Summary
Sirtuins are NAD-dependent protein deacetylases that connect metabolism and aging. In mammals, there are seven sirtuins (SIRT1-7), three of which are associated with mitochondria. Here we show that SIRT5 localizes in the mitochondrial matrix and interacts with carbamoyl phosphate synthetase 1 (CPS1), an enzyme, catalyzing the initial step of the urea cycle for ammonia detoxification and disposal. SIRT5 deacetylates CPS1 and up-regulates its activity. During fasting, NAD in liver mitochondria increases, thereby triggering SIRT5 deacetylation of CPS1 and adaptation to the increase in amino acid catabolism. Indeed, SIRT5 KO mice fail to up-regulate CPS1 activity and show elevated blood ammonia during fasting. Similar effects occur during long-term calorie restriction or a high protein diet. These findings demonstrate SIRT5 plays a pivotal role in ammonia detoxification and disposal by activating CPS1.
INTRODUCTION
Proteins with homology to yeast SIR2 are found in organisms ranging from bacteria to humans and have been termed sirtuins (Frye, 2000; Guarente, 2008). Yeast SIR2 and the mammalian ortholog SIRT1 were shown to be NAD-dependent deacetylases (Imai et al., 2000), thus linking their function to cellular metabolism (Guarente, 2006). SIRT1 has numerous nuclear substrates that are important metabolic regulators, including PGC-1α, LXR, NF-kB, p53, and forkhead proteins (Brunet et al., 2004; Li et al., 2007; Luo et al., 2001; Motta et al., 2004; Rodgers et al., 2005; Vaziri et al., 2001; Yeung et al., 2004). In yeast, C. elegans, and Drosophila, SIR2 orthologs slow aging and respond to metabolic conditions (Kaeberlein et al., 1999; Lin et al., 2002; Rogina and Helfand, 2004; Tissenbaum and Guarente, 2001; Wang et al., 2006). In mammals, genetic alterations in SIRT1 can affect at least some of the phenotypes associated with calorie restriction (Bordone et al., 2007; Chen et al., 2005).
There are six other SIR2 paralogs in mammals, SIRT2-7, of which SIRT3 and SIRT4 are mitochondrial (Ahuja et al., 2007; Cooper and Spelbrink, 2008; Haigis et al., 2006; Onyango et al., 2002; Schwer et al., 2002). SIRT3 and 4 have NAD-dependent deacetylase and ADP-ribosyl transferase activities, respectively, and have been shown to play roles in mitochondrial metabolism (Haigis and Guarente, 2006). SIRT3 deacetylates acetyl-CoA synthetase 2 (ACS2) at Lys-642 to regulate its activity (Hallows et al., 2006; Schwer et al., 2006). A recent report indicates that SIRT3 deacetylates a component of electron transport chain complex 1 and regulates energy homeostasis (Ahn et al., 2008). SIRT4 transfers ADP-ribose from NAD to glutamate dehydrogenase (GDH), an enzyme that converts glutamate to α-ketoglutarate, thereby repressing this enzymatic activity (Ahuja et al., 2007; Haigis et al., 2006). This repression limits the flow of carbon from amino acids into central metabolism, and thus regulates the use of amino acids for gluconeogenesis in the liver and as insulin secretagogues in pancreatic β-cells (Sener and Malaisse, 1980). Repression of GDH by SIRT4 is alleviated during calorie restriction, a time when amino acids can be an important energy source and can also function to induce insulin (Haigis et al., 2006).
Importantly, both SIRT3 and SIRT4 have been shown to protect cells from genotoxic stress (Yang et al., 2007). In stressed or starved cells, the NAD synthetic enzyme Nampt was shown to boost NAD levels in mitochondria to facilitate this protection.
In the case of SIRT5, the relevant biochemical activity and cellular functions have not been clearly determined, and reports on its location in mitochondria have been equivocal (Lombard et al., 2007; Michishita et al., 2005; Nakamura et al., 2008; Schlicker et al., 2008). Here we show that this sirtuin is a mitochondrial matrix NAD-dependent deacetylase that is specific for carbamoyl phosphate synthetase 1 (CPS1), the committed and regulated enzyme of the urea cycle (Haussinger, 1990; Meijer et al., 1990). CPS1 is critical in the detoxification of excess ammonia, and patients with CPS1 deficiency suffer from hyper-ammonemia, which can lead to mental retardation and death (Yefimenko et al., 2005). In normal individuals, excess ammonia is produced when amino acids are used as energy sources, for example during fasting (Meijer et al., 1990; Morris, 2002; Schimke, 1962b). We also show that SIRT5 regulates CPS1 activity in vivo and this sirtuin is activated upon fasting or during a prolonged high protein diet. The fact that the function of this sirtuin is dedicated to such a specific metabolic function as ammonia disposal underscores the strong connection between the activities of sirtuins and conditional metabolic adaptations.
RESULTS
SIRT5 is a broadly expressed mitochondrial protein
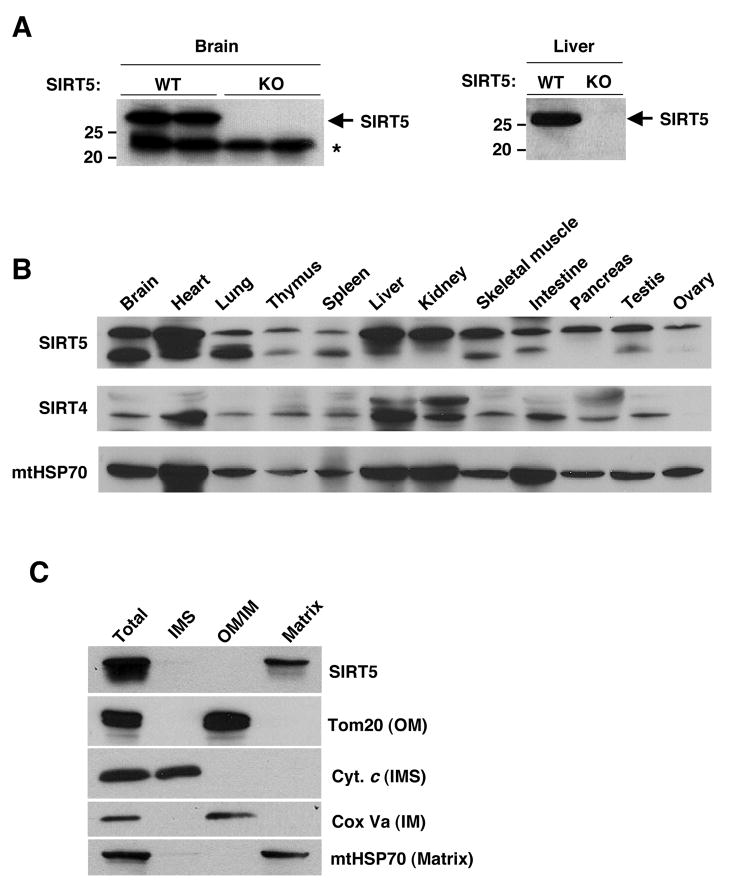
We raised a rabbit polyclonal serum specific for SIRT5 and identified the relevant protein band on a western blot by comparing total brain tissue lysates and liver mitochondria matrix lysates from SIRT5 KO mice and their wild type littermates (Fig. 1A). When blots were probed by anti-SIRT5 antibody, two bands appeared in the wild-type, and the lower band (~23kDa) persisted in the SIRT5 KO. Thus the upper band (~28kDa) corresponds to SIRT5, while the lower corresponds to a protein recognized non-specifically by the anti-SIRT5 antibody.
Figure 1. SIRT5 is broadly expressed and localized in the mitochondrial matrix.
(A) The SIRT5 antibody specificity was tested by western blotting of mouse total brain (left) and mouse liver mitochondria matrix (right) lysates from SIRT5 wild type and KO mice. Arrow indicates SIRT5 and asterisk indicates non-specific band.
(B) Total tissue lysates were subject to western blotting using anti-SIRT5, anti-SIRT4 and anti-mtHSP70 antibodies.
(C) Mitochondria were isolated from mice liver and subject to sub-mitochondrial fractionation. Blots are probed with antibodies to SIRT5, Tom20 - an outer membrane (OM) marker, Cytochrome c - an inter membrane space (IMS) marker, COX Va - an inner membrane (IM) marker and mtHSP70, a matrix marker.
