Abstract
The objectives of this study were: (i) to identify regions of the aged mouse brain in which advanced glycation end-products (AGEs) were increased, and (ii) assess the functional significance of AGEs by assessing the extent to which they could predict age-related brain dysfunction. Densitometric analyses of immunoblots for N epsilon-(carboxymethyl)lysine (CML), a predominant AGE, and receptor for AGE (RAGE), were performed in different brain regions of mice aged 8 or 25 months. The 25-month-old mice were tested for ability to perform on tests of cognitive and psychomotor function prior to assessment of CML or RAGE, to determine if immunostaining results could predict functional impairment among the older mice. The amounts of CML increased with age in cortex, hippocampus, striatum and midbrain, but were unchanged in the brainstem and cerebellum. Increases in RAGE were evident in all brain regions but the hippocampus, and were not linked to increased amounts of CML. Different statistical approaches each failed to reveal any strong association between the degree of age-related functional impairment among individual mice and amounts of CML or RAGE in any particular region of the brain. The findings from this study suggest that accrual of CML and expression of RAGE in different brain regions are time-related phenomena that do not account for individual differences in brain aging or cognitive decline.
Keywords: Advanced glycation end products, N epsilon-(carboxymethyl) lysine, Receptor for advanced glycation end products, Central nervous system, Aging, Motor function, Behavior
1. Introduction
Advanced glycation end products (AGEs) describe a group of glycated residues that are generated via nonenzymatic glycation and oxidation of proteins, a mechanism known as the Maillard reaction. The glycation of proteins may alter their biochemical properties and physical attributes leading to alterations in degradation rate (Verzijl et al., 2000) and in various functions (Kikuchi et al., 2003; Yan and Harding, 1997). Furthermore, glycated proteins are themselves a source of reactive oxygen species (ROS) (Lee et al., 1998; Yim et al., 2001) and they can accelerate oxidative damage (Qian et al., 1998; Sobal et al., 2000). Based on their accumulation in tissues and potential to produce or accelerate deleterious effects, AGEs have been implicated in the aging process and in the pathophysiology of atherosclerosis, diabetes, kidney disease, and arthritis (reviewed by Ramasamy et al., 2005; Sasaki et al., 1998).
N epsilon-(carboxymethyl) lysine (CML) is a predominant AGE found in vivo (Dei et al., 2002; Reddy et al., 1995). An increase in CML content as a function of age is commonly associated with long-lived proteins of the lens (Ahmed et al., 1997; Dunn et al., 1989), and skin collagen (Dunn et al., 1991; Dyer et al., 1993). However, age-related increases in CML also occur in regions of the brain such as the cerebral cortex (Pamplona et al., 2005) and hippocampus (Dei et al., 2002; Kimura et al., 1996), areas that have been linked to age-associated cognitive decline. Moreover, an enhanced accumulation of CML is associated with Alzheimer’s disease neuropathology (Castellani et al., 2001). These findings suggest that AGEs might play a key role in the decline of cognitive functions associated with aging or neurodegenerative disease.
A “receptor” system is present in various tissues that may represent a primary defense against AGE accumulation (Schmidt et al., 2000; Vlassara, 2001). However, one AGE receptor, receptor for advanced glycation end products (RAGE), may instead contribute to perturbation of cellular functions via activation of signal transduction cascades leading to generation of ROS, inflammatory processes, and cell death (reviewed in Ramasamy et al., 2005). RAGE expression in brain tissue appears to increase with age (Tohgi et al., 1999), suggesting that AGE-RAGE interactions could contribute deleterious effects in the aging brain, in addition to those conferred by AGEs, per se.
In spite of their accumulation in brain and potential to promote neuronal dysfunction, the potential causal roles of AGEs or RAGE in brain aging and cognitive impairment have not been addressed. This hypothesis was tested in the current study by determining if immunostaining for CML or RAGE expression could predict individual differences in the degree of cognitive impairment present in a group of aged mice. A comprehensive array of behavioral tests was administered to the mice in this study, so that potential associations of glycation status with impaired sensory or psychomotor performance could also be detected, in addition to the possible association with cognitive impairment.
