Abstract
Anti-lymphocyte antibodies (Abs) that suppress T-cell chemotactic and other responses to sphingosine 1-phosphate (S1P), but not to chemokines, were found in a lymphopenic patient with recurrent infections. Lymphocyte type 1 S1P receptor (S1P1) that transduces S1P chemotactic stimulation was recognized by patient Abs in Western blots of T cells, S1P1 transfectants, and S1P1-hemagglutinin purified by monoclonal anti-hemagglutinin Ab absorption. The amino terminus of S1P1, but not any extracellular loop, prevented anti-S1P1 Ab suppression of S1P1 signaling and T-cell chemotaxis to S1P. Human purified anti-S1P1 Abs decreased mouse blood lymphocyte levels by a mean of 72%, suppressed mouse T-cell chemotaxis to S1P in vivo, and significantly reduced the severity of dextran sodium sulfate-induced colitis in mice. Human Abs to the amino terminus of S1P1 suppress T-cell trafficking sufficiently to impair host defense and provide therapeutic immunosuppression.—Liao, J.-J., Huang, M.-C., Fast, K., Gundling, K., Yadav, M., Van Brocklyn, J. R., Wabl, M. R., Goetzl, E. J. Immunosuppressive human anti-lymphocyte autoantibodies specific for the type 1 sphingosine 1-phosphate receptor.
Keywords: inflammation, chemotaxis, sphingolipid, G protein- coupled
Sphingosine 1-phosphate (S1P) is a lipid mediator of diverse physiological functions, generated by mast cells, endothelial cells, some connective tissue cells, and erythrocytes that also store and release S1P in tissue microcirculatory beds (1,2,3,4,5,6,7). Mammalian blood, lymph, and other extracellular fluids have micromolar concentrations of S1P that chemotactically signal lymphocytes and other immune cells through their type 1 S1P G protein-coupled receptor (S1P1) (8,9,10,11,12,13). The S1P-S1P1 axis controls thymocyte and splenic T-cell emigration into blood, T-cell egress from lymph nodes and nonimmune tissues into lymph, B-cell distribution in splenic and other lymphoid compartments, and migration of antigen-presenting cells (13). S1P1 also transduces effects of S1P on some nonmigration functions of T cells (9).
Genetic and pharmacological alterations in blood and lymph concentrations of S1P, and in lymphocyte levels of expression of S1P1 disrupt normal immune system functions profoundly. S1P1-null mouse thymocytes matured to the CD4 and CD8 stages but failed to emigrate from the thymus in the absence of expression of S1P1 necessary for chemotaxis to higher concentrations of S1P in blood (10). Adoptive transfer of S1P1-null T cells into mouse blood revealed normal homing to lymph nodes in response to a variety of chemokines, but failure of lymphoid egress that requires S1P1-mediated chemotaxis from the low concentration of S1P in lymph nodes to the higher concentration in lymph (10, 11). The S1P-like drug FTY720 is an agonist of S1P1 and other S1P receptors that are capable of both maintaining long-lasting down-regulation of S1P1 and reducing the total S1P-like concentration gradient from lymph nodes to lymph (7, 14). One immunosuppressive dose of FTY720 prevented S1P1-mediated lymph node egress of T cells to a similar extent as for S1P1-null T cells (15). Recently developed S1P1-selective agonists also strikingly alter T-cell trafficking (16, 17). Numerous drugs alter S1P production or biodegradation, and consequently they decrease the positive S1P concentration gradients from lymph nodes to lymph and from spleen to blood. These drugs thereby suppress T-cell egress from lymphoid organs (11). However, no functionally relevant abnormalities of S1P metabolism or S1P1 expression have been identified to date in relation to any human disease.
It appears contradictory that genetic deficiency of lymphocyte S1P1 and pharmacological agonists of S1P1 similarly suppress lymphocyte egress from secondary lymphoid organs, whereas S1P1 pharmacological antagonists have no such inhibitory effect (18). Two sets of findings have elucidated this issue. First, the most effective S1P1 agonist inhibitors of lymphocyte egress from lymphoid organs, such as FTY720, act, in part, by prolonged down-regulation and intracellular proteolysis of lymphocyte S1P1, which results in an acquired state of lymphocyte S1P1 deficiency (14, 16). Second, exogenous S1P1 agonists act, in part, by reducing the endogenous S1P-like concentration gradients required to stimulate lymphocyte egress from lymphoid organs (7). The failure of S1P1 antagonists to inhibit lymphocyte traffic may be attributable to their sole dependence on blocking S1P1 occupancy or insufficient potency.
We now describe the first recognition of human functional anti-S1P1 autoantibodies in a patient with frequent severe infections and lymphopenia. The plasma polyclonal anti-S1P1 autoantibodies of this patient and a monoclonal anti-S1P1 antibody (Ab) generated by a clone of the patient’s B cells bind to the amino-terminal domain that is separate from the S1P-binding pocket. These anti-S1P1 Abs suppress T cell chemotaxis to S1P, but not chemokines, and thereby both decrease blood levels of lymphocytes and suppress tissue traffic of lymphocytes. Such Abs thus may be one cause of deficient adaptive immunity. The capacity of anti-S1P1 Abs to reduce the intensity of immunologically mediated colitis in mice suggests therapeutic potential for autoimmune diseases and transplant rejection.
MATERIALS AND METHODS
Patient clinical description
MAW is a 68-yr-old Caucasian female with a 15-yr history of scleritis, chronic bronchitis with hyperreactive airways, and recurrent ear infections, urinary tract infections, and pneumonias. Two years before our studies were performed, she had Mycobacterium avium complex and Nocardia species pneumonia, which was treated for 18 mo with combinations of levofloxacin, clarithromycin, minocycline, rifampin, and ethambutol. She also has hypertension and osteopenia. One daughter has ulcerative colitis. Physical examination showed patches of scleral pigmentation, scarring of the tympanic membranes, and diffuse wheezes with decreased end-expiratory flow rate and occasional lower lobe rhonchi symmetrically. Hematology and chemistry laboratory panels revealed mild anemia and persistently elevated sedimentation rate of 40 to 95 mm/h (age-corrected normal <30 mm/h). Chest X-rays and computerized tomographic studies documented right middle lobe bronchiectasis, mediastinal lymphadenopathy, and multiple small parenchymal nodules. Results of comprehensive testing for HIV were negative.
Abnormal values for constituents of the immune system over the past 4.5 yr were blood levels of the CD4+ subset of T cells = 209–339/μl (normal=410–1590/μl) and significantly diminished in vitro proliferative responses of blood lymphocytes to phytohemagglutinin-P, concanavalin A, and pokeweed mitogen. In vitro proliferative responses of blood lymphocytes to Candida albicans and tetanus toxoid were marginal relative to concurrent normal controls. Blood levels of CD8+ T cells, B cells, NK cells, other leukocytes, serum proteins by electrophoresis, all classes of immunoglobulins and complement components were normal. Her Ab responses to a booster dose of tetanus toxoid and a primary dose of Pneumovax were normal.
The patient was on no medications at the times of our studies. Eight months after the initial study, repeat relative quantification of anti-lymphocyte Abs by labeling of normal T cells with a series of dilutions of plasma showed a greater than 70% decrease in their concentration. The patient has had only one episode of mild bronchitis during that time.
