Abstract
Astaxanthin (ATX) is a dietary carotenoid of crustaceans and fish that contributes to their coloration. Dietary ATX is important for development and survival of salmonids and crustaceans and has been shown to reduce cardiac ischemic injury in rodents. The purpose of this study was to examine whether ATX can protect against ischemic injury in the mammalian brain. Adult rats were injected intracerebroventricularly with ATX or vehicle prior to a 60-min middle cerebral artery occlusion (MCAo). ATX was present in the infarction area at 70-75 min after onset of MCAo. Treatment with ATX, compared to vehicle, increased locomotor activity in stroke rats and reduced cerebral infarction at 2 d after MCAo. To evaluate the protective mechanisms of ATX against stroke, brain tissues were assayed for free radical damage, apoptosis, and excitoxicity. ATX antagonized ischemia-mediated loss of aconitase activity and reduced glutamate release, lipid peroxidation, translocation of cytochrome c, and TUNEL labeling in the ischemic cortex. ATX did not alter physiological parameters, such as body temperature, brain temperature, cerebral blood flow, blood gases, blood pressure, and pH. Collectively, our data suggest that ATX can reduce ischemia-related injury in brain tissue through the inhibition of oxidative stress, reduction of glutamate release, and antiapoptosis. ATX may be clinically useful for patients vulnerable or prone to ischemic events.—Shen, H., Kuo, C.-C., Chou, J., Delvolve, A., Jackson, S. N., Post, J., Woods, A. S., Hoffer, B. J., Wang, Y., Harvey, B. K. Astaxanthin reduces ischemic brain injury in adult rats.
Keywords: antioxidant, stroke, neuroprotection, apoptosis, glutamate
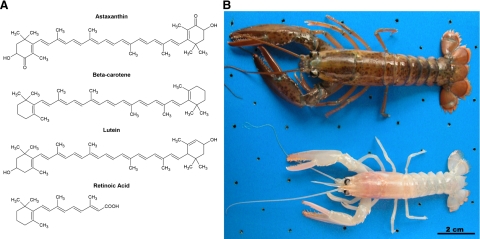
Astaxanthin (ATX; see chemical structure in Fig. 1) is a dark red pigment found in algae, crustaceans, and fish. Lobsters grown in the absence of dietary ATX appear white and devoid of coloration (Fig. 1). While ATX is a primary component of coloration, this pigment has other physiological roles in these organisms. In algae, ATX synthesis is partially controlled by light (1, 2) and has protective effects against ultraviolet (UV) light-induced damage (3, 4) and oxidative stress (5). In crustaceans, dietary ATX improves survival and growth (6,7,8), as well as increases resistance to physical and chemical stressors (9,10,11,12). In fish, dietary ATX alters liver function (13) and liver lipid profile (14), and it improves defenses against oxidative stress (15,16,17). Taken together, these data suggest that ATX is a protective agent in marine plants and fish.
Figure 1.
ATX is a carotenoid and a primary pigment in crustaceans. A) Chemical structures of carotenoids ATX, β-carotene, lutein, and the retinoid, retinoic acid. B) Juvenile lobsters (Homarus americanus) fed an ATX-depleted diet exhibit minimal pigmentation (bottom) compared to an older lobster fed an ATX-containing diet (top). Size difference in the two lobsters is a consequence of age, not the lack of ATX. Photo courtesy of Dr. Michael Tlusty, New England Aquarium, Boston, MA, USA. Scale bar = 2 cm.
ATX may have beneficial effects in mammalian cells and tissues as well. ATX was more effective than β-carotene or cantaxanthin at protecting against UV light-induced damage to human dermal fibroblasts in vitro (18). ATX prevented the UV light-mediated increase in DNA damage and superoxide dismutase activity, as well as the decrease in cellular glutathione levels in IBR-3 cells, a human skin fibroblast cell line (19). ATX attenuated myocardial damage induced by ischemia (20,21,22) or strenuous exercise (23) and protected tissues from oxidative insults (24). A recent study demonstrated that ATX has protective effects on retinal cells (25). One study has shown that oral administration of ATX, at a dose of 550 mg/kg, 1 h before a 20-min bilateral common carotid occlusion, improved Morris Water maze performance in mice (26). Because no biochemical or histological analyses were examined, the mechanisms and actions of ATX in the brain are still not known.
Although ATX is structurally similar to β-carotene and lutein (Fig. 1), it is a 5 to 15 times more potent antioxidant than these compounds, based on free radical scavenging assays of purified compounds (27, 28). The beneficial effects of naturally occurring antioxidants have been reported in rodent models of stroke. For example, pretreatment with antioxidants, such as vitamin E (29, 30), ebselen (31, 32), Ginkgo biloba extract (33, 34), α-lipoic acid (33), and lycopene (35) reduced ischemic brain damage in rodents. Our laboratory has found that rats that received antioxidant-enriched diets for 1 mo had a significant reduction in cerebral infarction and caspase-3 activity and an increase in poststroke locomotor activity after middle cerebral artery occlusion (MCAo) (36). The protective effects of antioxidants have also been reported in clinical observations; for example, intake of an antioxidant-enriched diet is associated with lower risk for cerebral infarction in male patients (37). Similarly, low antioxidant activity in plasma is associated with greater lesion volumes and neurological impairments in stroke patients (38). Taken together, these data suggest that compounds that possess antioxidant properties can reduce ischemia-mediated neurodegeneration in the brain. Whether ATX can reduce ischemic brain injury through an antioxidant property is still not known.
The purpose of the current study was to examine the protective effects of ATX in the CNS using an MCAo model in rats. Our data show that ATX pretreatment reduces ischemia-induced free radical damage, apoptosis, and cerebral infarction.
MATERIALS AND METHODS
Animals and MCAO surgery
Adult male Sprague-Dawley rats (250-300 g) were used. Animals were anesthetized with chloral hydrate (0.4 g/kg, i.p.). ATX [20 μl of 0.1 mM ATX dissolved in 10% (v/v) dimethyl sulfoxide, DMSO in saline; Sigma-Aldrich, St. Louis, MO, USA] or vehicle [20 μl of 10% (v/v) DMSO in saline] was administered into the left lateral ventricle (i.c.v.; 0.8 mm posterior, 1.5 mm lateral to the bregma and 3.7 mm below dura) through a 25-μl Hamilton syringe. Ten to 15 min after i.c.v. administration, the right middle cerebral artery (MCA) was ligated and the common carotids (CCAs) were bilaterally clamped for 60 min to generate focal ischemia in the cerebral cortex (39,40,41). Core body temperature was maintained at 37°C.
Detection of ATX in stroke brain using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOFMS)
Animals were injected with vehicle or ATX as described above. Ten to 15 min after release of ligature from the MCA or 8 h after onset of MCAo, animals were perfused with phosphate-buffered saline (PBS) at room temperature. The brains were removed, and the cerebellum and anterior 4 mm were cut away using a brain block. The remaining tissue was immediately frozen in isopentane on dry ice and stored at −80°C. Frozen brains were sectioned (18 μm) and processed as described previously (42, 43). Briefly, brain sections near the site of injection (4-5 mm from rostral end) were collected onto MALDI sample plates. The matrix used was 2,5-dihydroxybenzoic acid (DHB; Sigma) prepared in 50% ethanol. Matrix was sprayed on the tissue section using an artistic airbrush. The tissue section was imaged in positive ion mode using a MALDI TOF-TOF 4700 from Applied Biosystems (Framingham, MA, USA). Analysis of the data was conducted using TissueView (Applied Biosystems). This software allows for the generation of images based on the intensity of individual ions. Using a circular region of interest (ROI) feature of the TissueView software, we obtained an average mass spectrum from the average ion count for mass/charge range of 614-620 within the lesioned area. Images were adjusted equivalently for brightness and contrast for optimal visualization of signal.
