Abstract
Calsequestrin-1 (CASQ1) is a moderate-affinity, high-capacity Ca2+-binding protein in the sarcoplasmic reticulum (SR) terminal cisternae of skeletal muscle. CASQ1 functions as both a Ca2+-binding protein and a luminal regulator of ryanodine receptor (RYR1)-mediated Ca2+ release. Mice lacking skeletal CASQ1 are viable but exhibit reduced levels of releasable Ca2+ and altered contractile properties. Here we report that CASQ1-null mice exhibit increased spontaneous mortality and susceptibility to heat- and anesthetic-induced sudden death. Exposure of CASQ1-null mice to either 2% halothane or heat stress triggers lethal episodes characterized by whole-body contractures, elevated core temperature, and severe rhabdomyolysis, which are prevented by prior dantrolene administration. The characteristics of these events are remarkably similar to analogous episodes observed in humans with malignant hyperthermia (MH) and animal models of MH and environmental heat stroke (EHS). In vitro studies indicate that CASQ1-null muscle exhibits increased contractile sensitivity to temperature and caffeine, temperature-dependent increases in resting Ca2+, and an increase in the magnitude of depolarization-induced Ca2+ release. These results demonstrate that CASQ1 deficiency alters proper control of RYR1 function and suggest CASQ1 as a potential candidate gene for linkage analysis in families with MH/EHS where mutations in the RYR1 gene are excluded.—Dainese, M., Quarta, M., Lyfenko, A. D., Paolini, C., Canato, M., Reggiani, C., Dirksen, R. T., Protasi, F. Anesthetic- and heat-induced sudden death in calsequestrin-1-knockout mice.
Keywords: excitation-contraction coupling, environmental heat stroke, malignant hyperthermia, muscle disease, ryanodine receptor
Excitation-contraction (EC) coupling, the process that controls release of Ca2+ from the sarcoplasmic reticulum (SR) during muscle activation (1), is coordinated by several interacting proteins localized in intracellular junctions named Ca2+ release units (CRUs). Sarcolemma depolarization during an action potential is sensed by dihydropyridine receptors (DHPRs or L-type Ca2+ channels) in the T-tubule membrane that in turn activate SR Ca2+ release channels or ryanodine receptors (RYR1) in the SR (1). Correct functioning of the EC coupling machinery is crucial for normal muscle function and health, since altered control of intracellular Ca2+ levels leads to muscle dysfunction and disease (2, 3).
Calsequestrin (CASQ) is an acidic, high-capacity Ca2+-binding protein located within the SR terminal cisternae that serves to concentrate exchangeable Ca2+ ions near sites of RYR1-mediated Ca2+ release (4, 5, 6). Two CASQ isoforms, products of two different genes, are found in mammalian muscle: a skeletal muscle isoform (CASQ1) and a cardiac muscle isoform (CASQ2; refs. 7, 8). Cardiac muscle exclusively expresses CASQ2, independent of developmental stage. On the other hand, both isoforms are present in skeletal muscle but are differentially expressed during development. In adult muscle, CASQ1 is the only isoform expressed in fast-twitch fibers and 75% of all CASQ expressed in slow-twitch muscle (9, 10).
In addition to being an important determinant of SR Ca2+ store content, CASQ has also been shown to modulate RYR-mediated SR Ca2+ release (11, 12). CASQ is connected to RYRs via triadin and junctin, forming a quaternary RYR-triadin-junctin-CASQ complex that is hypothesized to modulate RYR-Ca2+ release in relation to luminal Ca2+ (13). However, the precise regulatory function of CASQ on RYR channel activity remains unclear. While some studies (12, 14) report that RYR channels are activated by CASQ, others (15, 16) provide evidence for CASQ-mediated inhibition. A recently published study (17) shows that down-regulation of CASQ2, but not of CASQ1, in C2C12 skeletal myotubes leads to impaired SR Ca2+ storage and release. We found that ablation of CASQ1 in mice affects CRU structure and both the amount of Ca2+ released and the kinetic parameters (time-to-peak and half-relaxation time) of contractile responses evoked by electrical stimulation and caffeine application (18).
Several muscle diseases are associated with defects in proteins involved in EC coupling (2). Loss-of-function mutations in the CASQ2 gene (19) are linked to catecholaminergic polymorphic ventricular tachycardia (CPVT), a rare arrhythmogenic disorder characterized by physical/emotional stress-induced syncopal events and sudden cardiac death. Interestingly, CVPT can result from either loss-of-function mutations in CASQ2 (19,20,21) or gain-of-function mutations in the cardiac RYR (RYR2) (22).
So far, no specific skeletal myopathy has been associated with mutations and/or deletions in the CASQ1 gene. However, by analogy to cardiac CVPT, we hypothesized that CASQ1 deficiency in mice might result in increased susceptibility to malignant hyperthermia (MH) and exertional/environmental heat stroke (EHS) similar to that observed for gain-of-function mutations in RYR1 (23,24,25,26,27). Malignant hyperthermia susceptibility (MHS) in humans is a life-threatening hypermetabolic response to volatile anesthetics (halothane, isofluorane, etc.) in which RYR1 mutations lead to release channels that exhibit increased SR Ca2+ leak and sensitivity to uncontrolled activation by volatile anesthetics (28, 29). Interestingly, hypermetabolic episodes similar to MH crises can also result from emotional stress and mating (in pigs), as well as vigorous exercise and prolonged elevations in ambient temperature (in mice and humans; refs. 24, 25, 30,31,32,33,34,35). Therefore, we investigated whether CASQ deficiency in mice results in an MH/EHS-like phenotype characterized by lethal stress-induced (i.e., exposure to halothane or high temperatures) hypermetabolic crises and sudden death.
MATERIALS AND METHODS
In vivo experiments
CASQ1-null mice
CASQ1-null mice were generated as described previously (18). Mice were housed in microisolator cages at 20°C, 12-h light-dark cycle, with free access to water and food.
Age-dependent survival analysis of male and female wild-type (WT) and CASQ1-null mice housed under standard conditions
The rate of spontaneous mortality of CASQ1-null and WT mice in standard housing conditions was evaluated across the entire colony up to 10 mo of age (300 d) using the Kaplan-Meier method. Age-dependent probability of survival is shown in Fig. 1A.
Figure 1.