Leukocyte isolation
Microbeads bearing mouse monoclonal Abs to human CD14, CD4, and CD8 (Miltenyi Biotec, Inc., Auburn, CA, USA) were used for positive immunomagnetic isolation of human blood monocytes, CD4 T cells, and CD8 T cells, respectively, whereas human NK and NKT cells were recovered from mixed blood mononuclear leukocytes by sequential incubation with mouse biotinylated anti-human CD56 Ab (Southern Biotechnology, Birmingham, AL, USA) and streptavidin microbeads (Miltenyi Biotec) before positive immunomagnetic adsorption chromatography. For some studies of T-cell functional or biochemical responses, the total populations of human blood T cells or mouse splenic CD4 T cells were purified by negative immunomagnetic adsorptive removal of all other types of mononuclear leukocytes (Miltenyi Biotec). Levels of lymphocytes and other leukocytes in EDTA-anticoagulated mouse blood were determined with a Hemavet 950FS system (Drew Scientific, Inc., Oxford, CT, USA).
Flow cytometry and immunocytochemistry
For flow-cytometric detection of human anti-lymphocyte Abs, replicate suspensions of 105 patient and control healthy human T cells were fixed in 1% paraformaldehyde for 15 min at room temperature, washed, and resuspended in 100 μl of Ca2+- and Mg2+-free Dulbecco’s PBS with 1% fetal bovine serum (FBS), incubated without and with 1/30 to 1/1,000 dilutions of different plasmas and 0.01 to 3 μg/ml of Sepharose-protein A/G (Pierce Biotechnology, Inc., Rockford, IL, USA)-purified immunoglobulins (Igs) or huMab-S1P1-1 IgM for 1 h at 4°C, washed, and then incubated for 1 h at 4°C with a 1/200 dilution of fluorescein isothiocyanate (FITC)-conjugated affinity-purified F(ab′)2 of donkey anti-human IgG (H+L chain-specific) Abs (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) or an FITC-conjugated mouse monoclonal anti-Ig isotype-selective Ab (Southern Biotechnology). For some analyses, T cells were pretreated with human Fcγ receptor-binding inhibitor cocktail (eBioscience, San Diego, CA, USA). In other analyses, immunoglobulins were removed from 20-μl portions of plasma by dilution to 200 μl with binding buffer, absorption with 50 μl of Sepharose-protein A/G gel for 16 h at 4°C, and dialysis against PBS before incubation with human T cells. F(ab′)2 fragments were prepared by isolation of Igs from 0.5 ml of plasma on a 2 ml column of Sepharose-protein A/G gel, dialysis against 20 mM sodium acetate (pH 4.5), digestion for 6 h with agarose-immobilized pepsin (Thermo Scientific, Rockford, IL, USA), and purification by removal of Fc fragments and undigested Igs on a Sepharose-protein A/G column prior to incubation with human T cells. For each sample, 104 propidium iodide-negative cells were analyzed in a FACScan system (Becton Dickinson, Franklin Lakes, NJ, USA).
For immune cytochemical staining, lymphocytes were allowed to adhere to poly-l-lysine-coated glass slides (BD Biocoat, Bedford, MA, USA) for 1 h at 37°C, washed twice with PBS, fixed in 4% paraformaldehyde for 30 min at 4°C, and pretreated with 5 g % BSA in TBS-0.1% Tween-20 for 30 min at 4°C to reduce unspecific binding. Fixed adherent lymphocytes were incubated first with patient or control healthy human intact Igs, F(ab′)2, huMab-S1P1-1, or buffer alone overnight at 4°C and then FITC-conjugated F(ab′)2 of donkey anti-human IgG (H and L chain-specific) Abs or an FITC-labeled mouse monoclonal anti-human Ig isotype-selective Ab for 1 h at 4°C. Images were obtained with an Olympus BX51 photomicroscope equipped with an X-Cite-120 Fluorescence Illumination System (Photonic Solutions, Inc., Mississauga, ON, Canada).
Anti-lymphocyte Ab characterization
The isotypes of anti-lymphocyte Abs were determined by staining patient T cells and those of healthy donors preincubated in various plasmas or Igs with FITC-labeled mouse monoclonal anti-human IgG1, IgG2, IgG3, IgG4, IgM, IgA1, and IgA2 Abs (Southern Biotechnology) prior to flow cytometric analyses. The immune cell specificity of anti-lymphocyte Abs was assessed by separate staining of purified populations of CD4 T cells, CD8 T cells, NK/NKT cells, and monocytes.
Quantification of binding of 32P-S1P
[32P]S1P was prepared, and binding assays were performed as described (19), with minor modifications. HEK293 human embryonic kidney cells were transiently transfected separately with a c-myc epitope-tagged construct of human wild-type S1P1 or vector alone using lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Two days later, transfectants were washed twice in Tris-buffered saline (pH 7.4, TBS) and preincubated for 10 min on ice in TBS containing 4 mg/ml of fatty acid-free BSA with and without 10 μM unlabeled S1P. [32P]S1P was then added, and transfectants were incubated for 30 min on ice, washed twice in TBS containing 0.4 mg/ml fatty acid-free BSA, and lysed in 0.5% SDS for quantitation of binding by scintillation counting.
Lymphocyte functional and biochemical studies
To assess chemotaxis, each upper insert of Transwell permeable supports with a 5-μm diameter pore filter (Corning Life Sciences, Lowell, MA, USA) received 1.2 × 106 T cells in 0.1 ml and was placed over 0.6 ml of RPMI 1640 with 10% charcoal- and dextran-extracted fetal bovine serum (lipid-free FBS) [University of California, San Francisco (UCSF) Cell Culture Facility] without or with a stimulus in the lower compartment. The filters had been preincubated in human type IV collagen for 8 h and then washed and dried overnight, as described previously (20). The T cells had been preincubated with patient or healthy control plasma or Igs or with huMab-S1P1-1 IgM for 30 min at 37°C, and the stimuli were 10 nM to 1 μM S1P and 10 nM to 100 nM CCL21 or CCL5 (Peprotech, Inc., Rocky Hill, NJ, USA). After 4 h of incubation at 37°C, the number of T cells in each lower compartment was determined by microscopic counting and expressed as a percentage of the initial number added to the upper insert. For quantification of proliferation and generation of IFN-γ, replicate 0.6-ml suspensions of 1–2 × 106 CD4 T cells or total T cells/ml of RPMI 1640 with 10% lipid-free FBS, 100 U/ml of penicillin G, and 50 μg/ml of streptomycin were preincubated with patient or healthy control plasma or Igs or with huMab-S1P1-1 for 30 min at 37°C and cultured without or with 10−7 M to 3 × 10−6 M S1P in 24-well plates that had been precoated with 2 μg each of mouse anti-human CD3 and anti-human CD28 Abs (eBioscience). Aliquots of supernatant were removed at 24 h and 48 h for determination of the concentration of human IFN-γ by ELISA (eBioscience). At 48 h, 2 μCi of 3H-thymidine (Perkin-Elmer Corp., Boston, MA, USA) was added to each well, and incubation was continued for 16–24 h, and radioactivity incorporated into lymphocytes was extracted and counted.