Locomotor measurements
Each animal was individually placed in a 42- × 42- × 31-cm activity chamber 1 d before as well as on d 1 and 2 after MCAo. The monitor contained 16 horizontal and 8 vertical infrared sensors spaced 2.5 cm apart. The vertical sensors were situated 10.5 cm from the floor of the chamber. Motor activities, such as horizontal activity (total number of beam interruptions that occurred in the horizontal sensors), total distance traveled, number of horizontal or vertical movements, movement and rest time, were calculated by the number of beams broken by the animals from 0-30 min after placement in the chamber (39,40,41, 44). Poststroke locomotor activity was normalized to prestroke values.
Triphenyltetrazolium chloride (TTC) staining
TTC staining was performed as described previously to determine infarction size (36, 41). Briefly, at 2 d after reperfusion, rats were decapitated, and the brains were removed and sliced into 2.0-mm-thick sections. The brain slices were incubated in a 2% TTC solution (Sigma-Aldrich) for 15 min at room temperature and then transferred into a 4% paraformaldehyde solution for fixation. The area of infarction in each slice was measured with a digital scanner and Imagetools programs (University of Texas Health Sciences Center, Houston, TX, USA). The volume of infarction in each animal was obtained from the product of average slice thickness (2 mm) and sum of infarction areas in all brain slices examined.
Aconitase assay
The third (4-6 mm) and fourth (6-8 mm) 2-mm brain sections were harvested at 8 h (41) after MCAo. The fourth section contains the largest infarction area after stroke (45). Brain tissues were homogenized in aconitase assay buffer (0.2 mM sodium citrate, 50 mM Tris-HCl, pH 7.4). The suspensions were centrifuged at 800 g for 10 min at 4°C. The cytoplasmic supernatant was centrifuged again at 20,000 g for 10 min at 4°C. The mitochondrial pellet was resuspended in 200 μl aconitase buffer and aconitase activity was measured using a Bioxytech Aconitase-340 kit (Oxis International, Inc., Foster City, CA, USA). Total protein concentration was determined using the DC Protein Assay (Bio-Rad Laboratories, Hercules, CA, USA).
Malondialdehyde assay
Brain sections (2 mm) from stroke rats were harvested at 24 h after MCAo (46). Animals were perfused with 0.9% (w/v) saline containing heparin (1 U/ml). The cortex was removed from the third through fifth 2-mm sections (4-10 mm from rostral end), which contains the maximal infarction areas and provides sufficient tissue for the MDA assay. The tissue was frozen in isopentane on dry ice and stored at −80°C. Samples were thawed and homogenized in PBS with 5 mM beta-hydroxytoluene (BHT; Sigma). Approximately 10 μl was used to determine protein concentration of the homogenate using the DC assay (Bio-Rad Laboratories), and 100 μl of homogenate was used to measure MDA according to the manufacturer’s instructions (MDA-586 kit; Oxis International). The concentration of MDA was normalized to protein levels measured by the DC assay.
In vivo microdialysis and glutamate measurements
Rats were anesthetized, and ATX or vehicle was administered i.c.v. as described above. The microdialysis probe was stereotaxically placed in the parietal cerebral cortex (−4.0 mm posterior, 4.5 mm lateral to bregma, 3.0 mm below brain surface) equivalent to the region of the fourth TTC-stained section. Artificial cerebrospinal fluid (aCSF) was perfused through the probe at 2 μl/min. Ischemia-induced glutamate release occurs within 10-20 min after the onset of ischemia (44); therefore, samples were collected every 20 min from 60 min before to 60 min after MCAo. In a subset of animals, hydrogen peroxide (0.03 and 0.3% in aCSF) or aCSF was perfused through the microdialysis probe.
The concentration of glutamate in the microdialysis samples was determined using high performance liquid chromatography (HPLC). The mobile phase consisted of 13% acetonitrile (v/v), 100 mM NaH2PO4, and 0.1 mM EDTA, pH 5.9. A reverse-phase column was used for separation, and precolumn derivatization was performed with o-phthalaldehyde using an ESA Model 542 autosampler (Magellan Biosciences, Chelmsford, MA, USA). Glutamate was detected using a fluorescence spectrophotometer with an excitation wavelength of 336 nm and an emission wavelength of 420 nm.
Western blot analysis of cytochrome c
Brain tissue from stroke rats was harvested at 8 h after the onset of reperfusion for Western blot analysis of mitochondrial cytochrome c (41). The fourth 2-mm coronal section (6-8 mm from rostral end) from each brain, which contains the largest area of infarction after MCAo (45), was dissected and homogenized in lysis buffer (20 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, 1 mM EDTA, 1 mM EGTA, 250 mM sucrose, 0.1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, and proteinase inhibitor). The brain tissue homogenates were centrifuged at 600 g for 10 min at 4°C. The supernatant was centrifuged at 14,000 g for 15 min at 4°C to obtain the mitochondria-enriched pellet. The mitochondrial pellet was resuspended with lysis buffer, and 50 μg of protein was separated using 15% SDS/PAGE. Proteins were electrotransferred onto PVDF membranes, and the membranes were blocked for 1 h in blocking buffer (Rockland, Gilbertsville, PA, USA) and then probed overnight at 4°C using mouse anti-cytochrome c antibody (1:200; BD Pharmingen, San Diego, CA, USA) or anti-cytochrome c oxidase (anti-COX IV 20E8, 1:5000; Invitrogen, Carlsbad, CA, USA). Detection of target proteins was performed using IRdye anti-mouse antibody (1:2000; Rockland) for 1 h and scanning by an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA). Protein bands were quantified by a densitometry program (Scion Image for Windows 4.0.3.2; Scion Corp., Frederick, MD, USA). Cytochrome c levels were normalized to cytochrome c oxidase.
TUNEL histochemistry
Animals were sacrificed at 2 d (47) after ischemia, and the brains were removed, sectioned (25-μm thickness), and mounted on slides. Sections were taken in the region equivalent to the third 2-mm TTC-stained section, which has previously been shown to have the maximal TUNEL labeling (48). A standard TUNEL procedure with minor modifications was performed (49). Briefly, slide-mounted sections from rat brain were rinsed in 0.5% Triton X-100 in 0.01 M PB for 20 min at 80°C. TUNEL labeling buffer (100 μl) mixture was added onto each sample and incubated for 60 min at 37°C in a humidified chamber. Control procedures were carried out as described in the manufacturer’s manual (Roche Applied Science, Indianapolis, IN, USA). Photomicrographs were acquired in similar regions from each brain sample with a Qimage Retiga Exi camera (QImaging, Surrey, BC, Canada) attached to a Nikon 80i microscope (Nikon, Tokyo, Japan). Identical acquisition settings were used for each image. Density of TUNEL labeling was analyzed using Metamorph software (Molecular Devices, Sunnyvale, CA, USA); the density of TUNEL pixels was quantified in core and penumbra in the lesioned side cortex and averaged from three consecutive sections in all brains samples by blinded observers.
Cerebral blood flow (CBF)
Local CBF was continuously measured using a laser-Doppler flowmeter (PF-5010; Perimed, Järfälla, Sweden), as described previously (44). A burr hole was made in the right frontoparietal region. An ultrafine blood flow probe (0.45 mm in diameter, Probe 411; Perimed) was stereotaxically placed in the cortex, 1.0 mm below dura, near the first bifurcation of MCA above the zygomatic arch. CBF was measured every second from 30 min before MCAo to 15 min after the onset of reperfusion.
