Abstract
Genetically susceptible rodents exposed to low nontoxic levels of inorganic mercury (Hg2+) develop idiosyncratic autoimmune disease associated with defective T-cell function. However, the molecular mechanisms underlying this phenomenon remain mostly unexplained. Brief exposure of T cells to micromolar concentrations of Hg2+ leads to physiologically relevant nontoxic cellular mercury burdens, and as we have previously reported, attenuates T-cell receptor (TCR) signal strength by ∼50%. We have found this to be the result of an inadequate activation of the tyrosine kinase ZAP-70, which is hypophosphorylated following TCR stimulation in Hg2+ burdened cells when compared to untreated controls. In T cells, ZAP-70 phosphorylation is dependent on lymphocyte-specific protein tyrosine kinase (Lck) activity, which in turn is either positively or negatively regulated by the phosphorylation of specific Lck tyrosine residues. In particular, the general belief is that Lck is negatively regulated by phosphorylation of tyrosine 192 (Y192). We now demonstrate by Western blotting that, in Jurkat T cells, TCR signal transduction (and ZAP-70 phosphorylation) was positively associated with a rapid transient phosphorylation of Y192, which was inhibited in cells that were briefly (5 min) exposed to 5 μM Hg2+. Thus, Hg2+ inhibits a critical activating role played by Lck Y192 during the most proximal events of the TCR-induced cell signaling.—Ziemba, S. E., Menard, S. L., McCabe, Jr., M. J., Rosenspire, A. J. T-cell receptor signaling is mediated by transient Lck activity, which is inhibited by inorganic mercury.
Keywords: Hg2+, ZAP-70, lymphocyte, signal transduction
Mercury is a ubiquitous xenobiotic that is well known to be toxic to many different cell types at moderate exposure levels. Much less is known about mercury at lower nontoxic exposure levels. However, one system that seems to be sensitive to low nontoxic mercury exposure is the immune system. It has been shown that, depending on chemical form and specific dosage, exposure to mercury at low levels is related to either autoimmune or immunosuppressive syndromes (1,2,3,4).
Toxicants such as inorganic mercury (Hg2+) can potentially exert a powerful effect on the T-cell receptor (TCR) signaling pathway. The Ras-Raf-ERK-MAP-kinase signaling cascade is an important element of the TCR signaling network (5). We have found previously that exposure of T cells to low and nontoxic concentrations of inorganic mercury prior to TCR stimulation did not affect ERK-MAP kinase by itself but attenuated upstream activation of Ras and subsequent ERK MAP kinase activation during TCR signaling. As activation of ERK-MAP kinase is associated with successful negative selection of T cells (6,7), this finding led us to suggest that diminished Ras/ERK activity in the presence of Hg2+ might be a central feature of mercury-induced autoimmunity by virtue of interference with TCR-mediated negative selection (8).
Ras and ERK MAP kinase activation are connected to TCR stimulation through a signaling pathway mediated by membrane-associated and cytosolic protein tyrosine kinases (PTKs) (9, 10). After antigen engagement, the TCR complex, without tyrosine kinase activity of its own, serves as a docking port for the Src family tyrosine kinase Lck and the Syk family CD3ζ chain-associated protein kinase of 70 kDa (ZAP-70) (11). The binding of Lck and Zap-70 to the TCR complex are proximal events in the TCR signaling cascade, mediating the phosphorylation of the scaffolding protein LAT. Phosphorylation of LAT is rapidly followed by LAT-centered signalosome formation, and then Ras/ERK activation (10, 12,13,14).
Previous reports have indicated that Hg2+ can alter phosphorylation profiles of numerous T-cell proteins, and in so doing trigger functional consequences, including cell death (15,16,17,18). By way of explanation of the effect of Hg2+ on attenuating activation of ERK-MAP Kinase, we have reported previously that T-cell exposure to inorganic mercury was followed by decreased phosphorylation of LAT when cells were subsequently activated with antigen. We also found that in this case hypophosphorylation of LAT was a direct result of upstream hypophosphorylation of ZAP-70 (19). In this report, we look directly at early TCR signaling elements that affect ZAP-70 phosphorylation and activation.
After TCR stimulation, Lck normally phosphorylates TCR zeta chains (11, 20). ZAP-70 then docks with the phosphorylated zeta chains, allowing Zap-70 to be phosphorylated and activated by Lck (21, 22). Thus, Lck has been recognized as an early central element in the TCR signaling cascade, which is directly responsible for the activation of ZAP-70. However, Lck itself is also regulated by the phosphorylation of several tyrosine residues (reviewed in ref. 23). We now show that on TCR stimulation, there is a rapid and transitory phosphorylation of the Lck regulatory tyrosine Y192 that is concomitant with Lck phosphorylation of zeta chains and ZAP-70. Furthermore, Hg2+ likely attenuates the ability of Lck to phosphorylate CD3ζ and activate ZAP-70 in response to TCR triggering by interfering with this transitory phosphorylation of Y192.
MATERIALS AND METHODS
Reagents
Solutions of mercuric chloride (HgCl2) were prepared in RPMI 1640 medium (HyClone, Logan, UT, USA). Antibodies were obtained from Santa Cruz Technology (Santa Cruz, CA, USA) [α-LAT (rabbit polyclonal IgG), α-Lck (rabbit polyclonal IgG), α-pLck (Tyr192/Ser-194, rabbit monoclonal IgG), α-CD3-ζ (mouse polyclonal IgG), goat α-mouse IgG-HRP]; Southern Biotechnology Associates, Birmingham, AL, USA [α-pTyr (PY20 clone)-HRP]; BD-Transduction Labs/Pharmigen, San Diego, CA, USA [α-pTyr (PY20 clone)] and Cell Signaling Technology, Beverly, MA, USA [α-ZAP-70 (rabbit monoclonal IgG), rabbit α-pZAP-70 (Tyr319, rabbit monoclonal IgG), rabbit α-pZAP-70 (Tyr493, rabbit monoclonal IgG)]. α-CD3 (OKT3) came from Ortho Pharmaceuticals (Raritan, NJ, USA). SuperSignal West Pico chemiluminescent detection kit was purchased from Pierce (Rockford, IL, USA). Reagents used for protein electrophoresis and transfer to nitrocellulose were prepared following published protocols (24, 25). All reagents and chemicals used were obtained from Sigma-Aldrich, St. Louis, MO, USA, or Fisher Scientific, Fair Lawn, NJ, USA, and were of analytical grade.
