Abstract
Omega-3-polyunsaturated fatty acids (ω-3-PUFAs) have well-documented protective effects that are attributed not only to eicosanoid inhibition but also to the formation of novel biologically active lipid mediators (i.e., resolvins and protectins). In this study, we examined their effects on ob/ob mice, an obesity model of insulin resistance and fatty liver disease. Dietary intake of ω-3-PUFAs had insulin-sensitizing actions in adipose tissue and liver and improved insulin tolerance in obese mice. Genes involved in insulin sensitivity (PPARγ), glucose transport (GLUT-2/GLUT-4), and insulin receptor signaling (IRS-1/IRS-2) were up-regulated by ω-3-PUFAs. Moreover, ω-3-PUFAs increased adiponectin, an anti-inflammatory and insulin-sensitizing adipokine, and induced AMPK phosphorylation, a fuel-sensing enzyme and a gatekeeper of the energy balance. Concomitantly, hepatic steatosis was alleviated by ω-3-PUFAs. A lipidomic analysis with liquid chromatography/mass spectrometry/mass spectrometry revealed that ω-3-PUFAs inhibited the formation of ω-6-PUFA-derived eicosanoids, while triggering the formation of ω-3-PUFA-derived resolvins and protectins. Moreover, representative members of these lipid mediators, namely resolvin E1 and protectin D1, mimicked the insulin-sensitizing and antisteatotic effects of ω-3-PUFAs and induced adiponectin expression to a similar extent that of rosiglitazone, a member of the thiazolidinedione family of antidiabetic drugs. Taken together, these findings uncover beneficial actions of ω-3-PUFAs and their bioactive lipid autacoids in preventing obesity-induced insulin resistance and hepatic steatosis.—González-Périz, A., Horrillo, R., Ferré, N., Gronert, K., Dong, B., Morán-Salvador, E., Titos, E., Martínez-Clemente, M., López-Parra, M., Arroyo, V., Clària, J. Obesity-induced insulin resistance and hepatic steatosis are alleviated by ω-3 fatty acids: a role for resolvins and protectins.
Keywords: adiponectin, fatty liver disease, adipose tissue, lipid mediators
Nonalcoholic fatty liver disease (NAFLD) is rapidly becoming the most common cause of chronic liver disease (1,2,3,4). NAFLD is no longer regarded as a neutral and innocent bystander but rather as a premorbid condition that comprises a morphological spectrum of liver injury, ranging from simple TG accumulation in the cytoplasm of hepatocytes (steatosis) to inflammatory and hepatocellular injury (nonalcoholic steatohepatitis), which can eventually lead to fibrosis and cirrhosis (1,2,3,4). Although the pathophysiology of NAFLD is complex and not completely understood, much of this hepatic complication is driven by obesity and especially by insulin resistance in the setting of the so-called metabolic syndrome (5, 6). Not surprisingly, the prevalence of metabolic syndrome and of NAFLD in the general population are coincidental (22 and 20%, respectively), supporting the notion that NAFLD is the hepatic manifestation of the metabolic syndrome (3, 4).
White adipose tissue (WAT) plays a critical role in regulating energy homeostasis (7). Since WAT functional integrity is required for the balanced body metabolism of a healthy organism, deregulation of WAT functions, for example, in obesity, leads to the obesity-associated pathologies of the metabolic syndrome, such as dyslipidemia, glucose intolerance, insulin resistance, and NAFLD (8). In these circumstances, the contribution of WAT to insulin resistance and fatty liver is mediated to some extent by several adipocyte-derived factors, collectively known as adipokines (9). Moreover, obesity is characterized by a low-grade inflammatory state, which aggravates WAT function and associated pathologies (7, 9). Therefore, the modulation of adipokine secretion and actions and the control of the low-grade inflammatory state in WAT are two emerging targets in the prevention of obesity-related insulin resistance and fatty liver disease.
Omega-3 polyunsaturated fatty acids (ω-3-PUFAs), such as docosahexaenoic acid (DHA; C22:6n-3) and its precursor, eicosapentaenoic acid (EPA; C20:5n-3), are of therapeutic value for their anti-inflammatory and protective actions in a number of illnesses, such as rheumatoid arthritis, cystic fibrosis, ulcerative colitis, asthma, atherosclerosis, cancer, and cardiovascular disease (10). The beneficial actions of ω-3-PUFAs were initially believed to be mediated by a decrease in the production of classic inflammatory mediators such as arachidonic acid-derived eicosanoids and inflammatory cytokines (10). However, in recent years, ω-3-PUFAs have been demonstrated to serve as substrates for the conversion to a novel series of lipid mediators designated resolvins and protectins, which mediate the protective and beneficial actions underlying the effects of ω-3-PUFAs (11,12,13,14,15). Indeed, these novel ω-3-PUFA-derived lipid mediators have been shown to display potent protective actions in experimental colitis, peritonitis, brain ischemia-reperfusion, and corneal injury (16,17,18,19). Of particular interest is resolvin E1, a representative member of these novel lipid autacoids, which is the most effective drug candidate of the growing family of endogenous resolvins and the compound with the most developed biology (14,15,16,17).
In a recent study (20), we demonstrated that ω-3-PUFAs and their bioactive derived lipid mediators exert protective actions in the liver by preventing necroinflammatory injury in this organ. The current study was aimed to specifically explore whether ω-3-PUFAs can alter adipose tissue function and protect the liver from insulin resistance and hepatic steatosis in a murine model of obesity. In addition, with lipidomic analysis, we characterized the profile of eicosanoids and ω-3-PUFA-derived lipid mediators generated in WAT and tested the direct effects in vivo and ex vivo of the ω-3-PUFA-derived lipid autacoids, resolvins, and protectins.
MATERIALS AND METHODS
Materials
Male ob/ob mice (B6.V-Lepob/J) were from Charles River (Saint Aubin les Elseuf, France). Deuterated leukotriene B4 (LTB4), synthetic DHA, resolvin E1, and rosiglitazone were purchased from Cayman Chemicals (Ann Arbor, MI, USA). Ketamine (Ketolar) and xylazine (Rompun) were from Parke Davis-Pfizer (Dublin, Ireland) and Bayer (Leverkusen, Germany), respectively. Optimal cutting temperature (OCT) compound and OCT cryomolds were purchased from Sakura Finetek (Tokyo, Japan). The Micro BCA protein assay kit was from Pierce (Rockford, IL, USA); 40% acrylamide/bis and polyvinylidene difluoride (PVDF) membranes were from Bio-Rad (Hercules, CA, USA). The ECL detection system was from GE Healthcare (Chalfont St. Giles, UK). The high-capacity cDNA archive kit and TaqMan expression assays were from Applied Biosystems (Foster City, CA, USA). Primary antibodies against AMPK and phospho-AMPK, F4/80, and adiponectin were from Cell Signaling Technology (Beverly, MA, USA), Serotec (Oxford, UK), and Abcam (Cambridge, UK), respectively. The Vectastain ABC kit was from Vector (Burlingame, CA, USA). Alexa Fluor 488 secondary antibody and ProLong Gold reagent with DAPI were from Molecular Probes (Eugene, OR, USA). All other reagents were purchased from Sigma (St. Louis, MO, USA).