CASQ1-null male mice exhibit a high incidence of spontaneous mortality and enhanced sensitivity to heat stress (41°C) and halothane exposure (2%), which are prevented by dantrolene pretreatment. A) Age-dependent survival analysis of male and female WT and CASQ1-null mice housed under standard conditions evaluated using the Kaplan-Meier method. B) Incidence of sudden death in WT and CASQ1-null mice as a result of halothane exposure (2% for 1 h). C) Incidence of sudden and delayed (within 24 h after challenge) death in WT and CASQ1-null mice as a result of heat challenge (41°C for 30 min). D) Dantrolene administration (4 mg/kg) protects male CASQ1-null mice from heat- and halothane-induced sudden death (compare & and # in B–D). *P < 0.01 vs. WT (see Supplemental Table 1).
Halothane sensitivity test
To determine sensitivity to volatile halogenated anesthetics, male and female CASQ1-null and WT mice were exposed to an air mixture containing halothane (Sigma Aldrich, Milan, Italy) at concentrations sufficient to induce stage 3 anesthesia (2% halothane, with more added as necessary to induce and maintain this level of anesthesia) using an Isotec 3 evaporator (Datex-Ohmeda; GE Healthcare, Wauwatosa, WI, USA). The procedure used was similar to that described by Chelu et al. (24; see Supplemental Material for additional details). The number of mice tested and experimental outcomes are reported in Fig. 1B and Supplemental Table 1.
Heat-stress test
The effects of heat challenge were studied in male and female CASQ1-null and WT animals by placing animals in a temperature-controlled environmental chamber in which the ambient temperature was maintained at 41°C, using a procedure similar to that used by Chelu et al. (24). For core body temperature measurements, the initial absolute internal temperature (t0), as well as the temperature every minute thereafter, was determined using a rectal thermometer taped to the tail of the animal (see Supplemental Material). The number of mice tested and experimental outcomes are reported in Fig. 1C, Supplemental Table 1, and Fig. 2.
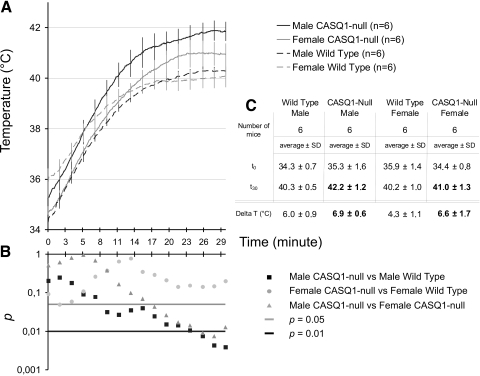
Figure 2.
Time-dependent increase in core temperature during heat challenge is greater in CASQ1-null mice. A) Increase in internal temperature during heat stress (41°C, 30 min) in CASQ1-null and WT male and female mice. B) Semilog plot showing results of Student’s t test performed during 30 min of exposure to heat (calculated every 2 min). C) Table summarizing absolute and relative changes in internal core temperatures in male and female WT and CASQ1-null mice at beginning (t0) and end (t30) of heat stress protocol.
Dantrolene pretreatment
CASQ1-null male mice were pretreated with 4 mg/kg dantrolene intraperitoneally before either heat-stress or halothane exposure. A protective effect of dantrolene was tested in male CASQ1-null animals since they were found to be more susceptible to heat- and halothane-induced lethal episodes. The number of mice tested and experimental outcomes are reported in Fig. 1D.
Histology and electron microscopy (EM)
Immediately after heat stress-induced lethal episodes, extensor digitorum longus (EDL) and soleus muscles were carefully dissected from CASQ1-null male mice. EDL and soleus muscles were also removed from WT male mice subjected to identical treatment after killing the animals by cervical dislocation. Muscles were fixed, embedded, and analyzed using standard procedures (18; see Supplemental Material for additional details). The structural damage and number of muscles/fibers analyzed are described in Fig. 3 and Supplemental Table 2.
Figure 3.
Rhabdomyolysis in CASQ1-null mice after heat stress. A, B) Damaged EDL fibers are frequent (B, asterisks) in male CASQ1-null mice after heat stress-induced sudden death but very rare in male WT mice exposed to the same treatment (A) (see Supplemental Table 2). C) Areas of excessive contracture (solid arrows) and overstretched sarcomeres (open arrows) shown in a representative damaged fiber (inset, asterisk) from a post-trigger CASQ1-null male mouse. Scale bars= : 10 μm (A, B); 2 μm (C).
Ex vivo and in vitro experiments
Temperature and caffeine sensitivity of isolated intact muscles
EDL and soleus muscles were dissected from hind limb of male WT and CASQ1-null mice in warm oxygenated Krebs solution and mounted between a force transducer (KG Scientific Instruments, Heidelberg, Germany) and a micromanipulator-controlled shaft in a small chamber where oxygenated Krebs solution was continuously circulated and temperature maintained at 25°C (see Supplemental Material). For heat stress, muscles were exposed to an increase in temperature of 2°C every 5 min while being continually stimulated with single pulses (single twitch). For caffeine contractures, muscles under continuous single twitch stimulation were exposed to increasing concentrations (0.5, 1, 2, 4, 6, 8, 10, 12, and 15 mM) of caffeine every 3 min (at 25°C). The number of mice/muscles tested and experimental outcomes are in reported in Fig. 4A, B and Supplemental Fig. 1.
Figure 4.
Temperature and caffeine dependence of basal muscle tension and resting cytosolic Ca2+ concentration in EDL muscles, single FDB fibers, and cultured primary myotubes. A) Temperature dependence of basal tension in EDL muscles isolated from male WT (○) and CASQ1-null (•) mice; n = 10; *P < 0.05. B) Percentage of EDL muscles from male WT (left) and CASQ1-null (right) mice exhibiting a contracture at caffeine concentration below 5 mM during exposure to increasing concentrations of caffeine (0.5, 1, 2, 4, 6, 8, 10, 12, and 15 mM). C, D) Temperature dependence of resting cytosolic Ca2+ concentration in single FDB muscle fibers (C) and cultured myotubes (D) from WT (○) and CASQ1-null (•) mice; n = 10; *P < 0.05; **P < 0.01.
Resting cytosolic Ca2+ concentration in primary cultures of myotubes and of single flexor digitorum brevis (FDB) muscle fibers
Primary cultures of myotubes were derived from newborn mice and single adult muscle fibers were enzymatically dissociated from FDB muscles using standard techniques (36, 37). The intracellular calcium concentration was measured in primary myotubes and FDB fibers loaded with the Ca2+-sensitive intracellular probe fluo-4 (Molecular Probes, Invitrogen, Eugene, OR, USA). After being loaded with dye, cells were moved to the stage of an upright Nikon TE-2000 microscope equipped with high numerical aperture oil immersion objectives (Nikon, Tokyo, Japan), and fluorescence was measured in a selected region of interest at increasing temperatures, as described in detail in Supplemental Material. The number of myotubes/fibers tested and experimental outcomes are in reported in Fig. 4C, D.