Cell cultures
The human Jurkat (clone E6) were obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were maintained in RPMI 1640 medium (HyClone) supplemented with 10% FBS (HyClone), 2 mM l-glutamine (Life Technologies, Rockville, MD, USA) and 10 g/ml gentamicin (Life Technologies) at 37°C in a humidified atmosphere of 5% CO2, passed every 3–4 d. Cell cultures used in assays reached average cell densities of ∼106 cells/ml and were at least 98% viable as determined by trypan blue dye exclusion after 4 d of incubation. The culture medium was removed by centrifugation, and the cells were suspended in an equal volume of fresh RPMI with supplements and incubated for an additional 24 h before experiments were performed.
Mercury exposure/TCR activation assays
Cell culture aliquots (1×106 cell/aliquot) had their culture medium removed by centrifugation and were washed once with warmed RPMI and then suspended in 200 μl warmed RPMI, with or without 5 μM HgCl2. Aliquots were incubated at 37°C for 10 min before α-CD3 (3–4 μg OKT3/aliquot) was added to activate the TCRs. Reactions were stopped by the addition (∼5 ml) of ice-cold phosphate-buffered saline (PBS), followed by centrifugation to pellet the cells. Supernatant fluid was removed by suction, and cell pellets were quick-frozen in an iced (−25°C) ethanol bath and held frozen until processed for SDS-PAGE.
SDS-PAGE and Western blot analysis
Thawed cell pellets were mixed (vortex) with 250 μM 2.5× nonreducing electrophoresis sample buffer [NRSB: 6 ml 1 M Tris-HCl (pH 6.8), 50 ml 50% glycerol, 20 ml 10% SDS, 10 ml 1% bromphenol blue, 114 ml ddH20]. This produced whole-cell lysates with 6 × 104 cell equivalents per each 15 μl sample loaded onto an SDS-PAGE gel. Lysates were boiled at 100°C for 3 min and pulled through a 23-gauge needle 3–4 times to shear the DNA. 2-Mercaptoethanol (5 μl) was added to each lysate, and all were vortexed briefly and boiled again for 2 min. Processed lysates were centrifuged (14,000 g, 1 min), and samples taken from the top portion of each lysate preparation were loaded onto 7, 10, or 12% polyacrylamide gels. Proteins were resolved by SDS-PAGE in a minigel electrophoresis system (Hoefer, San Francisco CA, USA). All electrophoretic separations were run at constant voltage (175 V) and at room temperature. Proteins were transferred to nitrocellulose membranes (Pall Life Sciences, Pensacola, FL, USA) with an electroblotting system (Bio-Rad Life Science Products, Hercules, CA, USA) at constant voltage (100 V) for 45 min at 0–4°C. Transfer membranes were blocked 2–4 h at room temperature or overnight at 4°C with 1% bovine serum albumin (Sigma-Aldrich) Tris blocking buffer and probed once with specific antibodies as indicated in Results. Immunoblots were then washed in dH2O; stripped for 10 min in 0.1 M glycine with 0.1% SDS, pH 2.0, at room temperature; and washed once with dH2O, twice in Tris buffer, and blocked 2–3 h at room temperature. Stripped immunoblots were reprobed with other antibodies; this strip-and-reprobe procedure was repeated 1–3 more times, depending on the proteins examined.
Lck and pTyr protein immunoprecipitation assays
In each experiment, 2 to 4 × 106 cells were exposed to mercury and then activated with 6–7 μg OKT3 for timed periods as described previously. The cell pellets were lysed on ice in 100 μl Tris buffer containing protease and phosphatase inhibitor cocktail (Sigma-Aldrich), and supernatants were collected following 20 min centrifugation at 18,000 g, 4°C. Following the addition of α-Lck or α-pTyr, supernatants were rotated 3 h at 4°C. Pansorbin (Calbiochem, San Diego, CA, USA) was then added, and rotation at 4°C continued overnight. Immunoprecipitates were then collected and processed for SDS-PAGE and subsequent Western blotting (25).
Data acquisition and analysis
Immunoblotted proteins were detected using appropriate primary or secondary antibodies coupled to horseradish peroxidase (HRP) and SuperSignal West Pico chemiluminescent substrate (Pierce). Kodak BioMax ML imaging films (Eastman Kodak Co., Rochester, NY, USA) were used to collect the chemiluminescence data, which were then scanned and converted into digital image files. In selected experiments, Image J (available at http://rsb.info.nih.gov/ij/) and Microsoft Excel© (Microsoft, Redmond, WA, USA) software were used to analyze the images.
Quantification of digitized images
For each image, the density of the specific band of interest in each lane was integrated over the band area to arrive at a metric that was used to assess the degree of signal (phosphorylation). To normalize the results between different experiments, for each experiment the band of interest with the absolute least density (minimum phosphorylation signal) at the 0 time point was identified. The absolute amount of tyrosine phosphorylation of the bands was somewhat variable between experiments, and depending on the particular experiment, the 0 time point band with the least initial phosphorylation could be either from the Hg2+-treated cells or from the untreated controls. In either event, the integrated density value for the least-dense band was taken as the background or basal phosphorylation level. The band was assigned a relative value of zero, and the actual numerical density value was subtracted from the integrated density of the remaining bands of interest to adjust for background signal. Next, the band with the greatest adjusted density (maximum phosphorylation signal) was assigned a value of 100%. In most cases, this band came from control cells not exposed to Hg2+. The percentage of this maximum value was then determined for the band of interest in all other lanes of the same experiment. Finally, the percentage of maximum response for all time points (in the presence or absence of Hg2+) in all replicate experiments was then averaged and plotted.