Physiological measurements
Physiological parameters were measured as described previously (41). Brain temperature was continuously monitored through a fine thermo-probe (PF-5020; Perimed, Sweden). A polyethylene catheter was inserted into the right femoral artery. Mean arterial pressure was monitored by a blood pressure recorder (Windo-Graf 930; Gould Inc., Valley View, OH, USA). Arterial blood was analyzed by a blood gas analyzer (GEM Premier 3000; Instrumentation Laboratory, Lexington, MA, USA).
Statistics
Student’s t test and 1- or 2-way ANOVA plus post hoc Newman-Keuls test were used for statistical comparison. Data are presented as means ± se.
RESULTS
Distribution of ATX in brain
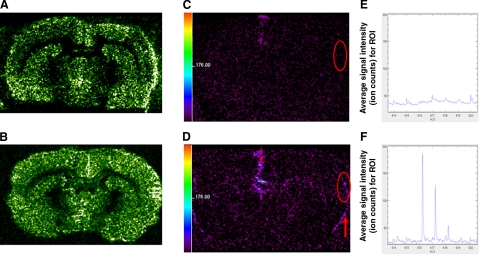
Using MALDI-TOFMS, we analyzed the tissue distribution of ATX in stroke brains. A glycerophosphocholine species (P32:0+H) was used to demonstrate the anatomical location of brain slices (Fig. 2A, B). In brain pretreated with ATX, an ATX molecular ion was found at a mass/charge (m/z) of 616 Da (Fig. 2F), corresponding to ATX + K+− H2O or 596 + 39 − 18, at 10-15 min after the onset of reperfusion, (or 70-75 min post-MCAo). Tandem MS analysis of this peak at 616 Da from tissue confirmed this assignment (Supplemental Fig. 1). ATX (616 Da) was prominently detected at the site of injection and the cortical surface of both hemispheres, as compared to the vehicle-treated group (Fig. 2C, D). ATX (m/z 616) was also detectable within the future infarction area in ATX-treated brain compared to vehicle-treated brain (Fig. 2E, F). The mass spectra in Fig. 2E, F represent an average mass spectrum for the region of interest within the future infarction area. Similar analysis at 8 h post-MCAo revealed that ATX can be detected in the lesioned cortex (data not shown). These data indicate that ATX injected into the lateral ventricle is transported to the cortical surface and infarction area at 10-15 min after reperfusion and remains there for at least 8 h.
Figure 2.
Distribution of ATX in stroke brain. Animals received vehicle (veh) or ATX at 10-15 min before a 60-min MCAo. Tissue was collected at 10-15 min after reperfusion began for ATX analysis using MALDI-TOFMS. Distribution of a glycerophosphocholine species (P32:0+H) was used as an anatomical marker for veh (A) and ATX (B) treatments. Same sections were imaged in the mass range of m/z 616 Da, [ATX+K+−H2O]+ or 596 + 39 − 18, for veh (C) and ATX (D) treatments. The 616-Da signal (ATX) was evident at injection site (small red arrow, D) and cortical surface (large arrow, D) in the ATX-treated group. The average mass spectrum for a region of interest in the future infarction area (red circle, C, D) demonstrated detectable average ion counts for 616 Da in ATX-treated group (F) but not the vehicle-treated group (E). Scale bar = signal intensity (ion count) for all peaks in m/z range of 614-620 (C, D).
Poststroke locomotor activity
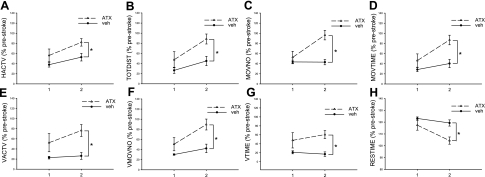
Animals (n=28) were individually placed into the activity chamber for 30 min at 1 (n=12) and 2 d (n=16) after MCAo (Fig. 3). Rats that received ATX pretreatment (n=14) had a significant increase in locomotor activity, compared with the vehicle stroke animals (n=14; Fig. 3). For example, horizontal activity (Fig. 3A; P=0.01, F1,24=7.927, 2-way ANOVA), total distance traveled (Fig. 3B; P=0.006, F1,24=9.016), movement number (Fig. 3C; P=0.001, F1,24=14.131), horizontal movement time (Fig. 3D; P=0.002, F1,24=11.750), vertical activity (Fig. 3E; P=0.006, F1,24=9.016), vertical movement number (Fig. 3F; P=0.006, F1,24=9.016), and vertical movement time (Fig. 3G; P=0.006, F1,24=9.016), were all significantly enhanced by ATX, whereas immobility (“rest time,” Fig. 3H; P=0.002, F1,24=11.750) was reduced.
Figure 3.
Locomotor activity in animals treated with ATX or vehicle at 1 and 2 d after MCAo. Animals received vehicle (veh) or ATX followed by a 60-min MCAo. Locomotor activity was measured in 30-min sessions at 1 and 2 d after MCAo. Animals receiving ATX had a significant increase in horizontal (A–D) and vertical (E–G) activity compared to vehicle controls. There was also less rest time in stroke animals treated with ATX (H). HACTV, horizontal activity; TOTDIST, total distance; MOVNO, movement number; MOVTIME, movement time; VACTV, vertical activity; VMOVNO, vertical movement number; Vtime, vertical movement time; RESTIME, rest time. Data are presented as percentage of prestroke activity. *P < 0.05; 2-way ANOVA.
Brain infarction
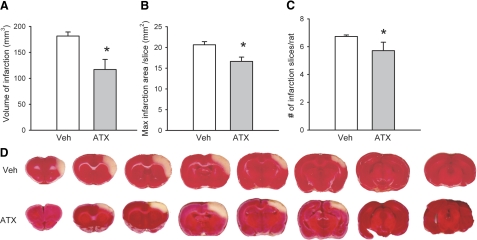
Fourteen rats were sacrificed and the brains were removed for infarction analysis by TTC staining on d 2 after MCAo. Of these 14 rats, 7 were treated with ATX and 7 were treated with vehicle. All animals receiving vehicle developed prominent infarction of the ischemic cortex. The size of infarction was analyzed using three parameters: the volume of infarction, the area of the largest infarction per slice, and the number of infarcted slices from each rat (Fig. 4). All of these parameters were significantly reduced in animals pretreated with ATX (P<0.05, Student’s t test), indicating a protective effect.
Figure 4.
Effects of ATX on cortical infarction induced by MCAo and reperfusion. Right middle cerebral artery was occluded for 60 min after bilateral common carotid ligation. Two days after MCAo, tissue was collected in 2-mm sections and stained with TTC. A) Volume of infarction = sum of infarction area (mm2) × 2-mm slice thickness for the 7 infarcted slices. B, C) Area of largest infarction per slice (B) and number of infarcted slices from each rat (C) were all significantly decreased in animals receiving ATX. *P < 0.05; Student’s t test. D) Representative TTC-stained sections of brains from vehicle (veh)-treated and ATX-treated animals.
