Abstract
Amyotrophic lateral sclerosis (ALS) is an adult-onset neurodegenerative disease characterized by selective degeneration of motor neurons and glial activation. Cell-specific transcriptional regulation induced by oxidative stress may contribute to the survival and activation of astrocytes in the face of motor neuron death. In the present study, we demonstrate an age-dependent increase in Bcl-xL and Ets-2 immunoreactivity that correlates with an increase of glial fibrillary acidic protein (GFAP)-positive cells in the ventral horn of the spinal cord in both ALS transgenic mice [mutant SOD1 (G93A)] and affected humans. Chromatin immunoprecipitation (ChIP) analysis verified that Ets-2 preferentially occupies the Ets-2 binding element in the promoter of Bcl-xL in primary astrocytes under oxidative stress conditions as well as in G93A spinal cords. Ets-2 small-interfering RNA down-regulated the transcriptional activity of Bcl-xL. In primary glial cultures, Bcl-xL overexpression and mutant SOD1 (G93A) both conferred resistance to oxidative stress-induced cell death. Our findings suggest that Ets-2 transcription factor activation of Bcl-xL gene may protect glia from constitutive oxidative stress that is thought to be a key mechanism contributing to the pathogenesis of ALS. This survival pathway may contribute to the glial survival and activation seen in the spinal cord of ALS patients.—Lee, J., Kannagi, M., Ferrante, R. J., Kowall, N. W., Ryu, H. Activation of Ets-2 by oxidative stress induces Bcl-xL expression and accounts for glial survival in amyotrophic lateral sclerosis.
Keywords: transcription, mitochondria, astrocytes, neurodegeneration, motor neurons
Amyotrophic lateral sclerosis (ALS) is an adult-onset neurodegenerative disease characterized by selective degeneration of motor neurons located in the spinal cord, brain stem, and motor cortex, resulting in progressive atrophy and paralysis of limb, bulbar, and respiratory muscles (1,2,3). The incidence of ALS is 1–2/100,000/yr, with a prognosis of death within 2–5 yr from disease onset due the denervation of the respiratory muscles and diaphragm (1,2,3). Approximately 5–10% of ALS cases are familial (autosomal-dominant), and no genetic association has been identified in 90–95% of all cases of “sporadic” ALS (3). Approximately 25% of the familial cases have been found to contain missense mutations in the gene encoding copper-zinc superoxide dismutase (SOD1) (4). Transgenic mice expressing human mutant SOD1 (mSOD1) develop age-dependent clinical and pathological features closely mirroring those found in human ALS and thus provide a comprehensive model to study the mechanisms of pathogenesis that may underlie the human disease (5,6,7,8). One of the most common mutations of SOD1 is the substitution of glycine by alanine at residue 93 (G93A) (3). The expression of mtSOD1 (G93A) is directly linked to an increased reactive oxygen species (ROS) level in neuronal tissues, creating oxidative stress that is responsible for the ALS-like symptoms. As a gain-of-function mutation, increased oxidative modification of macromolecules has been found in the spinal cord of mtSOD1-related familial ALS (FALS) patients. The mtSOD1 (G93A) transgenic mouse model has shown similar pathological features that were found in FALS (7). mtSOD1 (G93A) transgenic mice significantly develop motor neuron disease. In this regard, the mtSOD1 (G93A) mouse model has been contributed and utilized to better understand the neuropathological mechanisms of ALS.
Oxidative stress is also implicated in the pathogenesis of ALS and is linked to defects in oxidative phosphorylation. Metabolic processes involving the mitochondrial electron transport chain are known to contribute to the formation of harmful ROS. Superoxide anion, an unavoidable by-product of respiration, is a toxic radical that has been implicated in cellular damage such as DNA oxidation, protein oxidation, and lipid peroxidation (9). In the cytoplasm, the superoxide anion is neutralized by SOD1, an antioxidant enzyme that catalyzes the conversion of two superoxide radicals to hydrogen peroxide and molecular oxygen. The reactive hydrogen peroxide is then further broken down by the enzyme catalase to yield two relatively benign products. When a cell loses its ability to effectively neutralize ROS, oxidation of vital cellular structures disrupts homeostasis and a state of oxidative stress ensues. In ALS, motor neurons are particularly vulnerable to oxidative stress, a phenomena attributed to a low level of antioxidant enzymes, a high content of easily oxidized substrates, and an inherently high flux of ROS generated during energy metabolism (1,2,3). Therefore, based on the well-characterized and essential function of SOD1 in limiting free radical accumulation, previous studies have examined the associations between SOD1 mutations, the generation of pathological oxidative damage, and subsequent motor neuron degeneration. Mutant SOD1 transgenic mice show progressive accumulation of 8-hydroxy-2-deoxyguanosine, a marker of oxidative DNA damage, and elevated levels of mitochondrial oxidative damage (10, 11). Oxidative damage to spinal cord proteins has also been shown to occur in human ALS (12). These data support a prominent role for oxidative stress in ALS pathogenesis.
In contrast to progressive neuronal death, another prominent feature of ALS pathology is the generation and migration of new cells, specifically astrocytes, within and around damaged regions (2). Astrocytes respond to damage by proliferating and adopting a reactive phenotype characterized by the development of long and thick processes with an increased content of glial fibrillary acidic protein (GFAP). Interestingly, a similar increase in GFAP immunoreactivity was found when cultured spinal cord astrocytes were treated under oxidative stress inducing conditions, suggesting that such morphological changes may be provoked by oxidative stress (2).
Bcl-xL is a mitochondrial protein and known anti-apoptotic member of the Bcl-2 family. In addition, Bcl-xL has been shown to protect the mitochondrial membrane potential against insult, prevent apoptotic cell death, and protect against hydrogen peroxide-induced cell death when overexpressed in astrocytes (13). Expression of the Bcl-xL gene is specifically regulated by many transcription factors (14). Interestingly, a recent gene microarray analysis found that the GFAP and Bcl-xL genes are correlatively elevated in ALS (G93A) mice (15). These observations suggested to us that the up-regulation of Bcl-xL expression may be an astrocytic response to ALS progression. We hypothesized that the induction of Bcl-xL in astrocytes constitutes a survival pathway utilized under conditions of oxidative stress. Therefore, in the present study, we examined the possibility of a transcriptional activation mechanism for the Bcl-xL gene occurring within astrocytes and further characterized the sequence of events leading from oxidative stress to subsequent Bcl-xL induction. We found that Bcl-xL gene expression is strongly induced by oxidative stress and confirmed that the Ets-2 transcription factor mediates this effect in both in vitro and in vivo models of ALS. Furthermore, we demonstrated that Bcl-xL confers protection from cell death to astrocytes and thus represents a prosurvival pathway under oxidative stress.
MATERIALS AND METHODS
Animals
Male transgenic ALS mice of the G93A H1 high-expresser strain (The Jackson Laboratory, Bar Harbor, ME, USA) were bred with females with a similar background (B6/SJLF1). Offspring were genotyped using a polymerase chain reaction (PCR) assay on tail DNA. To ensure homogeneity of the cohorts tested, we have developed a standardized method to select mice. Body weights were taken at 20 days, and mice were equally distributed according to weight within each experimental cohort. Mice under 8 g at 20 days were excluded from the experiments (5). These experiments were carried out in accordance with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by both the Veterans Administration and Boston University Animal Care Committees.