RESULTS
Hg+2 attenuates the phosphorylation of multiple proteins in the range of 38 and 56 kDa in response to TCR stimulation
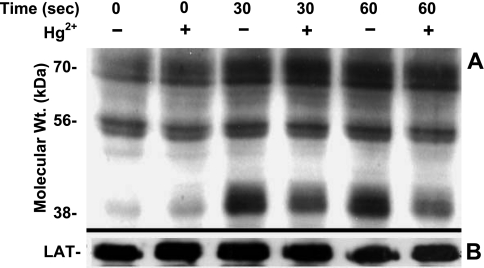
Figure 1A illustrates a representative example of experiments (n=3) in which Jurkat T cells were exposed or not to 5 μM Hg2+ for 10 min (conditions that we have previously determined to be optimal for Hg2+ dependent inhibition of TCR signaling; ref8) prior to the TCR being cross-linked and stimulated by an α-CD3 monoclonal antibody (OKT3) used as a surrogate T-cell antigen. At timed intervals following OKT3 addition, the cells were lysed, and the lysate proteins were resolved by SDS-PAGE. Proteins were transferred to nitrocellulose, and the membranes were blotted with an anti-phosphotyrosine antibody (α-pTyr). Most prominently, proteins in the range of 38 kDa are normally phosphorylated within 30 s on TCR stimulation, and Hg2+ clearly attenuates the signal. This result is consistent with earlier experiments where we have shown that TCR-induced tyrosine phosphorylation of the 38-kDa protein LAT is inhibited by Hg2+ (19). After staining for tyrosine-phosphorylated proteins, membranes were stripped and then reprobed for total LAT. This second probe superimposed over the 38-kDa phosphorylated band in Fig. 1A and served as the gel-loading control (Fig. 1B).
Figure 1.
Hg2+ attenuates TCR-stimulated tyrosine phosphorylation. A) Jurkat T cells were incubated in RPMI with (+) or without (−) 5 μM HgCl2 for 10 min, followed by the addition of α-CD3 to activate TCRs. Cells were held at 37°C, and reactions were stopped at either 30 or 60 s. Cells were then lysed with SDS, and whole-cell lysates were assayed for tyrosine phosphorylated proteins by Western blotting with α-phosphotyrosine antibody. Lanes were normalized in that the volume of cell lysate loaded in each was derived from equivalent cell numbers. B) For control purposes, membranes were stripped and then probed for total LAT. Results shown are representative of 12 independent experiments.
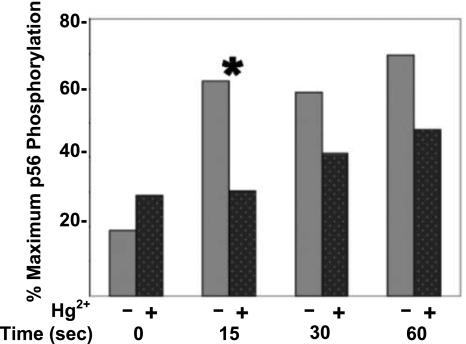
Whereas many proteins are potentially phosphorylated on tyrosine residues when the TCR is stimulated by α-CD3, in Fig. 1, in addition to the 38-kDa band, our attention was drawn to the phosphorylated band at 56 kDa. Although the effect is not as dramatic as it is for the 38-kDa LAT band, the 56-kDa band also seems to respond to TCR stimulation by increased phosphorylation. Like the LAT signal, the signal here also appears to be attenuated by Hg2+. A more detailed analysis of the effect of Hg2+ on tyrosine phosphorylation of the 56-kDa band is shown in Fig. 2. The figure plots the average percentage maximum tyrosine phosphorylation at 56 kDa in the presence or absence of Hg2+ at time points from 0 to 60 s after TCR stimulation, obtained from 12 independent experiments conducted as described in Fig. 1.
Figure 2.
Hg2+ rapidly and transiently attenuates the tyrosine phosphorylation of a phospho(tyrosine) protein of 56 kDa (p56). A quantitative analysis of the results depicted for the 56-kDa band in Fig. 1 was undertaken. For each of 12 independent experiments, Western blot images were scanned, and the density of the 56-kDa band was integrated over the band area to arrive at a measure of phosphorylation in cells that had been treated with mercury (dark gray, dotted bars) and those that had not (light gray bars). Within each experiment, the band with the greatest tyrosine phosphorylation was assigned a value of 100% and was considered maximally phosphorylated. The (0) time-point band with the least tyrosine phosphorylation (either with or without Hg2+) was assigned a value of 0%, and the relative maximum percentage of tyrosine phosphorylation in the other bands was determined. Maximum phosphorylation percentages determined for each time point were averaged over all experiments, and the results were plotted. Results between Hg2+-treated and untreated cells are only statistically different for the 15-s time point. *P < 0.05; paired Student’s t test.
Viewed in this way, we find that inorganic mercury attenuates TCR-dependent phosphorylation of the 56-kDa band proteins by ∼25% on average, from control values during the first minute after TCR stimulation. However, while these results are consistent with Fig. 1 and show that inorganic mercury attenuates the TCR signal at 15, 30, and 60 s poststimulation, only at 15 s poststimulation is the result statistically significant. Figure 2 also shows that, prior to TCR stimulation, Hg2+ may slightly augment the TCR signal, but this result is not statistically significant (P>0.05).
Hg+2 depresses transient Lck phosphorylation associated with TCR stimulation
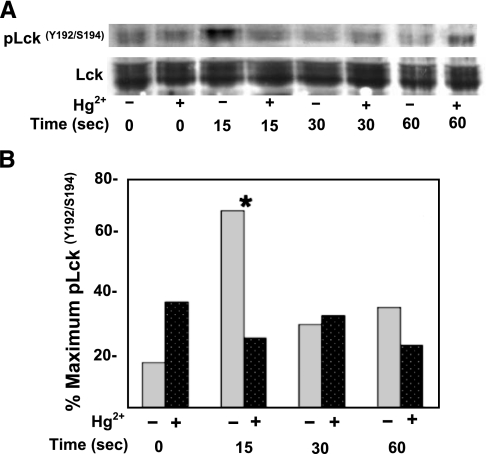
Lck is a 56-kDa Src family protein tyrosine kinase, which during TCR signal transduction is just upstream of ZAP-70 activation and necessary for the subsequent ZAP-70-induced phosphorylation of LAT. Therefore, in order to test whether the findings in Figs. 1 and 2 reflected an effect of Hg2+ on Lck, Jurkat T cells were treated or not with 5 μM Hg2+ and stimulated with α-CD3 for timed intervals. The cells were then lysed, and Western blot analysis on the lysates was performed with an antibody specific for Lck dually phosphorylated on tyrosine 192 and serine 194 (α-pLck). As a loading control, the membranes were then stripped and reblotted with an antibody binding total Lck. Figure 3 is a representative example of one such experiment (n=7). We find that, in the absence of Hg2+, Lck is maximally phosphorylated on Y192/S194 at 15 s post-TCR stimulation, returning to near baseline levels by 60 s, and that this transitory phosphorylation is inhibited by Hg2+. We also should note that the control antibody to total Lck detects a rather broad band. This is likely reflective of the fact that as discussed below, a fraction of the Lck is endogenously phosphorylated on Y505, and phosphorylated Lck isolates above native Lck in SDS gels.