Aconitase activity
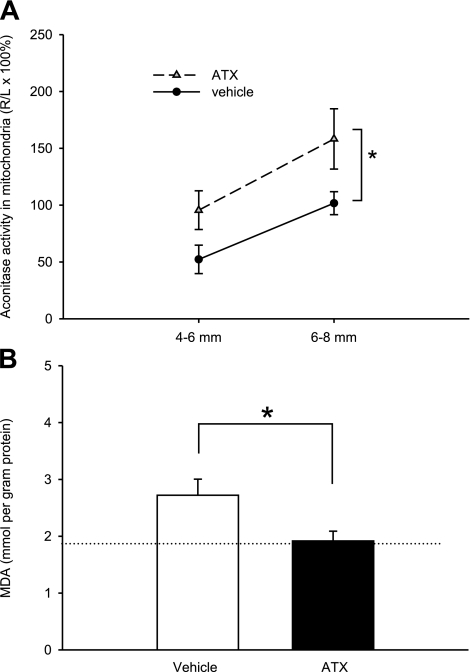
Superoxide inhibits aconitase activity, and the inhibition of cellular aconitase activity has been used as an indicator of oxidative stress (50,51,52). Thirteen rats were sacrificed for aconitase assay to determine the effects of ATX on stroke-induced decrease in aconitase activity. Brain sections from stroke rats were harvested at 8 h after MCAo. The third and fourth 2-mm coronal sections (i.e., 4-6 and 6-8 mm from the rostral end) from each brain were collected. The level of aconitase on the lesioned-side hemisphere was normalized by comparison to that from the nonlesioned side in the same animals. In the control animals (n=7), aconitase activity was significantly reduced by MCAo. Pretreatment with ATX (n=6) significantly attenuated the loss of aconitase by MCAo (Fig. 5A; P=0.008, F1,33=7.834, 2-way ANOVA).
Figure 5.
Aconitase enzymatic activity and lipid peroxidation in ATX-treated ischemic brain tissue. All animals received 60-min MCAo and reperfusion for 8-h (aconitase assay, A) or 24-h (MDA assay, B). Mitochondrial aconitase activity and MDA were normalized to total protein content. A) Aconitase data are presented as percentage of normalized aconitase activity in the ischemic hemisphere (R) compared to the normalized nonischemic contralateral hemisphere (L). *P < 0.05; 2-way ANOVA. B) MDA was also reduced after ATX treatment. Dotted line indicates MDA level in naive rat brain. *P < 0.05; Student’s t test.
Lipid peroxidation
Twelve rats were sacrificed for the MDA assay at 24 h after MCAo. The cortex was dissected from the fifth 2-mm section at 24 h after MCAo. Prestroke delivery of ATX (n=6) decreased the level of MDA in cortical tissue compared to vehicle-treated rats (n=6; P<0.05, Student’s t test; Fig. 5B).
Glutamate release during ischemia and perfusion of H2O2
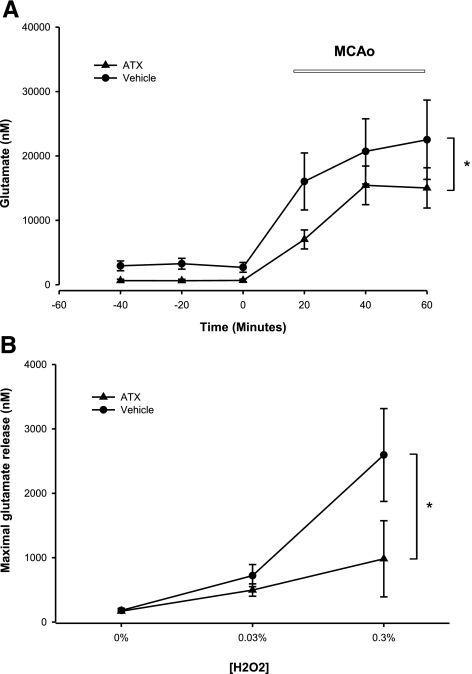
Glutamate levels were measured in the dialysates collected from 60 min before to 60 min after the onset of MCAo in 15 rats. MCAo significantly increased overflow of glutamate both in animals pretreated with ATX (Fig. 6A; n=8; P<0.001, F5,42=14.563; P<0.05, post hoc Newman-Keuls test) and vehicle (Fig. 6A; n=7; P<0.001, F5,36=6.279; P<0.05, post hoc Newman-Keuls test). However, 2-way ANOVA indicates that ATX significantly reduced glutamate release induced by MCAo compared to vehicle-treated stroke animals (P=0.032, F1,39=4.920).
Figure 6.
ATX alters ischemia and H2O2-induced glutamate release in the cortex. A) ATX suppresses ischemia-induced glutamate release in cerebral cortex. Glutamate levels were measured from the dialysates from 60 min before to 60 min after the onset of MCAo. B) ATX suppresses H2O2-induced glutamate release in cerebral cortex. Glutamate levels were measured from the dialysates for 60 min before and after H2O2 infusion. Pretreatment with ATX significantly reduced H2O2-mediated glutamate release. *P < 0.05; 2-way ANOVA.
A second set of animals was used to examine the interaction of ATX and H2O2 on glutamate release. H2O2 was used to simulate ischemia-induced free radical generation. H2O2 (0.03% for 60 min then 0.30% for 60 min, 2 μl/min) was infused through the microdialysis probe into parietal cortex of nonstroke rats; samples were collected every 20 min. In rats pretreated with vehicle (n=6), infusion of H2O2 resulted in a significant release of glutamate (Fig. 6B; P=0.003, F2,15=8.756, 1-way ANOVA). The peak of glutamate release dose-dependently correlated with the concentration of H2O2 used. Administration of ATX (n=6) significantly attenuated H2O2-mediated glutamate release (P=0.011, F1,30=7.402, 2-way ANOVA; Fig. 6B).
Mitochondrial cytochrome c
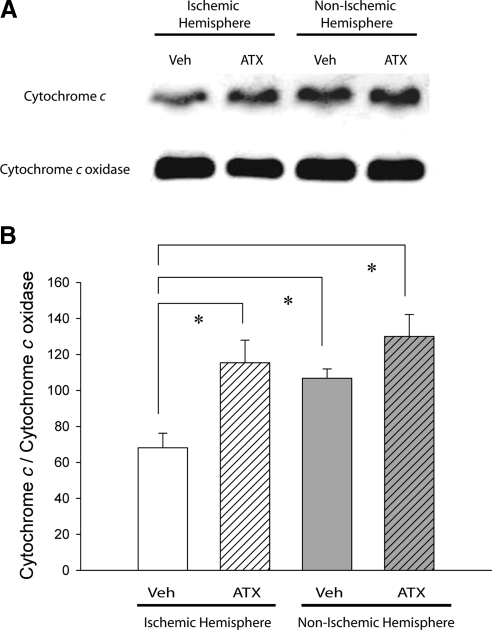
Eight rats were sacrificed at 8 h after MCAo for mitochondrial cytochrome c measurements. The cortical tissue of the fourth 2-mm coronal section (6-8 mm from rostral end) was dissected for Western blot analysis. The level of mitochondrial cytochrome c protein in the ischemic hemisphere, compared to the contralateral nonischemic hemisphere, was significantly reduced in 4 rats receiving vehicle (Fig. 7; P=0.007, F3,15=6.541, 1-way ANOVA; P<0.05, post hoc Fisher LSD test). In another 4 rats, pretreatment with ATX significantly antagonized the reduction of cytochrome c levels in the ischemic cortical hemisphere (Fig. 7B; P<0.05, F3,15=6.541, 1-way ANOVA + post hoc Fisher LSD test). There was no significant difference between the normalized cytochrome c levels in the ATX-treated ischemic hemisphere and the nonischemic hemispheres (P>0.05). These data suggest that ATX attenuates the ischemia/reperfusion-induced translocation of cytochrome c from the mitochondria.
Figure 7.
Mitochondrial cytochrome c levels are higher in ischemic cortex treated with ATX compared to vehicle. Animals pretreated with vehicle (veh) or ATX were sacrificed at 8 h after MCAo. A) Mitochondrial protein fractions from cortical tissue of the ischemic and nonischemic brain hemispheres were analyzed by Western blot. Ischemia/reperfusion decreases the level of mitochondrial cytochrome c protein but not cytochrome c oxidase protein. B) Densitometric analysis of changes in cytochrome c protein levels normalized to cytochrome c oxidase protein levels. *P < 0.05; 1-way ANOVA.