Animal studies
Male ob/ob and wild-type mice were housed in plastic cages on wood-chip bedding and provided free access to water and standard mouse chow AIN-93G, which is the growth diet recommended by the American Institute of Nutrition (21) for an acclimation period of 1 wk. Subsequently, ob/ob mice were assigned to two groups that were fed either a control diet (control group; n=8) or an ω-3-PUFA-enriched diet (experimental group; n=16) for 5 wk. These two diets had an equivalent amount of fat (8.4% fat by weight) compared to the standard mouse chow (7.2% fat by weight), but in the experimental group, 6% of the total lipid content was provided by ω-3-PUFAs. Fatty acids were incorporated into a base fabricated diet composed of starch, sucrose, cellulose, casein, l-cystine, lipids, salt mixture, vitamin mixture, and choline bitartrate as described previously (20). This diet has been used previously as a dietary ω-3-PUFA supplement in different animal models of disease (22, 23). Diets were stored at −20°C and provided fresh daily. Body weight and food intake were monitored throughout the study. Two additional groups of ob/ob mice (n=10) and their respective controls (n=10) received intraperitoneal injections of DHA at a dose of 4 μg/g body weight every 12 h during 4 d (see below) or resolvin E1 at a dose of 1.2 ng/g body weight every 24 h during 4 d. At the end of the intervention periods, the mice were anesthetized under a mixture of 0.1 mg ketamine/g body weight and 0.01 mg xylazine/g body weight via intraperitoneal injection. Blood was collected, and serum was obtained by centrifugation at 3000 g for 10 min. Liver was excised, rinsed in Dulbecco’s phosphate-buffered saline (DPBS), fixed in 10% formalin, and embedded in paraffin or placed in OCT, immersed in cold 2-methylbutane on dry ice, and kept at −80°C. Adipose tissue was fixed in 10% formalin and embedded in paraffin. In addition, portions of liver, adipose, and muscle tissues were snap-frozen in liquid nitrogen for further analysis. All experimental studies were conducted in accordance with the criteria of the Investigation and Ethics Committee of the Hospital Clínic and the European Community laws governing the use of experimental animals.
Analysis of hepatic lipid content by Oil Red O staining
OCT-embedded liver samples were cut at 5 μm sections and stained with Oil Red O to evaluate the hepatic lipid content as described previously (24). Briefly, cryosections were fixed in 60% isopropanol for 10 min and stained with 0.3% Oil Red O in 60% isopropanol for 30 min and subsequently washed with 60% isopropanol. Sections were counterstained with Gill’s hematoxylin, washed with acetic acid solution (4%), and mounted with aqueous solution. Sections were visualized under a Nikon Eclipse E600 microscope (Nikon, Kawasaki, Japan) at ×100, and relative areas of steatosis (expressed as percentage Oil Red O staining) were quantified by histomorphometry using a computerized image analysis system (AnalySIS; Soft Imaging System, Munster, Germany). A minimum of 20 independent fields per sample was evaluated.
Detection of F4/80 by immunohistochemistry
Liver paraffin sections were deparaffinized, rehydrated, and pretreated with 0.05% trypsin/0.1% CaCl2 for 20 min at 37°C to unmask the antigen, followed by incubation with 3% H2O2 for 25 min at room temperature and dark conditions to block endogenous peroxidase activity and 2% BSA for 20 min at room temperature to avoid unspecific binding of the primary antibody. The sections were then incubated overnight at 4°C with the primary rat anti-mouse F4/80 antibody (1:100), followed by incubation for 90 min at room temperature with a biotinylated rabbit anti-rat IgG secondary antibody (1:200) and incubation with ABC for 45 min at room temperature. Color was developed using the DAB substrate, and sections were counterstained with hematoxylin.
Detection of adiponectin by immunofluorescence
Sections of adipose tissue were deparaffinized, rehydrated, and pretreated with 0.05% trypsin/0.1% CaCl2 for 20 min at 37°C to unmask the antigen, followed by incubation with 2% BSA for 20 min at room temperature to avoid unspecific binding of the primary antibody. The sections were then incubated overnight at 4°C with the primary rabbit anti-adiponectin antibody (1:500), followed by incubation for 90 min at room temperature with a donkey anti-rabbit Alexa Fluor488 secondary antibody (1:400). Samples were mounted using ProLong Gold Reagent with DAPI and visualized under a Leica DMI 6000 fluorescence microscope using the software LAS AF (Leica Microsystems, Solms, Germany).
Insulin tolerance tests (ITTs)
Similar to previous studies in ob/ob mice (25) to evaluate the effects of ω-3-PUFAs on glucose homeostasis, we performed ITT curves in 5 ob/ob mice after short-term intraperitoneal injections (4 μg/g body weight, every 12 h during 4 d) of DHA, the most representative ω-3-PUFA in our diet. DHA was complexed with BSA as described elsewhere (26). Wild-type mice (n=4) and a placebo group of ob/ob mice (n=5) received saline. Subsequently, mice received an intraperitoneal injection of recombinant insulin (0.0075 U/g body weight), and blood samples were collected from the tail 0, 15, 30, 45, and 60 min later for serum glucose determination using the Accu-Chek Aviva system (Roche Diagnostics, Manheim, Germany). At the end of the experiment, tissue samples were collected and snap-frozen in liquid nitrogen for further analysis.
Biochemical analyses
Serum concentrations of cholesterol, TG, and free fatty acids, as well as alanine aminotransferase (ALT) activity, were determined by standard laboratory procedures. The total hepatic content of TG was determined by standard laboratory procedures after lipid extraction with isopropyl alcohol-hexane as described previously (24). Briefly, tissue samples (100 mg) were homogenized in 10 ml isopropyl alcohol-hexane mixture (3:2) and placed on an orbital shaker overnight at the maximum speed (250-300 rpm). Subsequently, 3 ml of anhydrous natrium sulfate (0.47 M) was added, and the samples were shaken again for 15 min. After centrifugation (200 g for 5 min), the upper phase was placed into a clean borosilicate tube, evaporated with liquid nitrogen, and resuspended in 0.5 ml of sodium cholate.
Ex vivo experiments in adipose tissue explants
Adipose tissue explants were obtained from the epididymal fat pads of ob/ob mice. Under sterile conditions, samples of adipose tissue were placed in a P60 plate with DPBS containing penicillin (100 U/ml) and streptomycin (100 mg/ml) prewarmed at 37°C. Connective tissue and blood vessels were removed by dissection before the tissue was cut into 40 mg pieces. Explants were washed with DPBS at 37°C by centrifugation during 1 min at 400 g to remove blood cells and pieces of tissue containing insufficient adipocytes to float. Then explants were incubated for 12 h in 12-well plates (40 mg/well) in 1 ml DMEM in the presence of vehicle (0.5% EtOH), protectin D1 (100 and 250 nM), and rosiglitazone (10 μM). Treatments were performed in duplicate. At the end of the incubation period, supernatants were collected and frozen at −80°C. Explants were placed directly into TRIzol reagent for immediate homogenization and frozen at −80°C for further RNA extraction.