Figure 3.
Hg2+ inhibits Lck(Y192/S194) phosphorylation after TCR stimulation. A) Jurkat T cells were incubated in RPMI with (+) or without (−) μM HgCl2, followed by the addition of α-CD3 antibody to activate TCRs. Aliquots containing equivalent cell numbers were incubated at 37°C, and reactions were stopped at the times indicated. Cells were then lysed in SDS, and phosphorylated Lck was detected by Western blotting with an antibody specific to Lck dually phosphorylated on tyrosine 192 and serine 194. The blots were then stripped and probed for total Lck. Results are representative of 7 independent experiments. B) A quantitative analysis of pLck(Y192/S194) after TCR signaling in cells that had been treated with mercury (dark gray, dotted bars) and those that had not (light gray bars) (performed as described for Fig. 2) was undertaken. Results from 7 independent experiments were averaged. Results between Hg2+-treated and untreated cells are only statistically different for the 15-s time point. *P < 0.05; paired Student’s t test.
To quantify these results and assess their statistical significance, the Western blots from all experiments (n=7) were scanned, and the results were digitized and plotted (Fig. 3B). Our findings are consistent with Fig. 3A: TCR stimulation is associated with a transitory increase in Lck phosphorylation (Y192/S194) at 15 s after the initiation of signal transduction, and Hg2+ inhibits this signal in a statistically significant manner (P<0.05). As in Fig. 3A, Hg2+ also appears to slightly increase basal phosphorylation of Y192/S194, but again this result is not statistically significant. Differences between Lck (Y192/S194) phosphorylation at 30 and 60 s post-TCR stimulation in the presence or absence of Hg2+ are also not statistically significant (P>0.05).
Hg+2 attenuates early ZAP-70 phosphorylation associated with Lck kinase activity
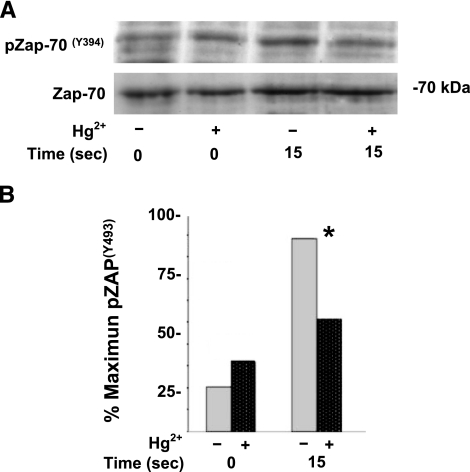
Activation of ZAP-70 is a critical early event in TCR signaling. Current thinking has been that initial ZAP-70 activation results from Lck-mediated phosphorylation of Y493 within the activation loop of ZAP-70 (reviewed in ref. 10). Accordingly, a series of experiments was performed to investigate the phosphorylation of ZAP-70 at Y493, after TCR triggering in T cells exposed or not to 5 μM Hg2+. Cells were triggered with OKT3 and then lysed after 15 s. Lysate proteins were resolved by SDS-PAGE, transferred to nitrocellulose, and probed with α-pZAP(Y493) antibody for Western blotting. Membranes were then stripped and blotted a second time with antibody to total ZAP-70 (α-ZAP-70). These blots served as protein loading controls. Figure 4A is a representative experiment showing that the amount of ZAP-70 specifically phosphorylated on Y493 after TCR stimulation in cells treated with Hg2+ is attenuated at 15 s (n=8).
Figure 4.
Hg2+ inhibits Lck-mediated phosphorylation of ZAP-70 (Y493). A) Jurkat T cells were preincubated in RPMI with (+) or without (−) 5 μM HgCl2 for 10 min, followed by the addition of α-CD3 antibody to activate TCRs. Cells were incubated at 37°C, and reactions were stopped after 15 s. Cells were lysed in SDS, and phosphorylated ZAP-70 was detected by Western blotting with an antibody specific to ZAP-70 phosphorylated on tyrosine 493. Blots were then stripped and reprobed with an antibody specific for total ZAP-70. Results are representative of 8 independent experiments. B) A quantitative analysis of pZap-70 (Y394) after TCR signaling in cells that had been treated with mercury. Bar graph illustrates a quantitative analysis of 8 independent experiments (performed as described for Fig. 2). Dark gray, dotted bars indicate cells that had been treated with mercury, and those that had not been treated are indicated by light gray bars. Results between Hg2+ treated and untreated cells are statistically different for the 15-s time point only. *P < 0.05; paired Student’s t test.
As in Figs. 2 and 3B, the Western blots from all experiments were scanned and digitized, and then the results were averaged and plotted as described previously. Similar to findings with respect to the phosphorylation of Lck (Y192/S194), Fig. 4B shows that Hg2+ significantly depressed phosphorylation of ZAP-70 (Y493) 15 s following antigen engagement of the TCR (P<0.03). The overall attenuation of ZAP-70 (Y493) phosphorylation in Hg2+-treated, antigen-stimulated cells averaged 40% of control values, similar to the attenuation by Hg2+ of pLck(Y192/S194). Hg2+ also seemed to have had a small attenuating effect on the basal level of Y493 phosphorylation, but this was not statistically significant (P>0.05).