Voltage-clamp experiments
The whole-cell patch-clamp technique was used to simultaneously measure the voltage dependence of L-type Ca2+ currents (L-currents) and intracellular Ca2+ transients in WT and CSQ1-null myotubes, as described previously (38; see Supplemental Material for additional details).
Electrically evoked and caffeine-induced Ca2+ release in myotubes
Electrically evoked and caffeine-induced Ca2+ transients were measured in intact myotubes loaded with Indo-1 AM (Molecular Probes) as described previously (38; see Supplemental Material for additional details).
Statistical analysis
Data in Figs. 4 and 5 and Supplemental Table 3 are given as means ± se. Data in Fig. 2 and Supplemental Table 2 are given as means ± sd. A χ2 test was used to evaluate statistical significance in experiments on halothane- and heat-induced mortality and dantrolene protection in WT and CASQ1-null mice (Fig. 1B–D), incidence of muscle damage after a lethal heat-induced event in CASQ1-null mice (Supplemental Table 2), and caffeine threshold sensitivity (Fig. 4B). Statistical significance for all other experiments was determined using a Student’s t test. For all analyses, differences were considered statistically significant at P < 0.05.
Figure 5.
Maximal voltage-gated Ca2+ calcium release is enhanced in myotubes from CASQ1-null mice. A) Representative L-type Ca2+ currents (bottom traces) and intracellular Ca2+ transients (top traces) elicited by 200 ms depolarization to the indicated test potential in WT (left) and CASQ1-null (right) myotubes. B, C) Average voltage dependence of peak L-type Ca2+ current density (B) and intracellular Ca2+ transients (C) in WT (•, n=21) and CASQ1-null (○, n=20) myotubes (see also Supplemental Table 3 and Supplemental Fig. 2).
RESULTS
In a recent study, we described the phenotype of mice with a null mutation for the skeletal muscle CASQ1 gene (18). Two lines of reasoning prompted us to investigate the phenotype of CASQ1-null mice under conditions of increased stress. First, compared with their wild-type counterparts, we observed an apparent increased mortality rate of male CASQ1-null mice placed in reproductive cages, particularly as animals progressed toward a more advanced age (after 6 mo of age). Second, loss-of-function mutations in CASQ2 result in CPVT, a stress-induced arrhythmogenic disorder characterized by syncopal events and sudden cardiac death (see introduction and refs. 19, 21).
High rate of spontaneous mortality in CASQ1-null male mice
We determined the rate of spontaneous mortality under standard housing conditions from birth to 10 mo of age in our CASQ1-null colony. The survival function of CASQ1-null and WT mice, both males and females, is provided in Fig. 1A. The mortality rate of male CASQ1-null mice was significantly (P<0.01) higher than that of females, as well as both male and female WT mice. A modest increase in mortality was also apparent in female CASQ1-null mice compared with female WT mice, although this trend was not statistically significant. Interestingly, most of the spontaneous deaths were registered in male CASQ1-null mice while they were housed in reproductive cages, suggesting a contribution of stress due to mating to the observed increase mortality of male CASQ1-null mice.
CSQ1-null mice exhibit lethal halothane-induced MH-like episodes
Halothane exposure triggers MH episodes in susceptible humans (29), MHS pigs (30), and heterozygous Y522S- and R163C-knockin mice (24, 25). Therefore, we determined the effect of halothane exposure on CASQ1-null mice. Male CASQ1-null animals exhibited a very high mortality rate during exposure to 2% halothane. Specifically, 80% (16/20) of male CASQ1-null mice died during halothane exposure, while the identical treatment was well-tolerated in all WT animals (Fig. 1B). Female CASQ1-null mice displayed a higher survival rate after halothane exposure compared with male CASQ1-null mice, but still lower than WT mice (Fig. 1B). However, increased mortality after halothane exposure was highly significant only for male CASQ1-null mice (P<0.01; see Supplemental Table 1).
CASQ1-null mice undergo lethal EHS-like episodes after exposure to heat stress
Since heat stress triggers fatal events in both pigs and mice carrying MH mutations in RYR1 (24, 25), we determined the heat sensitivity of CASQ1-null mice. WT and CASQ1-null mice were placed for up to 30 min in an environmental chamber in which the ambient temperature was maintained at 41°C. Under these conditions, >70% (17/24) CASQ1-null male mice underwent lethal episodes characterized by impaired movement, difficulty in breathing, and whole-body contractions that were followed by sudden death (Fig. 1C). Triggered events lasted no longer than a few minutes (5–10 min) and could easily be anticipated by increased agitation and movement of CASQ1-null mice before triggering. Identical heat challenge was well tolerated in WT mice, although 1 of the 20 WT animals tested died during heat challenge (Fig. 1C). While female CASQ1-null mice were more susceptible than either male or female WT mice, the difference in mortality after heat stress was highly significant (P<0.01) only within the male population. In addition, some delayed deaths (within 24 h after heat challenge) were also observed in surviving CASQ1-null mice after heat challenge (3 males, 2 females; Fig. 1C; see Supplemental Table 1).
In humans, MH episodes are associated with sustained muscle contractures accompanied by enhanced body temperature and metabolic rate (39, 40) Since a proportion of CASQ1-null mice responds to heat stress with MH/EHS-like episodes, we monitored rectal temperatures in male and female WT and CASQ1-null mice throughout the 30-min 41°C temperature challenge (Fig. 2). The results indicate that heat stress caused a larger time-dependent increase in core temperature in CASQ1-null male and female mice compared with WT animals. Specifically, the internal temperature of male CASQ1-null mice at the end of the heat stress protocol (42.2±1.2°C) was higher than the corresponding values observed in CASQ1-null female (41.0±1.3°C), WT female (40.2±1.0°C), and WT male (40.3±0.5°C) mice (Fig. 2A).
A Student’s t test performed during the 30-min heat exposure (Fig. 2B) indicates that absolute core temperature of male CASQ1-null mice was significantly (P<0.05) higher than that of both female CASQ1-null and male WT mice starting from ∼15 min after initiation of heat exposure, while the difference between WT and CASQ1-null female mice was not significant (Fig. 2B). The observed higher elevation in core temperature and greater susceptibility for muscle rigidity in male CASQ1-null mice are indicative of an increased metabolic rate in these animals during heat stress. The results presented in Figs. 2A and 1C reflect a strict correlation between hyperthermia susceptibility and mortality during heat challenge.