Analysis of mRNA expression by real-time RT-PCR
Total liver RNA was obtained with the RNAqueous kit. Total RNA from adipose and muscle tissues was obtained with the TRIzol reagent method. RNA concentration was assessed in an ultraviolet-spectrophotometer, and its integrity was tested on a 6000 LabChip in a 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). First-strand cDNA synthesis was performed by incubating 1 μg of total RNA with 2.5 μl of 10× RT buffer, 1 μl of 25× dNTPs, 2.5 μl of 10× primers, and 1.25 μl of reverse transcriptase (25 μl final volume) for 10 min at 25°C, followed by 2 h at 37°C in an MJ Research PTC-100 thermal cycler (Bio-Rad). Ready-to-use primer and probe sets predeveloped by Applied Biosystems (TaqMan Gene Expression Assays) were used to quantify adiponectin (ID: Mm00456425_m1), fatty acid synthase (FASN; ID: Mm00662319_m1), GLUT-2 (ID: Mm00446224_m1), GLUT-4 (ID: Mm00436615_m1), hormone-sensitive lipase (HSL; ID: Mm00495359_m1), interleukin-6 (IL-6; ID: Mm00446190_m1), insulin receptor substrate-1 (IRS-1; ID: Mm01278327_m1), IRS-2 (ID: Mm03038438_m1), monocyte chemoattractant protein-1 (MCP-1; ID: Mm00441242_m1), peroxisome proliferator-activated receptor-α (PPARα; ID: Mm00440939_m1), PPARγ (ID: Mm00440945_m1), resistin (ID: Mm00445641_m1), stearoyl-CoA-desaturase-1 (SCD-1; ID: Mm00772290_m1), sterol response element-binding protein-1c (SREBP-1c; ID: Mm00550338_m1), and tumor necrosis factor-α (TNF-α; ID: Mm00443258_m1) gene expression using β-actin (ID: Mm00607939_s1) as an endogenous control. Briefly, PCR reactions were performed in duplicate using the Universal TaqMan 2× PCR mastermix in a volume of 20 μl containing 1.25 μl cDNA. Real-time quantitative PCR was performed with an ABI Prism 7900 Sequence Detection System (Applied Biosystems) using the fluorescent TaqMan methodology. Real-time PCR results were analyzed with the Sequence Detector Software version 2.1 (Applied Biosystems). Relative quantitation of gene expression was performed using the 2−ΔΔCt method.
Analysis of AMP-activated protein kinase (AMPK) and phospho-AMPK protein expression by Western blot
Total proteins from liver and muscle tissues were extracted in homogenizing buffer containing 2 mM EGTA, 5 mM EDTA, 30 mM sodium fluoride, 10 mM sodium pyrophosphate decahydrate, 2 mM sodium orthovanadate, 40 mM β-glycerol phosphate, 0.5% Igepal, 20 mM MOPS, and protease inhibitors. For adipose tissue, the homogenizing buffer contained 50 mM HEPES, 20 mM β-glycerol, 2 mM EDTA, 1% Igepal, 10% glycerol, 1 mM MgCl2, 1 mM CaCl2, 150 mM NaCl, 10 mM sodium fluoride, 20 mM sodium pyrophosphate decahydrate, 2 mM sodium orthovanadate, and protease inhibitors. Tissue homogenates were incubated on ice for 15 min with frequent vortexing. Thereafter, homogenates were centrifuged at 16,100 g for 20 min at 4°C, and the supernatants were collected. AMPK and phospho-AMPK protein expression was analyzed by Western blot. Equal quantities of total protein (50 μg liver, 80 μg adipose tissue and 100 μg muscle tissue, determined by the Micro BCA protein assay kit) were resuspended in SDS-containing Laemmli sample buffer, heated for 5 min at 95°C, and separated by SDS-PAGE (12.5% for liver and muscle and 10% for adipose tissue). Proteins were electroblotted for 120 min at 100 V at 4°C onto PVDF membranes, and the efficiency of the transfer was visualized by Ponceau S solution staining. Membranes were then soaked for 1 h at room temperature in TBS (20 mM Tris-HCl, pH 7.4, and 0.5 M NaCl) containing 0.1% (v/v) Tween 20 (0.1% T-TBS) and 5% (w/v) nonfat dry milk. Blots were washed 3 times for 5 min each with 0.1% T-TBS and subsequently treated overnight at 4°C with primary rabbit anti-mouse antibodies against AMPK and phospho-AMPK (dilution 1:1000) in 0.1% T-TBS containing 5% BSA. After the blots were washed 3 times for 5 min each with 0.1% T-TBS, membranes were incubated for 1 h at room temperature with a horseradish-peroxidase-linked donkey anti-rabbit antibody (dilution 1:2000) in 0.1% T-TBS, and bands were visualized using an ECL detection system.
Lipidomic analysis by liquid chromatography/mass spectrometry/mass spectrometry (LC/MS/MS)
Adipose tissue samples (0.1 to 0.2 g) were placed in methanol/water (5 ml, 65:35, v/v, 4°C), and deuterated LTB4 (200 pg) was added as an internal standard. Samples were gently homogenized at 4°C with an Ultra-Turrax T 25 Basic homogenizer (IKA-Werke, Staufen, Germany) and extracted in Sep-Pak C18-ODS solid-phase columns as previously detailed (19, 27). In brief, homogenized liver suspensions were placed at −20°C for at least 1 h and centrifuged at 400 g for 10 min at 4°C. Supernatants were collected, brought to a final volume of 10 ml with HPLC-grade water at 4°C, acidified to pH 4.0 with HCl (1 N), transferred into syringes, and loaded onto C18-silica reverse-phase cartridges. Cartridges were washed with 10 ml of HPLC-grade water followed by hexane, and compounds were eluted in methyl formate followed by a final elution in methanol. The eluted methyl formate fraction was rapidly evaporated under a stream of nitrogen, resuspended in 100 μl of methanol, and kept at −80°C.
Endogenous levels of eicosanoids and ω-3-PUFA-derived lipid autacoids were analyzed by LC/MS/MS-based lipidomics using a triple quadruple linear ion trap LC/MS/MS system, which consisted of a DAD-HPLC (Agilent 1200) that was directly connected to the electrospray source of a hybrid triple quadrupole/linear ion trap mass spectrometer (MDS Sciex API 3200 QTRAP, Applied Biosystems). With the use of a LUNA C18-2 minibore column, lipid autacoids were eluted with a mobile phase consisting of methanol/water/acetate (65:35:0.03, v/v/v) that was run at a linear gradient to reach 100% methanol (methanol/acetate, 100:0.03, v/v) at a 0.35 ml/flow rate. Mass spectrometry analyses were carried out in negative ion mode using multiple reaction monitoring (MRM mode) of established specific transitions (lipid maps consortium; www. lipidmaps.org; refs. 28,29,30): PGE2 (351→271 m/z), PGF2α (353→193 m/z), TXB2 (369→169 m/z), LTB4 (335→195 m/z), PD1 (359→153 m/z), resolvin E1 (349→195 m/z), RvD1 (375→141 m/z), 7,17-diHDHA (359/199 m/z), 15-HETE (319→175 m/z), 12-HETE (319→179 m/z), 5-HETE (319→115 m/z), and 17-HDHA (343→245 m/z). Linear calibration curves (1-1000 pg), LC retention times, and MRM parameters for each compound were established and optimized with synthetic standards. Concentration of the lipid autacoids was calculated based on the integrated peak area for the specific transition ions and correction for the peak area of the internal deuterated standard d4-LTB4 (339→197 m/z).
Statistical analysis of the results was performed using the ANOVA and unpaired Student’s t test. Results were expressed as means ± se, and differences were considered significant at a value of P < 0.05.