Hg+2 attenuates zeta chain phosphorylation associated with activated Lck
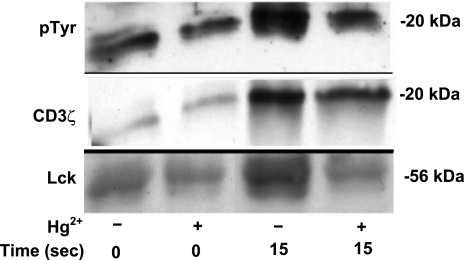
Shortly after TCR engagement of antigen, aside from phosphorylating ZAP-70, it has been shown that Lck rapidly phosphorylates two critically spaced tyrosine residues within the immunoreceptor tyrosine-based activation motifs (ITAMS) of the TCR zeta subunits (CD3ζ). Figure 5 illustrates representative results from experiments (n=3) during which T cells were or were not preincubated with 5 μM Hg2+ for 10 min and then briefly treated with OKT3 in order to stimulate phosphorylation of CD3ζ. After addition of OKT3, cells were collected at 15 s and lysed. In order to purify and concentrate phosphoproteins, lysates were immunoprecipitated with α-pTyr. Precipitated proteins were then resolved by SDS-PAGE, and Western blotting for phosphotyrosine was performed in order to visualize isolated proteins. A phosphorylated protein band in the vicinity of 20 kDa (likely a representative of CD3ζ) was detected (shown in Fig. 5, row 1). This band exhibited a basal level of phosphorylation independent of Hg2+ and, in the absence of Hg2+, clearly showed evidence of increased phosphorylation 15 s after TCR activation. Significantly, phosphorylation appears to be attenuated by Hg2+.
Figure 5.
Hg2+ inhibits the phosphorylation of CD3ζ during initial TCR signaling. Jurkat T cells were or were not incubated with Hg2+ and then treated with α-CD3 antibody to stimulate the TCR. After 15 s the cells were lysed, and tyrosine-phosphorylated proteins were immunoprecipitated with an α-pTyr-specific antibody. Precipitants were then resolved by SDS-PAGE, and pTyr-containing proteins were detected by Western blotting. A protein doublet at ∼20 kDa (likely representative of CD3ζ, p21, and p23) that was strongly phosphorylated after TCR stimulation was detected (row 1). The membranes were then stripped and reprobed for total CD3ζ (row 2). CD3ζ was detected as a broad superimposed band over the 20-kDa doublet described row 1. Finally, blots were stripped again and probed for total Lck (row 3). Results shown are representative of 3 independent experiments.
The membranes were then stripped and blotted for CD3ζ. This is shown in Fig. 5, row 2, where we found that the CD3ζ signal was exactly superimposed over the phosphoprotein band identified in row 1. However, more important, we also found that only small amounts of CD3ζ were immunoprecipitated from unstimulated cells, but much larger amounts were immunopreciptated from cells 15 s after TCR triggering, confirming increased levels of phosphotyrosine in CD3ζ after TCR signaling. Significantly, the amount of CD3ζ immunoprecipitated is reduced in the presence of Hg2+, which suggests that TCR-stimulated tyrosine phosphorylation of CD3ζ is attenuated by Hg2+. These results are consistent with our findings with respect to the phosporylation of ZAP-70. They imply that, like ZAP-70, CD3ζ exhibits a low basal level of tyrosine phosphorylation, which is significantly increased 15 s after the TCR is stimulated, but that the ability of the TCR to signal increased phosphorylation of CD3ζ is attenuated by Hg2+.
As a final control, the blots were stripped a second time and then blotted for Lck. The results are shown in Fig. 5, row 3, where it is seen that in unstimulated cells, little Lck is precipitated either in the presence or absence of Hg2+, implying a low basal level of Lck tyrosine phosphorylation that is independent of Hg2+ exposure. However, the amount of Lck precipitated at 15 s after TCR stimulation is greatly increased, but only in cells that have not been treated with Hg2+. This finding implies that Hg2+ inhibits TCR-induced phosphorylation of Lck 15 s after stimulation, which is in agreement with Fig. 3.
DISCUSSION
We have found previously that during TCR signaling, T cells exposed to Hg2+ show a depressed level of ZAP-70 tyrosine phosphorylation, implying a failure to properly activate the tyrosine kinase activity ZAP-70 (19). As ZAP-70 is itself a substrate of and activated by the Src family tyrosine kinase Lck (26, 27), in this study we have focused on the effect of Hg2+ on Lck functionality. In particular, we have looked at the effect of Hg2+ on the ability of Lck to phosphorylate ZAP-70 and the substrate CD3ζ. Lck has a mass of 56 kDa, and its activity is controlled by tyrosine phosphorylation (28). Thus, the relative attenuation of tyrosine phosphorylation of the 56-kDa band, seen in whole-cell lysates derived from T cells exposed to Hg2+ (Figs. 1 and 2), suggests that Hg2+ inhibits overall Lck phosphorylation during TCR signal transduction. Initially, at least, this finding seems consistent with the inhibitory effect of Hg2+ on the activation of ZAP-70, which results from upstream attenuation of Lck activity.
Unfortunately, a straightforward assessment of Lck activity by an analysis of total Lck tyrosine phosphorylation is complicated by the fact that Lck is both positively and negatively regulated by phosphorylated tyrosines, depending on the specific sites phosphorylated. Lck (as are other Src family kinases) is regulated by a C-terminal negative regulatory tyrosine (Y505 for Lck) that holds the molecule in a “closed” and inactive state. Current literature suggests that the tyrosine is endogenously phosphorylated by Csk, a cytoplasmic tyrosine kinase targeted to the internal face of the plasma membrane by its association with the membrane protein Cbp/PAG (phosphoprotein associated with glycosphingolipid-enriched microdomains) (29). Most models of T-cell signal transduction have generally assumed that activation of the TCR by antigen initially triggers dephosphorylation of Lck (Y505) by CD45. It is believed that dephosphorylation “opens” the molecule, which results in partial activation. According to this scenario, partial activation then allows auto phosphorylation of a second tyrosine residue in the activation loop (Y394), so as to promote full activation of the kinase (28).
However, problems exist with these models. First, much of the Lck in resting T cells is already dephosphorylated at Y505 and therefore should be in a partially activated state prior to TCR stimulation (30, 31). Second, while mutagenesis studies clearly show that Y394 phosphorylation is required for optimal Lck function (32), it has also been shown that although cross-linking of the TCR triggers downstream signaling events, including activation of ERK MAP kinase, TCR cross-linking does not in itself lead to changes in Y394 phosphorylation. This finding suggests that Lck recruitment, rather than activation, may be the critical initiation step during TCR signal transduction (33).