To examine effects of anti-lymphocyte antibodies on S1P stimulation of increases in T-cell phosphorylated JunD (P-JunD), replicate suspensions of 1.5–2 × 106 mouse splenic CD4 T cells that had been isolated by negative immunomagnetic selection (Miltenyi Biotec) were deprived of S1P by incubation for 6–16 h in 0.5 ml of RPMI 1640 with 10% charcoal- and dextran-extracted FBS, 100 U/ml of penicillin G, and 50 μg/ml of streptomycin in 48-well plates. Then the CD4 T cells were resuspended in 0.5 ml of the same medium containing PhosStop phosphatase inhibitor cocktail (Roche Applied Science, Mannheim, Germany), preincubated with patient or healthy control plasma or Igs or with huMab-1-S1P1 IgM for 30 min at 37°C, and then incubated without and with 10−7 M to 3 × 10−6 M S1P for 2 h before extraction of nuclear and cytosolic proteins. P-JunD was measured in nuclear and cytosolic extracts by ELISAs, according to protocol instructions, where AP-1 complexes bind to fixed oligonucleotides containing the 12-O-tetradecanoylphorbol-13- acetate-responsive element 5′-TGA(C/G)TCA-3′ and each individual AP-1 constituent is quantified by the binding of enzymatically linked reporter Abs to accessible epitopes (Active Motif, Carlsbad, CA, USA).
Mouse air-pocket model of in vivo chemotaxis
Five milliliters of micropore-filtered air was injected subcutaneously into the backs of 6- to 10-wk-old female C57BL/6 mice 2 days after they had been irradiated with 600 rad. Three days later, 2.5–3.0 × 106 wild-type mouse splenic T cells that had been preincubated for 15 min at 37°C with 5 mg of patient MAW or healthy control Igs or with 2 mg of huMab-S1P1-1 were introduced intravenously (i.v.) into each mouse. Ten min later, 0.2 ml of 10−5 M S1P or CCL21 was injected into each air pocket. Then 24 h later, each air pocket was drained and lavaged twice with 4 ml of Ca2+- and Mg2+-free PBS containing 0.5 g% of fatty acid-free BSA and 100 U/ml of heparin. The numbers of each type of leukocyte were determined with a Hemavet 950FS system.
Western blots of S1P1 receptors
Extracts enriched in plasma membrane proteins were prepared from HTC4 cell transfectants expressing human recombinant S1P1 with a hemagglutinin (HA) epitope tag (HTC4-S1P1-HA) and from normal human blood naive T cells using CelLytic MT reagent (Sigma, St. Louis, MO, USA). Twenty to thirty micrograms of extracted proteins, as determined by the BCA method (Pierce Biotechnology), was resolved by electrophoresis in 4% to 15% gradient polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA, USA) containing SDS and transferred to membranes for detection of S1P1. After blocking with 3% Blot-quickblocker reagent (Calbiochem/EMD, San Diego, CA, USA), blots were incubated in 0.1 μg/ml to 10 μg/ml of patient plasma Igs or huMab-S1P1-1 overnight, followed by 1.2 μg/ml of mouse anti-human IgG (H+L) in 3% quickblocker, and then in 2 ng/ml of horseradish peroxidase (HRP)-conjugated donkey F(ab′)2 anti-mouse IgG (H+L) (Jackson ImmunoResearch Laboratories) in 3% quickblocker. In some studies of huMab-S1P1-1, the second Ab was HRP-conjugated mouse anti-human IgM Ab. Portions of some HTC4-S1P1-HA extracts were preabsorbed with Sepharose-rat monoclonal anti-HA Ab (Roche Applied Science), and the absorbed protein recovered for electrophoresis and Western blot analysis using mouse anti-human IgG (H+L) and then HRP-conjugated donkey F(ab′)2 anti-mouse IgG (H+L) as above. Adherent Abs in antigen-Ab complexes were detected by SuperSignal Wet Pico substrate (Thermo Fisher Pierce, Rockford, IL, USA).
Synthetic substituent peptides of human S1P1
Peptide domains of human S1P1 representing the three extracellular loops (ECLs) and the 46 amino acid NH2-terminus, that are located in extracellular space available to Abs, were synthesized and purified for use in analyses of their competitive inhibition of anti-lymphocyte Ab binding to T cells and of effects of anti-lymphocyte Abs on T-cell functions. The relevant sequences are ECL 1, TTYKLTPAQWFLRE (108–121); ECL 2, SALSSCSTVLPLYHKH (186–201); and ECL 3, DVGCKVKTCDILFRAE (278–293). Each peptide was studied at 10−5 to 3 × 10−4 M in assays of prevention of anti-lymphocyte Ab inhibition of S1P-evoked chemotaxis of human T cells and of anti-lymphocyte Ab inhibition of S1P-induced increases in mouse T cell nuclear level of P-JunD.
Generation of patient MAW B-cell clones
Mononuclear leukocytes of patient MAW were isolated from 50 ml of venous blood anticoagulated with 100 U/ml of heparin by centrifugation of 10-ml portions on 10-ml cushions of Ficoll-Paque (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA) at 400 g for 30 min at room temperature. After being washed twice with Dulbecco’s Ca2+- and Mg2+-free PBS, the mononuclear leukocytes were incubated for 10 min at 4°C with a cocktail of biotinylated Abs to all mononuclear leukocytes other than B cells and 15 min at 4°C with antibiotin Ab-coated microbeads (human total B cell isolation kit; Miltenyi Biotec). Cell-microbead complexes were washed with 12 ml of chromatography buffer (CB) that consists of 0.5 g/100 ml of fatty acid-free bovine serum albumin and 0.01 M EDTA in Dulbecco’s Ca2+- and Mg2+-free PBS, resuspended in 0.5 ml of CB, and applied to a washed LS column (Miltenyi Biotec) in a magnetic field, from which B cells were recovered in 10 ml of CB wash after column retention of labeled other mononuclear leukocytes. As both IgG and IgM Abs were identified on T cells of patient MAW and normal control T cells preincubated in MAW plasma, the B cells were incubated for 15 min at 4°C with mouse anti-human IgG-coated microbeads (Miltenyi Biotec), washed with 8 ml of CB, resuspended in 0.5 ml of CB, and chromatographed on a washed MS column in a magnetic field (Miltenyi Biotec) that resolved IgG surface-negative (G−) (effluent) from IgG surface-positive (G+) (eluate) sets of B cells.
Epstein-Barr virus (EBV) transformation of both sets of B cells was performed as described (21) by incubating a 1-ml suspension of 3 × 104 to 8 × 104 G+ B cells or 4 × 105 to 6 × 105 G− B cells in standard RPMI 1640 medium containing 20% FBS, 100 U/ml of penicillin G, 50 μg/ml of streptomycin, and 2 μM glutamine with 1 ml of a suspension of EBV in the culture supernatant of B95.8 marmoset-transformed cells for 8 h at 37°C in the presence of 2.5 μg/ml of type B CpG oligonucleotide (ODN2006, InvivoGen, San Diego, CA, USA) and 1000 U/ml of human recombinant IL-2 (Peprotech, Inc., Rocky Hill, NJ, USA). The suspension then was diluted in standard RPMI 1640 medium-10% FBS with the same concentrations of CpG and IL-2, so that 75-μl aliquots with 100–400 G+ or 700-1000 G− B cells could be added to wells of 96-well plates. Each well contained a layer of 2 × 104 to 5 × 104 adherent irradiated (2000 rad) healthy human mixed mononuclear leukocytes in 75 μl of standard RPMI 1640 medium-10% FBS. After 14–21 days, when at least 10 B cell clusters/well were seen, supernatants were tested for human T cell-binding Igs by flow cytometry using an FITC-conjugated F(ab′)2 of donkey anti-human IgG (H+L chain-specific) as the second Ab to detect both IgG and IgM Ab products. B cells of each positive well were subcloned with feeder cells at least once in half of a 96-well plate and at least once in a 24-well plate before growing each set of CD19 cells producing anti-human T-cell Ab in 12-well plates without feeder cells.