Next, we analyzed various murine tissues by western blotting and determined that SIRT5 is a broadly expressed protein, with highest levels in brain, heart, liver and kidney (Fig. 1B). In order to determine the sub-cellular localization of SIRT5, we first obtained cell extracts from murine primary cultured hepatocytes and found SIRT5 to be associated with an organellar fraction (Fig. S1). A more systematic fractionation of murine liver mitochondria showed clear localization of SIRT5 to the mitochondrial matrix (Fig. 1C). Expression of amino-terminally Flag-tagged SIRT5 in 293T cells showed two bands, the faster migrating of which was missing the Flag tag, consistent with the idea that SIRT5 is processed at the amino terminus (Fig. S2A). N-terminal sequencing revealed that the first 36 amino acids were cleaved (Fig S2B) at a sequence (ARPSS) matching the consensus sequence cleaved by mitochondrial processing peptidase (Hendrick et al., 1989; von Heijne et al., 1989). These results all indicate that SIRT5 is a mitochondrial matrix protein.
Identification of SIRT5 protein substrates
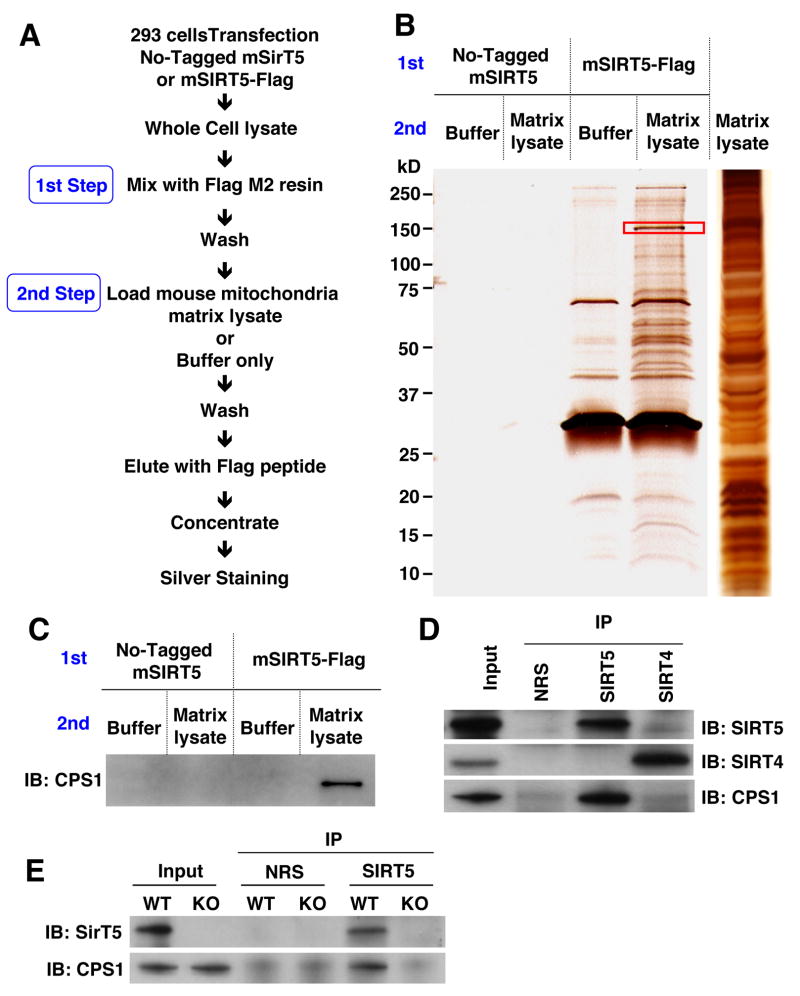
To identify SIRT5-interacting proteins, we adopted a biochemical approach. SIRT5 was Flag-tagged at the carboxyl-terminus and expressed in 293T cells to obtain large quantities of protein (Fig. S2). Lysates from these cells or control cells were mixed with the Flag M2 resin to generate a column of SIRT5-coupled beads (Fig. 2A). A mouse liver mitochondrial lysate (or buffer control) was applied to the SIRT5-coupled or control columns, repeatedly washed, and eluted with the Flag peptide. The eluents were concentrated and subjected to SDS-PAGE followed by silver staining. The control column did not bind any proteins at all, while the SIRT5 column eluted SIRT5 itself, as expected, and numerous other bands (Fig. 2B). Notably, there were several proteins present from the mitochondrial lysate that were absent in the buffer control. The most prominent of these, a protein of about 150 kDa (red box in the figure), was excised and analyzed by mass spectrometry. This analysis revealed peptides that covered 41.5% of the first and rate-limiting enzyme of the mitochondrial urea cycle, CPS1 (Fig. S3). In order to confirm that this protein species was CPS1, we probed a similarly prepared gel with antibodies to this protein, and observed a single band of 150 kDa (Fig. 2C).
Figure 2. SIRT5 interacts with CPS1.
(A) Strategy for in vitro Flag affinity purification. 293T cells were transfected with non-tagged SIRT5 or Flag-tagged SIRT5 and whole cell lysates were loaded onto Flag-M2 resin columns. After columns were washed, mitochondria matrix lysate from wild-type mice or a buffer control were loaded onto columns. Then columns were washed again, eluted by Flag peptides and concentrated.
(B) Samples were subjected to SDS-PAGE and silver staining. The band indicated by the red square was excised, analyzed by mass spectrometry for protein identification and determined to be CPS1. SIRT5-Flag is also indicated.
(C) These same samples were western blotted using anti-CPS1 to verify the identity of the 150 kDa band.
(D) Mitochondrial matrix lysates from SIRT5 wild-type were immuno-precipitated with normal rabbit serum (NRS), anti-SIRT4 or anti-SIRT5 antibody and blotted with anit-SIRT5, SIRT4 and CPS1 antibodies.
(E) Mitochondrial matrix lysates from SIRT5 wild-type and SIRT5 KO mice were immuno-precipitated with normal rabbit serum (NRS) or anti-SIRT5 antibody and blotted with anit-SIRT5 and CPS1 antibodies. The two proteins are shown to interact at endogenous levels.
We examined whether the interaction between SIRT5 and CPS1 observed on the beads was physiologically relevant and specific by carrying out immunoprecipitation of extracts from wild type or SIRT5 KO liver with antibodies to SIRT5 and SIRT4. CPS1 was co-immunoprecipitated by antibody to SIRT5 but not SIRT4 (Fig. 2D) and only from the wild type and not SIRT5 KO extract (Fig. 2E), demonstrating that these proteins interact specifically at endogenous levels.
SIRT5 activates CPS1 by deacetylation
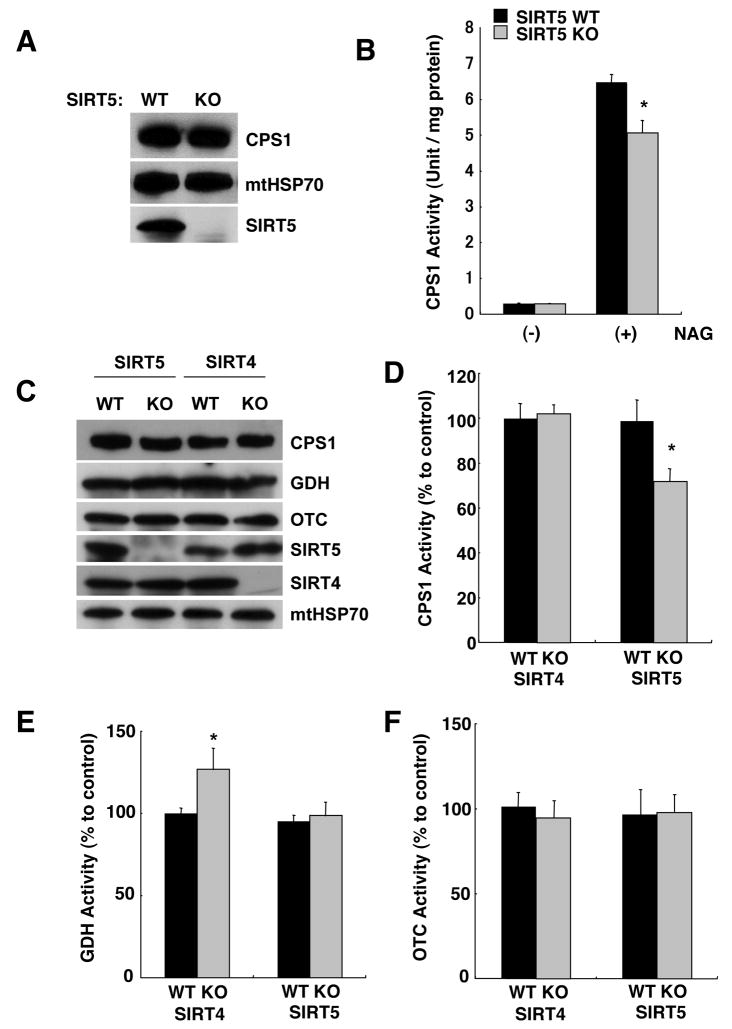
The interaction between SIRT5 and CPS1 suggested that this urea cycle enzyme might be a substrate for this sirtuin. Thus we determined whether CPS1 activity was altered in SIRT5 KO mice. These KO mice develop and grow normally, and display normal blood glucose (Fig S4). Liver mitochondrial extracts were prepared from wild type and SIRT5 KO fed mice, and CPS1 activity was determined by measuring conversion to citrulline colorimetrically (Nuzum and Snodgrass, 1976). As expected, activity was dependent on the essential cofactor, N-acetyl glutamate (NAG) (Fig. 3A and B) (McGivan et al., 1976). Activity was significantly lower in SIRT5 KO extracts, although the levels of CPS1 protein were not discernibly different (Fig. 3B).