The Src homology-2 (SH2) domain of Lck mediates the interaction of Lck with multiple substrates, including ZAP-70 and CD3ζ, and is required for the induction and maintenance of CD3ζ chain and ZAP-70 phosphorylation following TCR cross-linking (11, 20). It has been shown that phosphorylation of Y192 within the Lck SH2 domain inhibits the ability of Lck to bind to its substrates (34), which suggests that Y192 is a negative regulator of Lck. It has also been shown that phosphorylation of Y192 increases ∼15 min after the TCR is cross-linked (33, 35). The kinetic study in Fig. 3 now demonstrates that this “long-term” increase in phosphorylation is preceded by a phosphorylation event that is both rapid (within 15 s) and transitory, returning to baseline levels within 30 s. Figure 3 also shows that the brief TCR dependent increase in SH2 phosphorylation is significantly inhibited by Hg2+.
The transitory increase in Lck Y192 phosphorylation, as well as the attenuation of this phosphorylation by Hg2+, is functionally significant. Figure 4 shows, after cross-linking of the TCR, a concurrent rapid increase in phosphorylation of ZAP-70 (Y493), and this increase in phosphorylation is significantly attenuated by Hg2+. ZAP-70 (Y493) has generally been accepted as a proximal tyrosine target of Lck after TCR stimulation. Phosphorylation of ZAP-70 (Y493) is associated with activation of ZAP-70, and phosphorylation of Y493 is believed to relieve a negative inhibition of the ZAP-70 kinase site, allowing ZAP-70 to become fully activated by promoting the autophosphorylation of several other tyrosines, including Y319, another regulatory tyrosine (26). However, a more recent report suggests that Y319, rather than Y493, may in fact be the proximal tyrosine targeted by Lck, with Y493 phosphorylation resulting from phosphorylated Y319 stimulated autophosphorylation (36). As we have previously shown that Hg2+ inhibits Y319 phosphorylation (19), in either event it is clear that inhibition of TCR dependent phosphorylation of Lck (Y192/S194) is strongly correlated with an inhibition of ZAP-70 phosphorylation and activation.
A second target of Lck immediately after TCR stimulation is the CD3ζ ITAMs (37, 38). Figure 5 shows that, similar to ZAP-70 (Y493), CD3ζ is phosphorylated within 15 s after the TCR is cross-linked. Furthermore, phosphorylation is also attenuated in cells treated with Hg2+. Thus, in the presence of Hg2+, two of the primary substrates of Lck intimately connected to TCR signal transduction fail to become properly phosphorylated, which supports the notion that the brief transitory phosphorylation of Lck (Y192/S194) is necessary for TCR signaling. It thus appears that by interfering with the phosphorylation of Lck (Y192/S194), Hg2+ attenuates downstream signal transduction events in TCR signaling, including the phosphorylation of LAT and the activation of ERK MAP kinase.
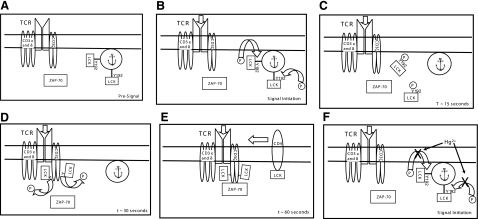
As we have mentioned, it has been shown that phosphorylation of Lck (Y192) terminates the high-affinity binding of Lck to ligands, so as to negatively regulate its participation in TCR signaling (34). Thus, the transitory phosphorylation of Lck (Y192/S194) associated with TCR signaling is likely not a classic activation event per se. We have also pointed out that substantial amounts of Lck are likely active prior to TCR stimulation (30, 31). It may be that in resting cells, endogenously active Lck is unable to phosphorylate either ZAP-70 or CD3ζ, by virtue of the fact that it is sequestered through SH2-mediated binding interactions. We postulate that antigen-induced stimulation of the TCR can then trigger phosphorylation of Lck (Y192/S194), disrupting SH2-mediated binding so as to free sequestered Lck. According to this scenario, the rapid dephosphorylation of Lck (Y192/S194) then allows endogenously active Lck to bind to and phosphorylate ZAP-70 and CD3ζ. Under conditions where the CD4 coreceptor is involved in signaling, additional CD4-associated Lck can then be brought into the signaling complex, leading to autophosphorylation of Lck (Y394) residues and full kinase activation. Hg2+, by preventing Lck (Y192/S194) phosporylation, ensures that Lck, although perhaps endogenously active, remains sequestered and unavailable to phosphorylate ZAP-70 and/or CD3ζ. This model is cartooned in Fig. 6A–E, where the times in the lower right corners indicate approximate time after initiation of the TCR signal. In the presence of Hg2+, phosphorylation of Lck (Y192) is inhibited, and signaling is attenuated (Fig. 6F).
Figure 6.
Cartoon depicting a possible function of Hg2+-sensitive Lck Y192 (transitory) phosphorylation in the initiation of TCR signal transduction. A) Presignal. B) Signal initiation. C) Time point ∼15 s. D) Time point ∼30 s. E) Time point ∼60 s. F) Signal initiation attenuated in the presence of Hg2+.
Studies with susceptible rodent strains have shown that exposure to subtoxic doses of inorganic mercury induces an autoimmune dysfunction characterized by the production of antinuclear antibodies, lymphoproliferation, and hyperglobulinemia (39,40,41). Although the details of the mechanism responsible for mercury-dependent autoimmune disease is unknown, autoimmune disease per se is the result of a breakdown in self: nonself discrimination, or immunological tolerance, which is in turn dependent on negative selection of self-reactive lymphocytes (42, 43). In the T-cell compartment, negative selection appears to be at least in part associated with the quality of the interaction between antigen and the TCR in developing cells. Strong antigen-TCR interactions lead to strong TCR signals, as measured by the level of ERK MAP kinase activation (6, 7). Developing T cells in the thymus are exposed primarily to self-antigens, and within the thymus, cells with high TCR avidity to self-antigens generate the strongest signals. These cells are subsequently targeted for elimination from the immune repertoire (42, 43). Hg2+, by attenuating TCR signal strength, may allow self-reactive cells to escape elimination.