Dantrolene pretreatment prevents halothane- and heat-induced sudden death
Dantrolene administration is the only pharmacological intervention for MH in humans (41) and has also been considered in the treatment of EHS (42). Therefore, we determined the efficacy of prior intraperitoneal injection of dantrolene (4 mg/kg) in preventing halothane- and heat-induced crises in male CASQ1-null mice. Dantrolene pretreatment protected male CASQ1-null mice from acute fatal events during both halothane exposure (10/10) and 30-min heat challenge (10/10; Fig. 1D), treatments that were lethal in ∼80% of untreated male CASQ1-null mice (Fig. 1B, C). While delayed (within 24 h) deaths were observed in two dantrolene-treated male CASQ1-null mice exposed to heat stress (Fig. 1D), dantrolene protection from halothane- and heat-induced mortality was highly significant (P<0.01).
Rhabdomyolysis in CASQ1-null mice after lethal heat stress
During lethal crises induced by high temperature, CASQ1-null mice exhibited impaired movement and spasmodic contractions, whereas WT animals in the same chamber moved normally. To assess possible muscle damage after lethal episodes, EDL muscles from male CASQ1-null mice were quickly dissected at the end of a lethal heat-induced episode and compared with those of WT mice killed after exposure to the same heat challenge. Male animals were used in these studies since they are more susceptible to heat-induced lethal episodes (Fig. 2C). After fixation, histological examination revealed that ∼40% (36/89) of fibers analyzed (3 EDL muscles from 3 different animals) from post-trigger CASQ1-null mice were severely damaged (rhabdomyolysis; Fig. 3B; Supplemental Table 2). Similar alterations were not found in surviving WT mice after exposure to the same treatment (Fig. 3A): only 1 disrupted fiber out of 44 (2 EDL muscles from 2 animals) was found in WT mice killed after 30 min of heat exposure (Supplemental Table 2). The increased incidence of EDL damage in male CASQ1-null mice after a lethal heat-induced event was highly significant (P<0.01). Whereas quantitative data were collected from light microscopy images (Fig. 3A, B), EM analysis of these areas revealed that damaged fibers exhibited regions of contractures and overstretched sarcomeres (Fig. 3C), Z-line misalignment, loss of M line, and SR swelling.
Enhanced temperature and caffeine sensitivity of skeletal muscles from CASQ1-null mice
The uncontrolled whole-body muscle contractures observed in CASQ1-null mice during heat challenge suggest that CASQ1 deficiency results in a temperature-induced impairment in skeletal muscle Ca2+ homeostasis. To test this hypothesis, we determined the effect of temperature challenge on in vitro basal tension of EDL and soleus muscles isolated from 4-mo-old male WT and CASQ1-null mice. Male animals were used since they were found to exhibit the greatest susceptibility to lethal heat-induced episodes (Fig. 2C). CASQ1-null EDL muscle showed an increase in basal tension at temperatures above 27°C, which was significantly greater than that observed in WT EDL muscles (P<0.05) in which basal tension did not increase until temperatures exceeded 35°C (Fig. 4A). Similar differences were not observed in soleus muscle (Supplemental Fig. 1), possibly reflecting compensation by CASQ2, which is expressed at moderate levels in slow-twitch muscle (9).
MH susceptibility is clinically diagnosed using a standardized in vitro contracture test (IVCT), which determines the contractile threshold of muscle biopsy samples to low concentrations of caffeine and halothane (43). Therefore, we compared the in vitro caffeine contracture threshold of EDL muscles isolated from male WT and CASQ1-null mice. Figure 4B shows that a higher percentage (50%) of EDL muscles from male CASQ1-null mice exhibit contractures at a significantly (P<0.05) lower caffeine threshold (<5 mM) compared with EDL muscles from WT male mice (10% <5 mM).
Finally, to determine if the increase in basal tension observed in the skeletal muscle of CASQ1-null mice results from a defect in Ca2+ homeostasis, the temperature-dependence of resting cytosolic Ca2+ concentration was determined in single FDB fibers (Fig. 4C) and myotubes (Fig. 4D) derived from WT and CASQ1-null mice. Compared with WT, resting Ca2+ was significantly (P<0.05) increased at temperatures ≥30°C in CASQ1-null fibers and myotubes.
Voltage-gated Ca2+ release is increased in CASQ1-null myotubes
We previously reported an increase in twitch tension and the twitch-to-tetanus ratio in EDL muscles from CASQ1-null mice (18). Moreover, others (16) have suggested that CASQ1 limits SR Ca2+ loss during EC coupling by reducing RYR activity when luminal Ca2+ levels drop during release. Thus, the observed effects of CASQ1 ablation of twitch tension and twitch-to-tetanus ratio could result from the loss of CSQ1-mediated inhibition of Ca2+ release during EC coupling. To test this hypothesis, we compared the magnitude and voltage dependence of depolarization-induced Ca2+ release in whole-cell voltage-clamp experiments of WT and CASQ1-null myotubes. Figure 5A shows representative L-type Ca2+ currents (top traces) and intracellular Ca2+ transients (bottom traces) elicited by 200 ms depolarization to the indicated potentials (−50, −10, 30, and 70 mV) in WT (left) and CASQ1-null myotubes (right). The magnitude and voltage dependence of L-type Ca2+ currents were similar in the two myotubes populations (Fig. 5B; Supplemental Table 3, I-V data). The magnitude and voltage dependence of depolarization-induced intracellular Ca2+ transients are summarized in Fig. 5C. While the voltage for half-maximal activation of voltage-gated Ca2+ release (VF1/2) did not differ between WT and CASQ1-null myotubes, maximal voltage-gated Ca2+ release was significantly increased (184%) in CASQ1-null myotubes (Supplemental Table 3, F-V data). We also found that maximal (30 mM) caffeine-induced Ca2+ release was significantly (P<0.01) reduced in CASQ1-null myotubes (see Supplemental Fig. 2A), consistent with prior results obtained in FDB fibers (18). Thus, the observed increase in voltage-gated Ca2+ release in Fig. 5C occurred despite the fact that the total RYR-releasable Ca2+ store content was actually reduced in CASQ1-null myotubes.
DISCUSSION
Background
CASQ1 is proposed to function both as a reservoir for storage of Ca2+ ions in the SR (4) and as a Ca2+ sensor that modulates RYR1 Ca2+ release channel activity (11, 16) Therefore, mutation/ablation of this critical SR Ca2+-binding protein would be expected to result in abnormal SR Ca2+ storage and altered regulation of RYR-mediated Ca2+ release, possibly leading to defects in muscle contractile function.
