallas C, Kruse M L, Groitl P, Lehn A, Hildt E, Held-Feindt J, Dobner T, Kabelitz D, Kronke M, Schutze S. Inhibition of TNF receptor 1 internalization by adenovirus 14.7K as a novel immune escape mechanism. J Clin Invest. 2006;116:2901–2913. doi: 10.1172/JCI23771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-alpha-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- Neumeyer J, Hallas C, Merkel O, Winoto-Morbach S, Jakob M, Thon L, Adam D, Schneider-Brachert W, Schutze S. TNF-receptor I defective in internalization allows for cell death through activation of neutral sphingomyelinase. Exp Cell Res. 2006;312:2142–2153. doi: 10.1016/j.yexcr.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Banno Y, Nakashima S, Takenaka K, Sakai H, Nishimura Y, Sakai N, Shimizu S, Eguchi Y, Tsujimoto Y, Nozawa Y. Ceramide formation leads to caspase-3 activation during hypoxic PC12 cell death. Inhibitory effects of Bcl-2 on ceramide formation and caspase-3 activation. J Biol Chem. 1998;273:6921–6927. doi: 10.1074/jbc.273.12.6921. [DOI] [PubMed] [Google Scholar]
- Varfolomeev E, Blankenship J W, Wayson S M, Fedorova A V, Kayagaki N, Garg P, Zobel K, Dynek J N, Elliott L O, Wallweber H J, Flygare J A, Fairbrother W J, Deshayes K, Dixit V M, Vucic D. IAP antagonists induce autoubiquitination of c-IAPs, NF-κB activation, and TNFα-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Wang L, Du F, Wang X. TNF-α induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Bertrand M J, Milutinovic S, Dickson K M, Ho W C, Boudreault A, Durkin J, Gillard J W, Jaquith J B, Morris S J, Barker P A. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]