In these experiments, we have determined a mechanism whereby Hg2+ attenuates TCR signaling in vitro and that may also be operative in vivo. Although the predominant environmental exposure to mercury is organic mercury, in these in vitro experiments we have chosen to work with Hg2+ because it has been shown that, while organic forms of mercury are generally associated with cell death, Hg2+ is associated with autoimmunity. Significantly, in vivo, organic forms of mercury have been shown to metabolize to Hg2+. Hence, for our experiments Hg2+ is the biologically relevant form of mercury (reviewed in ref. 44).
The exposure conditions that we are using are also biologically relevant. It is always difficult to reconcile in vitro toxicant exposures with in vivo exposures. However, in this case, the applicable metric is mercury burden (wt Hg/gm wet tissue wt), as control experiments in which Hg2+ was removed from the medium after the cells had been exposed but before the TCR was stimulated, give similar results to experiments in which Hg2+ remains in the medium after TCR triggering (not shown). Although 5 μM Hg2+ may perhaps at first seem to be a bit high, during these experiments cells have been exposed to mercury for only brief periods, 10 min or so. Additional control experiments based on cold vapor atomic absorption suggest that under these conditions the resulting mercury burden is 3 to 4 parts per million (not shown). This range is well within that of mercury burdens (averaging between 2 and 19 parts per million) reported for spleen, as derived from autopsy data within a random population (45).
Acknowledgments
This work was supported in part by NIH grants ES11000, ES12403 and ES01247.
References
- Bernier J, Brousseau P, Krzystyniak K, Tryphonas H, Fournier M. Immunotoxicity of heavy metals in relation to Great Lakes. Environ Health Perspect. 1995;103:23–34. doi: 10.1289/ehp.95103s923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M, Abedi-Valugerdi M. Mercuric chloride induces a strong immune activation, but does not accelerate the development of dermal fibrosis in tight skin 1 mice. Scand J Immunol. 2004;59:469–477. doi: 10.1111/j.0300-9475.2004.01415.x. [DOI] [PubMed] [Google Scholar]
- Hong R. Evaluation of toxic assault on the immune system. Mutat Res. 1999;428:109–114. doi: 10.1016/s1383-5742(99)00038-1. [DOI] [PubMed] [Google Scholar]
- Kazantzis G. Mercury exposure and early effects: an overview. Med Lav. 2002;93:139–147. [PubMed] [Google Scholar]
- Franklin R A, Tordai A, Patel H, Gardner A M, Johnson G L, Gelfand E W. Ligation of the T cell receptor complex results in activation of the Ras/Raf-1/MEK/MAPK cascade in human T lymphocytes. J Clin Invest. 1994;93:2134–2140. doi: 10.1172/JCI117209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommhardt U, Scheuring Y, Bickel C, Zamoyska R, Hunig T. MEK activity regulates negative selection of immature CD4+CD8+ thymocytes. J Immunol. 2000;164:2326–2337. doi: 10.4049/jimmunol.164.5.2326. [DOI] [PubMed] [Google Scholar]
- Daniels M A, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Hollander G A, Gascoigne N R, Palmer E. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- Mattingly R R, Felczak A, Chen C C, McCabe M J J, Rosenspire A J. Low concentrations of inorganic mercury inhibit Ras activation during T cell receptor-mediated signal transduction. Toxicol Appl Pharmacol. 2001;2001 176:162–168. doi: 10.1006/taap.2001.9272. [DOI] [PubMed] [Google Scholar]
- Finco T S, Kadlecek T, Zhang W, Samelson L E, Weiss A. LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- Huang Y, Wange R L. T cell receptor signaling: beyond complex complexes. J Biol Chem. 2004;279:28827–28830. doi: 10.1074/jbc.R400012200. [DOI] [PubMed] [Google Scholar]
- Straus D B, Chan A C, Patai B, Weiss A. SH2 domain function is essential for the role of the Lck tyrosine kinase in T cell receptor signal transduction. J Biol Chem. 1996;271:9976–9981. doi: 10.1074/jbc.271.17.9976. [DOI] [PubMed] [Google Scholar]
- Abraham R T, Weiss A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat Rev Immunol. 2004;4:301–308. doi: 10.1038/nri1330. [DOI] [PubMed] [Google Scholar]
- Zhang W, Sloan-Lancaster J, Kitchen J, Trible R P, Samelson L E. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- Zhang W, Irvin B J, Trible R P, Abraham R T, Samelson L E. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. Int Immunol. 1999;11:943–950. doi: 10.1093/intimm/11.6.943. [DOI] [PubMed] [Google Scholar]
- Rahman S M, Pu M Y, Hamaguchi M, Iwamoto T, Isobe K, Nakashima I. Redox-linked ligand-independent cell surface triggering for extensive protein tyrosine phosphorylation. FEBS Lett. 1993;317:35–38. doi: 10.1016/0014-5793(93)81486-j. [DOI] [PubMed] [Google Scholar]
- Nakashima I, Pu M-Y, Nishizaki A, Rosila I, Ma L, Katano Y, Ohkusu K, Rahman S M J, Isobe K, Hamaguchi M, Saga K. Redox mechanism as alteranative to ligand binding for receptor activation delivering disregulated cellular signals. J Immunol. 1994;152:1064–1071. [PubMed] [Google Scholar]
- Akhand A A, Kato M, Suzuki H, Miyata T, Nakashima I. Level of HgCl2-mediated phosphorylation of intracellular proteins determines death of thymic T-lymphocytes with or without DNA fragmentation. J Cell Biochem. 1998;71:243–253. doi: 10.1002/(sici)1097-4644(19981101)71:2<243::aid-jcb9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Parashar A, Akhand A A, Rawar R, Furuno T, Nakanishi M, Kato M, Suzuki H, Nakashima I. Mercuric chloride induces increases in both cytoplasmic and nuclear free calcium ions through a protein phosphorylation-linked mechanism. Free Radic Biol Med. 1999;26:227–231. doi: 10.1016/s0891-5849(98)00169-5. [DOI] [PubMed] [Google Scholar]