RESULTS
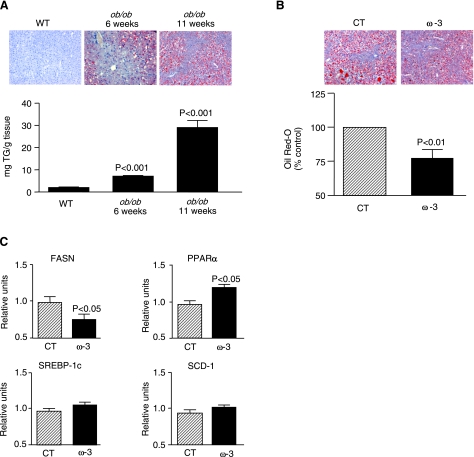
The study was performed in ob/ob mice, an experimental model of obesity-induced insulin resistance and fatty liver disease. As described in Table 1, ob/ob mice showed increased body, liver, and epididymal fat weight; increased liver and epididymal fat-to-body weight ratios; and higher serum cholesterol levels and ALT activity with respect to wild-type mice. Serum TG levels were decreased in ob/ob mice (Table 1). In addition to severe obesity and hypercholesterolemia, ob/ob mice showed progressive hepatic steatosis, as revealed by histomorphometrical analysis of Oil Red O-stained liver sections and by the presence of increased hepatic TG content (Fig. 1A). The degree of hepatic steatosis in obese ob/ob mice was significantly alleviated by the dietary intake of an ω-3-PUFA-enriched diet for 5 wk (Fig. 1B). This antisteatotic effect was associated with changes in the expression of genes governing hepatic lipogenesis and fatty acid oxidation. In fact, ω-3-PUFAs down-regulated FASN, a lipogenic factor, and up-regulated PPARα, a transcription factor that functions as a gatekeeper of fatty acid oxidation (Fig. 1C). The expression of SREBP-1c and SCD-1 remained unchanged (Fig. 1C). Mice consumed between 4.6 and 6.2 g food/d, and there were no significant differences in body, liver, and epididymal fat weight between control and experimental groups of ob/ob mice at the end of the intervention period (Table 1). Interestingly, serum cholesterol levels were significantly reduced in ob/ob mice fed an ω-3-PUFA-enriched diet (Table 1).
TABLE 1.
Body, liver, and epididymal fat weight and serum biochemistry values in wild-type mice and ob/ob mice receiving either control or ω-3-PUFA-enriched diets
| Parameter | Wild-type (n = 5) | ob/ob control diet (n=8) | ob/ob ω-3 diet (n=16) |
|---|---|---|---|
| Body weight (g) | 27.3 ± 0.21 | 43.4 ± 1.8*** | 41.9 ± 0.5** |
| Liver weight (g) | 1.24 ± 0.04 | 2.66 ± 0.24*** | 2.56 ± 0.11** |
| Liver/body weight ratio (%) | 4.55 ± 0.22 | 6.06 ± 0.35** | 6.07 ± 0.22** |
| Epididymal fat weight (g) | 0.32 ± 0.01 | 3.13 ± 0.19*** | 2.90 ± 0.08** |
| Epididymal fat/body weight ratio (%) | 1.18 ± 0.07 | 7.27 ± 0.35*** | 6.91 ± 0.20** |
| Serum cholesterol (mg/dl) | 81.6 ± 3.4 | 180.7 ± 4.6*** | 151.6 ± 6.8**,† |
| Serum TG (mg/dl) | 95.0 ± 5.0 | 60.57 ± 8.1* | 56.5 ± 4.1* |
| Serum ALT (U/L) | 33.5 ± 3.4 | 173.6 ± 31.1* | 156.6 ± 15.4* |
| Serum FFA (μM) | ND | 1811.0 ± 223.6 | 1406.2 ± 183.3 |
Data are expressed as means ± se. FFA, free fatty acids; ND, not determined.
P < 0.05,
P < 0.01,
P < 0.001 vs. wild-type.
P < 0.005 vs. control diet group.
Figure 1.
Hepatic steatosis in ob/ob mice is alleviated by ω-3-PUFAs. A) Representative photomicrographs of liver sections stained with Oil Red O (top) and hepatic TG levels (bottom) in liver samples from wild-type (n=3) and ob/ob mice (n=14) at different weeks of age. B) Representative photomicrographs and histomorphometrical analysis of liver sections stained with Oil Red O from ob/ob mice receiving either control (CT; n=8) or ω-3-PUFA-enriched (ω-3; n=16) diets for 5 wk. C) Expression of key genes involved in hepatic lipid metabolism (i.e., FASN, PPARα, SREBP-1c, and SCD-1) was determined by real-time RT-PCR in liver samples from ob/ob mice after receiving control or experimental diets. Results are expressed as means ± se; P values vs. wild-type (WT) group (A) or CT group (B, C).
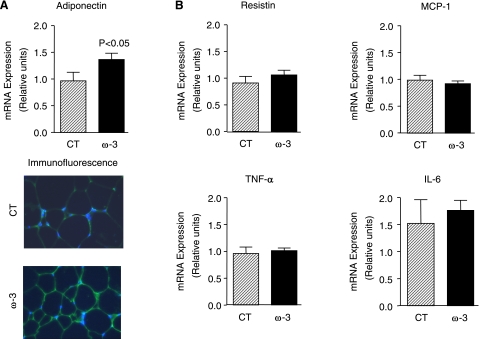
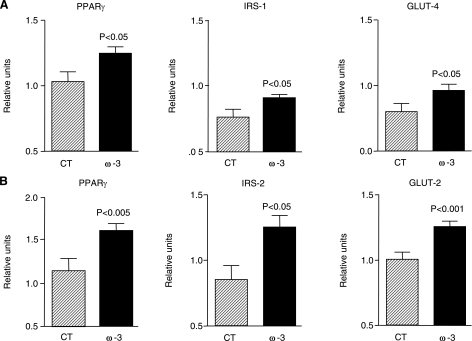
Adipose tissue is an important factor in the regulation of insulin resistance and fatty liver disease (8, 9). Since many of the interactions between adipose tissue, insulin resistance, and hepatic steatosis are orchestrated by soluble adipocyte-derived factors, we examined the effects of ω-3-PUFAs on selected adipokines known to participate in the regulation of insulin sensitivity and hepatic steatosis. As shown in Fig. 2A, the mRNA expression and the immunofluorescence labeling of adiponectin, an adipokine with antidiabetic, antilipogenic, and anti-inflammatory properties, were significantly increased in adipose tissue from ob/ob mice receiving ω-3-PUFAs. In contrast, no changes were observed in resistin, MCP-1, TNF-α, and IL-6 (Fig. 2B). Increased adiponectin expression in mice fed ω-3-PUFAs was associated with up-regulation in the adipose tissue of insulin-sensitizing genes (i.e., PPARγ) and genes coding for insulin receptor signaling (i.e., IRS-1, the substrate protein for the insulin receptor) as well as glucose transport (i.e., GLUT-4, the glucose transporter; Fig. 3A). Similar insulin-sensitizing actions in response to ω-3-PUFAs were observed in the liver with the induction of PPARγ, IRS-2 and GLUT-2 expression (Fig. 3B).
Figure 2.
Effects of ω-3-PUFAs on adipokines in adipose tissue from ob/ob mice. A) Adipose tissue was obtained from ob/ob mice after receiving either a control diet (n=8) or a diet enriched with ω-3-PUFAs (n=16) for 5 wk. Adiponectin expression was assessed by real-time-RT-PCR, and adiponectin production was assessed by immunofluorescence labeling as described in Materials and Methods. B) Expression of resistin, MCP-1, TNF-α, and IL-6 was determined by real-time-RT-PCR. Results are expressed as means ± se; P values vs. CT group.
Figure 3.
ω-3-PUFAs up-regulate the expression of insulin-sensitizing genes in adipose tissue and liver in ob/ob mice. Expression of PPARγ, IRS-1, and GLUT-4 in adipose tissue (A) and their counterparts in the liver (i.e., PPARγ, IRS-2, and GLUT-2) (B) was determined by real-time RT-PCR in samples from ob/ob mice receiving either a control diet (n=8) or a diet enriched with ω-3-PUFAs (n=16). Results are expressed as means ± se; P values vs. CT group.