MH/EHS-like phenotype of CASQ1-null mice
In this study, we report a striking MH/EHS-like phenotype in CASQ1-null mice. Specifically, exposure of CASQ1-null mice to either 2% halothane or heat stress triggers lethal episodes of whole-body contractures, hyperthermia, and prominent rhabdomyolysis, which are prevented by prior systemic administration of dantrolene (Figs. 123). EDL muscles of CASQ1-null mice exhibit increased sensitivity to temperature- and caffeine-induced contractures. We also found that intracellular Ca2+ levels are increased at physiological temperatures in FDB fibers and myotubes of CASQ1-null mice (Fig. 4). Together, these results strongly indicate that the anesthetic and heat sensitivity of CASQ1-null mice closely resemble that of pig and mouse models of MH/EHS based on gain-of-function RYR1 gene mutations (23, 25, 30, 31). Furthermore, our results reinforce a parallel between MHS and EHS and support the notion that the two syndromes may in some cases arise from a common pathogenic mechanism, i.e., lack of proper regulation of SR Ca2+ release (31,32,33, 35, 44, 45).
Spontaneous and stress-induced mortality
We found that adult male CASQ1-null mice not only exhibit anesthetic-induced MH-like crises but also display an unusually high rate of spontaneous mortality. The exact reasons for this increase in spontaneous mortality are not yet clear. However, other animal models of MH also exhibit anesthetic-independent events triggered by emotional stress (e.g., transportation in pigs), mating, vigorous exercise, and prolonged elevations in ambient temperature (23,24,25, 30, 31). Similarly, sudden death due to hyperthermia and/or strenuous exercise, but unrelated to anesthetic administration, has also been reported in humans (46,47,48,49). Two of these cases (47, 48) and another case of exercise-induced rhabdomyolysis (34) occurred in humans with family history of MH. A further indication that exercise-induced rhabdomyolysis and MH may be related syndromes comes from a study showing that 10 of 18 patients with a history of MH-like episodes induced by physical stress also exhibited a positive IVCT (50).
The significant increase in spontaneous death in male CASQ1-null mice was observed primarily for mice housed in reproductive cages, where mating may result in increased stress sufficient to trigger lethal episodes in these animals. Whereas this is a possible explanation, further investigation is needed to confirm the exact reasons for increased spontaneous mortality of male CASQ1-null mice.
Gender mortality differences of CASQ1-null mice
The striking difference in spontaneous and stress-induced mortality between male and female CASQ-null mice was an unexpected observation of our study. However, a similar disproportionate male susceptibility has also been observed in a murine MHS/EHS model (RYR1Y522S/wt mice; S. L. Hamilton, personal communication) and also reported in epidemiologic studies of MH in humans. Specifically, Strazis and Fox (51) reported a male-to-female ratio of 2.2:1 for MH susceptibility in humans with males exhibiting a far greater fatality rate. Other studies found males representing 78% of the 181 MH cases in the North American MH Registry (NAMHR) (52) and 73% of the 308 NAMHR patients included in a recent MH recrudescence study (53). A similar male prevalence (∼4:1) was observed in an epidemiological study of 383 MH cases in Japan from 1961 to 2004 (54). Moreover, EHS is also reported to be much more common in young men and quite rare in women (55). In light of these studies (51,52,53,54,55), the similar increased male prevalence for heat- and halothane-induced crises in both CASQ1-null mice and human MHS populations provides additional support for a relation between CASQ1 deficiency and MHS. However, the precise mechanisms responsible for the increased susceptibility of male CASQ1-null mice (or increased protection in female CASQ1-null mice) are unclear and will clearly require further investigation.
CASQ1 deficiency results in defects in proper SR Ca2+ handling
Similar to that reported for knockin mice harboring MH point mutations in RYR1 (24, 25, 31), the MH/EHS-like phenotype of CASQ1-null mice likely arises from defects in SR Ca2+ handling. The specific mechanisms by which gain-of-function mutations in RYR1 and CASQ1 deficiency alter SR Ca2+ handling are likely to be fundamentally different. In MH/EHS due to mutations in RYR1, channel activity is directly altered in a manner that leads to increased SR Ca2+ leak (24, 25) and subsequent RYR1 S-nitrosylation increases the temperature sensitivity of the channel for activation (31). Our finding that voltage-gated Ca2+ release is increased in CASQ1-null myotubes is consistent with the proposed role of CASQ1 as a luminal Ca2+ sensor that acts to limit SR Ca2+ depletion during RYR1-mediated Ca2+ release (16, 56) In planar lipid bilayers studies, Beard et al. (16) found that CASQ binding to the RYR1 complex inhibits channel activity and dissociation results in a 10-fold increase in the duration of channel opening. Thus, CASQ1 ablation would be expected to remove this inhibitory influence on RYR1-mediated Ca2+ release. Loss of CASQ1 would also be expected to increase SR Ca2+ leak by reducing total releasable SR Ca2+ content (18) while increasing free SR Ca2+ (57) and, by analogy to CASQ2 in the heart (21), cause a leftward shift in the SR Ca2+ leak-load relation. As CASQ1 is the major Ca2+-binding protein in the SR lumen, CASQ1 deficiency may also impair SR Ca2+ reuptake (58) in a manner that further contributes to an increase in resting myoplasmic Ca2+ under conditions that stress the cellular Ca2+ regulatory control mechanisms. Thus, the combination of loss of proper CASQ1-mediated RYR1 inhibition, increased SR Ca2+ leak, and reduced SR Ca2+ reuptake in CASQ1-null muscle would lead to increased resting Ca2+ and uncontrolled Ca2+ flux across the SR during caffeine/halothane exposure, heat stress, and, possibly, also physical/emotional stress due to mating. It will be important in future work to determine if RYR1 S-nitrosylation underlies increased heat sensitivity of CASQ1-null mice as has been shown to occur in RYR1-knockin mice (31).