Primary glia cultures from ALS transgenic (G93A) mouse
Astrocytes were cultured from newborn wild-type and ALS transgenic (G93A) mouse pups (1 day old). Cortexes were isolated and minced. A single-cell suspension was obtained by trypsin digestion and mechanical dissociation. The cells were further passed sequentially through 135 μm and then 20 μm pore size sterile nylon meshes. The mixture was diluted to 1 hemisphere/75 cm2 culture flask (Nunc, Rochester, NY, USA). After seeding, the cells were incubated in a humidified incubator under 5% CO2/95% air at 37°C. The culture medium was Eagle basal medium containing Glutamax-I and supplemented with 10% heat-inactivated fetal bovine serum, glucose (33 mM), penicillin (20 UI/ml), and streptomycin (20 μg/ml). The medium was renewed twice a week. Astrocytes were collected after 10–12 days of incubation when the cells became confluent. Microglia, oligodendrocytes, and other nonadherent cells were removed by shaking at 260 rpm overnight at 37°C in an orbital shaker. Before biochemical assays, astrocytes were subcultured onto 24-multiwell plates or 8-chamber slides for 24 h at different cell-plating densities per well. The cultures contained over 95% astrocytes. Astrocyte purity was assessed as previously published (16). After 80% confluence, cells were treated with H2O2 (0.05–1 mM) or with xanthine/xanthine-oxidase (X/XO) for 6, 12, or 24 h. After this treatment period, cells were incubated with a 10% heat-inactivated fetal bovine serum, and then cell injury and cell viability were determined by 3-(4,5-dimethyl-2-thizolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assays.
Plasmid DNA and RNA interference transfection
pCMV-Flag-control and pCMV-Flag-Ets-2 constructs were generous gifts from Dr. B. J. Graves (Department of Oncological Sciences, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA). pcDNA3-Flag-Sp1 and pcDNA3-NF-κB p65 plasmids were used for the transient transfection. Cultured primary astrocytes were transfected with plasmid DNA or small-interfering RNA (siRNA) as described previously (16,17,18,19) with some modifications. Briefly, astrocytes were maintained in DMEM supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). Cells were seeded in 48-well plates for cell viability assay and 8-chamber slides for confocal microscopy. For transfection, plasmid DNA and RNA interference (RNAi) were diluted in Opti-MEM and mixed with DMRIE-C reagent (Invitrogen, Carlsbad, CA, USA). Plasmid DNAs were used at a final concentration of 1 mg/ml. Validated stealth control RNAi and Ets-2 RNAi were purchased (Invitrogen Life Technologies). Ets-2 RNAi sequence was as follows: 5′-GCAGCCAGUCUCUCUGCCUCAAUAA-3′. RNAi was used at a final concentration of 20 nM.
Immunofluorescence staining and confocal microscopy
Indirect labeling methods were used to identify Bcl-xL, GFAP, SMI32, and Ets-2 in primary astrocytes and spinal cord sections from ALS mice as described previously (17, 18). For the confocal microscopy, the specimens were incubated for 1 h with fluorescence (FITC)-conjugated goat anti-mouse IgG antibody (Vector, Burlingame, CA, USA) and Cy3-conjugated anti-rabbit IgG antibody (The Jackson Laboratory) after the incubation of primary antibody. Images were analyzed using a spinning disk confocal microscope (IX81-DSU; Olympus, Tokyo, Japan). Preabsorbtion with excess target protein or omission of primary antibody was used to demonstrate antibody specificity and background generated from the detection assay.
Western blotting
Tissue lysates and subcellular fractions from wild-type and G93A mice were prepared using an ice-cold cell extraction buffer containing 50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 2 mM EDTA; 1% Triton X-100; 1 mM PMSF; 10 mg/ml leupeptin; 1 mM pepstatin; 1 mM N-ethylmleimide; 2 mM Na3VO4; 20 mM sodium pyrophosphate; and 50 mM NaF). Lysates were centrifuged at 15,000 rpm at 4°C for 30 min, and the supernatant fraction was kept. The protein concentration was quantified, and the samples were boiled for 10 min with Laemmli buffer (100 mM Tris-HCl, pH 6.8; 4% SDS; 200 mM dithiothreitol; 20% glycerol; 2% SDS; 0.2% bromphenol blue; 10 mg/ml aprotinin; and 10 mg/ml leupeptin) at 100°C. In general, 30 mg of proteins was electrophoresed on 10% SDS-polyacrylamide gel and transferred to nitrocellulose membrane. Membranes were blocked in 5% skim milk in TBST (Tris, pH 7.4; 150 mM NaCl; and 0.05% Tween 20) for 30 min at room temperature. Blots were probed with primary antibodies overnight at 4°C. The antibodies used were the following; GFAP, Bcl-xL (Santa Cruz Biotechnology, Santa Cruz, CA, USA; dilution 1:500), Ets-2 (Santa Cruz Biotechnology; dilution 1:500), α-tubulin, and β-actin (Sigma) at 1:3000. Horseradish perioxidase-conjugated secondary IgG anti-rabbit IgG and anti-mouse IgG (Bio-Rad Laboratories, Richmond, CA, USA) were used at 1:5000 and 1:3000, respectively.
Reverse transcriptase-PCR analysis
The spinal cord was snap frozen on dry ice and total RNA extraction was immediately performed from the lumbar portion using RNeasy lipid tissue mini-kit (Qiagen, Valencia, CA, USA; ref. 17). RNA was measured in a spectrophotometer at 260 nm absorbance. Fifty nanograms of RNA was used as a template for quantitative reverse transcriptase (RT)-PCR amplification, using Superscript One-Step RT-PCR with platinum Taq (Invitrogen). Primers were standardized in the linear range of cycles before onset of the plateau. The sequence of the primers was as follows: Bcl-xL forward: 5′-TGGAGTAAACTGGGGGTCGCATCG-3′; Bcl-xL reverse: 5′-AGCCACCGTCATGCCCGTCAGG-3′; GFAP forward: 5′-GGCCACCAGTAACATGCAAGAGA-3′; GFAP reverse: 5′-TTTCCTGTAGGTGGCGATCTCGA-3′; 18S RNA forward: 5′-CCGAGATTGAGCAATAACAGG-3′; and 18S RNA reverse: 5′-AGTTCGACCGTCTTCTCAGG-3′.
The conditions of one-step RT-PCR for Bcl-2 primers were as follows: 30 min at 50°C, 2 min at 94°C, then 32 cycles of amplification for 15 s at 94°C for 15 s, 30 s at 68°C, 1 min at 70°C, 10 min at 72°C, and 4°C. GAPDH and 18S RNA primers were processed at 34 cycles and 55°C for the annealing step. Amplified cDNA was detected by ethidium bromide staining and quantified with ChemiImager 4400 (Alpha-Innotech, San Leandro, CA, USA). A Bcl-xL/18S ratio was calculated for each sample. Experiments were run twice to verify the results.