Mouse model of colitis
Colitis mediated principally by the innate immune system and inflammation was induced by oral dextran sodium sulfate (DSS) (22). Six- to 8-wk-old female C57BL/6 mice were given 3.5% DSS in their drinking water for 5 days and observed for a total of 12 days. When weight loss reached 15% or bloody diarrhea was continuous, mice were euthanized, and the colon was removed for measurement of length, histopathologic studies, quantification of cytokine generation, and isolation of immune cells (23).
RESULTS
Anti-lymphocyte Abs in a patient with recurrent infections and lymphopenia
A 68-yr-old Caucasian female with a long history of otic, urinary, and respiratory infections resulting in sustained pulmonary airway damage and parenchymal lesions more recently was treated for Mycobacterium avium complex and Nocardia species pneumonia. Laboratory evaluations of her immune system have shown isolated CD4 lymphopenia for over 4.5 yr.
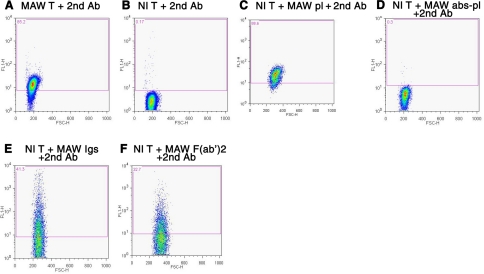
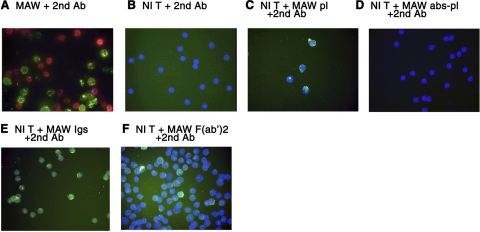
Flow-cytometric analyses of blood T cells stained with FITC-a-IgG-H+L revealed prominent binding of endogenous immunoglobulins (Igs) to most T cells of the patient, but not to T cells of normal subjects (Fig. 1A, B). Incubation of normal T cells with patient plasma prior to staining with FITC-a-IgG-H+L showed binding of patient Igs similar to that for patient T cells (Fig. 1C vs. A). Protein A/G absorption of patient plasma removed T-cell binding Igs (Fig. 1D). To confirm the Ab nature of T-cell binding patient Igs, F(ab′)2 from patient Igs were shown to bind to normal T cells in a pattern resembling that of intact patient Igs (Fig. 1E, F). Flow-cytometric analyses of patient T-cell surface Igs with a panel of isotype-specific fluorescein-labeled second Abs revealed similarly prominent binding of IgM and IgG1 (Table 1). The results of immunocytochemical analyses supported the presence of anti-T-cell Abs in patient plasma. Patient T cells but not normal T cells were stained directly with FITC-a-IgG-H+L (Fig. 2A, B). Preincubation of normal T cells with patient plasma, isolated Igs or F(ab′)2 demonstrated T-cell binding of patient Abs, which were removed from patient plasma by protein A/G (Fig. 2C–F).
Figure 1.
Flow-cytometric analyses of immunoglobulins on patient MAW and healthy control T cells. T-cell suspensions were incubated with plasma or Igs and/or FITC-conjugated affinity-purified F(ab′)2 of donkey anti-human IgG (H+L chain-specific) Abs (FITC-a-IgG-H+L), termed the 2nd Ab. Number in top left corner of each frame is percentage of T cells staining positive at a level above that of the background control. A) MAW T cells + FITC-a-IgG-H+L alone. B) Normal T cells + FITC-a-IgG-H+L alone. C) Normal T cells + MAW plasma 1:100 + FITC-a-IgG-H+L. D) Normal T cells + MAW plasma 1:100 after protein A/G absorption + FITC-a-IgG-H+L. E) Normal T cells + MAW purified Igs, 1 μg/ml + FITC-a-IgG-H+L. F) Normal T cells + MAW purified F(ab′)2, 0.3 μg/ml + FITC-a-IgG-H+L.
TABLE 1.
Immunoglobulin isotype distribution of autologous anti-lymphocyte antibodies on patient MAW blood T cells as assessed by flow cytometry
| IgG1 | IgG2 | IgG3 | IgG4 | IgM | IgA1 | IgA2 | |
|---|---|---|---|---|---|---|---|
| MAW T cells | 13.10 | 0.04 | 0.05 | 0.06 | 15.60 | 0.02 | 0.02 |
Each value is the mean of three determinations of the percentage of patient MAW isolated and washed T cells that stained with FITC-a-IgG-H + L antibodies.
Figure 2.
Immunocytochemical microscopic analyses of immunoglobulins on patient MAW and healthy control T cells. All slide-adherent T cells were treated with the same reagents as in Fig. 1. A) MAW T cells + FITC-a-IgG-H+L alone. B) Normal T cells + FITC-a-IgG-H+L alone. C) Normal T cells + MAW plasma 1:100 + FITC-a-IgG-H+L. D) Normal T cells + MAW plasma 1:100 after protein A/G absorption (abs-pl) + FITC-a-IgG-H+L. E) Normal T cells + MAW purified Igs 1 μg/ml + FITC-a-IgG-H+L. F) Normal T cells + MAW purified F(ab′)2, 0.1 μg/ml + FITC-a-IgG-H+L.
Effects of anti-lymphocyte Abs on T-cell functions in vitro
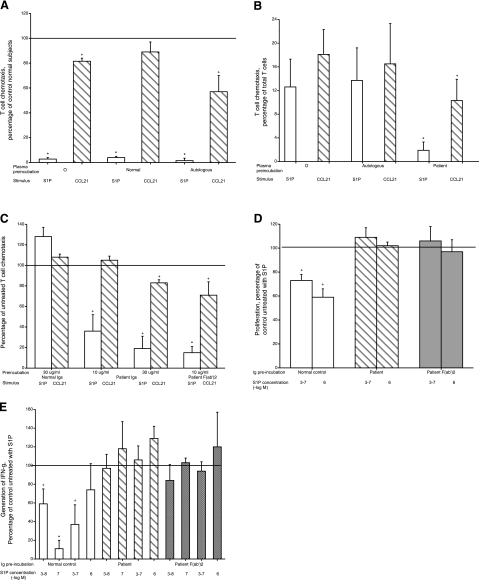
Chemotaxis of patient MAW blood T cells was evaluated in parallel with that of T cells from healthy donors. The response of patient T cells to an optimal stimulatory concentration of S1P was very significantly lower than that of the normal control T cells and was not altered by preincubation in autologous or normal control plasma (Fig. 3A). In contrast, patient T-cell chemotaxis to the chemokine CCL21 was only modestly lower than that of normal control T cells. In one of the studies, patient T-cell chemotaxis to CCL5 was a mean of 83% of the normal control responses. S1P-evoked chemotaxis of T cells from healthy subjects was suppressed strikingly by preincubation with patient MAW plasma, but not autologous plasma, whereas CCL21-elicited chemotaxis of a similar magnitude was only moderately suppressed by patient plasma (Fig. 3B). This specificity of inhibition suggested that Abs in patient plasma interact with S1P1, which is the principal transducer of S1P stimulation of T cell chemotaxis. It is likely that the much lower suppression of T cell chemotaxis to chemokines observed is attributable to loss of S1P enhancement of chemokine-evoked chemotaxis, which also is mediated by the S1P1 receptor being affected by patient Abs. Absorption of patient plasma with Sepharose-protein A/G, under conditions which deleted detectable anti-lymphocyte Ab staining (Figs. 1, 2), removed the Abs that suppressed S1P chemotaxis. Patient-purified Igs significantly inhibited normal T-cell chemotaxis to S1P at two concentrations and at the higher concentration suppressed to a lesser extent chemotaxis to CCL21 (Fig. 3C). At the higher concentration, normal control Igs had no chemotactic inhibitory effects. At an equimolar concentration, patient F(ab′)2 suppressed normal T cell chemotaxis to S1P and CCL21 in a pattern similar to the higher level of Igs (Fig. 3C).