An increase in the magnitude of depolarization-induced calcium transients in CASQ1-null myotubes was another unexpected finding of our study (Fig. 5). This increase occurred despite the fact that total RYR1-releasable SR Ca2+ store content assessed by caffeine application was reduced >50% in CASQ1-null myotubes (Supplemental Fig. 2A), consistent with results observed in adult muscle fibers (18). We also found that the first derivative of the fluo-4 fluorescence transient during depolarization, which provides an approximation of the maximal rate of Ca2+ release in myotubes (59), was significantly increased in CASQ1-null myotubes (Supplemental Fig. 2B). Finally, the rate of electrically evoked (action potential-induced) Ca2+ transient decay was not significantly different between intact WT and CASQ1-null myotubes (data not shown). Together, these results suggest that the observed increase in depolarization-induced Ca2+ transients in CASQ1-null myotubes results from enhanced release channel activity rather than from differences in store content and/or removal. An increase in RYR1-mediated Ca2+ release during depolarization in CASQ1-null myotubes could result from the loss of CASQ1 inhibition of RYR1 channel activity (16, 60). Alternatively, Royer et al. (61) recently found that an increase in SR “evacuability” (i.e., ability of the store to empty its contents) increases if either release channel permeability increases or SR Ca2+ buffering power and store content decrease. Since both SR Ca2+ store content and total SR Ca2+ buffering decrease in CASQ1-null myotubes, then this property could also contribute to the increase in voltage-gated Ca2+ release observed in CASQ1-null myotubes.
CASQ: a new candidate gene for MH linkage studies
While the majority of MHS kindreds are linked to mutations in the RYR1 gene, causative mutations in this gene are not found in ∼30% of families with a clear clinical MHS diagnosis (26, 27) and have been reported for only a few cases of EHS (34, 47, 48, 50). In the absence of an RYR1 mutation, MH (and potentially EHS) most likely arises from mutations to other proteins that coordinate and regulate SR Ca2+ handling and EC coupling in skeletal muscle. Indeed, at least five other genetic loci (on chromosomes 1, 3, 5, 7, and 17) have been implicated in MH (see ref. 27 for review), one of which involves confirmed mutations to the α1-subunit of DHPR (62) that enhance RYR1 sensitivity to activation by endogenous and exogenous triggers (63). Our demonstration that CASQ1 deficiency results in MHS and EHS in mice: 1) validates the potential role of loss-of-function mutations in CASQ1 as an alternative mechanism for MHS where mutations in RYR1 are excluded, and 2) suggests an alternative genetic locus (CASQ1) for linkage studies in those MHS patients (∼30%) in which mutations in RYR1 have been excluded. However, in the absence of a confirmed human kindred, we can not rule out the possibility that CASQ1 deficiency results in MHS/EHS susceptibility in mice but not humans.
CONCLUSIONS
Our results demonstrate that CASQ1 deficiency leads to MH/EHS in mice, and together with prior results obtained for CASQ2 (19, 21), support the notion that disruption of CASQ function represents an important and common pathogenic mechanism that underlies stress-induced sudden death in a range of genetically inherited cardiac (CPVT) and skeletal muscle (MH and EHS) disorders.
Supplementary Material
Acknowledgments
We thank Michele Scorzeto for the precious contribution of fluorescence microscopy in part of the experiments of Fig. 4. We also thank Paul D. Allen and Ronit Hirsh (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA) for providing the primary myotubes used for some of the experiments presented in Fig. 5. This study was supported by research grant GGP08153 from the Italian Telethon Foundation, by research funds from the University G. d’Annunzio of Chieti (to F.P.), and by U.S. National Institutes of Health grants AR44657 (to R.T.D.) and 5P01AR052354 (to F.P. and R.T.D.).
References
- Rios E, Ma J J, Gonzalez A. The mechanical hypothesis of excitation-contraction (EC) coupling in skeletal muscle. J Muscle Res Cell Motil. 1991;12:127–135. doi: 10.1007/BF01774031. [DOI] [PubMed] [Google Scholar]
- MacLennan D H. Ca2+ signalling and muscle disease. Eur J Biochem. 2000;267:5291–5297. doi: 10.1046/j.1432-1327.2000.01566.x. [DOI] [PubMed] [Google Scholar]
- Dirksen R T, Avila G. Distinct effects on Ca2+ handling caused by malignant hyperthermia and central core disease mutations in RyR1. Biophys J. 2004;87:3193–3204. doi: 10.1529/biophysj.104.048447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan D H, Wong P T. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1971;68:1231–1235. doi: 10.1073/pnas.68.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K P, MacLennan D H, Jorgensen A O, Mintzer M C. Purification and characterization of calsequestrin from canine cardiac sarcoplasmic reticulum and identification of the 53,000 dalton glycoprotein. J Biol Chem. 1983;258:1197–1204. [PubMed] [Google Scholar]
- Franzini-Armstrong C, Kenney L J, Varriano-Marston E. The structure of calsequestrin in triads of vertebrate skeletal muscle: a deep-etch study. J Cell Biol. 1987;105:49–56. doi: 10.1083/jcb.105.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegel L, Ohnishi M, Carpenter M R, Khanna V K, Reithmeier R A, MacLennan D H. Amino acid sequence of rabbit fast-twitch skeletal muscle calsequestrin deduced from cDNA and peptide sequencing. Proc Natl Acad Sci U S A. 1987;84:1167–1171. doi: 10.1073/pnas.84.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B T, Simmerman H K, Collins J H, Nadal-Ginard B, Jones L R. Complete amino acid sequence of canine cardiac calsequestrin deduced by cDNA cloning. J Biol Chem. 1988;263:8958–8964. [PubMed] [Google Scholar]
- Damiani E, Volpe P, Margreth A. Coexpression of two isoforms of calsequestrin in rabbit slow-twitch muscle. J Muscle Res Cell Motil. 1990;11:522–530. doi: 10.1007/BF01745219. [DOI] [PubMed] [Google Scholar]
- Sacchetto R, Volpe P, Damiani E, Margreth A. Postnatal development of rabbit fast-twitch skeletal muscle: accumulation, isoform transition and fibre distribution of calsequestrin. J Muscle Res Cell Motil. 1993;14:646–653. doi: 10.1007/BF00141561. [DOI] [PubMed] [Google Scholar]