Figure 3. CPS1 activity is decreased in SIRT5 KO mice, but not in SIRT4 KO mice.
(A) Liver mitochondria matrix lysates were prepared from SIRT5 KO and wild type littermate controls, and subjected to western blotting with anti-SIRT5, anti-CPS1 and anti-mtHSP70 antibodies.
(B) Liver mitochondria matrix lysates from SIRT5 KO and wild type mice fed ad libitum were assayed for CPS1 activity in the presence or absence of 10mM N-acetyl glutamate (NAG), an allosteric activator. CPS1 activity is shown as unit/mg protein. Error bars represent standard deviations. (n=8 for each groups).
(C) Immunoblots show SIRT5, SIRT4, GDH, CPS1, OTC and mtHSP70 protein levels in liver mitochondria matrix lysates prepared from SIRT5 KO, SIRT4 KO and their wild type littermate controls fed ad libitum. Neither SIRT KO affected the protein levels of the urea cycle enzymes or each other.
(D) CPS1 activity was measured in liver mitochondria matrix lysates from SIRT4 KO, SIRT5 KO and their wild type littermate controls fed ad libitum. CPS1 activity is shown as % of wild type. Error bars represent standard deviations. (n=8 for each groups)
(E) GDH activity was measured by monitoring NADH 340nm absorbance (Haigis et al., 2006). Liver mitochondria matrix lysates from SIRT4 KO, SIRT5 KO and their littermate controls fed ad libitum were assayed. GDH activity is shown as % of wild type. Error bars represent standard deviations. (n=6 for each groups)
(F) OTC activity was determined by measuring converted citrulline by the colorimetric method. Liver mitochondria matrix lysates from SIRT4 KO, SIRT5 KO and their control mice fed Ad libitum were assayed. OTC activity is shown as % to control. Error bars represent standard deviations. (n=4 for each groups)
When amino acids are catabolized for energy, the amino groups are transferred to α-ketoglutarate and converted to glutamate by aminotransferases (Haussinger, 1990; Meijer et al., 1990). Glutamate is then converted back to α–ketoglutarate by GDH releasing the excess ammonia for detoxification by the urea cycle (Stanley, 2004). Since SIRT4 is known to ADP-ribosylate and inhibit GDH (Ahuja et al., 2007; Haigis et al., 2006), it seemed possible that SIRT4 might also regulate CPS1. However, extracts from SIRT4 KO mice showed normal levels of CPS1 activity (Fig. 3C and D). As expected the activity of the SIRT4 substrate GDH was higher in SIRT4 KO extracts. Further validating the functional independence of SIRT4 and SIRT5, GDH activity was not altered in SIRT5 KO extracts (Fig. 3E).
We also checked CPS1 and GDH activity in SIRT3 KO mice, since this mitochondrial sirtuin is also a deacetylase, but did not observe any differences in GDH (Fig. S5) or CPS1 (Fig. S6) activities. These results suggest that the regulation of CPS1 in vivo is specific to SIRT5 and not other sirtuins.
While the first and second steps of the urea cycle occur in the mitochondrial matrix, subsequent steps occur cytoplasmically (Morris, 2002). The second step is mediated by ornithine transcarbamylase (OTC) (Wraith, 2001). To determine whether SIRT5 also regulates OTC, we monitored OTC activity in SIRT5 KO mice, but observed no difference in activity between wild type and SIRT5 KO (Fig. 3F). OTC activity was not affected in SIRT4 or SIRT3 KO mice. (Fig. 3F and S5)
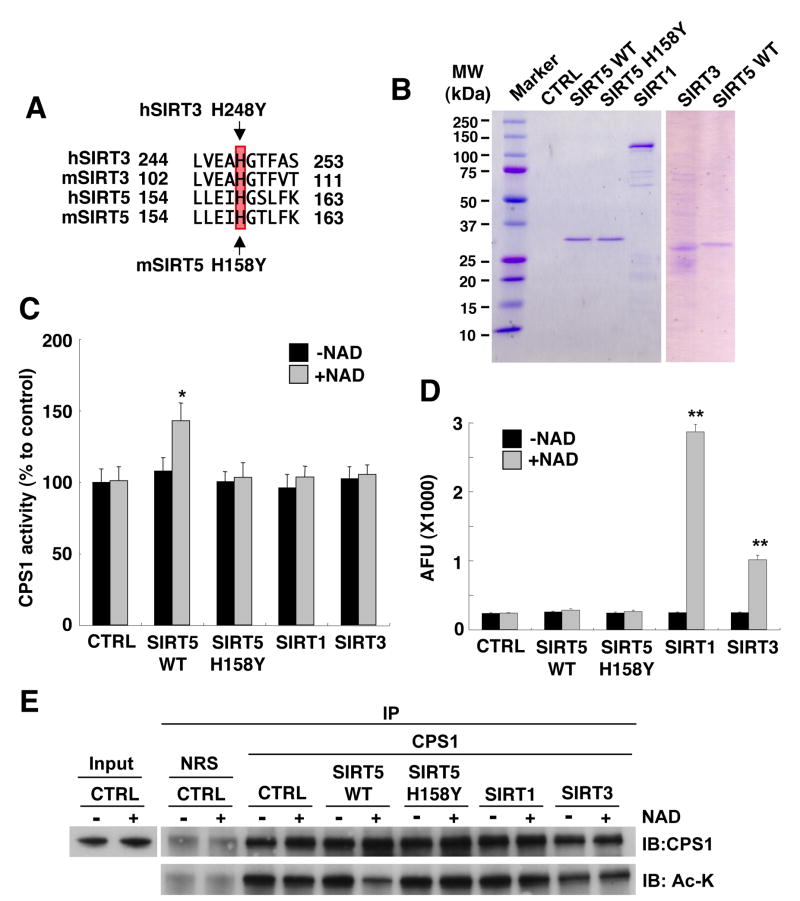
Next, we determined whether SIRT5 could activate CPS1 in vitro. Because mitochondrial CPS1 was found to be acetylated (Kim et al., 2006), SIRT5 could up-regulate CPS1 activity via deacetylation. For this purpose, we purified Flag-tagged mouse SIRT5 using the Flag M2 resin (Fig 4B), and incubated it with a mitochondrial extract from SIRT5 KO liver at 37° C in the presence of 0.5mM NAD. Significant dose-dependent activation of CPS1 by SIRT5 was observed, which was NAD-dependent (Fig. 4C). By homology to other sirtuins, we reasoned that a SIRT5 H158Y mutant would lose catalytic ability (Fig. 4A) (Schwer et al., 2002). Indeed, this mutant protein, purified like the wild type protein above, completely lost the ability to activate CPS1 (Fig. 4C).
Figure 4. SIRT5 deacetylates and activates CPS1 in vitro.
(A) Comparison of partial amino acid sequence between human SIRT3 (hSIRT3), mouse SIRT3 (mSIRT3), human SIRT5 (hSIRT5), and mouse SIRT5 mSIRT5). H248 in human SIRT3 is also conserved in mouse and human SIRT5 as H158.
(B) Flag affinity purified mouse wild-type SIRT5-Flag (SIRT5 WT), mouse catalytic mutant SIRT5-Flag (SIRT5 H158Y), human SIRT3, human SIRT1 recombinant protein and Mock IP control were subjected to SDS-PAGE and stained with Coomasie Brilliant Blue.
(C) Flag affinity purified Flag affinity purified wild-type SIRT5, catalytic mutant SIRT5 H158Y, SIRT1, SIRT3 recombinant protein or Mock IP buffer (CTRL) were assayed for deacetylation using the p53 peptide-fluorescence based SIRT1 activity assay (BIO MOL). Error bars represent standard deviations from triplicate experiments. SIRT1 is shown to deacetylate this peptide, while SIRT5 does not.