2. Materials and Methods
2.1. Animals
Male C57BL/6JNia mice aged 8 months (young, n=11) or 25 months (old, n =24) were obtained from the National Institute on Aging and housed in groups of 3 or 4 in clear polycarbonate cages (12 × 17 × 12.5 cm) in the University of North Texas Health Science Center vivarium. The mice were maintained at 23 ± 1°C, under a 12-hour light/dark cycle, with lights on at 0700 h. Mice were given ad libitum access to food and water. Over a 5-week period, beginning after 2 weeks of acclimation, the old mice received a battery of behavioral tests (Table 1) as described recently by Sumien et al. (2006) with previously established sensitivity for detecting age-related impairments in several domains of cognitive and psychomotor function in C57BL/6 mice (de Fiebre et al., 2006; Forster et al., 1996; Sumien et al., 2004). A week after the end of behavioral testing, the mice were euthanized by cervical dislocation and brain tissue samples were quickly dissected and frozen for later analysis. All procedures involving the mice were approved by the Institutional Animal Care and Use Committee of the University of North Texas Health Science Center at Fort Worth.
Table 1.
Descriptive statistics on behavioral measures for 25-month-old C57BL/6 mice
| Behavior Measure | Group Mean | Range | Standard Deviation |
|---|---|---|---|
| Psychomotor/Reflex | |||
| Wire tread (s) | 23.80 | 56.68 | 17.94 |
| Wire fall (s) | 25.07 | 39.24 | 10.21 |
| Bridge fall (s) | 30.50 | 39.60 | 9.66 |
| Rotorod fall (s) | 371.17 | 468.32 | 111.61 |
| Swim speed (s) | 14.26 | 7.30 | 2.07 |
| Auditory startle1 (force units) | 47.66 | 138.58 | 31.91 |
| Shock startle2 (force units) | 537.70 | 778.80 | 223.75 |
| Reaction time (s) | 65.77 | 64.80 | 16.65 |
| Learning/Memory | |||
| Swim Maze | |||
| Total learning index (cm) | 471.20 | 416.90 | 107.90 |
| Acquisition learning index (cm) | 483.34 | 498.40 | 119.34 |
| Reversal learning index (cm) | 459.09 | 476.30 | 145.73 |
| Maximum spatial performance (cm) | 336.36 | 578.70 | 126.95 |
| Target quadrant (% time spent) | 33.42 | 63.20 | 13.23 |
| Annulus 40 (% time spent) | 23.54 | 43.50 | 8.82 |
| Annulus 20 (% time spent) | 5.60 | 17.80 | 4.35 |
| Platform entries (counts) | 1.00 | 3.00 | 0.91 |
| Avoidance learning | |||
| Session 1 (number of trials) | 15.00 | 19.00 | 5.96 |
| Session 2 (number of trials) | 14.62 | 18.00 | 4.81 |
Response amplitude after 140-dB auditory stimulus
Response amplitude after 0.64-mA shock stimulus
2.2. Psychomotor/reflex assessment
2.2.1. Wire suspension
Mice were administered a wire suspension test for four consecutive days (2 trials/day). For each trial (lasting a maximum of 60 s), the mouse was allowed to grip a horizontal wire with the front paws when suspended 27 cm above a padded surface. There is a marked age-related decline in wire suspension performance when measured as the 4-session average latency to tread (a reflexive grasping of the wire with the hind legs) or the latency to fall (Sumien et al., 2004).
2.2.2. Bridge walking
Each mouse was tested for the latency to fall or reach a safe platform after being placed on one of four acrylic bridges, each mounted 50 cm above a padded surface. The bridges differed in diameter (small or large) and shape (round or square), providing four levels of difficulty. Each bridge was presented three times, and the measure of performance was the average latency to fall (up to a maximum of 60 s) across all bridges. The reliability and age sensitivity of this measure has recently been described for large reference groups of C57BL/6 mice tested under the conditions of this study (de Fiebre et al., 2006).