Promoter activity assay
To study the responsiveness of Bcl-xL to oxidative stress, we used Bcl-xL promoter constructs that were either responsive to κB (wild type) or mutated to be unresponsive to this factor as described previously (20, 21). We used the mouse Bcl-xL promoter-driven luciferase (Luc) reporter plasmid pGL2–3.2, which contained a 3.2-kbp fragment upstream of the Bcl-xL gene. pGL2 (−848), pGL2 (−822), and pGL2 (−631) were newly generated by insertion of DNA fragments of the Bcl-xL promoter, respectively, into the basic pGL2 vector containing a Luc reporter gene (Promega, Madison, WI, USA). pGL2κBM is a mutant of pGL2 (−848), carrying CC to GG mutations within the NF-κB binding site: the mutant construct (κBM) (GGGACTT[b]GG) for the kB DNA binding motif (GGGACTT[b]CC). Mutation in the ACC/GGT element −753/−751 in the Bcl-xL promoter was generated by site-directed mutagenesis (Stratagene, La Jolla, CA, USA). Plasmid integrity was confirmed by DNA sequencing. The primary astrocytes were transfected by the DMRIE-C solution (Invitrogen) with reporter plasmids. After 24 h of incubation, cells were treated with hydrogen peroxide for another 24 h and harvested, and Luc activity in the cellular lysates was measured with a luminometer. All promoter assays were performed in triplicate. Enzyme activities were normalized to the protein concentration of the extracts.
Chromatin immunoprecipitation (ChIP) analysis of the Bcl-xL promoter region with Ets2, NF-κB, and Sp1
ChIP analysis for the Bcl-xL promoter region was performed using a ChIP assay kit (Upstate Biotech, Lake Placid, NY, USA; refs. 17, 18). The 120-day-old G93A mouse spinal cord was homogenized and crossed-linked with 1% formaldehyde for 20 min at room temperature. After being washed twice with ice-cold PBS containing protease inhibitors by centrifugation, cell pellets were resuspended in SDS lysis buffer. After incubation for 10 min at 4°C, the lysates were sonicated 6 times, each time for 20 s, using Branson Sonifier 450 (Branson Ultrasonics, Danbury, CT, USA). After centrifugation, the supernatant was diluted in ChIP dilution buffer and then incubated overnight at 4°C with Ets2, NF-κB, and Sp1 antibodies. Immune complexes were recovered by the addition of 60 ml of salmon sperm DNA/protein A agarose-50% slurry and incubation for 2 h at 4°C with rotation. The beads were pelleted and washed with low and high salt buffer, LiCl buffer, and finally twice with Tris-EDTA (TE) buffer. The immune complexes were eluted by incubation for 15 min at 37°C with fresh elution buffer (1% SDS, 0.1 M NaHCO3). To reverse the cross-linking of DNA, 10 ml of 5 M NaCl was added to the combined elute (150 μl) and incubated overnight at 65°C. DNA was purified using QIA quick DNA purification spin column and eluted in 50 ml of nuclease-free water. PCR amplification was carried out for 35 cycles, and PCR products were separated on 2% agarose gels. Primer set for Bcl-xL was used. Forward primer was 5′-GGGAGTGGTCTTTCCGAA-3′, and reverse primer was 5′-CTCCATCGACCAGATCGA-3′.
Histopathological evaluation
Serially cut lumbar spinal cord tissue sections were stained for Nissl substance and immunostained for GFAP, Bcl-xL (Santa Cruz Biotechnology; dilution 1:500), Ets-2 (Santa Cruz Biotechnology; dilution 1:500), NF-κB p65 (Santa Cruz Biotechnology; dilution 1:500), and Sp1 (Santa Cruz Biotechnology; dilution 1:500), using a previously reported conjugated secondary antibody method in murine brain tissue samples (5). Preabsorption with excess target proteins, omission of the primary antibodies, and omission of secondary antibodies were performed to determine the amount of background generated from the detection assay.
Statistics
The data are expressed as means ± se. Statistical comparisons of data were performed using Student’s t test.
RESULTS
Pathogenic increase of GFAP- and Bcl-xL-positive cells in the spinal cord of ALS (G93A) mice
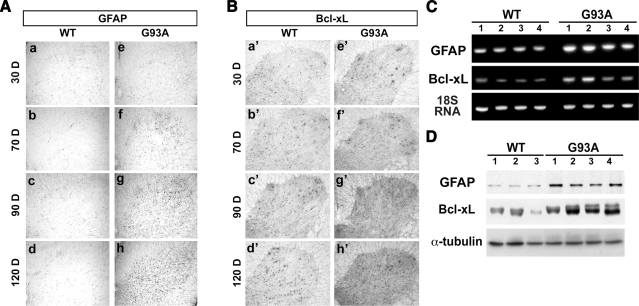
To characterize changes in ventral horn glial cell induction, lumbar spinal cord sections were prepared from wild-type and G93A animals when they reached 30, 70, 90, and 120 days of age (5). Immunohistochemistry demonstrated an age-dependent increase in GFAP-immunoreactive glial cells within the ventral horn of lumbar spinal cord sections from G93A mice, as compared with wild-type littermate controls (Fig. 1A). A significant increase of GFAP staining in astrocytes was seen at the 90- and 120-day time points (Fig. 1Ag, h) relative to control mice (Fig. 1Ac, d). In contrast, G93A spinal cord sections at the 30- and 70-day time points (Fig. 1Ae, f) did not reveal a significant difference in GFAP immunoreactivity as compared with the wild-type littermate controls (Fig. 1Aa, b). The control staining without GFAP primary antibody showed no signals for the immunoreactivity of GFAP in wild-type and G93A transgenic mice at 70 and 120 days (Supplemental Fig. 1). Therefore, the immunohistochemistry data with anti-GFAP antibody indicate the net increase of GFAP over time in G93A mice. Immunohistochemistry further demonstrated an age-dependent increase in Bcl-xL-immunoreactive glial cells within the ventral horn of lumbar spinal cord sections from G93A mice, as compared with wild-type littermate controls (Fig. 1B). A significant increase in Bcl-xL staining in astrocytes is seen at the 90- and 120-day time points (Fig. 1Bg′, h′) relative to control mice (Fig. 1Bc′, d′). In contrast, G93A spinal cord sections at the 30- and 70-day time points (Fig. 1Be′, f′) revealed no significant difference in Bcl-xL immunoreactivity as compared with control animals (Fig. 1Ba′, b′). The significant shift in Bcl-xL immunoreactivity from motor neurons at the 30-and 70-day time points to activated astrocytes at the 90- and 120-day time points was only found in G93A animals. As seen in Fig. 1B, the level of Bcl-xL immunoreactivity within the wild-type littermate control remained relatively constant at each time point.
Figure 1.
Glial activation and Bcl-xL expression in transgenic ALS (G93A) mice. A) Age-dependent (30, 70, 90, and 120 days) increase of GFAP immunoreactivity in the ventral horn of lumbar spinal cord sections in G93A mice (e–h) but not in wild-type (WT) littermate control mice (a–d). B) Age-dependent increase of Bcl-xL immunoreactivity in the ventral horn of lumbar spinal cord sections in G93A mice (e′–h′) compared with WT mice (a′–d′). C) Up-regulation of mRNA levels of GFAP and Bcl-xL in the lumbar spinal cord of G93A mice at 120 days. D) Increased levels of the GFAP and Bcl-xL protein in the spinal cords of G93A mice at 120 days. Each lane corresponds to a sample from an individual mouse.