(D) SIRT5 up-regulates CPS1 activity in vitro. Mitochondria matrix lysates from SIRT5 KO liver were incubated with Flag affinity purified wild-type SIRT5, catalytic mutant SIRT5 H158Y, SIRT1, SIRT3 recombinant protein or Mock IP buffer (CTRL) at 37 °C for 60 min in the presence or absence of NAD and subsequently subjected to the CPS1 activity assay. Error bars represent standard deviations from triplicate experiments. SIRT5 is shown to activate CPS1, while SIRT1 and SIRT3 does not.
(E) SIRT5 specifically deacetylates CPS1 in vitro. Mitochondria matrix lysates from SIRT5 KO liver were incubated with flag affinity purified wild-type SIRT5, catalytic mutant SIRT5 (H158Y), SIRT1, SIRT3 recombinant protein or mock IP buffer (CTRL) at 37 °C for 60 min in the presence or absence of NAD and subsequently subjected to immunoprecipitation with anti-CPS1 antibody or normal rabbit serum (NRS). Immuno-precipitates were analyzed by western blotting with anti-CPS1 and anti-pan acetylated lysine (Ac-K) antibodies.
Importantly, as observed in vivo CPS1 activation in vitro was specific for SIRT5. SIRT1 and SIRT3 were totally unable to activate CPS1 (Fig. 4C), even though they deacetylated the canonical p53-related substrate (Fig. 4D). Conversely, SIRT5 was completely inactive in the standard SIRT1 deacetylation assay (Fig. 4D). Finally we tested whether SIRT5 specifically deacetylated CPS1 in vitro. After treatment with sirtuins, CPS1 was immunoprecipitated and blotted with anti acetyl-lysine antibody. Consistent with its ability to activate the enzyme, SIRT5 deacetylated CPS1 in NAD-dependent manner. However, SIRT1 and SIRT3 did not deacetylate CPS1 (Fig. 4E). These data indicate SIRT5 specifically deacetylates CPS1 and up-regulates its activity in vitro, as it does in vivo.
CPS1 is regulated by SIRT5 in cultured primary hepatocytes and in mice
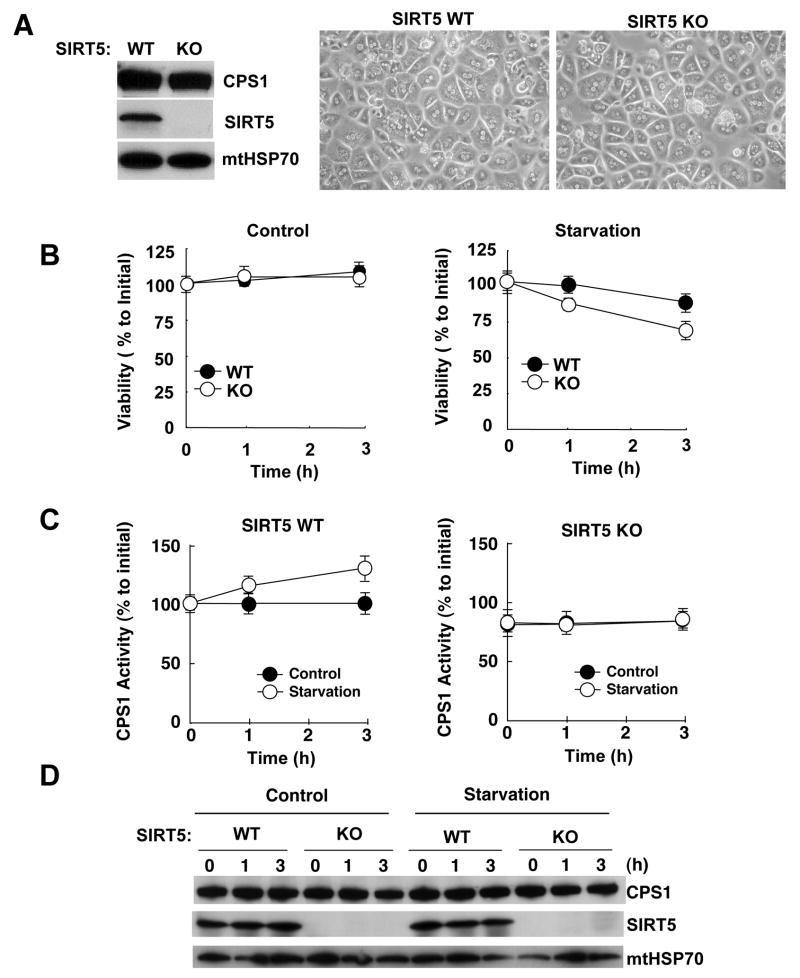
To determine whether SIRT5 regulates CPS1 in vivo, we first cultured primary murine hepatocytes from wild type and SIRT5 KO liver, and determined their responses to a nutrient depleted medium (HBSS medium) to mimic starvation in animals. Although SIRT5 KO hepatocytes appeared normal morphologically when grown in serum (Fig. 5A), their viability was significantly more sensitive to nutrient depletion (Fig. 5B and S7). Moreover, while CPS1 activity in wild type hepatocytes was induced by nutrient depletion, activity in SIRT5 KO cells was lower prior to nutrient depletion and unresponsive to this treatment (Fig. 5C). Nutrient depletion did not increase CPS1 protein levels in wild type or KO cells (Fig. 5D). These data suggest that SIRT5 is necessary to up-regulate CPS1 activity in liver cells.
Figure 5. Starvation induces CPS1 activity in primary cultured hepatocytes.
(A) Primary cultured hepatocytes were isolated from wild type and SIRT5 KO mice using the retro-perfusion technique. Hepatocytes were cultured in collagen coated 6-well plates. After 20 hours, whole cell lysates of hepatocytes were prepared and immunoblotted with anti-CPS1, anti-mtHSP70 and anti-SIRT5 antibodies (left). Images of hepatocytes from wild type and SIRT5 KO mice were taken with a Phase-contrast microscope at 100X magnification (right).
(B) After 20 hours from the isolation of hepatocytes, the media were changed to DMEM (Control) or HBSS (Starvation) and cell viability was measured by Cell Titer Blue reagent at 0, 1 and 3 hours (h) thereafter. Viability is indicated as % compare to time zero. Error bars represent standard deviations from triplicate experiments.
(C) Mitochondria matrix containing extracts were prepared at time 0, 1 and 3 hours (h) and assayed for CPS1 activity. Activity is shown as % of wild type at time zero. Error bars represent standard deviations from triplicate experiments.
(D) Mitochondria matrix containing extracts were prepared at time 0, 1 and 3 hours (h) and blotted with anti-CPS1, anti-mtHSP70 and anti-SIRT5 antibodies.
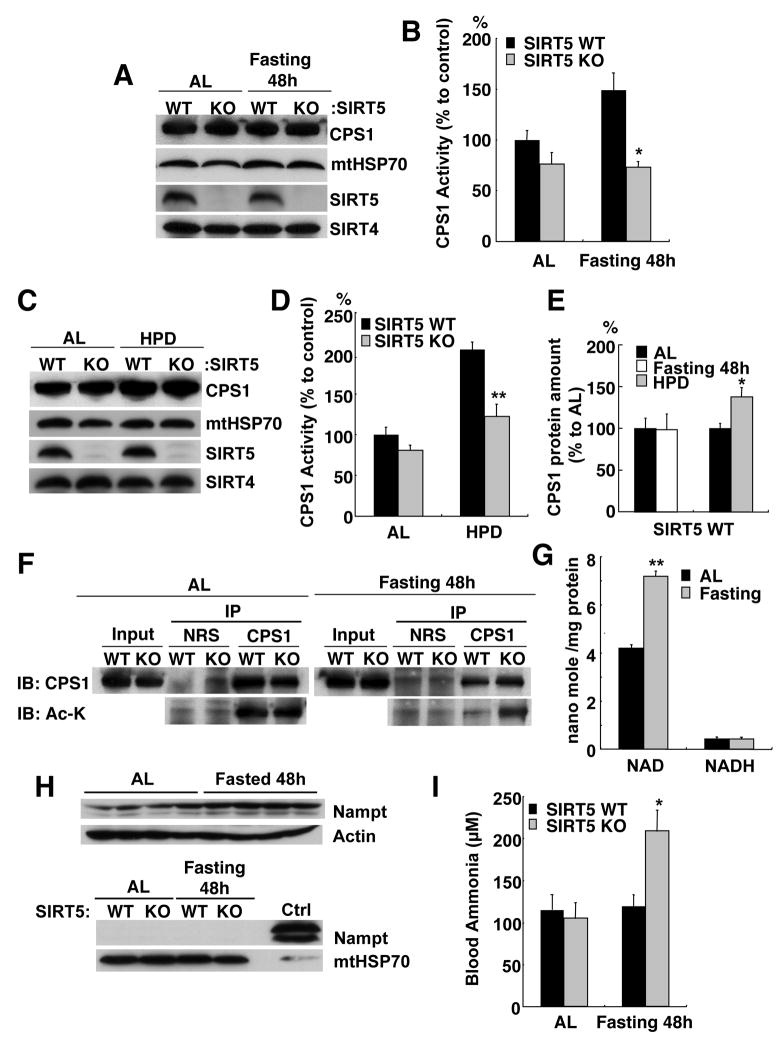
Next, we addressed the importance of SIRT5 in live animals. When mice are subjected to prolonged fasting, glycogen stores are rapidly depleted and fatty acids are subsequently catabolized (Exton et al., 1972). In addition, over more prolonged fasting, protein degradation in the muscle becomes an important source of carbon backbones for gluconeogenesis in the liver (Ruderman, 1975). Since this process generates the excess ammonia that requires detoxification by the urea cycle, we examined the effect of a 48-hour fast on liver mitochondrial CPS1 activity in wild type and SIRT5 KO mice. CPS1 activity was induced about 50% in the wild type liver, with no discernable change in total protein levels (Fig. 6A–B and S8). Induction of CPS1 activity in wild type liver was not apparent in a shorter, 24-hour fast (data not shown). In contrast, no induction of CPS1 activity by fasting was observed in SIRT5 KO mice. Levels of CPS1 protein were likewise unaffected by starvation in SIRT5 KO mice (Fig. 6A and B). Finally, CPS1 was also fully induced by fasting in SIRT4 KO and SIRT3 KO mice (Fig. S6).