Figure 3.
Effects of patient MAW anti-lymphocyte Abs on autologous and healthy normal human T-cell functions in vitro. A) Chemotaxis of patient MAW T cells to 10−7 M S1P or 3 × 10−8 M CCL21 after preincubation in medium alone, normal subject plasma 1:100, or patient MAW autologous plasma 1:100. Bars and brackets depict means ± range (n=2 in duplicate) of results vs. 2 normal subjects studied concurrently (100%). +P < 0.05, *P < 0.01; 1-tailed 2-sample t test. B) Chemotaxis of healthy normal subject T cells to 10−7 M S1P or 3 × 10−8 M CCL21 after preincubation in medium alone, normal subject autologous plasma 1:100, or patient MAW plasma 1:100. Bars and brackets represent means ± sd of results from 6 subjects. *P < 0.01; 2-tailed paired t test. C) Chemotaxis of healthy normal subject T cells to 10−7 M S1P or 3 × 10−8 M CCL21 after preincubation in normal subject Igs, patient MAW Igs, and patient MAW F(ab′)2. Bars and brackets depict means ± range (n=3 in duplicate) of results vs. cells preincubated in medium alone (100%). +P < 0.05, *P < 0.01; 1-tailed 2-sample t test. D) Reversal by patient MAW Igs and F(ab′)2 of S1P suppression of healthy normal subject T-cell proliferation. Purified normal blood T cells were preincubated with 10 μg/ml of Igs from normal subjects or patient MAW or with 30 μg/ml of patient F(ab′)2 prior to incubation on adherent anti-CD3 + anti-CD28 Abs without and with 3 × 10−7M and 10−6M S1P. Bars and brackets depict means ± range (n=3 in duplicate) of results vs. cells preincubated in medium alone (100%). +P < 0.05; 1-tailed 2-sample t test. E) Reversal by patient MAW Igs and F(ab′)2 of S1P suppression of healthy normal subject T-cell generation of IFN-γ. Preincubation of purified normal blood T cells with control and patient MAW Igs and with patient F(ab′)2 and their subsequent incubation with adherent anti-CD3 + anti-CD28 Abs and S1P was as in Fig. 3D, but S1P was introduced at 3 × 10−8 M and 10−7 M, as well as 3 × 10−7M and 10−6M. Bars and brackets depict means ± range (n=2 in duplicate) of results vs. cells preincubated in medium alone (100%). +P < 0.05, *P < 0.01; 1-tailed 2-sample t test.
The capacities of patient anti-lymphocyte Abs to block other effects of S1P on T-cell functions were examined next. At two concentrations, S1P suppressed significantly the proliferation of normal T cells that had been preincubated with control normal Igs (Fig. 3D). The same levels of patient Igs and F(ab′)2 prevented S1P suppression of T-cell proliferation completely. In parallel studies, generation of IFN-γ by normal T cells preincubated with control Igs was inhibited significantly by the usually effective range of 3 × 10−8 to 3 × 10−7 M S1P up to a maximum of more than 80% at 10−7 M S1P (Fig. 3E). At levels preventing S1P suppression of T-cell proliferation, patient Igs and F(ab′)2 also prevented S1P suppression of T-cell generation of IFN-γ.
Effects of anti-lymphocyte Abs on T cells in vivo
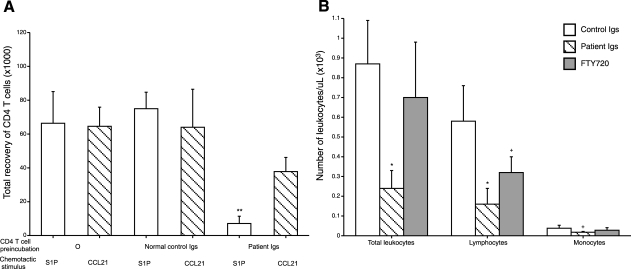
Mouse CD4 T cells were preincubated with normal and patient Igs before injection into irradiated mice with dorsal subcutaneous air pockets. Patient but not normal Igs labeled the T cells, as assessed by flow cytometry. Air pockets then received chemotactically active doses of S1P or CCL21. There was a similarly robust influx of control T cells in response to both chemotactic factors at 24 h (Fig. 4A). Patient Igs, but not normal control Igs, very significantly suppressed the influx of T cells in response to S1P without affecting responses to CCL21. Introduction of CD4 T cells together with patient Igs, but not normal Igs, into irradiated C57BL/6 mice significantly reduced circulating levels of total leukocytes and lymphocytes, with a marginal decrease in the level of monocytes, whereas the down-regulating S1P1 agonist FTY720 only significantly reduced the circulating level of lymphocytes after 24 h (Fig. 4B). In two studies, CD4 T cells and CD8 T cells were introduced together in equal number with patient Igs or normal Igs, and enumerated separately in blood after 24 h. Relative to their levels with normal Igs, patient Igs reduced CD4 counts by a mean of 67% and CD8 counts by only 22%.
Figure 4.
Effects of patient MAW anti-lymphocyte Abs on in vivo tissue chemotaxis and blood concentrations of CD4 T cells in C57BL/6 mice. Five days prior to introduction of lymphocytes with anti-lymphocyte Abs, mice were irradiated with 600 rad. A) Inhibition of S1P-evoked chemotaxis of mouse CD4 T cells into a dorsal subcutaneous air pocket by preincubation with patient MAW Igs, but not normal Igs. Each mouse received 2.5–3.0 × 106 CD4 T cells together with either 5 mg of patient Igs or 5 mg of normal Igs intravenously in 150 μl of PBS, followed in 10 min by 0.2 ml of 10−5 M S1P or CCL21 into the air pocket. Bars and brackets depict means ± sd of results of 3–5 studies analyzed after 24 h. **P < 0.001; 1-tailed 2-sample t test. B) Suppression of mouse blood lymphocyte level by FTY720 and patient MAW Igs, but not normal control Igs. Bars and brackets represent means ± sd; 6 mice/group. Previously irradiated mice received 3.0 × 106 CD4 T cells together with either 5 mg of patient Igs, 5 mg of normal Igs, or 20 μg/kg of FTY720 intravenously in 150 μl of PBS 24 h prior to blood counts. +P < 0.05, *P < 0.01; 2-tailed 2-sample t test.
T-cell binding and functional effects of an IgM anti-lymphocyte Ab (huMab-S1P1-1) produced by a B-cell clone of patient MAW
An EB virus-immortalized B-cell clone from patient MAW produced an IgM Ab (huMab-S1P1-1) that reacted with normal human T cells. Flow cytometric and immunocytochemical analyses demonstrated binding of patient plasma IgM and huMab-S1P1-1 IgM to human T cells (Supplemental Figs. S1 and S2). Normal T-cell chemotaxis to S1P was inhibited significantly by patient plasma and huMab-S1P1-1 (Supplemental Figs. S1–S3). At the same concentration, huMab-S1P1-1 only moderately suppressed normal T-cell chemotaxis to CCL21. HuMab-S1P1-1 appears to represent one subpopulation of anti-lymphocyte Abs in patient MAW plasma.