RT-PCR analysis was performed to detect GFAP, Bcl-xL, and 18S RNA in control mice and ALS (G93A) mice at 120 days as described in Materials and Methods. We found that transcripts of GFAP and Bcl-xL were significantly increased in ALS (G93A) mice (Fig. 1C). Quantitative real-time PCR further confirmed that GFAP and Bcl-xL increased 5.24- and 2.89-fold, respectively, in the lumbar spinal cord of G93A mice compared with control (Supplemental Fig. 2). Likewise, Western blot analysis revealed an increase in Bcl-xL and GFAP protein levels in ALS (G93A) mice as compared with wild-type control animals, whereas no differences were found in α-tubulin levels between groups (Fig. 1D).
Increase of Bcl-xL-positive astrocytes vs. decrease of Bcl-xL-positive motor neurons in the spinal cord of human and ALS (G93A) mice
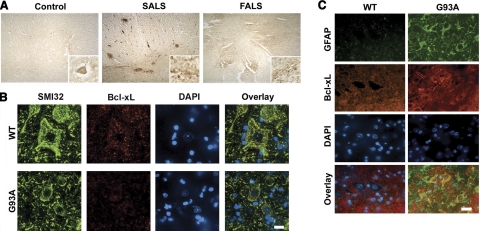
Immunohistochemistry demonstrated a reduction of Bcl-xL-immunoreactive motor neurons but a marked increase in Bcl-xL-immunoreactive glial cells within the ventral horn of lumbar spinal cord sections from sporadic ALS (SALS) and FALS patients as compared with age-matched controls (Fig. 2A). Similar to humans, control samples, the immunoreactivity of Bcl-xL was found in SMI32-positive motor neurons of control mice (Fig. 2B). The Bcl-xL immunoreactivity in motor neurons disappeared in ALS (G93A) mice as compared with the control mice. Otherwise, the increase of Bcl-xL immunoreactivity was localized and determined within GFAP-positive glial cells that infiltrated the ventral horn as seen by confocal microscopy (Fig. 2C).
Figure 2.
Decrease of Bcl-xL immunoreactivity in motor neurons vs. increase of Bcl-xL-positive astrocytes in the spinal cord of human ALS and transgenic ALS (G93A) mice. A) Gain of Bcl-xL immunoreactivity in glia but loss in motor neurons in the spinal cord of human SALS and FALS. B) Loss of Bcl-xL immunoreactivity in motor neurons (SMI32-positive cells) of G93A mice. Localization of SMI32 and Bcl-xL immunoreactivity in the ventral horn of lumbar spinal cord sections in 120-day WT and G93A mice. C) Colocalization of Bcl-xL immunoreactivity with GFAP-positive cells in G93A mouse. Scale bars = 10 μm.
Oxidative stress induces the transcriptional activation of Bcl-xL gene expression in glial cells
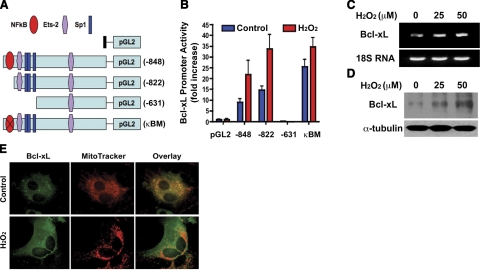
To investigate under what transcriptional mechanism Bcl-xL is induced in GFAP-positive glial cells in the pathogenesis of ALS, we mapped transcription factor binding elements in the mouse Bcl-xL promoter (Fig. 3A). Several consensus motifs for the binding of transcription factors such as Sp1, Ets-2, and NF-κB have been identified (22, 23). An NF-κB binding motif (GGGACTTCC) similar to the one in the immunoglobulin promoter was located at positions −848 to −840 of the Bcl-xL promoter in an inverted direction. To examine the contributions of these sites to oxidative stress-induced transactivation, a series of luciferase reporter constructs with several fragments of different lengths of 5′ deleted Bcl-xL promoters were prepared and transiently transfected into primary astrocytes and incubated for 24 h. Cells were treated with hydrogen peroxide and incubated another 24 h (Fig. 3B). The highest level of oxidative stress-induced Bcl-xL luciferase activity was observed in primary astrocytes transfected with the −822 construct. The Ets-2 and Sp1 binding site deletion construct pGL2 (−631) did not respond to oxidative stress. Furthermore, a mutated NF-κB-Luc plasmid, pGL2κBM, which had the same length as pGL2 (−848) but had a CC to GG mutation at positions −841 and −840 within the NF-κB motif, exhibited markedly increased levels of basal and oxidative stress-induced transactivation similar to pGL2 (−822). These results suggest that the NF-κB site located at positions −848 to −840 in the Bcl-xL gene promoter region is not the major response element of oxidative stress-induced transactivation of Bcl-xL.
Figure 3.
Transcriptional activation of Bcl-xL gene by oxidative stress in primary glial cells. A) A schematically illustrated series of Luc reporter plasmids based on pGL2 vectors containing fragments of 5′ flanking region of the Bcl-xL gene. Locations of the binding sites for transcription factors NF-κB, Ets-2, and Sp1 are indicated. pGL2κBM contained mutations within the NF-κB site as described in Materials and Methods. B) Oxidative stress induces transcriptional activity of Bcl-xL. Transfected glia were treated with H2O2 (50 μM) for 12 h. Luciferase activity was normalized to total protein concentration. C) Oxidative stress up-regulates Bcl-xL mRNA expression in primary astrocytes. D) Oxidative stress increases the protein level of Bcl-xL in primary astrocytes. E) Increased immunoreactivity of Bcl-xL in primary astrocytes in response to oxidative stress. H2O2 (50 μM) was treated for 12 h.
To examine whether the oxidative stress-induced increase of Bcl-xL transactivation is correlated with gene expression, we measured the mRNA levels of Bcl-xL in primary astrocytes. The result is shown in Fig. 3C. The mRNA level of Bcl-xL was increased after 12 h of hydrogen peroxide treatment. Expression of Bcl-xL protein was also increased by oxidative stress (Fig. 3D). In addition, confocal microscopy showed increased mitochondrial membrane potential in hydrogen peroxide treated astrocytes as well as an increase in Bcl-xL immunoreactivity (Fig. 3E). We consider that the fluorescence intensity change of MitoTracker was due to the increase of membrane potential and, in part, due to the change of mitochondrial shape. We found that hydrogen peroxide treatment affects the morphology of mitochondria in primary astrocytes. Especially, thread shapes of mitochondria were changed into more round shapes in response to oxidative stress.