Figure 6. Starvation and high protein diet up-regulate CPS1 activity in vivo.
(A) Liver mitochondria matrix lysates from wild type and SIRT5 KO mice fed ad libitum or after 48 hours fasting were analyzed by western blotting using antibodies to CPS1, mtHSP70, SIRT5 and SIRT4.
(B) CPS1 activity was measured in wild type and SIRT5 KO mice fed ad libitum or after 48 hours fasting. Error bars represent SEM. (n=8 for each groups). Starvation induces CPS1 activity in wild type but not SIRT5 KO mice.
(C) Liver mitochondria matrix lysates in wild type and SIRT5 KO mice fed normal chow or a high protein diet for 2 weeks were analyzed by western blotting using antibodies to CPS1, mtHSP70, SIRT5 and SIRT4. CPS1 protein levels were induced by the high protein diet in both wild type and SIRT5 KO mice.
(D) CPS1 activity was measured in wild type and SIRT5 KO mice fed normal chow diet or high protein diet for 2 weeks. Error bars represent SEM. (n=4 for each groups). High protein diet induces CPS1 in wild type and to a lesser degree in SIRT5 KO mice.
(E) CPS1 protein levels in mitochondria matrix in wild type mice fed ad libitum or fasted for 48 hours or fed High protein diet for 2 weeks. The intensity of CPS1 band relative to mtHSP70 was analyzed by NIH ImageJ. Data were shown as % to AL control. Error bars represent SEM. (n=4 for each groups).
(F) Liver mitochondria matrix lysates from wild type and SIRT5 KO mice fed ad libitum or after 48 hours fasting were immuno-precipitated with anti-CPS1 antibody or normal rabbit serum (NRS). Immuno-precipitates were analyzed by western blotting with anti-CPS1 and anti-pan acetylated lysine (Ac-K) antibodies. Fasting triggered deacetylation of CPS1 in wild type but not SIRT5 KO mice.
(G) NAD and NADH concentrations in liver mitochondria from wild type mice fed ad libitum or after 48 hr fasting were measured by the enzyme cycling method. The increase in NAD but not NADH shown is the average of four independent experiments. Error bars represent standard deviations
(H) Mice were fed ad libitum (AL) or starved 48-hours, and whole cell (upper) and mitochondrial (lower) extracts were blotted with antibodies to Nampt, actin and mtHSP70. Nampt was induced 2–3 fold by starvation in whole cell extracts (left), and was not associated with purified mitochondria (right).
(I) Blood ammonia was measured in wild type and SIRT5 KO mice fed ad libitum or after 48 hours fasting. Error bars represent SEM. (n=8 for each groups). Starvation caused hyper-ammonemia in SIRT5 but not wild type mice.
A high protein diet (HPD) is another condition that promotes the use of amino acids as energy sources (Schimke, 1962a). Wild type and SIRT5 KO mice were put on a HPD for 2 weeks and the activity of CPS1 was measured. Two-fold induction of CPS1 activity was observed in the wild type livers (Fig. 6C and D). In this case, an induction of CPS protein levels was also observed (Fig. 6E), indicating that at least some of the activation occurred at the level of CPS1 expression. Consistent with this surmise, a small induction of CPS1 activity was also observed in the SIRT5 KO mice, as was an induction in CPS1 protein levels similar to the wild type (Fig. 6C).
These findings suggested that starvation triggered CPS1 activation by SIRT5-mediated deacetylation of the urea cycle enzyme. To investigate whether CPS1 was indeed deacetylated upon fasting, CPS1 was immuno-precipitated from liver mitochondria of fed and fasted wild type and SIRT5 KO mice, and its acetylation status determined using anti-acetyl lysine antibodies. We observed that starvation caused deacetylation of CPS1 in wild type liver (Fig. 6F and S9). In striking contrast, no deacetylation was observed in starved livers of SIRT5 KO mice. We also found that CPS1 was deacetylated in SIRT5 WT mice fed the HPD, but not in SIRT5 KO mice fed the HPD (Fig S10).
We next studied what activates SIRT5 in the starved liver. The above experiment indicates that SIRT5 protein levels do not change upon starvation (Fig. 6A and Fig. S8). Yang et al (2007) showed that a 48-hour fast induces NAD levels in liver mitochondria by triggering Nampt transport to this organelle. Using a different method, we found an almost two-fold increase in mitochondrial NAD in liver mitochondria after fasting, with no change in NADH (Fig. 6G). In vitro, SIRT5 showed a steep activation by NAD in the 50–100 μM range (Fig. S11), consistent with the notion that the increase in mitochondrial NAD after starvation up-regulates SIRT5 in vivo.
Since NAD itself does not cross the mitochondrial membrane but the NAD precursor NMN does (Barile et al., 1996), we tested whether the NMN synthetic enzyme Nampt was up-regulated by 48-hour fasting. As was reported in rat liver (Yang et al., 2008), fasting induced the levels of this enzyme in whole cell extracts of mouse liver 2–3 fold (FIG. 6H, upper). However, unlike in rats, Nampt was not associated with the mitochondrial fraction at all (Fig. 6H, lower). Since the enzyme that converts NMN to NAD is found in mitochondria (Berger et al., 2005; Zhang et al., 2003), our findings suggest that the increase in Nampt in the starved liver cytosol increases the NMN pool for new NAD synthesis in mitochondria.
Functional importance of SIRT5 in ammonia detoxification in animals
Finally, we sought evidence that SIRT5 is physiologically important in live animals. Since defects in CPS1 are known to trigger hyper-ammonemia in humans (Yefimenko et al., 2005), we predicted that the deficiency in SIRT5 KO mice after 48-hour fasting might result in hyper-ammonemia. Thus, we subjected wild type and SIRT5 KO mice to 48 hours fasting and then determined their blood ammonia levels. In fed control mice, SIRT5 KO and wild type showed comparable blood ammonia levels. On the contrary, after 48 hours fasting, SIRT5 mice showed significantly elevated blood ammonia levels compared to wild type (Fig. 6I). We conclude that SIRT5 is critical to the proper disposal of ammonia during fasting.
