Abstract
To determine how endogenously secreted β-amyloid 42 (Aβ42) aggregates regulate synaptic functions, we examined effects of Aβ42 at the neuromuscular junction of Drosophila larvae. Voltage-clamp recordings of synaptic transmission and optical analysis of vesicle recycling at presynaptic terminals show that expression of Aβ42 in neurons leads to a reduction of neurotransmitter release. However, expression of Aβ42 in postsynaptic muscle cells enhanced neurotransmitter release. Both effects are neutralized by Aβ antibody, suggesting a role for secreted Aβ42 peptides. Application of exogenously prepared Aβ42 oligomers leads to a reduction in synaptic responses, whereas mixed Aβ42 aggregates with mainly fibrils elicit an opposite effect by increasing synaptic transmission. Further analysis of long-term depression (LTD) confirms differential effects of different Aβ42 aggregates. Taken together, our data suggest that Aβ42 is secreted from neurons primarily as oligomers that inhibit neurotransmitter release and exert no effect on LTD. Whereas larger-sized aggregates, possibly fibrils, are major components secreted from muscle cells, which enhance synaptic transmission and LTD. Thus, different types of cells may secrete distinct forms of Aβ42 aggregates, leading to different modulation of synaptic functions.—Chiang, H.-C., Iijima, K., Hakker, I., Zhong, Y. Distinctive roles of different β-amyloid 42 aggregates in modulation of synaptic functions.
Keywords: Alzheimer’s disease, Drosophila, neuromuscular junction, endogenous
Accumulating evidence has led to the hypothesis that toxicity of β-amyloid (Aβ) peptides, which are cleaved from APP by γ and β secretase activities, is largely conferred to its soluble aggregates (1,2,3). However, dystrophic neuritis is observed to surround large plaques of fibrillar Aβ in Alzheimer’s disease (AD) brains (4), and fibrillar Aβ deposits have been associated with synaptic abnormalities and breakage of neuronal braches in an AD mouse model (5). This finding suggests that fibrillar aggregates of Aβ might also contribute to neuronal injury.
In APP transgenic mice, Aβ accumulation leads to alterations in the expression and function of a range of molecules important for synaptic transmission and plasticity (6,7,8). It is, however, interesting to note that Aβ effects on synaptic function are remarkably different in different brain regions. Recording from hippocampal CA1 neurons, the basal level of synaptic transmission is reduced while long-term potentiation (LTP) remains unaffected in APP transgenic mice (9, 10). In contrast, recordings from the medial perforant pathway synapse within the dentate gyrus of the same transgenic mouse indicate that basal synaptic transmission is not affected but LTP is depressed (10). On the basis of recordings from CA1 neurons, it has been proposed that activity-dependent release of Aβ forms part of a negative feedback mechanism to control neuronal hyperactivity (11). However, this interpretation cannot explain the occurrence of epileptic seizures in a large fraction of AD patients (12, 13), a population subject to Aβ overproduction. This seizure activity and studies of the dentate gyrus have raised a network perspective in which aberrant increases in network excitability and compensatory inhibitory mechanisms in the hippocampus are proposed to contribute to Aβ-induced neurological deficits (10, 14, 15). However, how accumulation of Aβ leads to aberrant increases in network excitability remains to be determined, particularly in light of the proposed role for Aβ in controlling hyperexcitability (11). It is therefore critical to understand at the cellular level why Aβ modifies synaptic functions differentially among different populations of neurons.
Powerful genetic tools available in Drosophila may facilitate such understanding. An APP-like (APPL) gene is found in Drosophila, and the behavioral phenotype of APPL mutants can be rescued by expression of human APP (hAPP), suggesting conserved functions (16, 17). A recent publication claims that Aβ-like peptides are also cleaved from APPL, which can form amyloidogenic deposits and cause neurodegeneration in the Drosophila brain (18). Toxicity induced by human Aβ appears to be highly conserved across organisms, from Caenorhabditis elegans (19, 20), to Drosophila (21, 22), to mammals (23). In Drosophila, expression of hAPP together with β secretase leads to age-dependent neurodegeneration and amyloid plaque formation (24). Expression of hyperphosphorylated tau also triggers age-dependent neurodegeneration in Drosophila (25). In fact, Drosophila has been a powerful genetic model for studying major age-dependent neurodegenerative diseases, including Parkinson’s disease, Huntington’s disease, and AD (26,27,28).
In the present study, we focus on the synaptic effects of Aβ42. Our previous studies have shown that expression of secretary Aβ42 leads to age-dependent memory loss and severe neurodegeneration in Drosophila brain regions critical for memory formation (21, 22). To gain insights into the cellular mechanisms of such effects, this report investigates how synaptic transmission and synaptic plasticity are affected by expression of Aβ42 peptides at the larval neuromuscular junction (NMJ), the only preparation suitable for quantitative analysis of synaptic transmission at identifiable synapses in Drosophila.
Our analysis reveals that targeted expression of Aβ42 in the presynaptic neuron induces a synaptic modulation that is opposite to that induced by expression in postsynaptic muscle cells at the same synapse. Further studies suggest that different secreted Aβ42 aggregates exert distinct modulation on synaptic functions.