- Ikemoto N, Ronjat M, Meszaros L G, Koshita M. Postulated role of calsequestrin in the regulation of calcium release from sarcoplasmic reticulum. Biochemistry. 1989;28:6764–6771. doi: 10.1021/bi00442a033. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Kasai M. Regulation of calcium channel in sarcoplasmic reticulum by calsequestrin. Biochem Biophys Res Commun. 1994;199:1120–1127. doi: 10.1006/bbrc.1994.1347. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kelley J, Schmeisser G, Kobayashi Y M, Jones L R. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J Biol Chem. 1997;272:23389–23397. doi: 10.1074/jbc.272.37.23389. [DOI] [PubMed] [Google Scholar]
- Terentyev D, Viatchenko-Karpinski S, Gyorke I, Volpe P, Williams S C, Gyorke S. Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: mechanism for hereditary arrhythmia. Proc Natl Acad Sci U S A. 2003;100:11759–11764. doi: 10.1073/pnas.1932318100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L R, Suzuki Y J, Wang W, Kobayashi Y M, Ramesh V, Franzini-Armstrong C, Cleemann L, Morad M. Regulation of Ca2+ signaling in transgenic mouse cardiac myocytes overexpressing calsequestrin. J Clin Invest. 1998;101:1385–1393. doi: 10.1172/JCI1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard N A, Sakowska M M, Dulhunty A F, Laver D R. Calsequestrin is an inhibitor of skeletal muscle ryanodine receptor calcium release channels. Biophys J. 2002;82:310–320. doi: 10.1016/S0006-3495(02)75396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu L, Duan H, Pasek D A, Eu J P, Meissner G. Knocking down type 2 but not type 1 calsequestrin reduces calcium sequestration and release in C2C12 skeletal muscle myotubes. J Biol Chem. 2006;281:15572–15581. doi: 10.1074/jbc.M600090200. [DOI] [PubMed] [Google Scholar]
- Paolini C, Quarta M, Nori A, Boncompagni S, Canato M, Volpe P, Allen P D, Reggiani C, Protasi F. Reorganized stores and impaired calcium handling in skeletal muscle of mice lacking calsequestrin-1. J Physiol. 2007;583:767–784. doi: 10.1113/jphysiol.2007.138024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat H, Pras E, Olender T, Avidan N, Ben-Asher E, Man O, Levy-Nissenbaum E, Khoury A, Lorber A, Goldman B, Lancet D, Eldar M. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet. 2001;69:1378–1384. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi N, Liu N, Napolitano C, Nori A, Turcato F, Colombi B, Bicciato S, Arcelli D, Spedito A, Scelsi M, Villani L, Esposito G, Boncompagni S, Protasi F, Volpe P, Priori S G. Unexpected structural and functional consequences of the R33Q homozygous mutation in cardiac calsequestrin: a complex arrhythmogenic cascade in a knock in mouse model. Circ Res. 2008;103:298–306. doi: 10.1161/CIRCRESAHA.108.171660. [DOI] [PubMed] [Google Scholar]
- Knollmann B C, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann B E, Horton K D, Weissman N J, Holinstat I, Zhang W, Roden D M, Jones L R, Franzini-Armstrong C, Pfeifer K. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori S G, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli G A. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- Fujii J, Otsu K, Zorzato F, de Leon S, Khanna V K, Weiler J E, O'Brien P J, MacLennan D H. Identification of a mutation in porcine ryanodine receptor associated with malignant hyperthermia. Science. 1991;253:448–451. doi: 10.1126/science.1862346. [DOI] [PubMed] [Google Scholar]
- Chelu M G, Goonasekera S A, Durham W J, Tang W, Lueck J D, Riehl J, Pessah I N, Zhang P, Bhattacharjee M B, Dirksen R T, Hamilton S L. Heat- and anesthesia-induced malignant hyperthermia in an RyR1 knock-in mouse. FASEB J. 2006;20:329–330. doi: 10.1096/fj.05-4497fje. [DOI] [PubMed] [Google Scholar]
- Yang T, Riehl J, Esteve E, Matthaei K I, Goth S, Allen P D, Pessah I N, Lopez J R. Pharmacologic and functional characterization of malignant hyperthermia in the R163C RyR1 knock-in mouse. Anesthesiology. 2006;105:1164–1175. doi: 10.1097/00000542-200612000-00016. [DOI] [PubMed] [Google Scholar]
- Galli L, Orrico A, Lorenzini S, Censini S, Falciani M, Covacci A, Tegazzin V, Sorrentino V. Frequency and localization of mutations in the 106 exons of the RYR1 gene in 50 individuals with malignant hyperthermia. Hum Mutat. 2006;27:830. doi: 10.1002/humu.9442. [DOI] [PubMed] [Google Scholar]
- Robinson R, Carpenter D, Shaw M A, Halsall J, Hopkins P. Mutations in RYR1 in malignant hyperthermia and central core disease. Hum Mutat. 2006;27:977–989. doi: 10.1002/humu.20356. [DOI] [PubMed] [Google Scholar]
- Denborough M. Malignant hyperthermia. Lancet. 1998;352:1131–1136. doi: 10.1016/S0140-6736(98)03078-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg H, Davis M, James D, Pollock N, Stowell K. Malignant hyperthermia. Orphanet J Rare Dis. 2007;2:21. doi: 10.1186/1750-1172-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ording H, Hald A, Sjontoft E. Malignant hyperthermia triggered by heating in anaesthetized pigs. Acta Anaesthesiol Scand. 1985;29:698–701. doi: 10.1111/j.1399-6576.1985.tb02283.x. [DOI] [PubMed] [Google Scholar]
- Durham W J, Aracena-Parks P, Long C, Rossi A E, Goonasekera S A, Boncompagni S, Galvan D L, Gilman C P, Baker M R, Shirokova N, Protasi F, Dirksen R, Hamilton S L. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell. 2008;133:53–65. doi: 10.1016/j.cell.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins P M, Ellis F R, Halsall P J. Evidence for related myopathies in exertional heat stroke and malignant hyperthermia. Lancet. 1991;338:1491–1492. doi: 10.1016/0140-6736(91)92304-k. [DOI] [PubMed] [Google Scholar]
- Bouchama A, Knochel J P. Heat stroke. N Engl J Med. 2002;346:1978–1988. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- Davis M, Brown R, Dickson A, Horton H, James D, Laing N, Marston R, Norgate M, Perlman D, Pollock N, Stowell K. Malignant hyperthermia associated with exercise-induced rhabdomyolysis or congenital abnormalities and a novel RYR1 mutation in New Zealand and Australian pedigrees. Br J Anaesth. 2002;88:508–515. doi: 10.1093/bja/88.4.508. [DOI] [PubMed] [Google Scholar]
- Muldoon S, Deuster P, Brandom B, Bunger R. Is there a link between malignant hyperthermia and exertional heat illness? Exerc Sport Sci Rev. 2004;32:174–179. doi: 10.1097/00003677-200410000-00009. [DOI] [PubMed] [Google Scholar]