Calorie restriction up-regulates CPS1 activity through deacetylation
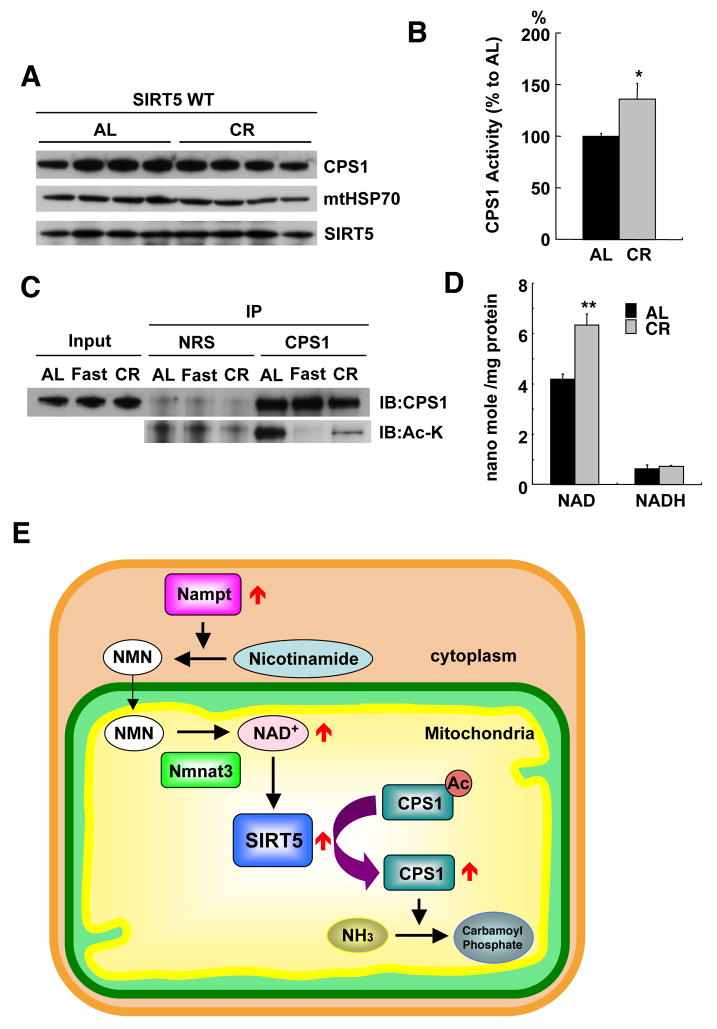
During long-term calorie restriction, as during fasting, the energy supply from carbohydrates is not sufficient, and amino acids are catabolized in liver and muscle (Hagopian et al., 2003a, b). As expected, CPS1 activity has been reported to increase during calorie restriction (Dhahbi et al., 2001). We thus assessed whether the mechanism of CPS1 activation by deacetylation occurs during this regimen by examining CPS1 in liver mitochondria of mice that were 40% calorie restricted for six months. Although CPS1 (and SIRT5) protein levels were not changed by calorie restriction (Fig. 7A), CPS1 activity was significantly increased in restricted animals compared to ad libitum fed controls (Fig. 7B). Moreover, CPS1 was highly deacetylated in calorie restricted mice, similar to what was observed after fasting (Fig. 7C and S12). Finally, mitochondrial NAD levels were significantly elevated in calorie restricted mice (Fig. 7D). These findings all suggest that SIRT5 also facilitates ammonia disposal during calorie restriction by deacetylating CPS1.
Figure 7. Calorie restriction up-regulates CPS1 activity through deacetylation.
(A) Liver mitochondria matrix lysates from wild type mice fed ad libitum (AL) or 40% calorie restricted (CR) for 6 months were analyzed by western blotting using antibodies to CPS1, mtHSP70 and SIRT5.
(B) CPS1 activity was measured in wild type mice fed ad libitum (AL) or 40% calorie restricted (CR) for 6 months. Error bars represent SEM. (n=4 for each groups).
(C) Liver mitochondria matrix lysates from wild type mice fed ad libitum (AL), after 48 hours fasting (Fast) or 40% calorie restricted (CR) for 6 months were immuno-precipitated with anti-CPS1 antibody or normal rabbit serum (NRS). Immuno-precipitates were analyzed by western blotting with anti-CPS1 and anti pan-acetylated lysine (Ac-K) antibodies.
(D) NAD and NADH concentrations in liver mitochondria from wild type mice fed ad libitum or calorie restricted were measured by the enzyme cycling method. The increase in NAD but not NADH shown is the average of four independent experiments. Error bars represent standard deviations
(E) A model for the role of SIRT5 on CPS1 regulation in liver. In response to fasting, Nampt is induced in the cytosol to increase NMN levels, thereby triggering de novo NAD synthesis and SIRT5 activation in mitochondria. Activated SIRT5 deacetylates CPS1 and up-regulates the detoxification of ammonia by the urea cycle. A similar induction likely occurs during calorie restriction.
DISCUSSION
In lower organisms, sirtuins function to extend life span. Because of their unique NAD requirement for activity, it was suggested that sirtuins respond to food limitation to program organisms for survival during dietary stress. Indeed, SIRT1 deacetylates regulators of numerous key metabolic pathways and may thus play an important role in the adaptation to calorie restriction. Here we study a novel sirtuin SIRT5, for which no functional information has been available. We show that SIRT5 is found in the mitochondrial matrix and deacetylates a specific mitochondrial enzyme, CPS1. This enzyme mediates the first and regulated step of the urea cycle, thereby linking SIRT5 to the major pathway of ammonia detoxification and disposal in organisms. Indeed, fasted SIRT5 KO mice become hyper-ammonemic. Since the need for ammonia disposal becomes acute during fasting, our findings provide a novel example of the importance of SIRT5 in adapting to food limitation.
Regulation of ammonia disposal by SIRT5
By linking SIRT5 to beads, we identified interacting proteins from mitochondrial extracts of murine livers. The most prominent of these was carbamoyl phosphate synthetase 1 (CPS1). CPS1 catalyzes the condensation of ammonia with HCO3− and ATP by generating carbamoyl phosphate, which is ultimately converted to urea, a molecule readily disposed from the body (Haussinger, 1990; Meijer et al., 1990). The urea cycle comes into play especially during fasting, when amino acids are catabolized for energy in the muscle to generate an excess of ammonia, which must be detoxified (Schimke, 1962b).
The interaction between SIRT5 and CPS1 is physiologically significant by several criteria. First, the proteins interact at endogenous levels in cells. Second, SIRT5 deacetylates and activates CPS1 efficiently in vitro, while not deacetylating the canonical p53-related SIRT1 substrate. Third, SIRT5 deacetylates CPS1 in vivo; prolonged starvation triggers deacetylation of CPS1 in wild type but not SIRT5 KO mice. Fourth, SIRT5 activates and regulates CPS1 enzymatic activity in vivo and in vitro; the activity of CPS1 in ad libitum fed animals was reduced in SIRT5 KO livers, and the normal up-regulation of activity by starvation did not occur in SIRT5 KO mice. Finally, the deacetylation and activation of CPS1 was specific to SIRT5 and not other mitochondrial sirtuins. Indeed, the other mitochondrial deacetylase SIRT3 did not affect the acetylation or activity of CPS1 in vivo or in vitro.
SIRT5 appears to regulate the urea cycle in a physiologically meaningful way, since the defect in CPS1 up-regulation during starvation of SIRT5 KO mice triggers hyper-ammonemia in blood. This defect in ammonia disposal is likely specific to CPS1, since the second enzymatic step of the urea cycle, matrix OTC, is not affected by SIRT5 and the remainder of the pathway lies in the cytoplasm.
Another condition in which the urea cycle becomes very important is a high protein diet (HPD) (Schimke, 1962a). Indeed, we observed deacetylation of CPS1 by SIRT5 in HPD mice, as well as a second level of regulation -- an increase in CPS1 protein levels. The up-regulation of CPS1 expression observed in the high protein condition may not occur during starvation, because energy for new synthesis is limited during starvation and activation by a post-translational mechanism may be most parsimonious. It will be interesting to see if high protein diets in humans, like the Atkins diet, trigger SIRT5-mediated activation of CPS1, increases in CPS1 protein levels, or both.
Mechanism of SIRT5 activation by starvation
SIRT5 protein levels do not change during starvation. However, we observed a doubling of NAD levels in liver mitochondria, with no change in NADH levels. Since we observe a steep activation of SIRT5 by NAD in vitro, we propose that this increase in NAD activates SIRT5 in the starved liver. Since NAD does not cross the mitochondrial membrane, the dramatic increase in mitochondrial NAD during starvation must be due to new synthesis (Yang et al., 2007). Indeed, we observed induction of Nampt, the enzyme that synthesizes the NAD precursor NMN, in the starved liver cytosol. Since NMN can be imported into mitochondria and then converted into NAD (Barile et al., 1996), we propose that starvation induces the urea cycle by increasing synthesis of NMN, which leads to de novo synthesis of NAD in mitochondria, SIRT5 activation, and CPS1 deacetylation (Fig. 7E).
Finally, long-term calorie restriction also gave rise to a significant increase in mitochondrial NAD levels without affecting NADH. Since this regimen also induced the deacetylation and activation of CPS1, we conclude that calorie restriction also triggers SIRT5 to up-regulate the urea cycle for ammonia disposal.