MATERIALS AND METHODS
Genetics
Transgenic fly lines used in this report have been previously described, including w1118(isoCJ1), UAS-Aβ40, UAS-Aβ42 (22), elav-Gal4C155, G7, and C57. G7 and C57 are muscle expression Gal4 drivers (29, 30). To ensure that Aβ42 and Aβ40 peptides were targeted to the secretory pathway, both peptides were linked to a rat preproenkephalin signal peptide (21, 22). Our previous publication showed that the Aβ peptides are precisely cleaved and can be secreted in the Drosophila brain (21). The flies were raised and maintained at room temperature (22–24°C).
Electrophysiology
Electrophysiological recordings of two-electrode voltage clamp were performed as described previously (31). In brief, wall-climbing third-instar larvae from large fresh bottles were chosen for dissection. Larvae were dissected at room temperature and in Ca2+-free hemolymph-like (HL-3) solution containing the following (mM): 70 NaCl, 5 KCl, 4 MgCl2, 10 NaHCO3, 5 trehalose, 5 HEPES, and 115 sucrose. All recordings were made at the longitudinal muscles of segments A4–A5, muscle fiber 12 with CaCl2 (concentrations are indicated in the text and the figure legends). The segmental nerve was stimulated at 1.5 times the stimulus voltage required for a threshold response for excitatory junction currents (EJCs). For recordings of long-term depression (LTD), the nerve was stimulated at baseline frequency of 0.05 Hz for 5 min and 30 Hz for induction of LTD. Current signals were amplified with an Axoclamp 2B amplifier (Molecular Devices, Palo Alto, CA, USA). The signals were filtered at 0.1 kHz online and converted to a digital signal using a Digidata 1320A interface (Molecular Devices) and acquired by pClamp 9.0 software (Molecular Devices).
FM1-43 dye imaging
This method is used widely for analyzing vesicle trafficking (32, 33). The preparation was performed as described previously (30), with some modification. Wall-climbing third-instar larvae were dissected in HL-3 solution as described in the preceding text. For loading, the preparation was incubated for 5 min in HL-3 solution with 90 mM K+ and Ca2+ (1.8 mM for G7-Gal4 group and 0.9 mM for Elav-Gal4 group) containing 10 μM FM1-43 dye (Molecular Probes, Eugene, OR, USA). The preparation was washed with HL-3 solution without Ca2+ for 10 min. NMJs were imaged using 2-photon imaging; a custom-built 2-photon laser scanning microscope was used as described previously (34). For unloading, larva were stimulated by incubating in 90 mM K+ with 0.4 mM Ca2+ for 20–30 s, followed by 10-min wash. Boutons of muscle fiber 12 on a section were circled, and after subtracting background fluorescence, mean intensity from loaded and unloaded conditions was compared.
Anatomical study
Thioflavin-S staining was performed as described previously (22, 35), with few modifications. Animals were fixed in 4% paraformaldehyde (EMS) overnight and permeabilized by 0.2% triton. They were then transferred to 0.25% thioflavin S (Sigma, St. Louis, MO, USA) in 50% ethanol for one night and destained for 10 min in 50% ethanol. After being washed 3 times, they were mounted using FocusClear (Pacgen Biopharmaceuticals Inc., Vancouver, BC, Canada), and coverslips were added. Slides were observed with a Zeiss LSM 510 confocal microscope (Carl Zeiss, Oberkochen, Germany). To detect amyloid deposits, we followed a well-established immunohistochemistry procedure (36): Fixed and permeabilized neuromuscular preparations were treated with formic acid (Sigma) followed by immunostaining with a mouse monoclonal anti-Aβ antibody with 1:500 dilution (Chemicon, Temecula, CA, USA).
Aβ preparations
Aβ1-42 peptides were purchased from Sigma. Aggregated Aβ1-42 was prepared based on protocols developed in previous studies (37). Aβ powder was initially dissolved to 1 mM in hexafluoroisopropanol (Sigma), then vacuumed in a SpeedVac (GMI, Ramsey, MN, USA) to remove hexafluoroisopropanol. The film was first resuspended in dry dimethyl sulfoxide (Me2SO, Sigma) to a concentration of 5 mM. For fibrillar conditions, 10 mM HCl was added to reach a final peptide concentration of 100 μM and incubated for 24 h at 37°C. For oligomeric conditions, Ham’s F-12 (phenol red-free; BioSource, Camarillo, CA, USA) was added to bring the peptide to a final concentration of 100 μM and incubated at 4°C for 24 h.
Western blot analysis
Samples were diluted in SDS sample buffer and separated by 10–20% Tris-Tricine gels (Invitrogen, Carlsbad, CA, USA) and transferred to nitrocellulose membranes (Invitrogen). The membranes were boiled in phosphate buffered saline (PBS) for 3 min, blocked with 5% nonfat dry milk (Nestlé, Vevey, Switzerland) and blotted with the 6E10 antibody.
Data analyses and statistics
To minimize variation, each experimental group was compared only to a dedicated control group that had a similar genetic background and was recorded in the same batch of experiments. To be compatible for different Ca2+ concentrations, all EJC amplitudes were normalized to the respective controls. Evoked and spontaneous responses were analyzed using the Mini Analysis Program (Synaptosoft, Decatur, GA, USA). All between-group comparisons were performed using t tests.
RESULTS
Synaptic transmission was determined by EJCs recorded via the two-electrode voltage-clamp method at the body-wall NMJ of third-instar larvae (38). Expression of the transgene encoding Aβ42 was driven mainly by either an elav-Gal4 pan-neuronal expression driver or by a G7-Gal4 muscle-specific expression driver. Additional drivers for expression were also used for confirmation. To control for nonspecific effects of genetic background, all genotypes were outcrossed with an isogenic line w1118(isoCJ1) for five generations.