Ets-2 drives the transcriptional activation of Bcl-xL in response to oxidative stress
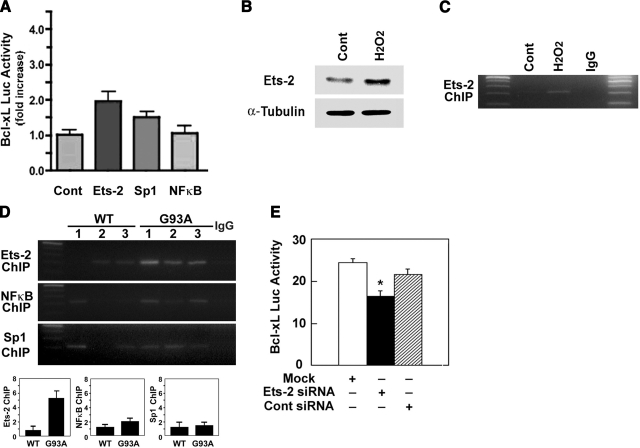
To determine which transcription factors are involved in Bcl-xL transactivation in astrocytes, we assessed the promoter activity of Bcl-xL using transient-transfection assays of candidate transcription factors. We overexpressed Ets-2, Sp1, and NF-κB in primary astrocytes using the pGL2 (−822) construct for 48 h and measured the luciferase activity. Interestingly, Ets-2 induced the highest (>2-fold) while Sp1 induced a 1.5-fold increase of Bcl-xL luciferase activity. However, NF-κB transactivation of Bcl-xL was almost equivalent to control cells (Fig. 4A). These results indicate that Ets-2 is a major factor in Bcl-xL transactivation in astrocytes, presumably under normal conditions and in the presence of oxidative stress. Thus, our data support a transcriptional activation mechanism for the Bcl-xL promoter occurring within astrocytes.
Figure 4.
Ets-2 induces transcription of Bcl-xL in response to oxidative stress. A) Ets-2 drives the transcriptional activity of Bcl-xL promoter. Primary glia was cotransfected with Ets-2, Sp1, NF-κB, and a control plasmid (pcDNA3) along with Bcl-xL promoter. Note that there is no change in the luciferase activity by NF-κB transfection. B) Oxidative stress (100 μM H2O2) activates and elevates the level of Ets-2 transcription factor in primary glia. C) ChIP confirms oxidative stress increases a robust occupancy of Bcl-xL promoter by Ets-2 transcription factor in primary glia. D) ChIP identifies the strongest occupancy of Bcl-xL promoter by Ets-2 transcription factor in the spinal cord of transgenic ALS (G93A) mouse. E) siRNA of Ets-2 down-regulates Bcl-xL transcriptional activity in primary glial cultures. *P < 0.05.
Using ChIP analysis, we further examined which transcription factors can actually occupy the putative element identified in the Bcl-xL promoter under oxidative stress in vitro and in vivo. Oxidative stress increased the Ets-2 level in primary astrocytes in vitro (Fig. 4B). In addition, as shown in Fig. 4C, a clear Ets-2 ChIP band was observed when primary astrocytes were treated with oxidative stress in vitro. Moreover, similar results were observed with ALS (G93A) mice. The Ets-2 DNA occupancy in the Bcl-xL promoter was most significantly increased (>5-fold) compared with Sp1 and NF-κB transcription factors in the spinal cord of ALS (G93A) mice (Fig. 4D). These data provide strong evidence that Bcl-xL gene expression induced by oxidative stress is mediated by Ets-2 in vivo. To further examine the contribution of Ets-2 to Bcl-xL promoter activity, primary astrocytes were transiently transfected with Ets-2 siRNA along with a reporter plasmid [pGL2 (−822)]. An RNAi study confirmed that Bcl-xL promoter activity is down-regulated (>30%) by Ets-2 siRNA but not by control siRNA (Fig. 4E).
To examine the effect of Ets-2 knockdown on glial survival, we transiently transfected control and Ets-2 RNAi in primary astrocyte cultures from wild-type and G93A transgenic mice and determined the cell viability (Supplemental Fig. 3). As shown in the Supplemental Fig. 3, loss-of-function of Ets-2 by means of RNAi leads to cell death phenotypes that are further affected by oxidative stress.
Mutation of Ets-2 DNA binding element abolishes oxidative stress-induced Bcl-xL promoter activity
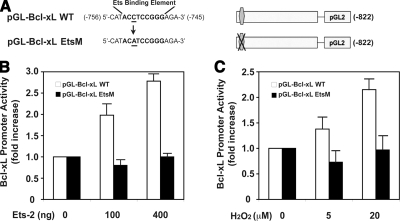
To confirm the functional Ets DNA binding element in the Bcl-xL promoter, we introduced a mutation by site-directed mutagenesis into CCT/GGA element sites, −753/−745, which was showing evident Ets-2 DNA binding activity by ChIP (Fig. 4). As we expected, the mutation in Ets-2 DNA binding sites of the Bcl-xL promoter abolished the promoter activity (Fig. 5A). A mutation of CCT/GGA element in the Bcl-xL promoter markedly decreased the basal luciferase activity ∼20% (Fig. 5B). In addition, the mutation abrogated Ets-2-induced luciferase activity of the Bcl-xL promoter, which is close to the basal expression level. Because we were interested in determining whether Ets-2 is involved in Bcl-xL expression subjected to oxidative stress, we further examined the promoter activity of mutated Bcl-xL on H2O2 treatment (Fig. 5C). Wild-type Bcl-xL promoter construct showed a dose-dependent luciferase activity in response to H2O2 as compared with its own control. The mutation in Ets-2 element reduced Bcl-xL promoter activity on H2O2 treatment. These data indicate that the −753/−745 position serves as a specific Ets-2 recognition site on oxidative stress. Therefore, we could conclude that the Ets-2 site is required both for the basal and oxidative stress-induced expression of Bcl-xL.
Figure 5.
A mutation in Ets-2 DNA binding site of Bcl-xL promoter abolishes the oxidative stress-induced promoter activity. A) Mutation in ACC/GGT element −753/−751 in the Bcl-xL promoter was generated by site-directed mutagenesis. Bold characters represent an Ets-2 DNA binding element (−753/−745). Bold underlined character is mutated (from ACC to ACA). B) Mutation in Ets-2 DNA binding element in Bcl-xL promoter abrogates Ets-2-induced reporter activity. C) Oxidative stress (5 and 20 μM H2O2) induced Bcl-xL promoter activity was reduced with the mutation. Luciferase activity was normalized with protein concentration of neuronal extract. Error bars = se of 3 experiments.
Increase of nuclear Ets-2 immunoreactivity in GFAP-positive cells
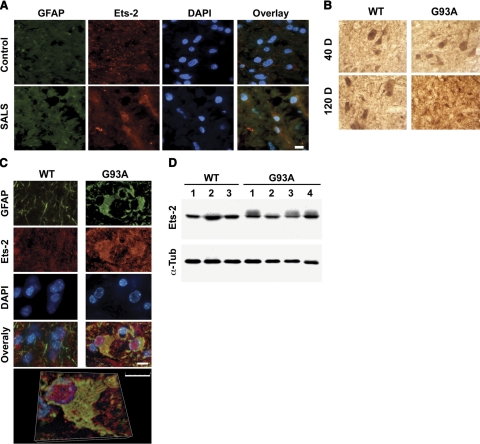
Confocal microscopy demonstrated a marked increase in Ets-2 and GFAP immunoreactivity in glial cells within the ventral horn of lumbar spinal cord sections from SALS as compared with age-matched controls (Fig. 6A). Immunohistochemistry also showed a marked increase in Ets-2-immunoreactive glial cells within the ventral horn of lumbar spinal cord sections from 120-day G93A mice as compared with wild-type littermate controls (Fig. 6B). No difference was found in Ets-2 staining between wild-type and G93A animals at the 40-day time point. Confocal microscopy determined that the increased Ets-2 immunoreactivity was primarily localized within glial cells that had infiltrated the ventral horn at 90 days (data not shown) and 120 days (Fig. 6C). Of note is the nuclear localization of Ets-2 in GFAP-positive cells in the ventral horn of ALS (G93A) spinal cord at 120 days. Western blot analysis showed increased protein levels of Ets-2 (phosphorylated form) in the spinal cord of G93A mice (Fig. 6D). The appearance of slow migrating Ets-2 bands in G93A spinal cord samples indicates that Ets-2 may be phosphorylated in vivo.