SIRT5 and SIRT4 in mitochondrial metabolism
SIRT4 represses another enzyme used during catabolism of amino acids, GDH, in this case by ADP-ribosylating it (Haigis et al., 2006). This repression is alleviated during calorie restriction, during which there is a significant lowering in SIRT4 protein levels in liver. We show here that unlike CPS1, GDH activity is not elevated during prolonged fasting (Fig. S6, S8 and S13). These results indicate that the level of GDH activity is not limiting in the adaptation to fasting. Since GDH is repressed both in fed and fasted mice, SIRT4, unlike SIRT5, must already be active in fed animals. We surmise that the lower level of NAD in fed mitochondria is sufficient to activate SIRT4, but not SIRT5. It will be of interest to determine whether, indeed, SIRT4 has a lower Km for NAD than does SIRT5.
In addition, the activation of CPS1 during fasting occurred normally in mice missing either SIRT4 or the other mitochondrial sirtuin, SIRT3. Thus SIRT5 is the only sirtuin regulating the urea cycle, and that the activities of SIRT4 on GDH and SIRT5 on CPS1 appear to be surprisingly uncoupled.
Substrate specificity of SIRT5 versus SIRT1
Previous studies on the enzymatic activity of sirtuins revealed, at best, a feeble deacetylase activity for SIRT5. The robust activity we observe in the case of CPS1 indicates a high level of substrate specificity of this deacetylase. In fact, SIRT5 was completely inactive on the SIRT1 p53-related substrate, while SIRT1 was inactive on CPS1. Since SIRT5 contains only very short amino and carboxyl terminal sequences flanking the conserved, catalytic sirtuin core, it is likely that residues within the conserved domain can dictate sirtuin substrate specificity. It would be of interest to create SIRT1-SIRT5 chimeric enzymes to pinpoint the basis of sirtuin substrate specificity.
Conclusion
Our findings indicate another important link between sirtuins and the adaptation to food scarcity. At present, CPS1 is the only bona fide SIRT5 substrate, although other substrates may emerge. Since SIRT5 appears keyed to the conditional use of amino acids as energy sources, it is possible that other methods may reveal additional SIRT5 mitochondrial substrates involved in energy production, for example enzymes of fatty acid oxidation. Our findings strengthen the ideas that sirtuins promote metabolic adaptations during dietary changes, and that small molecule sirtuin modulators will be an important approach to treat metabolic disorders.
EXPERIMENTAL PROCEDURES
Animal experiments
SIRT3 KO mice, SIRT4 KO mice and SIRT5 KO mice were described previously (Haigis et al., 2006; Lombard et al., 2007) and male 12–16 weeks littermates in 129Sv background were used for this study. Mice were housed under controlled temperature (25 °C) and dark-light cycle (12h:12h). Ad libitum mice were fed normal chow diet containing 19% protein. High protein diet contains 60% protein and the same amount of energy as control diet (Test Diet). Calorie restricted wild type mice were purchased from NIA. They are fed 40% reduced calories compared to control mice for 6 month. For fasting experiments, food was depleted for 48 hours, but mice could access water freely. After 48 h fasting, mice were euthanized by CO2 and tissues were harvested. For blood glucose assays, see Supplemental Data.
Western blotting and immunoprecipitation
Antibodies used for immunoblotting included anti-mtHSP70, CPS1, OTC (Abcam), GDH (US biological), Actin (Chemicon), Tom20 (Santa Cruz), Cytochrome c (Pharmingen), COX Va (Invitrogen), Nampt (Bethyl Laboratories), pan-acetylated lysine (Immunechem) and anti Flag M2 (Sigma-Aldrich). SIRT5 antisera were raised in rabbit against the C-Terminal peptide (GPCGKTLPEALAPHETERT). SIRT4 antisera was described previously (Haigis et al., 2006). HRP-conjugated secondary antibodies were obtained from Amersham Pharmacia. PVDF membrane (Millipore) was used for blotting and signals were revealed by ECL (Amersham Pharmacia)
For immunoprecipitation, liver mitochondria matrix supplemented with 150mM NaCl and 10mM Tris-HCl pH7.4 were incubated with anti-SIRT5, CPS1 antibody or normal rabbit serum overnight at 4 ° C. Then protein A sepharose (Amersham Pharmacia) were added and incubated for 1 h. After resins were washed, samples were boiled with 3X sample buffer and subjected to western blotting.
Mitochondria isolation and sub-fractionation
Mitochondria were isolated from the livers of SIRT5 KO mice, SIRT4 KO mice, SIRT3 KO mice and their control littermates, as described previously (Shimizu et al., 1998). Briefly, livers were homogenized with a glass-Teflon Potter homogenizer in medium containing 0.3 mM mannitol, 10 mM potassium HEPES (pH 7.4) and 0.2 mM EGTA. The mitochondria were washed twice and suspended in the same medium without EGTA. Mitochondria protein concentration was measured using protein DC assay kit (Bio Rad).
Mitochondrial sub-fractionation was performed as described elsewhere with some modifications (Cipolat et al., 2006). Mitochondria were incubated in hypotonic buffer (2mM KCl, 10 mM HEPES) at 2mg/ml for 20 min on ice. After centrifuging, supernatant was saved and pellets were washed with wash buffer (150mM KCl and 10mM HEPES) twice. All the supernatants were combined and subject to TCA precipitation and saved as inter membrane space fraction. Pellets were suspended in hypotonic buffer again and subjected to freeze and thaw cycle three times. Suspension were sonicated for 5 seconds three times on ice and centrifuged. Pellets were washed three times and saved as membrane fractions, and supernatants as matrix fractions.
Primary cultured hepatocytes isolation
Primary cultured hepatocytes were isolated from SIRT5 KO mice and their control littermates at 3–4 months of age using the retrograde two-step collagenase perfusion technique (Nakagawa et al., 2005). Details are described in Supplemental Data.
In vitro Flag affinity purification
293T cells were trasfected with pCAG-SIRT5 or pCAG-SIRT5-Flag plasmids. After 24 hours, cells were harvested and lysed in CHAPS buffer (2% CHAPS, 50mM Tris-HCl pH 7.4, 150mM NaCl and 1mM EGTA). The lysates were sonicated and centrifuged. The supernatants were mixed with Flag-M2 resin and washed with TBS buffer with NP40 (150 mM NaCl 10mM Tris-HCl pH 8.0 and 0.1% NP-40). Mitochondria matrix lysates from wild-type mice or buffer control were loaded onto columns, washed and eluted by 50 μg/ml Flag peptide. The eluted samples were concentrated using 10kDa-filtered microcon tube (Millipore) and subjected to SDS-PAGE and silver staining or Coomasie staining. The 150 kDa band was excised and analyzed by mass spectrometry for protein identification at Taplin Biological Mass Spectrometry Facility (Harvard Medical School, Boston).
Enzymatic activity assays
CPS1 activity and OTC activity were assessed by measuring converted citrulline by a colorimetric method (Nuzum and Snodgrass, 1976). GDH activity was determined as described (Haigis et al., 2006). To measure blood ammonia, blood was collected by eye-bleeding. Whole blood was immediately centrifuged at 4 °C and supernatant was saved as plasma at −80°C. Plasma ammonia concentration was measured at the Children’s Hospital Boston core laboratories (Boston, MA). For more details, see Supplemental Data.
In vitro deacetylation assays
Flag tagged SIRT5 protein and catalytic mutant SIRT5 (Figure S5) were purified by Flag M2 resin and eluted with Flag peptides in TBS buffer. To deplete endogenous NAD completely, liver mitochondria matrix lysate from SIRT5 KO mice were dialyzed against dialysis buffer (20mM Tris-HCl pH8.0, 0.2mM EDTA, 10% Glycerol and 50mM NaCl) using Slide-A-Lyzer 10K Dialysis Cassette (Pierce). For SIRT5 in vitro deacetylation assay, 20μg liver mitochondria matrix lysate from SIRT5 KO mice and 4 μg SIRT5-Flag were incubated in HDAC buffer (50mM Tris-HCl pH8.0, 4mM MgCl2 and 0.2mM DTT) in the presence or absence of 0.5mM NAD for 60 min at 37 °C. After the reaction, samples were subject to CPS1 activity assay. SIRT1 and SIRT3 in vitro deacetylation assay were performed using the p53 peptide Fluor de Lys substrate Assay kit (BIOMOL) according to supplier’s manual.
NAD/NADH measurement
NAD and NADH were measured by enzyme cycling method as described previously with a few modifications (Easlon et al., 2008). 5mg of whole mitochondria pellet were suspended in hypotonic buffer and subjected to sonication. 2X acid buffer was added for NAD extraction and 2X alkali buffer was added for NADH extraction. After 30 min incubation at 60°C, neutralization buffer were added to each samples to destroy any endogenous enzymatic activities that might interfere with the assay. After centrifuging, supernatants were saved and part of them were used for enzymatic cycling reaction. After the reaction, the concentration of nucleotides was measured fluorometrically with excitation at 365 nm and emission monitored at 460 nm.