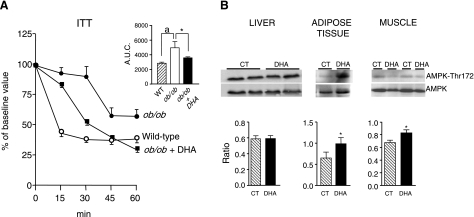
To confirm the insulin-sensitizing effects of ω-3-PUFAs at the functional level, we performed ITTs in obese ob/ob mice. As expected, as compared with lean wild-type mice, ob/ob mice showed a pronounced decrease in insulin sensitivity (Fig. 4A). The administration of the ω-3-PUFA DHA markedly improved insulin sensitivity in these obese mice (Fig. 4A). Given that AMPK is a fuel-sensing enzyme that responds to changes in cellular energy state (31), we next examined the effects of ω-3-PUFAs on AMPK activity by measuring AMPK phosphorylation by Western blot. As shown in Fig. 4B, AMPK activity was significantly increased by DHA in adipose and muscle tissues from ob/ob mice. No changes in body, liver, and epididymal fat weight and serum biochemistry values were noted in ob/ob mice receiving DHA (Table 2).
Figure 4.
Effects of the ω-3-PUFA DHA on insulin tolerance and AMPK phosphorylation in ob/ob mice. A) Insulin tolerance test curves were performed in wild-type mice (n=4) and in ob/ob mice (n=5) receiving saline and in ob/ob mice receiving DHA at a dose of 4 μg/g body weight (n=5) every 12 h for 4 d. Mice received an intraperitoneal injection of recombinant insulin (0.0075 U/g body weight), and blood samples were collected from the tail 0, 15, 30, 45, and 60 min later for serum glucose determination. Inset: analysis of area under the curve (AUC) for these experiments. Results are expressed as means ± se. aP < 0.05 vs. WT; *P < 0.05 vs. ob/ob group treated with saline. B) AMPK phosphorylation was determined in samples of liver, adipose, and muscle tissues obtained from control ob/ob mice (CT; n=8) and ob/ob mice receiving the ω-3-PUFA DHA (n=8). Equal quantities of total protein (50 μg liver, 80 μg adipose tissue, and 100 μg muscle tissue) were separated by SDS-PAGE and analyzed by Western blot. Top and bottom blots represent protein bands detected by specific anti-AMPK phosphorylated on the residue Thr172 or by specific anti-total AMPK antibodies, respectively. Densitometric analysis of the phosphorylated AMPK-to-total AMPK ratio from these blots is shown at bottom. Results are expressed as means ± se. *P < 0.05 vs. CT group.
TABLE 2.
Body, liver, and epididymal fat weight and serum biochemistry values in ob/ob mice receiving placebo, DHA, or resolvin E1
| Parameter | Placebo | DHA | Resolvin E1 |
|---|---|---|---|
| Body weight (g) | 38.8 ± 1.4 | 39.6 ± 1.2 | 35.1 ± 0.7 |
| Liver weight (g) | 2.64 ± 0.13 | 2.39 ± 0.25 | 2.32 ± 0.17 |
| Liver/body weight ratio (%) | 6.80 ± 0.18 | 5.98 ± 0.45 | 6.57 ± 0.40 |
| Epididymal fat weight (g) | 2.70 ± 0.21 | 3.09 ± 0.14 | 2.23 ± 0.08 |
| Epididymal fat/body weight ratio (%) | 6.88 ± 0.34 | 7.81 ± 0.40 | 6.34 ± 0.21 |
| Serum cholesterol (mg/dl) | 117.3 ± 5.2 | 106.0 ± 9.1 | 126.0 ± 6.2 |
| Serum TAG (mg/dl) | 44.4 ± 4.9 | 49.6 ± 8.6 | 33.3 ± 1.3 |
| Serum ALT (U/L) | 343.0 ± 40.9 | 236.0 ± 65.4 | 186.7 ± 11.3* |
| Serum FFA (μM) | 2044.0 ± 142.4 | 1914.0 ± 125.1 | 1798.0 ± 130.4 |
Data are expressed as means ± se; n = 10, n = 5, or n = 5 for placebo, DHA, and resolvin E1, respectively.
P < 0.05 vs. placebo group.
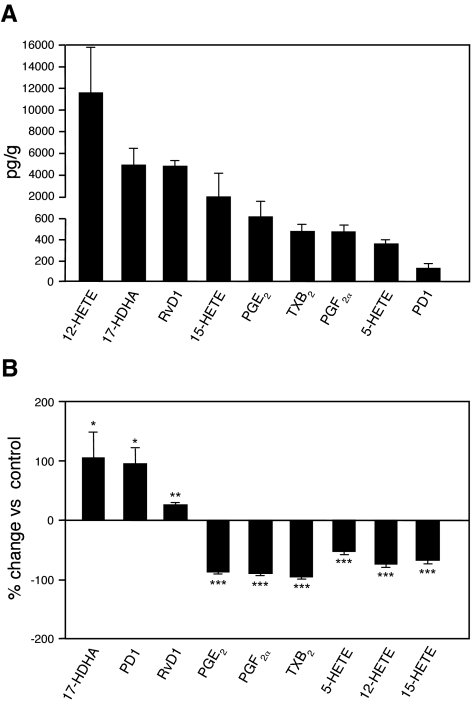
A novel family of bioactive lipid mediators has recently been uncovered (11,12,13,14,15,16,17). These novel ω-3-PUFA-derived autacoids, denoted resolvins and protectins, underlie most of the protective and beneficial actions of the ω-3-PUFAs (17,18,19,20). In this study, we monitored these novel lipid mediators using LC/MS/MS in samples of adipose tissue from ob/ob mice. As shown in Fig. 5A, the ω-3-PUFA-derived bioactive lipid mediators 17-HDHA, protectin D1, and resolvin D1 were detected under normal conditions in adipose tissue from obese mice. There were also significant levels of eicosanoids derived from the ω-6-PUFA arachidonic acid such as those produced through the cyclooxygenase [i.e., prostaglandin (PG) E2, PGF2α, and thromboxane (TX) B2] and lipoxygenase pathways [5-hydroxyeicosatetraenoic acid (5-HETE), 12-HETE, and 15-HETE; Fig. 5A]. Interestingly, products derived from the 12/15-LO were among the most abundant eicosanoids in the adipose tissue (Fig. 5A). Enriching ω-3-PUFAs through diet triggered the formation of 17-HDHA as well as the levels of protectin D1 and resolvin D1 in the adipose tissue of ob/ob mice (Fig. 5B). In addition, endogenous formation of ω-6-PUFA-derived inflammatory mediators, including the cyclooxygenase products PGE2, PGF2α, and TXB2, the 5-LO product 5-HETE, and the 12/15-LO products 12-HETE and 15-HETE, was inhibited in ob/ob mice fed the ω-3-PUFA diet (Fig. 5B).
Figure 5.
Lipidomic analysis of adipose tissue samples using LC/MS/MS. A) Levels of lipid mediators generated in adipose tissue of untreated ob/ob mice. Samples were extracted in C18-ODS solid-phase columns and analyzed by LC-MS/MS-based lipidomics (see Materials and Methods for details). B) Percentage change of tissue levels of ω-3-PUFA-derived docosanoids and ω-6-PUFA-derived eicosanoids in mice receiving ω-3-PUFA-enriched diet (n=8) compared to those receiving CT diet (n=4). Results are expressed as means ± se. *P < 0.05, **P < 0.005, ***P < 0.0005 vs. CT group.