Opposite synaptic effects resulting from neuronal vs. muscle expression of Aβ42
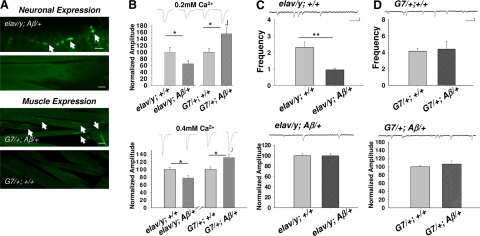
Aβ42 immunoreactivity was detected at motor nerve terminals innervating larval body-wall muscle fiber 12 (for nomenclature, see ref. 39) with a well-characterized antibody against the N terminus of Aβ peptide, 6E10, (see Materials and Methods) in elav-Gal4/y; UAS-Aβ42/+ transgenic larvae (Fig. 1A). The staining was also widely distributed in the larval ganglion within which motor neurons localized (not shown). In contrast, Aβ42 immunoreactivity was observed only in muscle fibers when driven by G7-Gal4 (bottom panels in Fig. 1A). Based on this observation, all electrophysiological recordings were obtained from muscle 12 in body-wall segments 4 and 5.
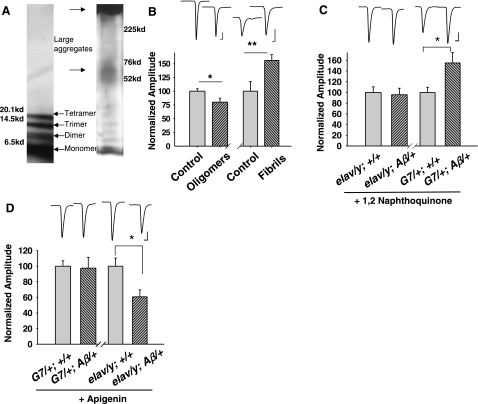
Figure 1.
Opposite synaptic effects resulting from expression of Aβ42 in neurons vs. muscle cells. A) Distribution of immunohistochemical staining of the Aβ42 peptide via antibody 6E10 in motor nerve terminals of elav-Gal4/y; UAS-Aβ/+ (elav/y; Aβ/+ in figure labels) larvae and in muscle cells of G7/+; Aβ/+ larvae. Elav is a pan-neuronal driver; G7 is a muscle-specific driver. Top panels: arrows indicate boutons of nerve terminals with positive anti-Aβ42 peptide staining. Bottom panels: arrows indicate fiber-like staining within muscle cells. Scale bars = 15 μm (top); 100 μm (bottom). B) EJCs were depressed in elav/y; Aβ/+ larvae while enhanced in G7/+; Aβ/+ larvae. EJC amplitude is normalized to the size of a respective control group (see Materials and Methods). Ca2+ concentrations are as indicated. n = 10, 11, 26, 24, 8, 8, 9, 10 for elav/y; +/+, elav/y; Aβ/+, G7/+; +/+, G7/+; Aβ/+ at 0.2 and 0.4 mM Ca2+, respectively. Scale bars = 5 nA (vertical, top); 10 nA (vertical, bottom); 7.5 ms (horizontal). C, D) Quantal analysis of effects of Aβ42 expression. Frequency of spontaneous mEJCs was significantly lower in neuronal expression of Aβ42 (elav/y; Aβ/+) vs. its control (C); frequency was similar in muscle expression of Aβ42 (G7/+; Aβ/+) (D). Amplitude of mEJCs remained unchanged in both cases. n = 10, 9, 20, 11 for elav/y; +/+, elav/y; Aβ/+, G7/+; +/+, G7/+; Aβ/+, respectively. Scale bars = 0.4 nA (vertical); 130 ms (horizontal). Error bars = se. *P < 0.05, **P < 0.01; test.
Evoked EJCs were reduced in elav-Gal4/y; UAS-Aβ42/+ larvae with expression of Aβ42 in neurons or presynaptic nerve terminals. This finding was true at a variety of external Ca2+ concentrations (Fig. 1B). Surprisingly, EJC amplitude was increased significantly in G7-Gal4/+; UAS-Aβ42/+ larvae with expression of Aβ42 in postsynaptic muscle cells (Fig. 1B). Here, elav-Gal4/y; + and G7/+; + served as corresponding controls. EJC peak amplitude was similar between these two controls (10.4±1.5 nA in elav-Gal4/y; + and 8.3±1 nA in G7/+; + at 0.2 mM Ca2+), which corresponds to data reported previously (30). Similar synaptic effects were observed in two independently isolated UAS-Aβ42 lines. These results raised the question of how synaptic transmission would be affected if Aβ42 were expressed in neurons and muscle cells simultaneously. Driven by a universal promoter (40), armadillo (arm)-Gal4, EJC amplitude was reduced (39.1±2.2 nA in arm-Gal4/+; +/+, 27.6±4 nA in arm-Gal4/+; UAS- Aβ42/+). It appeared that Aβ42 expressed in neurons played a dominant role, which might reflect a stronger expression in neurons vs. muscle fibers.