Figure 6.
Ets-2 induction in the glia of human SALS and transgenic ALS (G93A) mice. A) Elevation of Ets-2 immunoreactivity in the ventral horn of lumbar spinal cord sections from human SALS. B) Ets-2 immunoreactivity in the ventral horn of lumbar spinal cord sections at 40-and 120-day time points in WT and G93A mice. At 120 days, G93A mice show a decrease in motor neuron staining and the appearance of small, round, and densely stained forms indicative of astrocyte morphology, as compared with control WT mice. C) Colocalizaton of Ets-2 immunoreactivity with GFAP-positive cells in ALS (G93A) mouse. D) Increased level of the phosphorylated Ets-2 (p-Ets-2) protein in the spinal cords of G93A mice at 120 days. Scale bars = 10 μm.
To determine whether enforced expression of Ets-2 directly affects the level of Bcl-xL in primary astocytes, we transiently overexpressed pCMV-Flag and -Ets-2 plasmids in primary astrocytes. Confocal microscopic data indicated that Ets-2 overexpression elevated the level of Bcl-xL in primary astrocytes compared with control vector overexpression (Supplemental Fig. 4A, B). The analysis of xz and zy stacked images (originated from Supplemental Fig. 4Ab, f) using AQI-X-COMBO-CWF software (MediaCybernetics, Bethesda, MD, USA) confirms the increase in Bcl-xL in primary astrocytes. Bcl-xL density was increased 2-fold by Ets-2 overexpression.
Astrocytes from G93A mice and with Bcl-xl overexpression are both resistant to oxidative stress
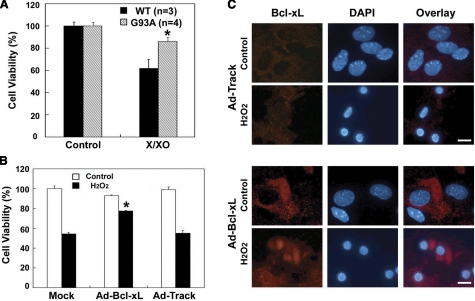
To examine whether astrocytes from G93A mice are less vulnerable to oxidative stress than wild-type mice, primary astrocyte cultures were treated with the X/XO method. When added to the cultures X/XO interact to produce the reactive chemical species hydrogen peroxide and thus can be used to model the cellular environment associated with oxidative stress. The results indicated that astrocytes obtained from ALS (G93A) mice are more resistant to cell death when exposed to oxidative stress compared with wild-type cells. Astrocytes from ALS (G93A) mice exhibited a >25% increase in cell viability compared with control cells (Fig. 7A). To further confirm the prosurvival effect by Bcl-xL, we used transient overexpression of Bcl-xL in primary cultured astrocytes using adenovirus. Astrocytes transduced with Ad-Bcl-xL were protected from oxidative stress (H2O2) by >20% compared with control astrocytes with Ad-Track (Fig. 7B). Confocal microscopy showed that astrocytes overexpressing Bcl-xL are more resistant to cell death induced by oxidative stress (Fig. 7C). These data indicate that Bcl-xL induction rescues primary astrocytes from oxidative stress-induced cell damage and death.
Figure 7.
Bcl-xL overexpressing and ALS (G93A) glia are resistant to oxidative stress-induced cell death. A) Primary astrocytes from ALS (G93A) mice (n=4) are more resistant against X/XO (100 μM/10 mU/ml)-induced cell death compared with WT (n=3). Cell viability measured 12 h after X/XO treatment. B) Primary astrocytes overexpressing Bcl-xL are more resistant against H2O2-induced cell death. Adeno-Track control and Adeno-Bcl-xL virus were infected in primary astrocytes for 24 h, and cell viability was measured 12 h after the treatment of 1mM H2O2. C) Confocal microscopy shows that astrocytes transduced with Adeno-Bcl-xL (Ad-Bcl-xL) virus are more resistant against oxidative stress-induced cell death compared with astrocytes transduced with control adenovirus. *P < 0.05. Scale bars = 10 μm.
DISCUSSION
While detrimental to motor neurons, oxidative stress is associated with anti-apoptotic and generative effects on astrocytes (2). The molecular events underlying decreased astrocytic vulnerability in ALS have not been elucidated. Previous studies (13, 22, 24, 25) have implicated the mitochondrial protein and known anti-apoptotic factor Bcl-xL as playing a pivotal role in glial survival. We have found that oxidative stress induces Ets-2-dependent transcriptional activation of Bcl-xL gene and up-regulates Bcl-xL mRNA and protein levels in astrocytes in vitro. Consistent with this, we confirmed a time-dependent increase in Bcl-xL expression as well as an elevated level of Bcl-xL protein in GFAP-positive cells in the spinal cord of ALS transgenic (G93A) mice. In addition, overexpression of Bcl-xL in primary mouse astrocytes resulted in increased cell survival when subjected to oxidative stress. These data delineate a plausible mechanism for the paradoxical anti-apoptotic and generative effects of oxidative stress on astrocytes in ALS.
The proposed mechanism correlates well with previous studies of Bcl-xL function and regulation. Bcl-xL is a protein of the Bcl-2 family and localizes to the outer mitochondrial membrane and has been shown to prevent glia from hydrogen peroxide-induced cell death (13) and to minimize 1,3-dinitrobenzene-induced mitochondrial dysfunction in astrocytes (23). In astrocytes, Bcl-xL overexpression prevents excess ROS accumulation by maintaining mitochondria membrane potential in response to glucose deprivation and the presence of hydrogen peroxide (13). The correlation of Bcl-xL expression with survival under oxidative stress in cultured astrocytes and the finding of increased Bcl-xL expression in GFAP-positive cells in the spinal cord G93A mice support the hypothesis that Bcl-xL mediates, in part, the astrocytic response to oxidative stress in ALS.
Several transcription factors are known to regulate the expression of Bcl-xL (21, 26, 27), but the mechanisms of transcriptional regulation in response to oxidative stress have not been studied. The murine Bcl-xL promoter contains NF-κB binding sites, as in rat PC12 cells, and can be activated through a direct interaction between the NF-κB transcription factor and the Bcl-xL promoter (14). Further, although less direct, associations between NF-κB-induced Bcl-xL expression have been shown to occur in human B lymphocytes, wild-type murine thymocytes, and rat hippocampal neurons (14, 20). However, our data demonstrate that NF-κB produces no significant induction of Bcl-xL promoter activity in primary mouse astrocytes. In addition to the NF-κB binding sites, the Bcl-xL promoter is known to contain binding sites for Sp1 and Ets-2 transcription factors (14). Notably, we found that a major induction of Bcl-xL promoter activity was shown by Ets-2 transcription factor relative to the control, NF-κB, and Sp1 plasmids. Our ChIP data establish a marked increase of Ets-2 binding to the putative binding element in the Bcl-xL promoter during oxidative stress in primary astrocyte cultures. Moreover, in the spinal cord of ALS mice, increased Ets-2 occupancy in the Bcl-xL promoter correlates with increased Bcl-xL expression. These findings suggest that the Ets-2 binding element plays an important role in oxidative stress-induced Bcl-xL gene activation. Our data indicated that the −753/−745 position serves as a specific Ets-2 recognition site on oxidative stress. The mutation in Ets-2 element reduced Bcl-xL promoter activity in respsone to oxidative stress. Thus, the Ets-2 site is required both for the basal and oxidative stress-induced expression of Bcl-xL. In addition, the findings of elevated nuclear Ets-2 in astrocytes from transgenic ALS mice and human ALS support our proposed mechanism of transcriptional activation of Bcl-xL by Ets-2 in response to oxidative stress in ALS.