Determination of the T-cell target antigen of patient anti-lymphocyte Abs
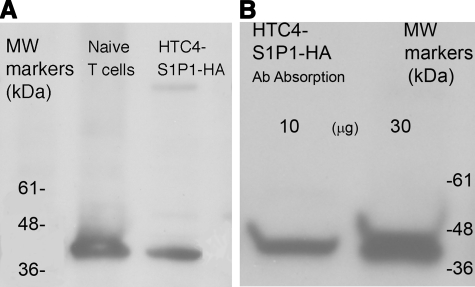
The ability of patient anti-lymphocyte Abs to suppress T-cell chemotaxis to S1P far more than to chemokines suggested the possibility of specific binding to S1P1 or a component of a signaling pathway coupled selectively to S1P1. Proteins extracted from HTC4 cells transfected with human recombinant S1P1 containing a hemagglutinin (HA) tag (HTC4- S1P1-HA) and from naive human blood T cells were resolved by SDS-polyacrylamide gel electrophoresis, blotted, and labeled with patient Igs followed by mouse anti-human IgG (H+L) and then horseradish peroxidase (HRP)-conjugated donkey F(ab′)2 anti-mouse IgG (H+L). One predominant band of 42 kDa was detected for both T cell and HTC4-S1P1-HA cell extracts (Fig. 5A). To establish the identity of the 42-kDa protein as S1P1, extracts of HTC4-S1P1-HA cells were absorbed with Sepharose-rat monoclonal anti-HA Ab, the absorbed proteins were resolved by electrophoresis, and the blot was developed with patient Igs followed by mouse anti-human IgG (H+L) and then HRP-conjugated donkey F(ab′)2 anti-mouse IgG (H+L). Only the 42-kDa protein was found, which has the properties of recombinant S1P1-HA (Fig. 5B). Recombinant S1P1-HA was absorbed by anti-HA Abs, so that its subsequent binding by patient plasma Igs confirms the presence of patient Abs specific for S1P1. One population of the patient’s functional anti-lymphocyte Abs, therefore, are specific for S1P1. Flow cytometric analyses of the binding specificity of patient Igs to S1P1 were performed with two types of HTC4 transfectants. Patient Abs in purified plasma Igs and F(ab′)2 preparations stained HTC4-S1P1 transfectants, but not HTC4- S1P4 transfectants.
Figure 5.
Western blots of SDS-polyacrylamide gradient gel electrophoretic patterns. A) Proteins extracted from normal human T cells (left lane) and S1P1-HA-transfected HTC4 cells (right lane) were electrophoresed, blotted, and labeled with patient MAW plasma Igs followed by mouse anti-human IgG (H+L) and then HRP-conjugated donkey F(ab′)2 anti-mouse IgG (H+L). B) Proteins extracted from S1P1-HA-transfected HTC4 cells were preabsorbed on Sepharose-rat monoclonal anti-HA Ab and eluted, and then 10 and 30 μg of absorbed proteins was electrophoresed, blotted, and labeled with patient MAW plasma Igs, followed by mouse anti-human IgG (H+L) and then HRP-conjugated donkey F(ab′)2 anti-mouse IgG (H+L).
To further investigate the nature of T-cell S1P1 binding by patient anti-lymphocyte Abs, chemotactic studies were designed to achieve more than 80% suppression of the T-cell response to S1P by patient Igs and assess any prevention of this suppression by preincubation of the T cells with S1P1 extracellular substituent peptides (Fig. S2A). The 46 amino acid amino-terminal peptide of S1P1 prevented anti-S1P1 Ab suppression of T cell chemotaxis to S1P in a concentration-dependent manner, whereas none of the three extracellular loop substituent peptides had any effect (Fig. S2A). Thus, most of the patient’s population of functional anti-S1P1 Abs appears to bind specifically to the amino terminus of S1P1. This finding was confirmed by results of parallel analyses of S1P1 peptide prevention of anti-S1P1 Ab suppression of S1P-evoked increases in T-cell nuclear content of P-JunD (Fig. S2B). Anti-S1P1 Abs suppressed S1P-enhanced nuclear levels of P-JunD by nearly 50%, and the amino-terminal peptide of S1P1, but none of the ECL peptides, prevented this suppressive effect of anti-S1P1 Abs. The lack of effect of patient anti-S1P1 Abs on binding of 32P-S1P to S1P1 in transfectants (Table 2) is consistent with their binding to the amino terminus of S1P1. Functionally inhibitory Abs to the amino terminus of S1P1 would not be expected to affect the intramembranous S1P-binding pocket, but instead to alter S1P1 conformation sufficiently to modify signal transduction (24).
TABLE 2.
Lack of effect of patient MAW purified Igs on S1P binding to S1P1
| Normal Igs (μg/ml)
|
Patient MAW Igs
|
|||||
|---|---|---|---|---|---|---|
| 40 | 200 | 1000 | 40 | 200 | 1000 | |
| Mean % of control | 107a | 107 | 105 | 98 | 105 | 105 |
Each replicate was incubated with 200 fmol of 32P-S1P (mean cpm=44,595) without and with a 1000-fold molar excess of nonradioactive S1P. In the absence of Igs, mean specific binding of S1P by S1P1-HEK293 cell transfectants was 16.9 fmol over a background of 1.0 fmol for HEK293 cells transfected with vector alone.
Mean value for one of three identical studies showing no significant effect of Igs on binding.
Suppression of DSS-induced colitis in C57BL/6 mice by patient MAW Igs
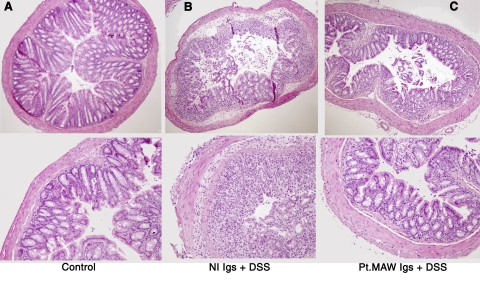
Introduction of anti-S1P1 Abs from patient plasma into mice before induction of colitis decreased colitis-associated blood levels of total T (CD3) cells and CD4 T cells significantly when contrasted with those in mice that received normal plasma Igs, which is consistent with the expected lymphoid trapping (Fig. S3A). Studies of colon histopathology showed markedly less mononuclear leukocyte infiltration and less destruction of glands and crypt structures in mice that received patient anti-S1P1 Abs compared to those that received normal Igs (Fig. 6). The consequent reduction in T-cell infiltration of the colon significantly alleviated the severity of colitis-associated weight loss (Fig. S3B).
Figure 6.
Histopathology of the mouse colon in DSS-induced colitis. A) Normal control colon tissue (hematoxylin-and-eosin stain). B) Colon tissue of mice that received normal human Igs before a course of DSS. C) Colon tissue of mice that received patient anti-S1P1 Abs before a course of DSS.
DISCUSSION
Regulation of lymphocyte traffic in lymphoid organs and other tissues by the extracellular S1P-lymphocyte S1P1 axis is separately dependent on factors influencing S1P concentration in each compartment and on mechanisms controlling expression of lymphocyte S1P1 receptors. Down-regulation of T-cell S1P1 by S1P or by stimulation of the T-cell antigen receptor and its re-expression are rapid events (8, 20, 25, 26). In contrast, FTY720 and its analog AFD-R, which are agonists for several S1P G protein-coupled receptors, and the S1P1-selective agonist CYM-5442 share the capacity to induce internalization, ubiquitination, and partial proteolysis of S1P1 (17). Down-regulation of S1P1 evoked by FTY720 and AFD-R is followed by very slow plasma membrane restitution of the residual S1P1 over many days (14). Both potent S1P1 agonism and prolonged down-regulation of S1P1 underlie the capacity of FTY720 and AFD-R to persistently diminish S1P1 signaling and thereby suppress thymocyte emigration and lymphocyte egress from lymph nodes (13, 15, 27). S1P1 antagonists do not mimic these effects of agonists that persistently down-regulate S1P1.