In addition, we also examined effects of expressing Aβ40. We observed no statistically significant difference in EJC amplitude when comparing elav-Gal4/y; UAS-Aβ40/+ and G7-Gal4/+; UAS-Aβ40/+ with their controls. This finding is consistent with the previously reported observation in which Aβ40 expressed in the adult brain causes no neurodegeneration and only very mild learning defects even with much higher levels of expression than Aβ42. Furthermore, no accumulation of oligomers or fibrils was observed at such high levels (21). Therefore, it is not surprising to see no synaptic effects at the Aβ40 larval NMJ. In the following analysis, we focus on Aβ42.
Altered exocytosis rates
Analysis of spontaneous miniature EJCs (mEJCs) indicated that amplitudes of mEJCs remained unaltered in both cases (Fig. 1C, D), while frequency of mEJCs was significantly decreased with neuronal expression of Aβ42 (Fig. 1C), suggesting possible presynaptic effects. Both minifrequency and amplitude were unaffected by muscle expression of Aβ42 (Fig. 1D), even though evoked EJCs were increased (Fig. 1B). Although minianalysis may provide hints on what happens at evoked synaptic transmission, often no direct causative relationship is found between these two. Therefore, we still cannot rule out the possibility of presynaptic effects.
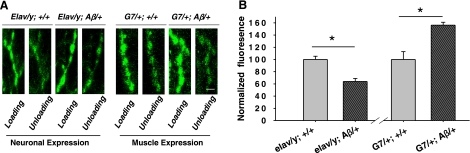
To confirm whether the observed effects were presynaptic, we examined exocytosis of synaptic vesicles at the nerve terminals through optical imaging analysis of evoked discharges of the fluorescent dye, FM1-43. FM1-43 was loaded into synaptic vesicles within boutons at which synapses are formed. This method is commonly used in studying vesicle recycling (32, 33). To achieve the dye loading, the neuromuscular preparation was treated with stimulation buffer containing a high potassium concentration (90 mM). The chosen loading regime showed no significant difference in fluorescence intensities of loaded dye at motor nerve terminals among different genotypes (Fig. 2A). Loaded dye in boutons was unloaded using a stimulation buffer with 90 mM K+ and 0.4 mM Ca2+. The unloading rate in response to stimulation was significantly reduced with neuronal expression of Aβ42 but increased with muscle expression (Fig. 2B). This result confirmed that evoked release of synaptic vesicles was altered in opposite directions in larvae with expression of Aβ42 in presynaptic terminals vs. postsynaptic muscle cells. In addition, imaging data suggest that regardless of whether Aβ42 was expressed pre- or postsynaptically, the effect was always on presynaptic functions.
Figure 2.
Exocytotic rates altered in opposite directions resulting from neuronal vs. muscle expression of Aβ42 peptides. A) Fluorescence imaging of motor nerve terminals stained with FM1-43 dye. Dye was loaded into synaptic vesicles through a period (5 min) of high potassium depolarization-induced vesicle recycling processes and then was unloaded using mild stimulation (see Materials and Methods). Changes in fluorescence intensity are proportional to exocytotic rates. Scale bar = 10 μm. B) Statistical analysis of fluorescence intensity changes (normalized; see Materials and Methods) in different genotypes. n = 4, 4, 5, 5 for elav/y; Aβ/+, elav/y; +/+, G7/+; +/+, G7/+; Aβ/+, respectively.
Distinct effects on LTD
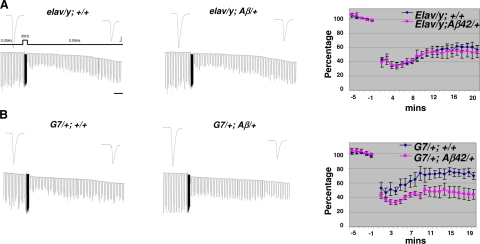
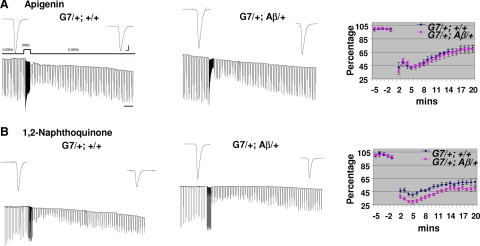
To further support the observation presented above, we examined LTD at the NMJ. We have shown previously that high frequency (30 Hz) stimulation of motor axons can induce LTD that lasts up to 1 h (31). Our recordings showed that LTD was not affected by neuronal expression of Aβ42 in elav/y; UAS-Aβ42 larvae (Fig. 3A). In contrast, LTD was significantly enhanced in Aβ42 in G7/+; UAS-Aβ42/+ larvae (Fig. 3B). Again, we showed that expression of Aβ42 could lead to very different effects on LTD, depending on where Aβ42 is expressed. The enhancement of LTD by muscle expression of Aβ42 is also observed by using another muscle driver line, C57-Gal4, see Supplemental Fig. 1.
Figure 3.
Enhancement of LTD induced by expression of Aβ42 peptide in muscle cells. A) No effects on LTD by neuronal expression of Aβ42 peptide in elav/y; Aβ/+ larvae. Stimulation paradigm for induction of LTD is depicted. EJCs were recorded in 0.4 mM Ca2+ saline. Horizontal bar = 2 min. B) LTD is enhanced in G7/+; Aβ/+ larvae with muscle expression of Aβ42 peptide. Same induction paradigm as in A. Top panels: representative EJC traces for each genotype. Bottom panels: averaged EJC amplitude normalized to basal level before titanic stimulation. n = 4, 4, 5, 5 for elav/y; +/+, elav/y; Aβ/+, G7/+; +/+ and G7/+; Aβ/+. Scale bars = 10 nA (vertical); 7.5 ms (horizontal).