TUNEL histochemistry
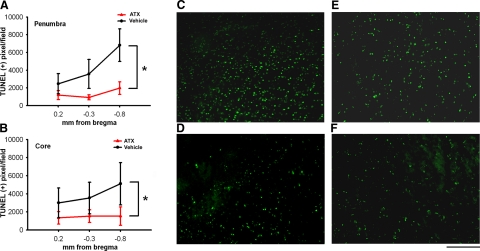
Brain tissue was collected from 11 rats (6 pretreated with ATX and 5 pretreated with vehicle) for TUNEL analysis on d 2 after MCAo. TUNEL(+) nuclei were found in the penumbra and core of infarction in the ischemic cortex (Fig. 8A, B). Animals pretreated with ATX had fewer TUNEL(+) nuclei in the penumbra (Fig. 8D) and core (Fig. 8F) than those treated with vehicle (penumbra, Fig. 8C; core, Fig. 8E). The density of TUNEL(+) cells was quantified from three sections (0.2 mm, −0.3 mm and −0.8 mm to bregma) in all brain samples. There was a significant reduction in the density of TUNEL(+) nuclei in animals receiving ATX pretreatment both in penumbra (Fig. 8A; P=0.003, F1,27=10.823, 2-way ANOVA) and core (Fig. 8B; P=0.043, F1,27=4.520, 2-way ANOVA).
Figure 8.
Density of TUNEL(+) cells is decreased in stroke animals receiving ATX compared to vehicle. A, B) Animals were pretreated with vehicle or ATX before MCAo. Two days after MCAo, density of TUNEL(+) cells was quantified in penumbra (A) and core (B) of the infarction at three locations relative to bregma. There was a significant reduction in density of TUNEL-positive cells in both core and penumbra of animals receiving ATX pretreatment. *P < 0.05; 2-way ANOVA. C–F) Representative fields of TUNEL(+) cells in −0.8-mm bregma sections for vehicle-penumbra (C), ATX-penumbra (D), vehicle-core (E) and ATX-core (F). Scale bar = 200 μm
CBF
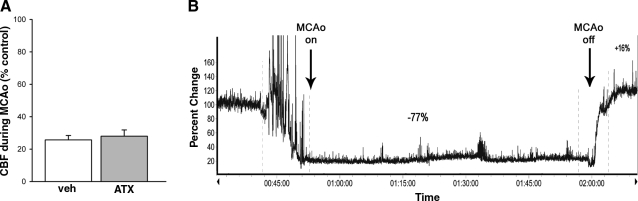
An ultrafine blood flow probe was used to measure real-time CBF at 1.0 mm below cortical surface in nonstroke (n=3 ATX, n=3 vehicle) and stroke (n=4 ATX, n=3 vehicle) animals. CBF was normalized to the mean blood flow before administration of chemicals or MCAo in each animal. ATX or vehicle did not alter CBF in the nonstroke rats. In all stroke rats, MCAo significantly reduced CBF. There was no difference in the decrease in CBF during MCAo between animals treated with vehicle or ATX (Fig. 9; P=0.666, Student’s t test). A typical CBF tracing is shown in Fig. 9B.
Figure 9.
CBF was not altered by ATX during ischemia. A) CBF was normalized (% control) by comparison to the mean blood flow before administration of MCAo in each animal. There was no difference in the decrease of CBF during MCAo between animals treated with vehicle or ATX (P=0.55; Student’s t test). B) Representative recording from a stroke animal treated with ATX.
Blood gases, pH, blood pressure, temperature
Physiological parameters were measured in rats treated with vehicle (n=6) or ATX (n=6). Blood pH and gas were examined before and 20-30 min after injection. There was no difference in blood pH, PaCO2, PaO2, blood pressure, brain temperature and body temperature between animals treated with ATX and vehicle (Table 1; P>0.05, Student’s t test).
TABLE 1.
Arterial blood gas, blood pressure, and brain temperature in animals treated with ATX or vehicle
| Parameter | ATX (n=6)
|
Vehicle (n=6)
|
||
|---|---|---|---|---|
| Before | After | Before | After | |
| pH | 7.37 ± 0.01 | 7.44 ± 0.02 | 7.40 ± 0.02 | 7.41 ± 0.02 |
| PaCO2 (mmHg) | 42.00 ± 1.79 | 40.67 ± 2.14 | 39.67 ± 1.23 | 41.83 ± 2.12 |
| PaO2 (mmHg) | 92.50 ± 4.02 | 83.83 ± 1.76 | 87.83 ± 2.55 | 87.17 ± 2.44 |
| BP (mmHg) | 99.67 ± 8.82 | 105.33 ± 10.93 | 97.00 ± 8.27 | 100.67 ± 8.19 |
| Brain temp. (°C) | 35.77 ± 0.56 | 36.3 ± 0.17 | ||
| Body temp. (°C) | 36.07 ± 0.22 | 36.38 ± 0.14 | ||
Data recorded before and 20-30 min after i.c.v. administration of ATX. Values are presented as means ± se. BP, blood pressure; temp., temperature.
DISCUSSION
In the current study, a naturally occurring dietary component of fish and crustaceans with potent antioxidant properties was examined in an animal model of stroke. Pretreatment with ATX reduced cerebral infarction and improved locomotor activity recovery from stroke in rats. These data suggest that ATX is a protective agent against cerebral ischemia.
Using MALDI-TOFMS imaging (42, 43), we observed that ATX was found in the ischemic cortex at 10-15 min after the onset of reperfusion and at 8 h after MCAo. These data indicate that ATX can be quickly transported to the lesioned area and remains there up to 8 h after MCAo.
There is a trend suggesting that ATX treatment improved vertical but not horizontal movement on d 1. A more prominent recovery of both vertical and horizontal movement was found on d 2, probably because bradykinesia induced by brain ischemia was less confounded by surgical trauma on d 2. These data suggest that ATX changes the dynamics of rate of recovery. Additional experiments, i.e., up to 1 mo or longer, may be useful to determine the full extent of behavioral recovery after ATX treatment.
Oxidative stress is a contributing factor to stroke-induced neurodegeneration. When produced in excessive quantities, reactive oxygen species, such as the superoxide anion radical O2·− or its dismutation product H2O2, are detrimental to metabolic functions. The O2·− radical oxidizes the 4Fe-4S center from dehydratases, such as aconitase, which releases one Fe(II). Fe(II) reacts with H2O2 to yield the potent oxidizing free radical species hydroxyl radical (OH·). These free radicals react with key organic substrates, such as DNA, proteins, and lipids, which disturb cell function and can lead to cell death. In our study, we found that ATX pretreatment reduces the loss of aconitase activity, an indirect marker for reactive oxygen species (ROS) production, as early as 8 h after MCAo and the level of MDA, a marker for lipid peroxidation, at 24 h after MCAo in ischemic brain. These data suggest that ATX can reduce ischemia/reperfusion-mediated oxidative stress and lipid peroxidation in stroke rats.
Ischemic injury can induce glutamate release (44). Pretreatment with glutamate antagonists prevents the damage induced by MCAo (53), suggesting that ischemia-mediated neurodegeneration also involves excitotoxicity. Ischemia also induces H2O2 generation. The overproduction of H2O2 further facilitates excitatory amino acid release (54) during ischemia. Similarly, exogenous H2O2 increases extracellular glutamate and excitotoxic damage in mouse cortical cultures (55). We found that MCAo or perfusion with H2O2 via microdialysis induced glutamate release in the cortex; both responses were antagonized by ATX pretreatment, suggesting that ATX-mediated protection may involve inhibition of glutamate overflow. On the other hand, in primary cortical neuron cultures, ATX did not antagonize glutamate-mediated toxicity, as measured by ATP levels, when given concomitantly or immediately after glutamate treatment (unpublished results). This suggests that ATX does not directly alter the toxicity of glutamate.