Previous data are consistent with the transcriptional activation of the Bcl-xL gene by Ets-2 (26, 27). Ets-2 is a member of a large family of transcription factors known as the ets family, and was first identified by its sequence homology to the v-Ets portion of the gag-Myb-Ets fusion protein of the E26 avian retrovirus (28, 29). An Ets domain of ∼85 amino acids recognizes a GGA/CCT consensus core sequence. Ets-2 is active in its monomeric form and interacts with other transcription factors binding to adjacent sites to activate transcription. Ets-2 activation induces neuronal apoptosis in response to oxidative stress (30, 31) but rescues macrophages from stress induced by colony-stimulating factor 1 depletion through a Bcl-xL-dependent mechanism (26). In differentiated myeloid cells, such as human U937 and HL60 cells, Ets-2 and Bcl-xL are coexpressed (26). Moreover, it has previously been found that phosphorylation of Ets-2 correlates with the expression of the anti-apoptotic Bcl-xL gene (26, 27). In addition, phosphorylation of Ets-2 and activation of target gene expression were found to correlate with increased macrophage survival. These results indicate that constitutive Ets-2 activity may contribute to the expression of genes that promote cell survival in macrophages. Ets-2 can inhibit apoptosis in the absence of a growth factor, and at least one mechanism of inhibition involves the capacity of Ets-2 to transactivate the Bcl-xL gene, resulting in cell survival. As in myeloid cells, our data show that glial survival is correlated with the elevation of Ets-2 transcription factor and with Bcl-xL expression. Therefore, the transcriptional activation of Bcl-xL by Ets-2 may compensate oxidative stress by preventing glia from apoptotic or necrotic cell death in the pathogenesis of ALS. Molecular mechanisms of Ets-2-mediated and Bcl-xL-dependent survival pathways may vary among different cell types. In particular, the role of transcriptional activation by Ets-2 among motor neurons remains to be investigated. Taken together, our findings suggest that the molecular signaling between glia and motor neurons may be triggered differently by mediators of oxidative stress. In this paradigm, due to increased oxidative stress or other mechanisms, a critical threshold is overcome and the pathological phenotype is initiated by glia that subsequently leads to a noncell autonomous death of motor neurons in ALS. As one of the plausible mechanisms, it is possible that activation of Ets-2 transcription factor improves glial survival but occurs too late to prevent earlier glial dysfunction, or perhaps Ets-2 induction allows dysfunctional glia to survive at late stages and could thereby contribute to the propagation of motor neuron toxicity. However, whether this might be expected to occur at an earlier stage, before Bcl-xL up-regulation by Ets-2 is reached its threshold, remains to be investigated in the future studies. This concept suggests that any therapeutic trial for the treatment of ALS should be aimed at cell-type specific interception of pro-oxidant signals.
In summary, we have demonstrated that oxidative stress enhances the expression of Bcl-xL through the Ets-2 transcription factor-dependent pathway in astrocytes and that the increased Bcl-xL gene expression is associated with survival of glial cells under oxidative stress. Our data indicate that Ets-2-mediated Bcl-xL induction may be an important mechanism for glial survival and activation in ALS.
Supplementary Material
Acknowledgments
We thank Cyd Khayter and Oh Kyu Kwon for technical assistance and the preparation of manuscript. J.L. is an awardee of a Les Turner ALS Foundation Grant. This work was supported by National Institutes of Health (NIH) NS-52724 (to H.R.), NIH P30 AG-13846 (to J.L.), NIH NS045242 and NS045806 (to R.J.F.), and a Department of Veterans Affairs (VA) merit grant (to J.L. and N.W.K.). This work was supported in part by a World Class University (WCU) grant from the Korea Science and Engineering Foundation (KOSEF)(to H.R.).
References
- Friedlander R M. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med. 2003;348:1365–1375. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- Barbeito L H, Pehar M, Cassina P, Vargas M R, Peluffo H, Viera L, Estevez A G, Beckman J S. A role for astrocytes in motor neuron loss in amyotrophic lateral sclerosis. Brain Res Rev. 2004;47:263–274. doi: 10.1016/j.brainresrev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Cleveland D W, Rothstein J D. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Neurosci Rev. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- Andersen P M, Sims K B, Xin W W, Kiely R, O'Neill G, Ravits J, Pioro E, Harati Y, Brower R D, Levine J S, Heinicke H U, Seltzer W, Boss M, Brown R H., Jr Sixteen novel mutations in the Cu/Zn superoxide dismutase gene in amyotrophic lateral sclerosis: a decade of discoveries, defects and disputes. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:62–73. doi: 10.1080/14660820310011700. [DOI] [PubMed] [Google Scholar]
- Ryu H, Smith K M, Camelo S I, Carreras I, Lee J, Iglesias A H, Dangond F, Cormier K A, Cudkowicz M E, Brown R H, Jr, Ferrante R J. Sodium phenylbutyrate prolongs survival and regulates expression of anti-apoptotic genes in transgenic amyotrophic lateral sclerosis mice. J Neurochem. 2005;93:1087–1098. doi: 10.1111/j.1471-4159.2005.03077.x. [DOI] [PubMed] [Google Scholar]
- Bruijn L I, Becher M W, Lee M K, Anderson K L, Jenkins N A, Copeland N G, Sisodia S S, Rothstein J D, Borchelt D R, Price D L, Cleveland D W. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- Gurney M E, Pu H, Chiu A Y, Dal Canto M C, Polchow C Y, Alexander D D, Caliendo J, Hentati A, Kwon Y W, Deng H X, Chen W, Zhai P, Sufit R L, Siddique T. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Rosen D R, Siddique T, Patterson D, Figlewicz D A, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan J P, Deng H X, Zohra Rahmani Z, Krizus A, Mckenna-Yasek D, Cayabyab A, Gaston S M, Berger R, Tanzi R E, Halperin J J, Herzfeldt B, Van Den Bergh R, Hung W Y, Bird T, Deng G, Mulder D W, Smyth C, Laing N G, Soriano E, Pericak-Vance M A, Haines J, Rouleau G A, Gusella J S, Horvitz H R, Brown R H., Jr Mutations in Cu/Zn superoxide dismutase are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Langley B, Ratan R R. Oxidative stress induced death in the nervous system: cell cycle dependent or independent? J Neurosci Res. 2004;77:621–629. doi: 10.1002/jnr.20210. [DOI] [PubMed] [Google Scholar]