Opposite synaptic effects induced by Aβ42 oligomers vs. fibrils
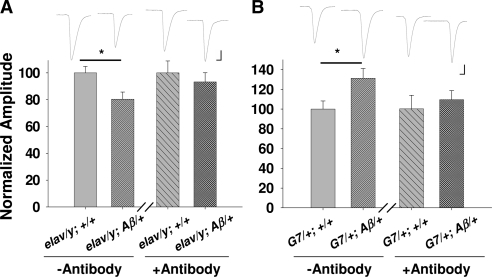
Since Aβ42 is targeted to the secretory pathway, it is possible that muscle cells expressing Aβ42 secrete Aβ42 that acts on presynaptic nerve terminals. To examine this possibility, an antibody against Aβ (6E10) was incubated with the NMJ preparation. Aβ42 effects were suppressed in both neuronal expression and muscle expression (Fig. 4), implying that the observed synaptic effects were caused by secreted Aβ42 no matter where it is expressed.
Figure 4.
Application of Aβ peptide antibody reverses synaptic modification resulting from both neuronal and muscle expression of Aβ42. Synaptic effects of incubation of Aβ42 antibody (0.5 μg/μl) for 30 min in neuromuscular preparations of larvae with expression of Aβ42 in neurons (elav/y; Aβ/+) (A) or in muscle cells (G7/+; Aβ/+) (B). [Ca2+] in saline is 0.4 mM. n = 14, 14, 13, 13, 10, 10, 8, 8 for elav/y; +/+, elav/y; Aβ/+, G7/+; +/+, G7/+; Aβ/+ without and with antibody treatment, respectively. Scale bars = 10 nA (vertical); 7.5 ms (horizontal).
Such a conclusion, however, raised an obvious concern: how Aβ42 could produce opposite effects at the same presynaptic terminals. Considering that Aβ42 expressed in the adult Drosophila brain is highly aggregating, but not Aβ40, we examined the idea that different Aβ42 aggregates were secreted from neurons vs. muscle fibers, which in turn, elicit differential synaptic effects.
We prepared oligomers as well as fibrils with synthetic Aβ42 from well-established protocols (37, 41, 42). Our Western blot analysis showed that dimer, trimer, and tetramer were produced when prepared with the oligomer protocol (see Materials and Methods), while a range of aggregates, including larger aggregates and fibrils (smear-like band on top in the right lane of Fig. 5A), were formed with the fibril procedure (Fig. 5A). Incubating the neuromuscular preparation with exogenously prepared fibrils (10 μM) for 30 min enhanced EJCs. In contrast, incubation with exogenously prepared oligomers (10 μM) depressed EJCs (Fig. 5B). This observation indicated that small Aβ42 oligomers inhibited synaptic transmission, while larger oligomers or fibrils enhanced synaptic transmission.
Figure 5.
The change in synaptic transmission resulting from endogenous secretion of Aβ42 can be produced by exogenous application of synthetic Aβ42 oligomers or fibrils and reversed by drug application. A) Representative Western blots of oligomeric (left) and fibrillar Aβ42 (right). Left panel: dimer, trimer, and tetramer of Aβ42 in oligomer-forming conditions; no indication of higher molecular weight aggregates and smears. Right panel: in fibril-forming conditions, large Aβ42 aggregates with higher molecular weight remain in the well. B) Application of synthetic oligomeric and fibrillar Aβ42 peptides (10 μM) for 30 min depressed and enhanced EJCs, respectively. Experiments were conducted in 0.4 and 0.2 mM Ca2+ for oligomers (n=11/treatment) and fibrils (n=8/treatment), respectively. C) Incubation with 1,2-naphthoquinone (0.15 μM) for 30 min rescued the synaptic transmission deficit in elav/y; Aβ/+ larvae. n = 6/genotype. D) Application of apegenin (15 μM) for 30 min reversed the enhancement of EJCs in G7/+; Aβ/+ larvae. n = 4/genotype. *P < 0.05; **P < 0.02. Scale bars = 10 nA (vertical); 7.5 ms (horizontal).
Pharmacological analysis of Aβ42-dependent regulation of synaptic functions
Taken together, data obtained from genetically targeted expression of Aβ42 and from synthetic Aβ42 support a scenario in which small oligomers are secreted from motor nerve terminals that inhibit EJCs and exert no effect on LTD, whereas large oligomers or fibrils are secreted from muscle fibers that enhance EJCs and LTD. To advance this hypothesis, we examined pharmacological effects.
Drugs that inhibit fibrillization and oligomerization of Aβ peptides have been intensively studied and tested because of their potential for treatment of AD. Among them, apigenin and 1,2-naphthoquinone have been shown to inhibit fibrillization and oligomerization, respectively (43). In concordance, we found that oligomerization inhibitor 1,2-naphthoquinone reversed the reduced EJCs seen with expression of Aβ42 in neurons but had no effect on synaptic transmission enhancement due to muscle expression of Aβ42 (Fig. 5C). However, fibrillization inhibitor, apigenin, neutralized enhanced EJCs caused by expression of Aβ42 in muscle but could not reverse the synaptic transmission depressed by expression of Aβ42 in the neuron (Fig. 5D). Furthermore, apigenin completely suppressed enhanced LTD (Fig. 6A) in larvae with muscle expression of Aβ42, but 1,2-naphthoquinone had little effect on enhanced LTD (Fig. 6B).