Apoptosis occurs following ischemia/reperfusion and can result from free radical damage and excessive glutamate release. We found that pretreatment with ATX reduced TUNEL labeling, suggesting that ATX inhibits DNA fragmentation in stroke brain. Furthermore, animals receiving ATX had higher levels of mitochondrial cytochrome c at 8 h after MCAo, indicating ATX reduces preapoptotic translocation of mitochondrial cytochrome c. Taken together, these data suggest that ATX pretreatment reduces apoptosis caused by transient MCAo.
Previous studies have indicated that chronic systemic treatment with ATX reduces blood pressure in spontaneously hypertensive rats (26). We found that acute intracerebroventricular administration of ATX did not alter blood pressure in Sprague-Dawley rats. It is possible that the antihypertensive effect of ATX reported earlier may be specific to hypertensive rats or the route of delivery. Using an ultrafine (0.45-mm diameter) laser-Doppler flowmeter, we found that ATX did not alter CBF in stroke and nonstroke rats. There were no changes in blood gases or temperature as a result of ATX administration as well. Taken together, our data suggest that ATX-induced protection in stroke brain is not secondary to an increase of CBF or changes in physiological parameters.
We demonstrated that ATX has antioxidant effects using two markers of free radical damage; aconitase activity and MDA levels were normalized by ATX treatment in stroke rats. These antioxidant effects can lead to a decrease in glutamate overflow and antiapoptosis and, furthermore, a reduction of cerebral infarction in stroke rats. Besides its antioxidant properties, several reports have indicated that ATX may induce protection through other mechanisms. For example, Bertram and Vine (56) found that ATX increased connexin 43 protein, a gap junction protein in a teratocarcinoma cell line. This upregulation does not depend on protein synthesis but may involve peroxisome proliferator-activated receptor-γ (56, 57). A separate study found that ATX reduced NF-κB translocation to the nucleus and the activation of inflammatory mediators (58). These data suggest a possible antioxidant-independent action of ATX. ATX is also structurally similar to retinoic acid (Fig. 1), which reduces ischemic injury in brain through the up-regulation of various trophic factors (59, 60). It is thus possible that ATX also interacts with retinoic acid receptors to confer protective effects. Further investigation is required to verify such interactions.
The association of antioxidants and stroke has been examined in several clinical studies. Low antioxidant activity in plasma was associated with higher lesion volumes and neurological impairments in stroke patients (38). Elevated intake of β-carotene or lycopene was found to be associated with decreased risk for cerebral infarction in male smokers (37). There was also a significant inverse association between the consumption of lutein, a carotenoid found in dark green leafy vegetables, and risk for stroke in patients, although other dietary factors may have contributed (61). In contrast, dietary flavonoids, vitamin C, or vitamin E did not significantly affect the risk of stroke (37, 61). These data suggest that certain, but not all, antioxidants have protective effects against stroke, possibly because of their antioxidant potency. In our current study, we found that ATX, which is 5-15 times more potent at scavenging free radicals compared to vitamin E, reduces stroke-induced damage in rodents. In addition to its strong antioxidant property, we found that ATX possesses additional properties, such as antiapoptosis and suppression of glutamate overflow, which may further contribute to its protective action during cerebral ischemia.
We demonstrated that ATX given prior to stroke can reduce stroke-related damage as indicated by multiple measurements. The prophylactic use of ATX against stroke has clinical utility to patients prone to ischemic events. A comprehensive study is needed to explore the poststroke effects using multiple time points and alternate behavioral endpoints to determine whether ATX can alter the neurological outcome when given after brain ischemia.
In conclusion, we found that ATX has protective effects against free radical damage and ischemia/reperfusion-induced neurodegeneration in vivo. The mechanism of protection involves suppression of ROS, inhibition of glutamate overflow, and prevention of apoptosis. A recent in vitro study indicated that ATX inhibits 6-hydroxydopamine-induced ROS production in human neuroblastoma SH-SY5Y cells (62). It is thus possible that ATX may have protective effects in other neurodegenerative disorders involving free radical toxicity as well. A randomized clinical trial found that 6 mg/d of ATX could safely be consumed by “healthy” adults (63). The effectiveness of ATX pretreatment for stroke may be clinically useful for patients vulnerable or prone to ischemic events.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program at the National Institute on Drug Abuse (USA). We thank Mr. Douglas Howard and Dr. Guann-Juh Chen for their technical assistance, Dr. Michael Tlusty for his photograph of the lobsters and his input on ATX in crustaceans, and Dr. Marc Halterman for his review and comments on the manuscript.
References
- Chaumont D, Thepenier C. Carotenoid content in growing cells of Haematococcus pluvialis during a sunlight cycle. J Appl Phycol. 1995;7:529–537. [Google Scholar]
- Steinbrenner J, Linden H. Light induction of carotenoid biosynthesis genes in the green alga Haematococcus pluvialis: regulation by photosynthetic redox control. Plant Mol Biol. 2003;52:343–356. doi: 10.1023/a:1023948929665. [DOI] [PubMed] [Google Scholar]
- Gorton H L, Vogelmann T C. Ultraviolet radiation and the snow alga Chlamydomonas nivalis (Bauer) Wille. Photochem Photobiol. 2003;77:608–615. doi: 10.1562/0031-8655(2003)077<0608:uratsa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Gorton H L, Williams W E, Vogelmann T C. The light environment and cellular optics of the snow alga Chlamydomonas nivalis (Bauer) Wille. Photochem Photobiol. 2001;73:611–620. doi: 10.1562/0031-8655(2001)073<0611:tleaco>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kobayashi M. In vivo antioxidant role of astaxanthin under oxidative stress in the green alga Haematococcus pluvialis. Appl Microbiol Biotechnol. 2000;54:550–555. doi: 10.1007/s002530000416. [DOI] [PubMed] [Google Scholar]
- Pangantihon-Kuhlmann M P, Millamena O, Chern Y. Effect of dietary astaxanthin and vitamin A on the reproductive performance of Penaeus monodon broodstock. Aquat Living Resour. 1998;11:403–409. [Google Scholar]
- Pan C H, Chien Y H, Cheng J H. Effects of light regime, algae in the water, and dietary astaxanthin on pigmentation, growth, and survival of black tiger prawn Penaeus monodon post-larvae. Zool Stud. 2001;40:371–382. [Google Scholar]