Statistical Analysis
Analysis was performed using an unpaired Student’s t test, and significant differences are indicated by single asterisk (*) when p < 0.05 and double asterisk (**) when p<0.01.
Supplementary Material
Acknowledgments
We are grateful to Eric Bell, Huan-chung Chang, Sergiy Libert and Gizem Donmez for reading the manuscript and Shin-ichiro Imai for valuable comments. We also thank Fred Alt, David Lombard and Raul Mostovslavsky for sharing the SIRT5 KO mice, Danica Chen for SIRT3 KO mice, Jun-ichi Miyazaki for the gift of pCAG plasmid and Su-Ju Lin for technical comments on NAD/NADH measurement. This work was supported by a grant from Human Frontier Science Program to T.N. and grants from the NIH and the Paul F. Glenn Foundation to L.G. L.G. is a consultant for Sirtris Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja N, Schwer B, Carobbio S, Waltregny D, North BJ, Castronovo V, Maechler P, Verdin E. Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. J Biol Chem. 2007;282:33583–33592. doi: 10.1074/jbc.M705488200. [DOI] [PubMed] [Google Scholar]
- Barile M, Passarella S, Danese G, Quagliariello E. Rat liver mitochondria can synthesize nicotinamide adenine dinucleotide from nicotinamide mononucleotide and ATP via a putative matrix nicotinamide mononucleotide adenylyltransferase. Biochem Mol Biol Int. 1996;38:297–306. [PubMed] [Google Scholar]
- Berger F, Lau C, Dahlmann M, Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J Biol Chem. 2005;280:36334–36341. doi: 10.1074/jbc.M508660200. [DOI] [PubMed] [Google Scholar]
- Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D’Adamio L, et al. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126:163–175. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Cooper HM, Spelbrink JN. The human SIRT3 protein deacetylase is exclusively mitochondrial. Biochem J. 2008;411:279–285. doi: 10.1042/BJ20071624. [DOI] [PubMed] [Google Scholar]
- Dhahbi JM, Mote PL, Wingo J, Rowley BC, Cao SX, Walford RL, Spindler SR. Caloric restriction alters the feeding response of key metabolic enzyme genes. Mech Ageing Dev. 2001;122:1033–1048. doi: 10.1016/s0047-6374(01)00230-5. [DOI] [PubMed] [Google Scholar]
- Easlon E, Tsang F, Skinner C, Wang C, Lin SJ. The malate-aspartate NADH shuttle components are novel metabolic longevity regulators required for calorie restriction-mediated life span extension in yeast. Genes Dev. 2008;22:931–944. doi: 10.1101/gad.1648308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton JH, Corbin JG, Harper SC. Control of gluconeogenesis in liver. V. Effects of fasting, diabetes, and glucagon on lactate and endogenous metabolism in the perfused rat liver. J Biol Chem. 1972;247:4996–5003. [PubMed] [Google Scholar]
- Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 2000;273:793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444:868–874. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- Guarente L. Mitochondria--a nexus for aging, calorie restriction, and sirtuins? Cell. 2008;132:171–176. doi: 10.1016/j.cell.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagopian K, Ramsey JJ, Weindruch R. Caloric restriction increases gluconeogenic and transaminase enzyme activities in mouse liver. Exp Gerontol. 2003a;38:267–278. doi: 10.1016/s0531-5565(02)00202-4. [DOI] [PubMed] [Google Scholar]
- Hagopian K, Ramsey JJ, Weindruch R. Influence of age and caloric restriction on liver glycolytic enzyme activities and metabolite concentrations in mice. Exp Gerontol. 2003b;38:253–266. doi: 10.1016/s0531-5565(02)00203-6. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Hallows WC, Lee S, Denu JM. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc Natl Acad Sci U S A. 2006;103:10230–10235. doi: 10.1073/pnas.0604392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussinger D. Nitrogen metabolism in liver: structural and functional organization and physiological relevance. Biochem J. 1990;267:281–290. doi: 10.1042/bj2670281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick JP, Hodges PE, Rosenberg LE. Survey of amino-terminal proteolytic cleavage sites in mitochondrial precursor proteins: leader peptides cleaved by two matrix proteases share a three-amino acid motif. Proc Natl Acad Sci U S A. 1989;86:4056–4060. doi: 10.1073/pnas.86.11.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- McGivan JD, Bradford NM, Mendes-Mourao J. The regulation of carbamoyl phosphate synthase activity in rat liver mitochondria. Biochem J. 1976;154:415–421. doi: 10.1042/bj1540415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer AJ, Lamers WH, Chamuleau RA. Nitrogen metabolism and ornithine cycle function. Physiol Rev. 1990;70:701–748. doi: 10.1152/physrev.1990.70.3.701. [DOI] [PubMed] [Google Scholar]
- Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16:4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SM., Jr Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr. 2002;22:87–105. doi: 10.1146/annurev.nutr.22.110801.140547. [DOI] [PubMed] [Google Scholar]
- Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Ogura M, Tanaka D, Inagaki N. Localization of mouse mitochondrial SIRT proteins: shift of SIRT3 to nucleus by co-expression with SIRT5. Biochem Biophys Res Commun. 2008;366:174–179. doi: 10.1016/j.bbrc.2007.11.122. [DOI] [PubMed] [Google Scholar]
- Nuzum C, Snodgrass P. Multiple Assays of the Five Urea-cycle Enzymes in Human Liver Homogenates. In: Grisolia S, Baguena R, Mayor F, editors. The Urea Cycle. New York: Wiley; 1976. pp. 325–349. [Google Scholar]
- Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Natl Acad Sci U S A. 2002;99:13653–13658. doi: 10.1073/pnas.222538099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman NB. Muscle amino acid metabolism and gluconeogenesis. Annu Rev Med. 1975;26:245–258. doi: 10.1146/annurev.me.26.020175.001333. [DOI] [PubMed] [Google Scholar]
- Schimke RT. Adaptive characteristics of urea cycle enzymes in the rat. J Biol Chem. 1962a;237:459–468. [PubMed] [Google Scholar]
- Schimke RT. Differential effects of fasting and protein-free diets on levels of urea cycle enzymes in rat liver. J Biol Chem. 1962b;237:1921–1924. [PubMed] [Google Scholar]
- Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CF, Steegborn C. Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J Mol Biol. 2008;382:790–801. doi: 10.1016/j.jmb.2008.07.048. [DOI] [PubMed] [Google Scholar]
- Schwer B, Bunkenborg J, Verdin RO, Andersen JS, Verdin E. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc Natl Acad Sci U S A. 2006;103:10224–10229. doi: 10.1073/pnas.0603968103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwer B, North BJ, Frye RA, Ott M, Verdin E. The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J Cell Biol. 2002;158:647–657. doi: 10.1083/jcb.200205057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener A, Malaisse WJ. L-leucine and a nonmetabolized analogue activate pancreatic islet glutamate dehydrogenase. Nature. 1980;288:187–189. doi: 10.1038/288187a0. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Eguchi Y, Kamiike W, Funahashi Y, Mignon A, Lacronique V, Matsuda H, Tsujimoto Y. Bcl-2 prevents apoptotic mitochondrial dysfunction by regulating proton flux. Proc Natl Acad Sci U S A. 1998;95:1455–1459. doi: 10.1073/pnas.95.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley CA. Hyperinsulinism/hyperammonemia syndrome: insights into the regulatory role of glutamate dehydrogenase in ammonia metabolism. Mol Genet Metab . 2004;81(Suppl 1):S45–51. doi: 10.1016/j.ymgme.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- von Heijne G, Steppuhn J, Herrmann RG. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989;180:535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Oh SW, Deplancke B, Luo J, Walhout AJ, Tissenbaum HA. C. elegans 14-3-3 proteins regulate life span and interact with SIR-2.1 and DAF-16/FOXO. Mech Ageing Dev. 2006;127:741–747. doi: 10.1016/j.mad.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Wraith JE. Ornithine carbamoyltransferase deficiency. Arch Dis Child. 2001;84:84–88. doi: 10.1136/adc.84.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yefimenko I, Fresquet V, Marco-Marin C, Rubio V, Cervera J. Understanding carbamoyl phosphate synthetase deficiency: impact of clinical mutations on enzyme functionality. J Mol Biol. 2005;349:127–141. doi: 10.1016/j.jmb.2005.03.078. [DOI] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Kurnasov OV, Karthikeyan S, Grishin NV, Osterman AL, Zhang H. Structural characterization of a human cytosolic NMN/NaMN adenylyltransferase and implication in human NAD biosynthesis. J Biol Chem. 2003;278:13503–13511. doi: 10.1074/jbc.M300073200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.