Figure 6.
Inhibition of fibrilization reverses enhanced LTD. Effects of preincubation of fibrilization inhibitor, apigenin (A), and oligomerization inhibitor, 1,2-naphthoquinone (B) with G7/+; Aβ/+ larvae. LTD induction paradigm is as indicated. Top panels: representative EJC traces. EJCs were recorded at 0.4 mM Ca2+. Normalized EJCs were plotted against time for statistical analysis (n=6/group). Scale bars = 10 nA (vertical); 7.5 ms (horizontal).
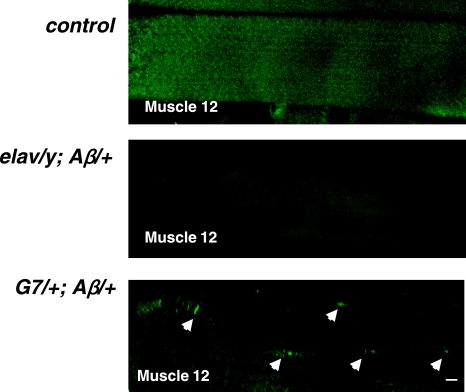
To visualize whether different aggregates are indeed formed from targeted expression of Aβ42, we performed thioflavin-S staining; this fluorescence dye has been used to stain Aβ fibrils, which are primarily composed of β sheets (4). Confocal images showed positive thioflavan-S staining in muscle fibers that express Aβ42 (arrows in Fig. 7). We found an average of 6 thioflavan-S-positive spots in each muscle fiber in G7/+; Aβ/+ larvae, while no signal was observed in motor nerve terminals of larvae that express Aβ42 in neurons. The thioflavan-S staining pattern in muscle fibers was similar to our immunostaining results (see arrows in Fig. 1A, muscle expression). This observation supports the notion that fibrils were formed in muscle cells expressing Aβ42 but not in motor neurons.
Figure 7.
Endogenous Aβ42 fibrils revealed by thioflavin-S staining in muscle fibers of G7/+; Aβ/+ larvae. Thioflavin-S-positive staining was detected in the muscle cells in G7/+; Aβ/+ larvae, but not in the ctrl and elav/y; Aβ/+ larvae. Arrowheads indicate fiber-like staining within muscle cells. Scale bar = 10 μm.
DISCUSSION
In the current study, we focused our analysis on the effects of Aβ peptides. We demonstrated that expression of Aβ42 could lead to two different types of synaptic modulation at the same synapses, depending on which cells expressed Aβ42. Neuronal expression induced a reduction in EJC amplitude and had no effects on LTD. In contrast, muscle expression enhanced EJC as well as LTD. We then showed, using synthetic peptide, that exogenously prepared small Aβ42 oligomers modulated EJC amplitude in a manner similar to neuronal expression, while exogenously prepared large Aβ42 aggregates, mainly fibrils, exerted effects similar to muscle expression. Although exogenously prepared Aβ42 fibrils also contained less-striking bands of small Aβ42 oligomers, enhanced synaptic effects likely resulted from larger aggregates or fibrils. This conclusion is well supported by the observation that pharmacological disruption of fibrilization, but not oligomization, leads to inhibition of synaptic effects elicited by muscle expression of Aβ42.
On the basis of these observations, we concluded that reduced synaptic transmission elicited by neuronal expression of Aβ42 is mediated primarily by released small oligomers (either dimer, trimer, or tetramer), while enhanced synaptic transmission and LTP induced by muscle expression of Aβ42 result from the release of large aggregates, such as fibrils. Thus, this in vivo study indicates that Aβ42 aggregates distinctively within different population of cells and this difference manifests in differential physiological functions.
In vitro studies of cultured vertebrate neurons have, in fact, shown that exogenously applied Aβ oligomers, protofibrils, or fibrils can produce qualitatively distinct effects on neuronal activities and cell death (44,45,46,47). In hAPP transgenic mice, it has been shown that excitatory synaptic transmission was reduced in the hippocampus (8, 11), while synaptic activity at inhibitory synapses was increased (10). Our in vivo observation at the Drosophila NMJ not only indicates that distinct synaptic modulations resulting from Aβ42 oligomers vs. fibrils are pathological but also provides a plausible mechanism: Different sizes of aggregates are formed in distinctive cell types.
Such an idea lends an intuitive explanation to apparently paradoxical observations. While Aβ peptide is shown to serve as a negative regulator of neuronal activity, seizure activities have been reported in both AD patients and in the AD mouse model (10, 12, 13). It is possible that Aβ indeed inhibits neuronal activity in CA1 neurons but might up-regulate synaptic activity in other populations of neurons. This may be responsible for the observed seizure activity.
Supplementary Material
Acknowledgments
We thank Dr. Iijima-Ando Kanae (Thomas Jefferson University, Philadelphia, PA, USA) for providing G7 flies and Dr. Shouzhen Xia (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, USA) for providing C57 flies, and Dr. Jennifer Beshel for reading and correcting the manuscript. This work was supported by grants from the U.S. National Institutes of Health (2R01 NS34779-06), the U.S. Army Neurofibromatosis Research Program (DAMD17-99-1-9500), and Dart Neuroscience (San Diego, CA, USA) to Y.Z.