- Rando T A, Blau H M. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defranchi E, Bonaccurso E, Tedesco M, Canato M, Pavan E, Raiteri R, Reggiani C. Imaging and elasticity measurements of the sarcolemma of fully differentiated skeletal muscle fibres. Microsc Res Tech. 2005;67:27–35. doi: 10.1002/jemt.20177. [DOI] [PubMed] [Google Scholar]
- Avila G, Dirksen R T. Functional effects of central core disease mutations in the cytoplasmic region of the skeletal muscle ryanodine receptor. J Gen Physiol. 2001;118:277–290. doi: 10.1085/jgp.118.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyfenko A D, Goonasekera S A, Dirksen R T. Dynamic alterations in myoplasmic Ca2+ in malignant hyperthermia and central core disease. Biochem Biophys Res Commun. 2004;322:1256–1266. doi: 10.1016/j.bbrc.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Treves S, Anderson A A, Ducreux S, Divet A, Bleunven C, Grasso C, Paesante S, Zorzato F. Ryanodine receptor 1 mutations, dysregulation of calcium homeostasis and neuromuscular disorders. Neuromuscul Disord. 2005;15:577–587. doi: 10.1016/j.nmd.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Wedel D J, Quinlan J G, Iaizzo P A. Clinical effects of intravenously administered dantrolene. Mayo Clin Proc. 1995;70:241–246. doi: 10.4065/70.3.241. [DOI] [PubMed] [Google Scholar]
- Hausfater P. Dantrolene and heatstroke: a good molecule applied in an unsuitable situation. Crit Care. 2005;9:23–24. doi: 10.1186/cc2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group T E M H. A protocol for the investigation of malignant hyperpyrexia (MH) susceptibility. The European Malignant Hyperpyrexia Group. Br J Anaesth. 1984;56:1267–1269. doi: 10.1093/bja/56.11.1267. [DOI] [PubMed] [Google Scholar]
- Bourdon L, Canini F. On the nature of the link between malignant hyperthermia and exertional heatstroke. Med Hypotheses. 1995;45:268–270. doi: 10.1016/0306-9877(95)90116-7. [DOI] [PubMed] [Google Scholar]
- Grogan H, Hopkins P M. Heat stroke: implications for critical care and anaesthesia. Br J Anaesth. 2002;88:700–707. doi: 10.1093/bja/88.5.700. [DOI] [PubMed] [Google Scholar]
- Pamukcoglu T. Sudden death due to malignant hyperthermia. Am J Forensic Med Pathol. 1988;9:161–162. doi: 10.1097/00000433-198806000-00015. [DOI] [PubMed] [Google Scholar]
- Ryan J F, Tedeschi L G. Sudden unexplained death in a patient with a family history of malignant hyperthermia. J Clin Anesth. 1997;9:66–68. doi: 10.1016/S0952-8180(96)00207-3. [DOI] [PubMed] [Google Scholar]
- Tobin J R, Jason D R, Challa V R, Nelson T E, Sambuughin N. Malignant hyperthermia and apparent heat stroke. JAMA. 2001;286:168–169. doi: 10.1001/jama.286.2.168. [DOI] [PubMed] [Google Scholar]
- Wyndham C H. Heat stroke and hyperthermia in marathon runners. Ann N Y Acad Sci. 1977;301:128–138. doi: 10.1111/j.1749-6632.1977.tb38192.x. [DOI] [PubMed] [Google Scholar]
- Wappler F, Fiege M, Steinfath M, Agarwal K, Scholz J, Singh S, Matschke J, Schulte Am Esch J. Evidence for susceptibility to malignant hyperthermia in patients with exercise-induced rhabdomyolysis. Anesthesiology. 2001;94:95–100. doi: 10.1097/00000542-200101000-00019. [DOI] [PubMed] [Google Scholar]
- Strazis K P, Fox A W. Malignant hyperthermia: a review of published cases. Anesth Analg. 1993;77:297–304. doi: 10.1213/00000539-199308000-00014. [DOI] [PubMed] [Google Scholar]
- Brandom B W, Larach M G. Reassessment of the safety and efficacy of dantrolene [Abstract] Anesthesiology. 2002;96:A1199. [Google Scholar]
- Burkman J M, Posner K L, Domino K B. Analysis of the clinical variables associated with recrudescence after malignant hyperthermia reactions. Anesthesiology. 2007;106:901–906. doi: 10.1097/01.anes.0000265148.86566.68. [DOI] [PubMed] [Google Scholar]
- Migita T, Mukaida K, Kawamoto M, Kobayashi M, Yuge O. Fulminant-type malignant hyperthermia in Japan: cumulative analysis of 383 cases. J Anesth. 2007;21:285–288. doi: 10.1007/s00540-006-0495-5. [DOI] [PubMed] [Google Scholar]
- Sidman R D, Gallagher E J. Exertional heat stroke in a young woman: gender differences in response to thermal stress. Acad Emerg Med. 1995;2:315–319. doi: 10.1111/j.1553-2712.1995.tb03229.x. [DOI] [PubMed] [Google Scholar]
- Wei L, Varsanyi M, Dulhunty A F, Beard N A. The conformation of calsequestrin determines its ability to regulate skeletal ryanodine receptors. Biophys J. 2006;91:1288–1301. doi: 10.1529/biophysj.106.082610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R M, Larkins N T, Mollica J P, Beard N A, Lamb G D. Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat. J Physiol. 2008;587:443–460. doi: 10.1113/jphysiol.2008.163162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer M W, Stephenson D G. Total and sarcoplasmic reticulum calcium contents of skinned fibres from rat skeletal muscle. J Physiol. 1996;493:357–370. doi: 10.1113/jphysiol.1996.sp021388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila G, Dirksen R T. Rapamycin and FK506 reduce skeletal muscle voltage sensor expression and function. Cell Calcium. 2005;38:35–44. doi: 10.1016/j.ceca.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Beard N A, Casarotto M G, Wei L, Varsanyi M, Laver D R, Dulhunty A F. Regulation of ryanodine receptors by calsequestrin: effect of high luminal Ca2+ and phosphorylation. Biophys J. 2005;88:3444–3454. doi: 10.1529/biophysj.104.051441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer L, Pouvreau S, Rios E. Evolution and modulation of intracellular calcium release during long-lasting, depleting depolarization in mouse muscle. J Physiol. 2008;586:4609–4629. doi: 10.1113/jphysiol.2008.157990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier N, Procaccio V, Stieglitz P, Lunardi J. Malignant-hyperthermia susceptibility is associated with a mutation of the alpha 1-subunit of the human dihydropyridine-sensitive L-type voltage-dependent calcium-channel receptor in skeletal muscle. Am J Hum Genet. 1997;60:1316–1325. doi: 10.1086/515454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R G, O'Connell K M, Flucher B E, Allen P D, Grabner M, Dirksen R T. Functional analysis of the R1086H malignant hyperthermia mutation in the DHPR reveals an unexpected influence of the III-IV loop on skeletal muscle EC coupling. Am J Physiol Cell Physiol. 2004;287:C1094–C1102. doi: 10.1152/ajpcell.00173.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.