Flow-cytometric and immunocytochemical detection of surface Igs on T cells of patient MAW, which failed to respond chemotactically to S1P despite near-normal responses to chemokines, suggested the presence of inhibitory Abs selectively directed to S1P1 or elements of its signaling pathway (Figs. 1, 2, 3A). These were polyclonal as evidenced by the identification of both IgG1 and IgM on patient T cells (Table 1). Patient MAW Abs bound to normal human T cells and concurrently inhibited their chemotactic responses to S1P, but not chemokines, and a monoclonal IgM anti-lymphocyte Ab generated by a B-cell line cloned from patient MAW had the same properties (Figs. 1, 2, 3, S1). Unlike FTY720, however, concentrations of patient Igs that maximally inhibited chemotaxis to S1P did not down-regulate S1P1. Patient MAW anti-lymphocyte Abs cross-reacted with mouse T cells by flow cytometry. Preincubation of mouse CD4 T cells with patient Igs, but not normal Igs, prior to intravenous injection into irradiated mice resulted in a lower circulating level of lymphocytes than did the down-regulating agonist FTY720 and diminished T-cell chemotaxis to S1P, but not chemokines, in subcutaneous air pockets (Fig. 4A, B).
The possibility that S1P-specific chemotactic inhibitory effects of patient Igs and the monoclonal IgM Ab were attributable to binding to S1P1 was supported by finding that HA-tagged recombinant S1P1 isolated immunospecifically with monoclonal anti-HA Ab from HTC4 cell transfectants was strongly recognized by patient MAW Abs in Western blots (Fig. 5). These Abs did not, however, prevent binding of 32P-S1P to S1P1 in a standard cell-based assay, which suggested lack of interaction with transmembrane components of the combining site (Table 2). As the predominant anti-lymphocyte Abs appeared to be reacting with cell-surface determinants that often clustered or capped (Fig. 2 and S1 and S2), it was postulated that the major epitope is either the extracellular amino terminus or one of the three extracellular loops. Introduction of high molar excesses of these substituents of S1P1 identified the extracellular amino terminus as the probable epitope. The intact amino-terminal peptide prevented patient MAW anti- S1P1 Abs from inhibiting T-cell chemotaxis to S1P (Fig. S2A). Similar concentrations of the intact amino-terminal peptide also prevented patient anti-S1P1 Abs from suppressing S1P augmentation of the nuclear level of P-JunD in T cells (Fig. S2B).
The functionally antagonistic anti-S1P1 Abs of patient MAW bind to S1P1 with sufficient avidity to block S1P-elicited lymphocyte chemotaxis significantly (Fig. 3B, C; Supplemental Figs. S1–S3) and some other functional responses to S1P in vitro (Fig. 3D, E), without down-regulating S1P1, which also has been observed with potent pharmacological antagonists of S1P1. The capacity of anti-S1P1 Abs and not small molecule antagonists of S1P1 to alter lymphocyte chemotaxis and trafficking in vivo (Fig. 4) may be attributable to higher effective avidity for S1P1, differences attributable to targeting a structural epitope rather than the S1P combining site, or greater resistance to biodegradation of Abs than pharmacological antagonists. These anti-S1P1 Abs with full effects on T cells had no apparent in vivo influence on cells of other organ systems, which also express similar levels of S1P1. This greater susceptibility of S1P chemotactic responses to anti-S1P1 Abs most probably reflects the requirement for a high cell-surface density of S1P receptors for detection of the concentration gradient of such a stimulus across the diameter of the T cell. Thus, modest reductions in effective T-cell levels of S1P1 by anti-S1P1 Abs in blood and other extracellular fluids of patient MAW would suppress S1P1-induced chemotaxis of T cells significantly without necessarily altering responses to S1P of nonmigratory cells in the cardiovascular, gastrointestinal, nervous, and other critical systems. The corollary of this hypothesis is that higher levels of anti-S1P1 Abs might, in fact, have physiologically significant effects on other S1P1-expressing cells.
Human anti-lymphocyte Abs have been discovered previously in recipients of renal or cardiac allografts, patients with chronic infections such as malaria and Dengue fever, and patients with abnormal immunity, including those with systemic lupus erythematosus or AIDS (28,29,30,31,32,33,34,35). Most of these anti-lymphocyte Abs were of the IgM or IgG isotype, reacted with a wide range of lymphocyte surface antigens, including gangliosides and isoforms of CD45, and had broadly negative effects on lymphocyte functions, encompassing inhibition of proliferation, chemotaxis to diverse chemokines, and cytokine production (36). Our finding of functional autoantibodies to S1P1 in a patient with increased susceptibility to serious infections is the first description of specificity for a chemotactic receptor and the first concrete evidence supporting a clear pathogenetic relationship between an altered S1P-lymphocyte S1P1 axis and impaired human host defense against microbial infections. The possibility that such anti-S1P1 autoantibodies may be therapeutically beneficial in human autoimmune diseases is suggested tentatively by their suppression of DSS-induced colitis in mice that received a single dose of patient anti-S1P1 Abs (Supplemental Fig. S3; ref. 6).
Supplementary Material
Acknowledgments
These studies were made possible by RO-1 grant HL-31809 from the National Institutes of Health (NIH) (E.J.G.), NIH grant RO1 AI041570 (M.R.W.), and the Rainin Post-Doctoral Training Fund (M.Y.). K.F. is supported by the UCSF Geriatric Program Training Grant T32 AG000212 from NIH.