- Ziemba S E, Mattingly R R, McCabe M J, Jr, Rosenspire A J. Inorganic mercury inhibits the activation of LAT in T-cell receptor-mediated signal transduction. Toxicol Sci. 2006;89:145–153. doi: 10.1093/toxsci/kfj029. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Takamatsu M, Iwashima M. The kinase, SH3, and SH2 domains of Lck play critical roles in T-cell activation after ZAP-70 membrane localization. Mol Cell Biol. 1996;16:7151–7160. doi: 10.1128/mcb.16.12.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashima M, Irving B A, van Oers N S, Chan A C, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- Lysechko T L, Ostergaard H L. Differential Src family kinase activity requirements for CD3 zeta phosphorylation/ZAP70 recruitment and CD3 epsilon phosphorylation. J Immunol. 2005;174:7807–7814. doi: 10.4049/jimmunol.174.12.7807. [DOI] [PubMed] [Google Scholar]
- Shephard E G, De Beer F C, von Holt C, Hapgood J. The use of Sulfosuccinimidyl-2(p-azidosalicylamido)-1,3′-dithiopropionate as a crosslinking reagent to identify cell surface receptors. Anal Biochem. 1988;168:306–313. doi: 10.1016/0003-2697(88)90323-5. [DOI] [PubMed] [Google Scholar]
- Bollag A G E S J. New York, NY, USA: Wiley-Liss; Protein Methods. 1991 [Google Scholar]
- Firestone G L, Winguth S D. Immunoprecipitation of proteins. Methods Enzymol. 1990;182:688–700. doi: 10.1016/0076-6879(90)82054-6. [DOI] [PubMed] [Google Scholar]
- Chan A C, Dalton M, Johnson R, Kong G H, Wang T, Thoma R, Kurosaki T. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 1995;14:2499–2508. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoGrasso P V, Hawkins J, Frank L J, Wisniewski D, Marcy A. Mechanism of activation for Zap-70 catalytic activity. Proc Natl Acad Sci U S A. 1996;93:12165–12170. doi: 10.1073/pnas.93.22.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios E H, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- Brdicka T, Pavlistova D, Leo A, Bruyns E, Korinek V, Angelisova P, Scherer J, Shevchenko A, Hilgert I, Cerny J, Drbal K, Kuramitsu Y, Kornacker B, Horejsi V, Schraven B. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J Exp Med. 2000;191:1591–1604. doi: 10.1084/jem.191.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard H L, Shackelford D A, Hurley T R, Johnson P, Hyman R, Sefton B M, Trowbridge I S. Expression of CD45 alters phosphorylation of the lck-encoded tyrosine protein kinase in murine lymphoma T-cell lines. Proc Natl Acac Sci U S A. 1989;86:8959–8963. doi: 10.1073/pnas.86.22.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahir McFarland E D, Hurley T R, Pingel J T, Sefton B M, Shaw A, Thomas M L. Correlation between Src family member regulation by the protein-tyrosine-phosphatase CD45 and transmembrane signaling through the T-cell receptor. Proc Natl Acad Sci U S A. 1993;90:1402–1406. doi: 10.1073/pnas.90.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron L, Abraham N, Pawson T, Veillette A. Structural requirements for enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Mol Cell Biol. 1992;12:2720–2729. doi: 10.1128/mcb.12.6.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdorf A D, Lee K H, Burack W R, Allen P M, Shaw A S. Regulation of Lck activity by CD4 and CD28 in the immunological synapse. Nat Immunol. 2002;3:259–264. doi: 10.1038/ni761. [DOI] [PubMed] [Google Scholar]
- Couture C, Songyang Z, Jascur T, Williams S, Tailor P, Cantley L C, Mustelin T. Regulation of the Lck SH2 domain by tyrosine phosphorylation. J Biol Chem. 1996;271:24880–24884. doi: 10.1074/jbc.271.40.24880. [DOI] [PubMed] [Google Scholar]
- Soula M, Rothhut B, Camoin L, Guillaume J L, Strosberg D, Vorherr T, Burn P, Meggio F, Fischer S, Fagard R. Anti-CD3 and phorbol ester induce distinct phosphorylated sites in the SH2 domain of p56lck. J Biol Chem. 1993;268:27420–27427. [PubMed] [Google Scholar]
- Brdicka T, Kadlecek T A, Roose J P, Pastuszak A W, Weiss A. Intramolecular regulatory switch in ZAP-70: analogy with receptor tyrosine kinases. Mol Cell Biol. 2005;25:4924–4933. doi: 10.1128/MCB.25.12.4924-4933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher L A, Ohashi P S, van Oers N S. T cell antagonism is functionally uncoupled from the 21- and 23-kDa tyrosine-phosphorylated TCR zeta subunits. J Immunol. 2003;171:845–852. doi: 10.4049/jimmunol.171.2.845. [DOI] [PubMed] [Google Scholar]
- Pitcher L A, van Oers N S. T-cell receptor signal transmission: who gives an ITAM? Trends Immunol. 2003;24:554–560. doi: 10.1016/j.it.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Bagenstose L M, Salgame P, Monestier M. IL-12 down-regulates autoantibody production in mercury-induced autoimmunity. J Immunol. 1998;160:1612–1617. [PubMed] [Google Scholar]
- Hu H, Moller G, Abedi-Valugerdi M. Mechanism of mercury-induced autoimmunity: both T helper 1- and T helper 2-type responses are involved. Immunology. 1999;96:348–357. doi: 10.1046/j.1365-2567.1999.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Monestier M. Inhibitory signal override increases susceptibility to mercury-induced autoimmunity. J Immunol. 2003;171:1596–1601. doi: 10.4049/jimmunol.171.3.1596. [DOI] [PubMed] [Google Scholar]
- Goodnow C C. Balancing immunity and tolerance: deleting and tuning lymphocyte repertoires. Proc Natl Acac Sci U S A. 1996;93:2264–2271. doi: 10.1073/pnas.93.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto H, Sprent J. The thymus and central tolerance. Clin Immunol. 2000;95:S3–S7. doi: 10.1006/clim.1999.4818. [DOI] [PubMed] [Google Scholar]
- Havarinasab S, Hultman P. Organic mercury compounds and autoimmunity. Autoimmun Rev. 2005;4:270–275. doi: 10.1016/j.autrev.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Kevorkian J, Cento D P, Hyland J R, Bagozzi W M, Van Hollebeke E. Mercury content of human tissues during the twentieth century. Am J Public Health. 1972;62:504–513. doi: 10.2105/ajph.62.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]