2.2.3. Coordinated running
Maximum running performance was measured using an accelerating rotorod test described previously (Forster and Lal, 1999). The apparatus was a motor-driven treadmill (Accuscan Instruments, Model # AIO411RRT525M) that consisted of a 3-cm diameter nylon cylinder mounted horizontally at a height of 35 cm above a well-padded surface. On each trial, the mouse was placed on the cylinder, which then began rotating with increasing speed until the animal fell to the padded surface. Ability of the mice to improve running performance was assessed in a series of training sessions (two per day), each consisting of four trials at 10-min intervals. The training sessions continued until the running performance (the average latency to fall from the cylinder) failed to show improvement over three consecutive sessions, which represented each mouse’s maximum stable level of performance. The average latency to fall on the final session, the dependent measure considered in the current study, is consistently shorter in aged when compared to young C57BL/6 mice (Sumien et al., 2004)
2.2.4. Sensory reactivity
The startle reflex to auditory or shock stimuli of various intensities was determined using a standard testing system (SA Lab, San Diego Instruments, San Diego CA). For the auditory startle test, each mouse was placed inside an acrylic cylinder and presented with a series of mixed-frequency noise bursts (0, 90, 100, 110, 120 or 140 dB). Each acoustic signal (lasting 20 ms) was presented 12 times in a counterbalanced series, for a total of 72 trials. For the shock startle test, a mouse was placed inside the same acrylic cylinder, and a series of shocks (0, 0.02, 0.04, 0.08, 0.12, 0.16, 0.24, 0.32, or 0.64 mA) was delivered. Each shock stimulus (100 ms in duration and scrambled across 8 inputs to the grid floor of the acrylic cylinder) was given five times, for a total of 45 trials. The amplitude of the startle reflex was defined as the peak response to each auditory or shock intensity within a 250-ms time window that began with the stimulus presentation. An age-related decline in the amplitude of the startle response to high intensity auditory stimuli occurs in C57BL/6 mice that is reflective of age-related hearing loss present in several mouse genotypes (Sumien et al., 2004; Sumien et al., 2006). A measure of longer “reaction time” used previously (Sumien et al., 2004; Sumien et al., 2006) was the latency to achieve the peak startle response following presentation of the 0.64 mA shock stimulus, an intensity that elicited a startle response of maximum amplitude in both young and old mice.
2.3. Learning/memory assessment
2.3.1. Spatial learning and memory
Spatial learning and memory were measured using a swim maze test as described previously (de Fiebre et al., 2006; Forster et al., 1996). On a given trial, the mouse was allowed to swim in a 120-cm diameter plastic tank filled to 34 cm from the top edge with colored water (non-toxic white paint) and maintained at 24 ± 1 °C. An escape was provided by means of a small 10 × 10-cm platform hidden from view 1.5 cm below the surface of the water. A computerized tracking system recorded the length of the path taken by the mouse to reach the platform, as well as the swimming speed (San Diego Instruments, San Diego CA, Model # SA-3).
During a pretraining phase, the tank was covered by a black curtain to prevent pre-exposure of the mice to visual cues present outside of the tank. In this way, mice learned the motor components of swimming and climbing onto the platform without learning its location in the tank. On each trial, the mouse was placed at one end of a 10 × 65-cm (W × L) straight alley that had a platform at the other end, and allowed to swim until it reached the platform or a maximum latency of 60 s had elapsed. The mice were given four sessions of pretraining (two per day), each consisting of five trials spaced at 5-min intervals.
After pretraining, the black curtain was removed from above the tank, and the mice were tested for their ability to learn the location of the platform using spatial cues. Testing was divided into three phases: acquisition (eight sessions with the platform in a fixed location), retention (two additional sessions after a 66-h delay interval), and reversal (four sessions with the platform at a new, fixed location). Each session consisted of five trials, at 10-min intervals, during which the mouse had to swim to the platform from one of four different starting points in the tank. Two sessions were conducted per day, separated by a period of at least 2 h, during which the mice were returned to the home cages. After the fifth trial of session 8, a probe trial was given in which the platform was submerged to a depth that prevented the mice from climbing onto it. The platform was raised after 30 s, and the trial was ended when the mouse successfully located it. On this trial, spatial bias for the platform location was evaluated in terms of the (i) percentage of time spent in the platform quadrant, (ii) percentage of time spent within 40- and 20-cm diameter annuli surrounding the platform location, and (iii) entries into the platform zone itself. A total learning index was defined as the average path length that each mouse took to reach the platform on sessions 2–4 during acquisition (acquisition learning index) and sessions 12–14 during reversal (reversal learning index). Maximum spatial learning was defined as the average path length over sessions 7 and 8, after spatial performance had reached a plateau.
2.3.2. Discriminated avoidance
A T-maze constructed of acrylic (black for the sides and clear for the top) was utilized for the discriminated avoidance task (Forster and Lal, 1992; McDonald et al., 2005). The maze was divided into three compartments: a start box (10 × 6.3 × 6 cm), a stem (17.5 × 6.3 × 6 cm), and two goal arms (14.5 × 6.3 × 6 cm), each separated by clear acrylic doors. The maze rested on a grid floor wired to deliver 0.27-mA scrambled shock to the feet.