- Chien Y H, Shiau W C. The effects of dietary supplementation of algae and synthetic astaxanthin on body astaxanthin, survival, growth, and low dissolved oxygen stress resistance of kuruma prawn, Marsupenaeus japonicus Bate. J Exp Mar Biol Ecol. 2005;318:201–211. [Google Scholar]
- Merchie G, Kontara E, Lavens P, Robles R, Kurmaly K, Sorgeloos P. Effect of vitamin C and astaxanthin on stress and disease resistance of postlarval tiger shrimp, Penaeus monodon (Fabricius) Aquac Res. 1998;29:579–585. [Google Scholar]
- Chien Y H, Pan C H, Hunter B. The resistance to physical stresses by Penaeus monodon juveniles fed diets supplemented with astaxanthin. Aquaculture. 2003;216:177–191. [Google Scholar]
- Pan C H, Chien Y H, Hunter B. The resistance to ammonia stress of Penaeus monodon fabricius juvenile fed diets supplemented with astaxanthin. J Exp Mar Biol Ecol. 2003;297:107–118. [Google Scholar]
- Flores M, Diaz F, Medina R, Re A D, Licea A. Physiological, metabolic and haematological responses in white shrimp Litopenaeus vannamei (Boone) juveniles fed diets supplemented with astaxanthin acclimated to low-salinity water. Aquac Res. 2007;38:740–747. [Google Scholar]
- Nakano T, Tosa M, Takeuchi M. Improvement of biochemical features in fish health by red yeast and synthetic astaxanthin. J Agric Food Chem. 1995;43:1570–1573. [Google Scholar]
- Page G I, Russell P M, Davies S J. Dietary carotenoid pigment supplementation influences hepatic lipid and mucopolysaccharide levels in rainbow trout (Oncorhynchus mykiss) Comp Biochem Physiol B Biochem Mol Biol. 2005;142:398–402. doi: 10.1016/j.cbpb.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Nakano T, Kanmuri T, Sato M, Takeuchi M. Effect of astaxanthin rich red yeast (Phaffia rhodozyma) on oxidative stress in rainbow trout. Biochim Biophys Acta. 1999;1426:119–125. doi: 10.1016/s0304-4165(98)00145-7. [DOI] [PubMed] [Google Scholar]
- Bell J G, McEvoy J, Tocher D R, Sargent J R. Depletion of alpha-tocopherol and astaxanthin in Atlantic salmon (Salmo salar) affects autoxidative defense and fatty acid metabolism. J Nutr. 2000;130:1800–1808. doi: 10.1093/jn/130.7.1800. [DOI] [PubMed] [Google Scholar]
- Amar E C, Kiron V, Satoh S, Watanabe T. Influence of various dietary synthetic carotenoids on bio-defence mechanisms in rainbow trout, Oncorhynchus mykiss (Walbaum) Aquac Res. 2001;32:162–173. [Google Scholar]
- Camera E, Matrofrancesco A, Fabbri C, Daubrawa F, Picardo M, Sies H, Stahl W. Astaxanthin, canthaxanthin and beta-carotene differently affect UVA-induced oxidative damage and expression of oxidative stress-responsive enzymes. [E-pub ahead of print] Exp Dermatol. 2009 doi: 10.1111/j.1600-0625.2008.00790.x. 2008 Sept, 18. [DOI] [PubMed] [Google Scholar]
- Lyons N M, O'Brien N M. Modulatory effects of an algal extract containing astaxanthin on UVA-irradiated cells in culture. J Dermatol Sci. 2002;30:73–84. doi: 10.1016/s0923-1811(02)00063-4. [DOI] [PubMed] [Google Scholar]
- Gross G J, Lockwood S F. Cardioprotection and myocardial salvage by a disodium disuccinate astaxanthin derivative (Cardax) Life Sci. 2004;75:215–224. doi: 10.1016/j.lfs.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Gross G J, Lockwood S F. Acute and chronic administration of disodium disuccinate astaxanthin (Cardax) produces marked cardioprotection in dog hearts. Mol Cell Biochem. 2005;272:221–227. doi: 10.1007/s11010-005-7555-2. [DOI] [PubMed] [Google Scholar]
- Lockwood S F, Gross G J. Disodium disuccinate astaxanthin (Cardax): antioxidant and antiinflammatory cardioprotection. Cardiovasc Drug Rev. 2005;23:199–216. doi: 10.1111/j.1527-3466.2005.tb00166.x. [DOI] [PubMed] [Google Scholar]
- Aoi W, Naito Y, Sakuma K, Kuchide M, Tokuda H, Maoka T, Toyokuni S, Oka S, Yasuhara M, Yoshikawa T. Astaxanthin limits exercise-induced skeletal and cardiac muscle damage in mice. Antioxid Redox Signal. 2003;5:139–144. doi: 10.1089/152308603321223630. [DOI] [PubMed] [Google Scholar]
- Wu T H, Liao J H, Hou W C, Huang F Y, Maher T J, Hu C C. Astaxanthin protects against oxidative stress and calcium-induced porcine lens protein degradation. J Agric Food Chem. 2006;54:2418–2423. doi: 10.1021/jf052651q. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Inokuchi Y, Shimazawa M, Otsubo K, Ishibashi T, Hara H. Astaxanthin, a dietary carotenoid, protects retinal cells against oxidative stress in-vitro and in mice in-vivo. J Pharm Pharmacol. 2008;60:1365–1374. doi: 10.1211/jpp/60.10.0013. [DOI] [PubMed] [Google Scholar]
- Hussein G, Nakamura M, Zhao Q, Iguchi T, Goto H, Sankawa U, Watanabe H. Antihypertensive and neuroprotective effects of astaxanthin in experimental animals. Biol Pharm Bull. 2005;28:47–52. doi: 10.1248/bpb.28.47. [DOI] [PubMed] [Google Scholar]
- Miki W. Biological fnctions and activities of animal carotenoids. Pure Appl Chem. 1991;63:141–146. [Google Scholar]
- Naguib Y M. Antioxidant activities of astaxanthin and related carotenoids. J Agric Food Chem. 2000;8:1150–1154. doi: 10.1021/jf991106k. [DOI] [PubMed] [Google Scholar]
- Hurtado O, De Cristobal J, Sanchez V, Lizasoain I, Cardenas A, Pereira M P, Colado M I, Leza J C, Lorenzo P, Moro M A. Inhibition of glutamate release by delaying ATP fall accounts for neuroprotective effects of antioxidants in experimental stroke. FASEB J. 2003;17:2082–2084. doi: 10.1096/fj.02-1086fje. [DOI] [PubMed] [Google Scholar]
- Khanna S, Roy S, Slivka A, Craft T K, Chaki S, Rink C, Notestine M A, DeVries A C, Parinandi N L, Sen C K. Neuroprotective properties of the natural vitamin E alpha-tocotrienol. Stroke. 2005;36:2258–2264. doi: 10.1161/01.STR.0000181082.70763.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai H, Masayasu H, Dewar D, Graham D I, Macrae I M. Ebselen protects both gray and white matter in a rodent model of focal cerebral ischemia. Stroke. 2001;32:2149–2154. doi: 10.1161/hs0901.095725. [DOI] [PubMed] [Google Scholar]
- Namura S, Nagata I, Takami S, Masayasu H, Kikuchi H. Ebselen reduces cytochrome c release from mitochondria and subsequent DNA fragmentation after transient focal cerebral ischemia in mice. Stroke. 2001;32:1906–1911. doi: 10.1161/01.str.32.8.1906. [DOI] [PubMed] [Google Scholar]
- Clark W M, Rinker L G, Lessov N S, Lowery S L, Cipolla M J. Efficacy of antioxidant therapies in transient focal ischemia in mice. Stroke. 2001;32:1000–1004. doi: 10.1161/01.str.32.4.1000. [DOI] [PubMed] [Google Scholar]
- Zhang W R, Hayashi T, Kitagawa H, Sasaki C, Sakai K, Warita H, Wang J M, Shiro Y, Uchida M, Abe K. Protective effect of ginkgo extract on rat brain with transient middle cerebral artery occlusion. Neurol Res. 2000;22:517–521. doi: 10.1080/01616412.2000.11740713. [DOI] [PubMed] [Google Scholar]
- Hsiao G, Fong T H, Tzu N H, Lin K H, Chou D S, Sheu J R. A potent antioxidant, lycopene, affords neuroprotection against microglia activation and focal cerebral ischemia in rats. In Vivo. 2004;18:351–356. [PubMed] [Google Scholar]