References
- Spiegel S, Milstien S. Sphingolipid metabolites: members of a new class of lipid second messengers. J Membr Biol. 1995;146:225–237. doi: 10.1007/BF00233943. [DOI] [PubMed] [Google Scholar]
- Lebman D A, Spiegel S. Cross-talk at the crossroads of sphingosine-1-phosphate, growth factors, and cytokine signaling. J Lipid Res. 2008;49:1388–1394. doi: 10.1194/jlr.R800008-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabe K, Paugh S W, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera A, Rivera J. Sphingolipids and the balancing of immune cell function: lessons from the mast cell. J Immunol. 2005;174:1153–1158. doi: 10.4049/jimmunol.174.3.1153. [DOI] [PubMed] [Google Scholar]
- Li X, Stankovic M, Bonder C S, Hahn C N, Parsons M, Pitson S M, Xia P, Proia R L, Vadas M A, Gamble J R. Basal and angiopoietin-1-mediated endothelial permeability is regulated by sphingosine kinase-1. Blood. 2008;111:3489–3497. doi: 10.1182/blood-2007-05-092148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanel P, Andreani P, Graler M H. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- Pappu R, Schwab S R, Cornelissen I, Pereira J P, Regard J B, Xu Y, Camerer E, Zheng Y W, Huang Y, Cyster J G, Coughlin S R. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- Graeler M, Goetzl E. Activation-regulated expression and chemotactic function of sphingosine 1-phosphate receptors in mouse splenic T cells. FASEB J. 2002;16:1874–1878. doi: 10.1096/fj.02-0548com. [DOI] [PubMed] [Google Scholar]
- Dorsam G, Graeler M H, Seroogy C, Kong Y, Voice J K, Goetzl E J. Transduction of multiple effects of sphingosine 1-phosphate (S1P) on T cell functions by the S1P1 G protein-coupled receptor. J Immunol. 2003;171:3500–3507. doi: 10.4049/jimmunol.171.7.3500. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo C G, Cinamon G, Lesneski M J, Xu Y, Brinkmann V, Allende M L, Proia R L, Cyster J G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Schwab S R, Pereira J P, Matloubian M, Xu Y, Huang Y, Cyster J G. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- Graler M H, Huang M C, Watson S, Goetzl E J. Immunological effects of transgenic constitutive expression of the type 1 sphingosine 1-phosphate receptor by mouse lymphocytes. J Immunol. 2005;174:1997–2003. doi: 10.4049/jimmunol.174.4.1997. [DOI] [PubMed] [Google Scholar]
- Rosen H, Goetzl E J. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- Graler M H, Goetzl E J. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 2004;18:551–553. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei G-J, Card D, Keohane C, Rosenbach M, Hale J, Lynch C L, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- Hale J J, Neway W, Mills S G, Hajdu R, Ann Keohane C, Rosenbach M, Milligan J, Shei G J, Chrebet G, Bergstrom J, Card D, Koo G C, Koprak S L, Jackson J J, Rosen H, Mandala S. Potent S1P receptor agonists replicate the pharmacologic actions of the novel immune modulator FTY720. Bioorg Med Chem Lett. 2004;14:3351–3355. doi: 10.1016/j.bmcl.2004.02.106. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cabrera P J, Jo E, Sanna M G, Brown S, Leaf N, Marsolais D, Schaeffer M T, Chapman J, Cameron M, Guerrero M, Roberts E, Rosen H. Full pharmacological efficacy of a novel S1P1 agonist that does not require S1P-like head-group interactions. Mol Pharmacol. 2008;74:1308–1318. doi: 10.1124/mol.108.049783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J. Immunology. The sources of a lipid conundrum. Science. 2007;316:208–210. doi: 10.1126/science.1142239. [DOI] [PubMed] [Google Scholar]
- Fieger C B, Huang M C, Van Brocklyn J R, Goetzl E J. Type 1 sphingosine 1-phosphate G protein-coupled receptor signaling of lymphocyte functions requires sulfation of its extracellular amino-terminal tyrosines. FASEB J. 2005;19:1926–1928. doi: 10.1096/fj.05-4476fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeler M H, Kong Y, Karliner J S, Goetzl E J. Protein kinase C epsilon dependence of the recovery from down-regulation of S1P1 G protein-coupled receptors of T lymphocytes. J Biol Chem. 2003;278:27737–27741. doi: 10.1074/jbc.C300147200. [DOI] [PubMed] [Google Scholar]
- Traggiai E, Becker S, Subbarao K, Kolesnikova L, Uematsu Y, Gismondo M R, Murphy B R, Rappuoli R, Lanzavecchia A. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med. 2004;10:871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz S, Neufert C, Weigmann B, Neurath M F. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- Fina D, Sarra M, Fantini M C, Rizzo A, Caruso R, Caprioli F, Stolfi C, Cardolini I, Dottori M, Boirivant M, Pallone F, Macdonald T T, Monteleone G. Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology. 2008;134:1038–1048. doi: 10.1053/j.gastro.2008.01.041. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Osborne D A, Walker M D, Wang D A, Bautista D A, Liliom K, Van Brocklyn J R, Parrill A L, Tigyi G. Identification of the hydrophobic ligand binding pocket of the S1P1 receptor. J Biol Chem. 2007;282:2374–2385. doi: 10.1074/jbc.M609648200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi J, Michel T. Agonist-modulated targeting of the EDG-1 receptor to plasmalemmal caveolae. eNOS activation by sphingosine 1-phosphate and the role of caveolin-1 in sphingolipid signal transduction. J Biol Chem. 2000;275:32363–32370. doi: 10.1074/jbc.M003075200. [DOI] [PubMed] [Google Scholar]
- Kohno T, Wada A, Igarashi Y. N-glycans of sphingosine 1-phosphate receptor Edg-1 regulate ligand-induced receptor internalization. FASEB J. 2002;16:983–992. doi: 10.1096/fj.01-0809com. [DOI] [PubMed] [Google Scholar]
- Wei S H, Rosen H, Matheu M P, Sanna M G, Wang S K, Jo E, Wong C H, Parker I, Cahalan M D. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat Immunol. 2005;6:1228–1235. doi: 10.1038/ni1269. [DOI] [PubMed] [Google Scholar]
- Lobo P I. Nature of autolymphocytotoxins present in renal hemodialysis patients. Their possible role in controlling alloantibody formation. Transplantation. 1981;32:233–237. doi: 10.1097/00007890-198109000-00010. [DOI] [PubMed] [Google Scholar]
- Gilbreath M J, Pavanand K, MacDermott R P, Wells R A, Ussery M A. Characterization of cold reactive lymphocytotoxic antibodies in malaria. Clin Exp Immunol. 1983;51:232–238. [PMC free article] [PubMed] [Google Scholar]
- Minota S, Winfield J B. Identification of three major target molecules of IgM antilymphocyte autoantibodies in systemic lupus erythematosus. J Immunol. 1987;139:3644–3651. [PubMed] [Google Scholar]
- Stricker R B, McHugh T M, Moody D J, Morrow W J, Stites D P, Shuman M A, Levy J A. An AIDS-related cytotoxic autoantibody reacts with a specific antigen on stimulated CD4+ T cells. Nature. 1987;327:710–713. doi: 10.1038/327710a0. [DOI] [PubMed] [Google Scholar]
- Warren R Q, Johnson E A, Donnelly R P, Lavia M F, Tsang K Y. Specificity of anti-lymphocyte antibodies in sera from patients with AIDS-related complex (ARC) and healthy homosexuals. Clin Exp Immunol. 1988;73:168–173. [PMC free article] [PubMed] [Google Scholar]
- Mimura T, Fernsten P, Jarjour W, Winfield J B. Autoantibodies specific for different isoforms of CD45 in systemic lupus erythematosus. J Exp Med. 1990;172:653–656. doi: 10.1084/jem.172.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggi T, Bauer R, Garofalo T, Kukel S, Lenti L, Massetti A P, Muller C, Sorice M, Pontieri G M. Autoantibodies against ganglioside GM3 represent a portion of anti-lymphocyte antibodies in AIDS patients. Scand J Immunol. 1994;40:77–82. doi: 10.1111/j.1365-3083.1994.tb03436.x. [DOI] [PubMed] [Google Scholar]
- Przybylowski P, Balogna M, Radovancevic B, Frazier O H, Susskind B, Van Buren C, Katz S, Kahan B D, Kerman R. The role of flow cytometry-detected IgG and IgM anti-donor antibodies in cardiac allograft recipients. Transplantation. 1999;67:258–262. doi: 10.1097/00007890-199901270-00012. [DOI] [PubMed] [Google Scholar]
- Lobo P I, Schlegel K H, Spencer C E, Okusa M D, Chisholm C, McHedlishvili N, Park A, Christ C, Burtner C. Naturally occurring IgM anti-leukocyte autoantibodies (IgM-ALA) inhibit T cell activation and chemotaxis. J Immunol. 2008;180:1780–1791. doi: 10.4049/jimmunol.180.3.1780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.