References
- McLean C A, Cherny R A, Fraser F W, Fuller S J, Smith M J, Beyreuther K, Bush A I, Masters C L. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Walsh D M, Klyubin I, Fadeeva J V, Cullen W K, Anwyl R, Wolfe M S, Rowan M J, Selkoe D J. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe D J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Urbanc B, Cruz L, Le R, Sanders J, Ashe K H, Duff K, Stanley H E, Irizarry M C, Hyman B T. Neurotoxic effects of thioflavin S-positive amyloid deposits in transgenic mice and Alzheimer’s disease. Proc Natl Acad Sci U S A. 2002;99:13990–13995. doi: 10.1073/pnas.222433299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J, Grutzendler J, Duff K, Gan W B. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci. 2004;7:1181–1183. doi: 10.1038/nn1335. [DOI] [PubMed] [Google Scholar]
- Almeida C G, Tampellini D, Takahashi R H, Greengard P, Lin M T, Snyder E M, Gouras G K. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005;20:187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Chin J, Palop J J, Puoliväli J, Massaro C, Bien-Ly N, Gerstein H, Scearce-Levie K, Masliah E, Mucke L. Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2005;25:9694–9703. doi: 10.1523/JNEUROSCI.2980-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia A, Masliah E, McConlogue L, Yu G, Tatsuno G, Hu K, Kholodenko D, Malenka R C, Nicoll R A, Mucke L. Plaque-independent disruption of neuroal circuits in Alzheimer’s disease mouse models. Proc Natl Acad Sci U S A. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop J J, Chin J, Roberson E D, Wang J, Thwin M T, Bien-Ly N, Yoo J, Ho K O, Yu G Q, Kreitzer A, Finkbeiner S, Noebels J L, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Romanelli M F, Morris J C, Ashkin K, Coben L A. Advanced Alzheimer’s disease is a risk factor for late-onset seizures. Arch Neurol. 1990;47:847–850. doi: 10.1001/archneur.1990.00530080029006. [DOI] [PubMed] [Google Scholar]
- Lozsadi D A, Larner A J. Prevalence and causes of seizures at the time of diagnosis of probable Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;22:121–124. doi: 10.1159/000093664. [DOI] [PubMed] [Google Scholar]
- Graf R A, Kater S B. Inhibitory neuronal activity can compensate for adverse effects of beta-amyloid in hippocampal neurons. Brain Res. 1998;786:115–121. doi: 10.1016/s0006-8993(97)01451-0. [DOI] [PubMed] [Google Scholar]
- Palop J J, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443:768–773. doi: 10.1038/nature05289. [DOI] [PubMed] [Google Scholar]
- Luo L, Tully T, White K. Human amyloid precursor protein ameliorates behavioral deficit of flies deleted for Appl gene. Neuron. 1992;9:595–605. doi: 10.1016/0896-6273(92)90024-8. [DOI] [PubMed] [Google Scholar]
- Torroja L, Packard M, Gorczyca M, White K, Budnik V. The Drosophila beta-amyloid precursor protein homolog promotes synapse differentiation at the neuromuscular junction. J Neurosci. 1999;19:7793–7803. doi: 10.1523/JNEUROSCI.19-18-07793.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmine-Simmen K, Proctor T, Tschäpe J, Poeck B, Triphan T, Strauss R, Kretzschmar D. Neurotoxic effects induced by the Drosophila amyloid-beta peptide suggest a conserved toxic function. Neurobiol Dis. 2009;33:274–281. doi: 10.1016/j.nbd.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link C D. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1995;92:9368–9372. doi: 10.1073/pnas.92.20.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J, Link C D, Butterfield D A. Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid beta-peptide (1-42) in a transgenic Caenorhabditis elegans model. Neurobiol Aging. 2003;24:415–420. doi: 10.1016/s0197-4580(02)00225-7. [DOI] [PubMed] [Google Scholar]
- Iijima K, Liu H P, Chiang A S, Hearn S A, Konsolaki M, Zhong Y. Dissecting the pathological effects of human Abeta40 and Abeta42 in Drosophila: a potential model for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101:6623–6628. doi: 10.1073/pnas.0400895101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima K, Chiang H C, Hearn S A, Hakker I, Gatt A, Shenton C, Granger L, Leung A, Iijima-Ando K, Zhong Y. Abeta42 mutants with different aggregation profiles induce distinct pathologies in Drosophila. PLoS ONE. 2008;3:e1703. doi: 10.1371/journal.pone.0001703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D L, Tanzi R E, Borchelt D R, Sisodia S S. Alzheimer’s disease: genetic studies and transgenic models. Annu Rev Genet. 1998;32:461–493. doi: 10.1146/annurev.genet.32.1.461. [DOI] [PubMed] [Google Scholar]
- Greeve I, Kretzschmar D, Tschäpe J A, Beyn A, Brellinger C, Schweizer M, Nitsch R M, Reifegerste R. Age-dependent neurodegeneration and Alzheimer-amyloid plaque formation in transgenic Drosophila. J Neurosci. 2004;24:3899–3906. doi: 10.1523/JNEUROSCI.0283-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann C W, Wszolek M F, Shulman J M, Salvaterra P M, Lewis J, Hutton M, Feany M B. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science. 2001;293:711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- Chan H Y, Bonini N M. Drosophila models of human neurodegenerative disease. Cell Death Differ. 2000;7:1075–1080. doi: 10.1038/sj.cdd.4400757. [DOI] [PubMed] [Google Scholar]