- Wang Y, Chang C F, Chou J, Chen H L, Deng X, Harvey B K, Cadet J L, Bickford P C. Dietary supplementation with blueberries, spinach, or spirulina reduces ischemic brain damage. Exp Neurol. 2005;193:75–84. doi: 10.1016/j.expneurol.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Hirvonen T, Virtamo J, Korhonen P, Albanes D, Pietinen P. Intake of flavonoids, carotenoids, vitamins C and E, and risk of stroke in male smokers. Stroke. 2000;31:2301–2306. doi: 10.1161/01.str.31.10.2301. [DOI] [PubMed] [Google Scholar]
- Leinonen J S, Ahonen J P, Lonnrot K, Jehkonen M, Dastidar P, Molnar G, Alho H. Low plasma antioxidant activity is associated with high lesion volume and neurological impairment in stroke. Stroke. 2000;31:33–39. doi: 10.1161/01.str.31.1.33. [DOI] [PubMed] [Google Scholar]
- Chen S T, Hsu C Y, Hogan E L, Maricq H, Balentine J D. A model of focal ischemic stroke in the rat: reproducible extensive cortical infarction. Stroke. 1986;17:738–743. doi: 10.1161/01.str.17.4.738. [DOI] [PubMed] [Google Scholar]
- Tomac A C, Agulnick A D, Haughey N, Chang C F, Zhang Y, Backman C, Morales M, Mattson M P, Wang Y, Westphal H, Hoffer B J. Effects of cerebral ischemia in mice deficient in Persephin. Proc Natl Acad Sci U S A. 2002;99:9521–9526. doi: 10.1073/pnas.152535899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chang C F, Morales M, Chiang Y H, Harvey B K, Su T P, Tsao L I, Chen S, Thiemermann C. Diadenosine tetraphosphate protects against injuries induced by ischemia and 6-hydroxydopamine in rat brain. J Neurosci. 2003;23:7958–7965. doi: 10.1523/JNEUROSCI.23-21-07958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S N, Ugarov M, Egan T, Post J D, Langlais D, Albert Schultz J, Woods A S. MALDI-ion mobility-TOFMS imaging of lipids in rat brain tissue. J Mass Spectrom. 2007;42:1093–1098. doi: 10.1002/jms.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H Y J, Jackson S N, Post J D, Woods A S. Imaging of glycerophospholipids and sphingolipids in rat brain sections. Int J Mass Spectrom. 2008;278:143–149. doi: 10.1016/j.ijms.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Chen G J, Harvey B K, Bickford P C, Wang Y. Inosine reduces ischemic brain injury in rats. Stroke. 2005;36:654–659. doi: 10.1161/01.STR.0000155747.15679.04. [DOI] [PubMed] [Google Scholar]
- Chang C F, Morales M, Chou J, Chen H L, Hoffer B, Wang Y. Bone morphogenetic proteins are involved in fetal kidney tissue transplantation-induced neuroprotection in stroke rats. Neuropharmacology. 2002;43:418–426. doi: 10.1016/s0028-3908(02)00092-8. [DOI] [PubMed] [Google Scholar]
- Tsai S K, Hung L M, Fu Y T, Cheng H, Nien M W, Liu H Y, Zhang F B, Huang S S. Resveratrol neuroprotective effects during focal cerebral ischemia injury via nitric oxide mechanism in rats. J Vasc Surg. 2007;46:346–353. doi: 10.1016/j.jvs.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Chen G J, Harvey B K, Shen H, Chou J, Victor A, Wang Y. Activation of adenosine A3 receptors reduces ischemic brain injury in rodents. J Neurosci Res. 2006;84:1848–1855. doi: 10.1002/jnr.21071. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chang C F, Morales M, Chou J, Chen H L, Chiang Y H, Lin S Z, Cadet J L, Deng X, Wang J Y, Chen S Y, Kaplan P L, Hoffer B J. Bone morphogenetic protein-6 reduces ischemia-induced brain damage in rats. Stroke. 2001;32:2170–2178. doi: 10.1161/hs0901.095650. [DOI] [PubMed] [Google Scholar]
- Deng X, Wang Y, Chou J, Cadet J L. Methamphetamine causes widespread apoptosis in the mouse brain: evidence from using an improved TUNEL histochemical method. Brain Res. 2001;93:64–69. doi: 10.1016/s0169-328x(01)00184-x. [DOI] [PubMed] [Google Scholar]
- Flint D H, Tuminello J F, Emptage M H. The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J Biol Chem. 1993;268:22369–22376. [PubMed] [Google Scholar]
- Gardner P R, Nguyen D D, White C W. Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc Natl Acad Sci U S A. 1994;91:12248–12252. doi: 10.1073/pnas.91.25.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackensen G B, Patel M, Sheng H, Calvi C L, Batinic-Haberle I, Day B J, Liang L P, Fridovich I, Crapo J D, Pearlstein R D, Warner D S. Neuroprotection from delayed postischemic administration of a metalloporphyrin catalytic antioxidant. J Neurosci. 2001;21:4582–4592. doi: 10.1523/JNEUROSCI.21-13-04582.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S Z, Chiou A L, Wang Y. Ketamine antagonizes nitric oxide release from cerebral cortex after middle cerebral artery ligation in rats. Stroke. 1996;27:747–752. doi: 10.1161/01.str.27.4.747. [DOI] [PubMed] [Google Scholar]
- Haskew-Layton R E, Mongin A A, Kimelberg H K. Hydrogen peroxide potentiates volume-sensitive excitatory amino acid release via a mechanism involving Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2005;280:3548–3554. doi: 10.1074/jbc.M409803200. [DOI] [PubMed] [Google Scholar]
- Mailly F, Marin P, Israel M, Glowinski J, Premont J. Increase in external glutamate and NMDA receptor activation contribute to H2O2-induced neuronal apoptosis. J Neurochem. 1999;73:1181–1188. doi: 10.1046/j.1471-4159.1999.0731181.x. [DOI] [PubMed] [Google Scholar]
- Bertram J S, Vine A L. Cancer prevention by retinoids and carotenoids: independent action on a common target. Biochim Biophys Acta. 2005;1740:170–178. doi: 10.1016/j.bbadis.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Vine A L, Leung Y M, Bertram J S. Transcriptional regulation of connexin 43 expression by retinoids and carotenoids: similarities and differences. Mol Carcinog. 2005;43:75–85. doi: 10.1002/mc.20080. [DOI] [PubMed] [Google Scholar]
- Lee S J, Bai S K, Lee K S, Namkoong S, Na H J, Ha K S, Han J A, Yim S V, Chang K, Kwon Y G, Lee S K, Kim Y M. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I(κ)B kinase-dependent NF-κB activation. Mol Cells. 2003;16:97–105. [PubMed] [Google Scholar]
- Harvey B K, Shen H, Chen G J, Yoshida Y, Wang Y. Midkine and retinoic acid reduce cerebral infarction induced by middle cerebral artery ligation in rats. Neurosci Lett. 2004;369:138–141. doi: 10.1016/j.neulet.2004.07.086. [DOI] [PubMed] [Google Scholar]
- Shen H, Lou Y, Kuo C C, Deng X, Chang C F, Harvey B K, Hoffer B J, Wang Y. 9-cis-retinoic acid reduces ischemic brain injury in rodents via bone morphogenetic protein. J Neurosci Res. 2009;87:545–555. doi: 10.1002/jnr.21865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascherio A, Rimm E B, Hernan M A, Giovannucci E, Kawachi I, Stampfer M J, Willett W C. Relation of consumption of vitamin E, vitamin C, and carotenoids to risk for stroke among men in the United States. Ann Intern Med. 1999;130:963–970. doi: 10.7326/0003-4819-130-12-199906150-00003. [DOI] [PubMed] [Google Scholar]
- Liu X, Osawa T. Cis astaxanthin and especially 9-cis astaxanthin exhibits a higher antioxidant activity in vitro compared to the all-trans isomer. Biochem Biophys Res Commun. 2007;357:187–193. doi: 10.1016/j.bbrc.2007.03.120. [DOI] [PubMed] [Google Scholar]
- Spiller G A, Dewell A. Safety of an astaxanthin-rich Haematococcus pluvialis algal extract: a randomized clinical trial. J Med Food. 2003;6:51–56. doi: 10.1089/109662003765184741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.