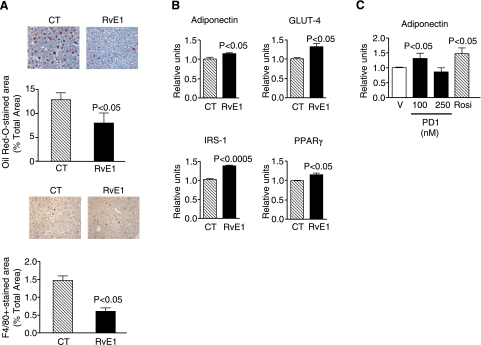
Resolvins and protectins are bioactive lipid mediators with potent protective, anti-inflammatory, and proresolution properties (13,14,15,16,17,18,19,20). Their contribution to the regulation of lipid deposition and inflammatory cell recruitment in the liver as well as their role in insulin signaling and adipokine secretion in the adipose tissue has not been investigated. As shown in Fig. 6, representative members of DHA- and EPA-derived lipid mediators, namely PD1 and resolvin E1, mimicked the beneficial actions of dietary-enriched ω-3-PUFAs in ob/ob mice. In fact, intraperitoneal injection of resolvin E1 conferred significant protection against hepatic steatosis (Fig. 6A), decreased serum ALT levels, a serum marker of liver injury (Table 2), and consistently reduced hepatic F4/80 immunostaining, a macrophage marker (Fig. 6A). Since the F4/80 monoclonal antibody used in our experiments cannot distinguish between recruited and resident macrophages, our results cannot establish whether resolvin E1 decreases macrophage infiltration or this lipid mediator affects Kupffer cells, the liver resident macrophages. On the other hand, resolvin E1 elicited significant insulin-sensitizing effects by inducing adiponectin, GLUT-4, IRS-1, and PPARγ expression in the adipose tissue (Fig. 6B). Furthermore, protectin D1 increased adiponectin expression in WAT explants from ob/ob mice to a similar extent as that of the insulin-sensitizing agent rosiglitazone (Fig. 6C). These findings coincide with our dietary ω-3-PUFA results and indicate that the protective effects of ω-3-PUFAs against insulin resistance and hepatic steatosis may be mediated, in part, by resolvins and protectins.
Figure 6.
Effects of resolvins and protectins on liver and adipose tissue from ob/ob mice. A) Representative photomicrographs of liver sections stained with Oil Red O (top) or incubated with an F4/80 specific antibody (bottom) in ob/ob mice receiving intraperitoneal saline as a control (n=5) or resolvin E1 (RvE1) (n=5) for 4 d. B) Adiponectin, GLUT-4, IRS-1, and PPARγ mRNA expression in adipose tissue samples obtained from ob/ob mice treated with RvE1 mice was determined by real-time RT-PCR. C) Adipose tissue explants from ob/ob mice were incubated for 12 h in the presence of vehicle (V; 0.5% EtOH), protectin D1 (PD1; 100 and 250 nM), or rosiglitazone (10 μM). Four different experiments with duplicates were performed. At the end of the incubation period, RNA was extracted, and adiponectin expression was determined by real-time RT-PCR. Results are expressed as means ± se; P values vs. CT group (A, B) or V group (C).
DISCUSSION
In the present study, we demonstrate that increased intake of ω-3-PUFAs alleviates obesity-induced insulin resistance and advanced hepatic steatosis in obese mice. These beneficial effects were associated with up-regulation of genes involved in insulin sensitivity (i.e., PPARγ), glucose transport (i.e., GLUT-2 and GLUT-4), and insulin receptor signaling (i.e., IRS-1 and IRS-2) in both adipose tissue and liver. In addition, ω-3-PUFAs induced the expression and the production of the potent anti-inflammatory, antisteatotic, and insulin-sensitizing adipokine, adiponectin, and induced the phosphorylation of AMPK. These beneficial responses were associated with a decrease in the formation of ω-6-PUFA-derived eicosanoids (i.e., PGE2 and 5-HETE) and a concomitant increase in the generation of protective ω-3-PUFA-derived lipid mediators (i.e., protectins and resolvins), which mimicked the insulin-sensitizing and antisteatotic effects exerted by ω-3-PUFAs. Interestingly, the effects of resolvins and protectins appeared to be more potent than their ω-3 precursors. To our knowledge, this is the first study reporting the effect of these lipid mediators on adipose tissue function and liver steatosis.
NAFLD is unique among the liver diseases because its etiology is closely related to the metabolic syndrome. Much of the increased prevalence of NAFLD is driven by obesity and especially by insulin resistance. Indeed, it has been reported that in the absence of obesity, even in patients with total lipodistrophy, insulin resistance leads to hepatic steatosis (3, 5). Although the mechanisms underlying the association of insulin resistance to hepatic steatosis remain unclear, altered insulin sensitivity has been shown to increase hepatic de novo lipogenesis and to induce lipolysis of adipocyte TGs and the flux of free fatty acids to the liver (7, 32). Therefore, our results showing a significant improvement in insulin tolerance test curves in obese mice treated with DHA, presumably by induction of insulin-signaling and glucose uptake components, contribute to explain the antisteatotic actions of ω-3-PUFAs. It is important to note that adipose and hepatic tissues were similarly affected by the insulin-sensitizing actions, since IRS-1 and GLUT-4 in adipose tissue and their counterparts in the liver, IRS-2 and GLUT-2, were up-regulated to a similar extent by ω-3-PUFAs. In addition to the insulin-sensitizing actions, a significant reduction in the expression of FASN, a key enzyme in hepatic lipogenesis, was induced by ω-3-PUFAs. Moreover, ω-3-PUFAs up-regulated PPARα, a masterpiece in the control of hepatic peroxisomal β-oxidation of fatty acids.
Altered expression of PPARγ is a frequent finding in insulin resistance (33). PPARγ is a member of the nuclear hormone receptor superfamily that binds to specific DNA response elements as heterodimers with the retinoid X receptor (34). PPARγ activation results in insulin sensitization, and this nuclear factor is the cognate receptor and the established target for the thiazolidinenione class of antidiabetic agents, of which rosiglitazone is a representative member (33, 34). In a previous investigation (20), we reported that ω-3-PUFAs, especially DHA and the derivative 17-HDHA, are potent PPARγ agonists. The significance of these previous findings appears to be pertinent in the context of this study, since levels of 17-HDHA were triggered in adipose tissue after dietary supplementation of ω-3-PUFAs (Fig. 5). Taken together, these findings suggest that induction of PPARγ expression and activation of this nuclear receptor by DHA-derived products contribute to the insulin-sensitizing actions exerted by ω-3-PUFAs. More to the point, the finding that putative metabolites of DHA are strong PPARγ activators has stirred much interest in developing ω-3-PUFA derivatives as potent antidiabetic agents targeting PPARγ (35). Nevertheless, it is important to note that ω-3-PUFA-derived mediators also bind specific surface G-protein-coupled receptors. In this regard, a specific G protein-coupled receptor for resolvin E1, namely ChemR23 or CMKLR1 (chemokine-like receptor 1), has been described in mouse and human tissues (17, 36, 37). In particular, the resolvin E1 receptor is highly expressed in mouse and human adipocytes and a peptide ligand for this receptor (chemerin) has been shown to be a potent adipokine associated with obesity (38,39,40).
Another mechanism whereby ω-3-PUFAs may influence insulin sensitivity is by modifying the profile of adipokines secreted from adipose tissue. Among the different adipokines, adiponectin appears to be the best-characterized candidate to mediate the insulin-sensitizing effects of ω-3-PUFAs. Adiponectin is produced mainly by adipocytes and interacts with at least two different cellular receptors, the activation of which results in a reduction of insulin resistance as well as in the regulation of many biological processes including inflammation and immunity (9). In obese animals, treatment with adiponectin decreases hyperglycemia and improves insulin sensitivity (9). In the liver, adiponectin has beneficial effects in NAFLD by decreasing steatosis and liver injury and by attenuating fibrogenesis (41,42,43). Therefore, in our study, induction of the expression and the immunofluorescence labeling of adiponectin in adipose tissue by ω-3-PUFAs (Fig. 2) contributes to explain the insulin-sensitizing and antisteatotic effects of these compounds. Moreover, in parallel with increased adiponectin, ω-3-PUFAs induced the phosporylation of AMPK (Fig. 4), a fuel-sensing enzyme downstream the adiponectin receptor that acts as a gatekeeper of the systemic energy balance by regulating glucose and lipid homeostasis in adipose, liver, and muscle tissues (31). AMPK responds to changes in the cellular energy state, so when the AMP-to-ATP ratio is increased this enzyme is phosphorylated and becomes active to restore the energy levels by inhibiting ATP-consuming pathways and activating ATP-producing pathways (31). Moreover, the contribution of adiponectin to insulin sensitivity appears to be mediated by a mechanism involving AMPK-dependent PPARγ activation (44).