- Mattiazzi M, D'Aurelio M, Gajewski C D, Martushova K, Kiaei M, Beal M F, Manfredi G. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J Biol Chem. 2002;277:29626–29633. doi: 10.1074/jbc.M203065200. [DOI] [PubMed] [Google Scholar]
- Warita H, Hayashi T, Murakami T, Manabe Y, Abe K. Oxidative damage to mitochondrial DNA in spinal motoneurons of transgenic ALS mice. Mol Brain Res. 2001;89:147–152. doi: 10.1016/s0169-328x(01)00029-8. [DOI] [PubMed] [Google Scholar]
- Niebroj-Dobosz I, Dziewulska D, Kwiecinski H. Oxidative damage to proteins in the spinal cord in amyotrophic lateral sclerosis (ALS) Folia Neuropathol. 2004;42:151–156. [PubMed] [Google Scholar]
- Ouyang Y B, Carriedo S G, Giffard R G. Effect of Bcl-x(L) overexpression on reactive oxygen species, intracellular calcium, and mitochondrial membrane potential following injury in astrocytes. Free Radic Biol Med. 2002;33:544–551. doi: 10.1016/s0891-5849(02)00912-7. [DOI] [PubMed] [Google Scholar]
- Glasgow J N, Wood T, Perez-Polo J R. Identification and characterization of nuclear factor kappaB binding sites in themurine bcl-x promoter. J Neurochem. 2000;75:1377–1389. doi: 10.1046/j.1471-4159.2000.0751377.x. [DOI] [PubMed] [Google Scholar]
- Yoshihara T, Ishigaki S, Yamamoto M, Liang Y, Niwa J, Takeuchi H, Doyu M, Sobue G. Differential expression of inflammation and apoptosis related genes in spinal cords of a mutantSOD1 transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem. 2002;80:158–167. doi: 10.1046/j.0022-3042.2001.00683.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Ryu H, Ferrante R J, Morris S M, Jr, Ratan R R. Translational control of inducible nitric oxide synthase expression by arginine can explain the “arginine paradox”. Proc Natl Acad Sci U S A. 2003;100:4843–4848. doi: 10.1073/pnas.0735876100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Hagerty S, Cormier K A, Ferrante R J, Andrew L, Kung A L, Ryu H. Monoallele deletion of CBP leads to pericentromeric heterochromatin condensation through ESET expression and histone H3 (K9) methylation. Hum Mol Genet. 2008;17:1774–1782. doi: 10.1093/hmg/ddn067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu H, Lee J H, Olofsson B A, Mwidau A, Deodoglu A, Escudero M, Flemington E, Azizkhan-Clifford J, Ferrante R J, Ratan R R. Histone deacetylase inhibitors prevent oxidative neuronal death independent of expanded polyglutamine repeats via an Sp1-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:4281–4286. doi: 10.1073/pnas.0737363100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu H, Lee J, Impey S, Ratan R R, Ferrante R J. Antioxidants modulate mitochondrial protein kinase A and increase CREB binding to D-loop DNA of the mitochondrial genome in neurons. Proc Natl Acad Sci U S A. 2005;102:13915–13920. doi: 10.1073/pnas.0502878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara T, Kannagi M, Ohashi T, Kato H, Arai M, Nunez G, Iwanaga Y, Yamamoto N, Ohtani K, Nakamura M, Fujii M. Induction of Bcl-x(L) expression by human T-cell leukemia virus type 1 Tax through NF-kappaB in apoptosis-resistant T-cell transfectants with Tax. J Virol. 1999;73:7981–7987. doi: 10.1128/jvi.73.10.7981-7987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillot D A, Gonzalez Garcia M, Ekhterae D, Duan L, Inohara N, Ohta S, Seldin M F, Nunez G. Genomic organization, promoter region analysis, and chromosome localization of the mouse bcl-x gene. J Immunol. 1997;158:4750–4757. [PubMed] [Google Scholar]
- Ouyang Y B, Giffard R G. Bcl-xL maintains mitochondrial function in murine astrocytes deprived of glucose. J Cereb Blood Flow Metab. 2003;23:275–279. doi: 10.1097/01.WCB.0000055774.06337.F6. [DOI] [PubMed] [Google Scholar]
- Phelka A D, Sadoff M M, Martin B P, Philbert M A. BCL-XL expression levels influence differential regional astrocytic susceptibility to 1,3-dinitrobenzene. Neurotoxicology. 2006;27:192–200. doi: 10.1016/j.neuro.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Xu L, Lee J E, Giffard R G. Overexpression of bcl-2, bcl-XL or hsp70 in murine cortical astrocytes reduces injury of co-cultured neurons. Neurosci Lett. 1999;277:193–197. doi: 10.1016/s0304-3940(99)00882-4. [DOI] [PubMed] [Google Scholar]
- Xu L, Koumenis I L, Tilly J L, Giffard R G. Overexpression of bcl-xL protects astrocytes from glucose deprivation and is associated with higher glutathione, ferritin, and iron levels. Anesthesiology. 1999;91:1036–1046. doi: 10.1097/00000542-199910000-00024. [DOI] [PubMed] [Google Scholar]
- Sevilla L, Aperlo C, Dulic V, Chambard J C, Boutonnet C, Pasquier O, Pognonec P, Boulukos K E. The Ets2 transcription factor inhibits apoptosis induced by colony-stimulating factor 1 deprivation of macrophages through a Bcl-xL-dependent mechanism. Mol Cell Biol. 1999;19:2624–2634. doi: 10.1128/mcb.19.4.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla L, Zaldumbide A, Carlotti F, Dayem M A, Pognonec P, Boulukos K E. Bcl-xL expression correlates with primary macrophage differentiation, activation of functional competence, and survival and results from synergistic transcriptional activation by Ets2 and PU.1. J Biol Chem. 2001;276:17800–17807. doi: 10.1074/jbc.M008270200. [DOI] [PubMed] [Google Scholar]
- Gunther C V, Graves B J. Identification of ETS domain proteins in murine T lymphocytes that interact with the Moloney murine leukemia virus enhancer. Mol Cell Biol. 1994;14:7569–7580. doi: 10.1128/mcb.14.11.7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther C V, Nye J A, Bryner R S, Graves B J. Sequence-specific DNA binding of the proto-oncoprotein ets-1 defines a transcriptional activator sequence within the long terminal repeat of the Moloney murine sarcoma virus. Genes Dev. 1990;4:667–679. doi: 10.1101/gad.4.4.667. [DOI] [PubMed] [Google Scholar]
- Sanij E, Hatzistavrou T, Hertzog P, Kola I, Wolvetang E J. Ets-2 is induced by oxidative stress and sensitizes cells to H(2)O(2)-induced apoptosis: implications for Down’s syndrome. Biochem Biophys Res Commun. 2001;287:1003–1008. doi: 10.1006/bbrc.2001.5680. [DOI] [PubMed] [Google Scholar]
- Helguera P, Pelsman A, Pigino G, Wolvetang E, Head E, Busciglio J. Ets-2 promotes the activation of a mitochondrial death pathway in Down’s syndrome neurons. J Neurosci. 2005;25:2295–2303. doi: 10.1523/JNEUROSCI.5107-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.