- Marsh J L, Thompson L M. Drosophila in the study of neurodegenerative disease. Neuron. 2006;52:169–178. doi: 10.1016/j.neuron.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Ho I S, Hannan F, Guo H F, Hakker I, Zhong Y. Distinct functional domains of neurofibromatosis type 1 regulate immediate versus long-term memory formation. J Neurosci. 2007;27:6852–6857. doi: 10.1523/JNEUROSCI.0933-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V, Koh Y H, Guan B, Hartmann B, Hough C, Woods D, Gorczyca M. Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron. 1996;17:627–640. doi: 10.1016/s0896-6273(00)80196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renden R B, Broadie K. Mutation and activation of Galpha s similarly alters pre- and postsynaptic mechanisms modulating neurotransmission. J Neurophysiol. 2003;89:2620–2638. doi: 10.1152/jn.01072.2002. [DOI] [PubMed] [Google Scholar]
- Guo H F, Zhong Y. Requirement of Akt to mediate long-term synaptic depression in Drosophila. J Neurosci. 2006;26:4004–4014. doi: 10.1523/JNEUROSCI.3616-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic G. Exocytosis in bovine chromaffin cells: studies with patch-clamp capacitance and FM1-43 fluorescence. Biophys J. 2002;83:849–857. doi: 10.1016/S0006-3495(02)75213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. The optically determined size of exo/endo cycling vesicle pool correlates with the quantal content at the neuromuscular junction of Drosophila larvae. J Neurosci. 1999;19:1557–1565. doi: 10.1523/JNEUROSCI.19-05-01557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendvai B, Stern E A, Chen B, Svoboda K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature. 2000;404:876–881. doi: 10.1038/35009107. [DOI] [PubMed] [Google Scholar]
- Fay D S, Fluet A, Johnson C J, Link C D. In vivo aggregation of β-amyloid peptide variants. J Neurochem. 1998;71:1616–1625. doi: 10.1046/j.1471-4159.1998.71041616.x. [DOI] [PubMed] [Google Scholar]
- Lahey T, Gorczyca M, Jia X X, Budnik V. The Drosophila tumor suppressor gene dlg is required for normal synaptic bouton structure. Neuron. 1994;13:823–835. doi: 10.1016/0896-6273(94)90249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren K N, Manelli A M, Stine W B, Jr, Baker L K, Krafft G A, LaDu M J. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- Jan L Y, Jan Y N. Properties of the larval neuromuscular junction in Drosophila melanogaster. J Physiol. 1976;262:189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J, Halpern M E, Keshishian H. Axonal guidance and the development of muscle fiber-specific innervation in Drosophila embryos. J Neurosci. 1989;9:4318–4332. doi: 10.1523/JNEUROSCI.09-12-04318.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P A, Sanson B, Vincent J P. Compartments, wingless and engrailed: patterning the ventral epidermis of Drosophila embryos. Development. 1996;122:4095–4103. doi: 10.1242/dev.122.12.4095. [DOI] [PubMed] [Google Scholar]
- Abad M A, Enguita M, DeGregorio-Rocasolano N, Ferrer I, Trullas R. Neuronal pentraxin 1 contributes to the neuronal damage evoked by amyloid-beta and is overexpressed in dystrophic neurites in Alzheimer’s brain. J Neurosci. 2006;26:12735–12747. doi: 10.1523/JNEUROSCI.0575-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert M P, Barlow A K, Chromy B A, Edwards C, Freed R, Liosatos M, Morgan T E, Rozovsky I, Trommer B, Viola K L, Wals P, Zhang C, Finch C E, Krafft G A, Klein W L. Diffusible, nonfibrillar ligands derived from Aβ1-42 are potent central nervous system neurotoxins, Proc. Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Necula M, Kayed R, Milton S, Glabe C G. Small molecule inhibitors of aggregation indicate that amyloid beta oligomerization and fibrillization pathways are independent and distinct. J Biol Chem. 2007;282:10311–10324. doi: 10.1074/jbc.M608207200. [DOI] [PubMed] [Google Scholar]
- Fu H, Li W, Lao Y, Luo J, Lee N T, Kan K K, Tsang H W, Tsim K W, Pang Y, Li Z, Chang D C, Li M, Han Y. Bis(7)-tacrine attenuates beta amyloid- induced neuronal apoptosis by regulating L-type calcium channels. J Neurochem. 2006;98:1400–1410. doi: 10.1111/j.1471-4159.2006.03960.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Schubert D. Steroid hormones block amyloid fibril-induced 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) formazan exocytosis: relationship to neurotoxicity. J Neurochem. 1998;71:2322–2329. doi: 10.1046/j.1471-4159.1998.71062322.x. [DOI] [PubMed] [Google Scholar]
- Wang H W, Pasternak J F, Kuo H, Ristic H, Lambert M P, Chromy B, Viola K L, Klein W L, Stine W B, Krafft G A, Trommer B L. Soluble oligomers of beta amyloid (1-42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 2002;924:133–140. doi: 10.1016/s0006-8993(01)03058-x. [DOI] [PubMed] [Google Scholar]
- Ye C, Walsh D M, Selkoe D J, Hartley D M. Amyloid beta-protein induced electrophysiological changes are dependent on aggregation state: N-methyl-D-aspartate (NMDA) versus non-NMDA receptor/channel activation. Neurosci Lett. 2004;366:320–325. doi: 10.1016/j.neulet.2004.05.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.