One of the most important findings of our study was that increased intake of ω-3-PUFAs inhibited the formation of eicosanoids derived from the ω-6-PUFA arachidonic acid (Fig. 5). Culp et al. (45) were the first to identify that ω-3-PUFAs reduce lipoxygenase and cyclooxygenase activities and the formation of ω-6-PUFA-derived eicosanoids. Importantly, in our study, ω-3-PUFAs reduced the formation of eicosanoids derived from 5-lipoxygenase, a major pathway of arachidonic acid metabolism recently established as a potent steatogenic factor in obese ob/ob mice (24). More importantly, in our investigation, inhibition of arachidonic acid-derived eicosanoids was accompanied by an increased biosynthesis of bioactive intermediaries from ω-3-PUFAs, such as 17-HDHA, resolvin D1, and protectin D1. Although we did not detect resolvin E1 in our samples because of our limits of detection, we selected this compound as a representative member of these novel lipid mediators in subsequent studies, because it is the most effective drug candidate of the growing family of endogenous resolvins and the compound with the most developed biology (36, 46,47,48). These novel oxygenated products originated from ω-3-PUFAs evoke potent protective actions demonstrable at the nanomolar and picomolar ranges, many of which are related to the resolution of unremitting inflammation (12, 16,17,18,19,20, 36, 46,47,48). Given that obesity is defined as a state of low-grade inflammation (8, 9), in our study resolution of local inflammation by ω-3-PUFAs and their derived lipid mediators is likely to underlie the beneficial effects of these compounds on insulin resistance and hepatic steatosis in obese mice. Our findings are consistent with a recent study by Merched et al. (49) who demonstrated that a deficit in the biosynthesis of ω-3-PUFA-derived lipid autacoids is involved in the progression of atherosclerosis, a disease also closely related to the metabolic syndrome.
The results of the current study strongly support the concept that increased intake of ω-3-PUFAs would contribute to the prevention of metabolic liver disease similar to the improved outcomes reported in arthritis, cystic fibrosis, IgA nephropathy, diabetes, ulcerative colitis, Crohn’s disease, asthma, and sepsis (10). Our results are consistent with previous investigations reporting that 1) essential fatty acid deficiency modulates very low density lipoprotein secretion in hepatocytes, 2) fish oil feeding decreases the propensity for hepatic TG storage, 3) PUFAs ameliorate adipose tissue inflammation in diabetic mice, and 4) PUFAs prevent fatty liver and mitochondrial dysfunction in rats fed with alcohol (50,51,52,53). The sum of the findings of the current study with results reported in the literature, together with our previous findings showing that ω-3-PUFAs reduce the incidence of necroinflammatory liver injury (20), provides a strong rationale for dietary supplementation with ω-3-PUFAs in patients with liver disease. This approach could especially have potential implications for the use of marginal steatotic liver donors, which are currently excluded because of the presence of macrovesicular steatosis (54, 55).
Acknowledgments
This work was supported by a grant from the Ministerio de Educación y Ciencia (MEC; SAF 06/03191) to J.C. and by a grant from the National Eye Institute (EY01613604) to K.G. CIBERehd is funded by the Instituto de Salud Carlos III. A.G.-P. (BES-2004-5054) was supported by MEC. M.L.-P., E.T., and N.F. had contracts with Instituto de Salud Carlos III, CIBERehd, and MEC Juan de la Cierva, respectively. M.M.-C. (BES-2007-16147) and E.M.-S. (AP2007-02004) received fellowships from MEC. R.H. is supported by Generalitat de Catalunya-European Social Funds (2006FI-00091).
References
- Clark J M, Brancati F L, Diehl A M. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- Falck-Ytter Y, Younossi Z M, Marchesini G, McCullough A J. Clinical features and natural history of nonalcoholic steatosis syndromes. Semin Liver Dis. 2001;21:17–26. doi: 10.1055/s-2001-12926. [DOI] [PubMed] [Google Scholar]
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- Sanyal A J. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- Ginsberg H N. Is the slippery slope from steatosis to steatohepatitis paved with triglyceride or cholesterol? Cell Metab. 2006;4:179–181. doi: 10.1016/j.cmet.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Qureshi K, Abrams G A. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2007;13:3540–3553. doi: 10.3748/wjg.v13.i26.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilherme A, Virbasius J V, Puri V, Czech M P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol Scand. 2005;184:285–293. doi: 10.1111/j.1365-201X.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- Tilg H, Moschen A R. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- Calder P C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- Serhan C N, Hong S, Gronert K, Colgan S P, Devchand P R, Mirick G, Moussignac R L. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Gronert K, Devchand P R, Moussignac R L, Serhan C N. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- Serhan C N, Gotlinger K, Hong S, Arita M. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis. Prostaglandins Other Lipid Mediat. 2004;73:155–172. doi: 10.1016/j.prostaglandins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke T,E. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C N. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- Arita M, Yoshida M, Hong S, Tjonahen E, Glickman J, Petasis N A, Blumberg R S, Serhan C N. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis N A, Serhan C N. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheselli V L, Hong S, Lukiw W J, Tian X H, Gronert K, Musto A, Hardy M, Gimenez J M, Chiang N, Serhan C N, Bazan N G. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and proinflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- Gronert K, Maheshwari N, Khan N, Hassan I R, Dunn M, Laniado Schwartzman M. A role for the mouse 12/15- lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem. 2005;280:15267–15278. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- González-Périz A, Planagumà A, Gronert K, Miquel R, López-Parra M, Titos E, Horrillo R, Ferré N, Deulofeu R, Arroyo V, Rodés J, Clària J. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J. 2006;20:2537–2539. doi: 10.1096/fj.06-6250fje. [DOI] [PubMed] [Google Scholar]
- Reeves P G, Nielsen F H, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Rousseau D, Hélies-Toussaint C, Raederstorff D, Moreau D, Grynberg A. Dietary n-3 polyunsaturated fatty acids affect the development of renovascular hypertension in rats. Mol Cell Biochem. 2001;225:109–119. doi: 10.1023/a:1012266005428. [DOI] [PubMed] [Google Scholar]
- Rousseau D, Hélies-Toussaint C, Moreau D, Raederstorff D, Grynberg A. Dietary n-3 PUFAs affect the blood pressure rise and cardiac impairments in a hyperinsulinemia rat model in vivo. Am J Physiol Heart Circ Physiol. 2003;285:H1294–H1302. doi: 10.1152/ajpheart.00651.2002. [DOI] [PubMed] [Google Scholar]
- López-Parra M, Titos E, Horrillo R, Ferré N, González-Périz A, Martínez-Clemente M, Planagumà A, Masferrer J L, Arroyo V, Clària J. Regulatory effects of arachidonate 5-lipoxygenase on hepatic MTP activity and VLDL-TG and ApoB secretion in obese mice. J Lipid Res. 2008;49:2513–2523. doi: 10.1194/jlr.M800101-JLR200. [DOI] [PubMed] [Google Scholar]
- Song X M, Fiedler, Galuska M, D, Ryder J W, Fernström M, Chibalin A V, Wallberg-Henriksson H, Zierath J R. 5-Aminoimidazole-4-carboxamide ribonucleoside treatment improves glucose homeostasis in insulin-resistant diabetic (ob/ob) mice. Diabetologia. 2002;45:56–65. doi: 10.1007/s125-002-8245-8. [DOI] [PubMed] [Google Scholar]
- Kielar M L, Jeyarajah D R, Zhou X J, Lu C Y. Docosahexaenoic acid ameliorates murine ischemic acute renal failure and prevents increases in mRNA abundance for both TNF-alpha and inducible nitric oxide synthase. J Am Soc Nephrol. 2003;14:389–396. doi: 10.1097/01.asn.0000045047.44107.0b. [DOI] [PubMed] [Google Scholar]
- Gronert K, Clish C B, Romano M, Serhan C N. Transcellular regulation of eicosanoid biosynthesis. Transcellular regulation of eicosanoid biosynthesis. Methods Mol Biol. 1999;120:119–144. doi: 10.1385/1-59259-263-5:119. [DOI] [PubMed] [Google Scholar]
- Murphy R C, Barkley R M, Zemski Berry K, Hankin J, Harrison K, Johnson C, Krank J, McAnoy A, Uhlson C, Zarini S. Electrospray ionization and tandem mass spectrometry of eicosanoids. Anal Biochem. 2005;346:1–42. doi: 10.1016/j.ab.2005.04.042. [DOI] [PubMed] [Google Scholar]
- Masoodi M, Mir A A, Petasis N A, Serhan C N, Nicolaou A. Simultaneous lipidomic analysis of three families of bioactive lipid mediators leukotrienes, resolvins, protectins and related hydroxy-fatty acids by liquid chromatography/electrospray ionisation tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:75–83. doi: 10.1002/rcm.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C N, Lu Y, Hong S, Yang R. Mediator lipidomics: search algorithms for eicosanoids, resolvins, and protectins. Methods Enzymol. 2007;432:275–317. doi: 10.1016/S0076-6879(07)32012-0. [DOI] [PubMed] [Google Scholar]
- Long Y C, Zierath J R. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y H, Ginsberg H N. Adipocyte signaling and lipid homeostasis: sequelae of insulin-resistant adipose tissue. Circ Res. 2005;96:1042–1052. doi: 10.1161/01.RES.0000165803.47776.38. [DOI] [PubMed] [Google Scholar]
- Semple R K, Chatterjee V K, O'Rahilly S. PPAR gamma and human metabolic disease. J Clin Invest. 2006;116:581–589. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass C K. Going nuclear in metabolic and cardiovascular disease. J Clin Invest. 2006;116:556–560. doi: 10.1172/JCI27913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Itoh T, Abe D, Shimizu M, Kanda T, Koyama T, Nishikawa M, Tamai T, Ooizumi H, Yamada S. Identification of putative metabolites of docosahexaenoic acid as potent PPARgamma agonists and antidiabetic agents. Bioorg Med Chem Lett. 2005;15:517–522. doi: 10.1016/j.bmcl.2004.11.053. [DOI] [PubMed] [Google Scholar]
- Campbell E L, Louis N A, Tomassetti S E, Canny G O, Arita M, Serhan C N, Colgan S P. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 2007;21:3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- Arita M, Ohira T, Sun Y P, Elangovan S, Chiang N, Serhan C N. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- Goralski K B, McCarthy T C, Hanniman E A, Zabel B A, Butcher E C, Parlee S D, Muruganandan S, Sinal C J. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282:28175–28188. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- Roh S G, Song S H, Choi K C, Katoh K, Wittamer V, Parmentier M, Sasaki S. Chemerin–a new adipokine that modulates adipogenesis via its own receptor. Biochem Biophys Res Commun. 2007;362:1013–1018. doi: 10.1016/j.bbrc.2007.08.104. [DOI] [PubMed] [Google Scholar]
- Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, Walder K, Segal D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148:4687–4694. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- Xu A, Wang Y, Keshaw H, Xu L Y, Lam K S, Cooper G J. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y, Tamura S, Kiso S, Matsumoto H, Saji Y, Yoshida Y, Fukui K, Maeda N, Nishizawa H, Nagaretani H, Okamoto Y, Kihara S, Miyagawa J, Shinomura Y, Funahashi T, Matsuzawa Y. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology. 2003;125:1796–1807. doi: 10.1053/j.gastro.2003.08.029. [DOI] [PubMed] [Google Scholar]
- Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M, Yoshimatsu H. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology. 2004;40:177–184. doi: 10.1002/hep.20282. [DOI] [PubMed] [Google Scholar]
- Nawrocki A R, Rajala M W, Tomas E, Pajvani U B, Saha A K, Trumbauer M E, Pang Z, Chen A S, Ruderman N B, Chen H, Rossetti L, Scherer P E. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- Culp B R, Titus B G, Lands W E. Inhibition of prostaglandin biosynthesis by eicosapentaenoic acid. Prostaglandins Med. 1979;3:269–278. doi: 10.1016/0161-4630(79)90068-5. [DOI] [PubMed] [Google Scholar]
- Connor K M, SanGiovanni J P, Lofqvist C, Aderman C M, Chen J, Higuchi A, Hong S, Pravda E A, Majchrzak S, Carper D, Hellstrom A, Kang J X, Chew E Y, Salem N, Jr, Serhan C N, Smith L E. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth O, Cernadas M, Yang R, Serhan C N, Levy B D. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J M, Chiang N, Arita M, Serhan C N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merched A J, Ko K, Gotlinger K H, Serhan C N, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22:3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner A, Havinga R, Bos T, Bloks V W, Kuipers F, Verkade H J. Essential fatty acid deficiency in mice is associated with hepatic steatosis and secretion of large VLDL particles. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1150–1158. doi: 10.1152/ajpgi.00456.2004. [DOI] [PubMed] [Google Scholar]
- Levy J R, Clore J N, Stevens W. Dietary n-3 polyunsaturated fatty acids decrease hepatic triglycerides in Fischer 344 rats. Hepatology. 2004;39:608–616. doi: 10.1002/hep.20093. [DOI] [PubMed] [Google Scholar]
- Todoric J, Löffler M, Huber J, Bilban M, Reimers M, Kadl A, Zeyda M, Waldhäusl W, Stulnig T M. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n-3 polyunsaturated fatty acids. Diabetologia. 2006;49:2109–2119. doi: 10.1007/s00125-006-0300-x. [DOI] [PubMed] [Google Scholar]
- Song B J, Moon K H, Olsson N U, Salem N., Jr Prevention of alcoholic fatty liver and mitochondrial dysfunction in the rat by long-chain polyunsaturated fatty acids. J Hepatol. 2008;49:262–273. doi: 10.1016/j.jhep.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwayn I P, Andersson C, Zauscher B, Gura K, Nosé V, Puder M. Omega-3 fatty acids improve hepatic steatosis in a murine model: potential implications for the marginal steatotic liver donor. Transplantation. 2005;79:606–608. doi: 10.1097/01.tp.0000150023.86487.44. [DOI] [PubMed] [Google Scholar]
- El-Badry A M, Moritz W, Contaldo C, Tian Y, Graf R, Clavien P A. Prevention of reperfusion injury and microcirculatory failure in macrosteatotic mouse liver by omega-3 fatty acids. Hepatology. 2007;45:855–863. doi: 10.1002/hep.21625. [DOI] [PubMed] [Google Scholar]