The test consisted of two sessions separated by 1 h. On each training trial, the mouse was placed in the start box, and the start door was removed to signal the beginning of the trial. On the first trial of the first session, the mouse received shock in the first arm entered and was permitted to escape shock by running to the opposite arm, which was then designated the correct arm for the remainder of the session. On subsequent trials, shock was initiated 5 s after the opening of the start door if the mouse had not entered the correct goal arm, or immediately upon entry into the incorrect arm. In either case, the shock continued until the correct goal arm was entered or a maximum of 60 s had elapsed. Upon the mouse’s entry into the correct arm, the door was closed (to prevent departure) and, after 10 s, the mouse was removed (by detaching the goal arm) and allowed to enter a holding cage for 1 min. Training in this fashion continued at 1-min intervals until the mouse had met the criterion of a correct avoidance (defined as running directly to the correct arm within 5 s) on four of the last five training trials. The second session of avoidance training was a reversal such that the mice were required to run to the goal arm opposite that to which they had been trained on the previous session. Ability to learn the avoidance problem was considered inversely proportional to the number of trials required to reach criterion in each of the sessions. Previous investigations indicated consistent age-related impairments in performance of the reversal component of this test, suggesting the presence of cognitive inflexibility in older C57BL/6 mice (Forster and Lal, 1992; McDonald et al., 2005).
2.4. Detection and densitometric quantification of CML content and RAGE expression
An anti- CML antibody was provided by Mark Smith, Ph.D., Case Western University, OH. This antibody was raised in rabbit to CML-modified keyhole limpet hemocyanin as described by Castellani et al (2001). Goat anti-RAGE antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Each mouse brain was dissected into six regions: cerebral cortex, cerebellum, midbrain, brain stem, striatum, and hippocampus. Each brain region from the young and old mice was homogenized in antioxidant buffer (10 mM sodium phosphate, 0.9% sodium chloride, 100 µM DTPA and 1mM BHT) containing a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) and 0.1% Triton-X 100. After the assay for protein content, the homogenates were resolved by SDS-PAGE (10% w/v) followed by electrophoretic gel transfer to nitrocellulose membranes (Pierce, Rockford, IL) with a Mini-Trans-Blot electrophoretic transfer cell (Bio-Rad, Richmond, CA). Transference was carried out at 100 V (constant voltage) for 1 h at 4°C in a buffer containing 25 mM Tris, 192 mM glycine, 10% methanol (v/v), pH 8.3. The blots were incubated in 5% nonfat dried milk (w/v) at room temperature for 2 h, followed by 10 min washing, with Tris-buffered saline that contained 0.1% Tween-20 (TBST). Blots were then incubated overnight at 4°C with either anti-CML antibodies (1:5,000) or anti-RAGE antibodies (1:2,000) in TBST containing 0.2% BSA. The primary antibody was removed and the blots were washed three times with TBST. The blots were then incubated in horseradish peroxidase-conjugated goat anti-rabbit IgG (1:50,000) (Zymed, San Francisco, CA) or bovine anti-goat IgG (1:10,000) (Santa Cruz Biotechnology, Santa Cruz, CA) in TBST containing 0.2% BSA for at least 1 h at room temperature. After washing the blots with TBST five times (10 min each), CML modified proteins and RAGE were visualized with an enhanced chemiluminescence kit. The blots were stripped by incubating the blots with stripping buffer (100 mM 2-Mercaptoethanol, 2% SDS, 62.5 mM Tri-HCL pH 6.7) at 45°C for 50 min. After washing the blots with TBST twice (10 min each), the blots were blocked and probed in the same manner as stated above. Anti-alpha tubulin (1:10,000) (Abcam, Cambridge, MA) was used as primary antibody and horseradish peroxidase-conjugated goat anti-mouse IgG (1: 10,000) (Zymed, San Francisco, CA) was used as the secondary antibody.
All immunoblot images were scanned by an Epson Perfection 1670 scanner and densitometric quantifications were performed using Scion Image software (version 4.0.3). Total CML was quantified using the intensity of the whole lane, whereas RAGE protein expression was quantified using the intensity of doublet. Alpha-tubulin intensity was used to normalize CML or RAGE intensity. Additionally, the number of visible bands on CML immunoblots was determined to provide a qualitative assessment of the effect of age and brain region on CML-reactive proteins.
2.5. Statistical analysis of data
The effects of age on CML and RAGE immunostaining, as well as the number of CML-reactive bands in various regions were considered in two-way analyses of variance (ANOVA), with Age as a between-groups factor and Brain Region as a within-groups factor. Planned individual comparisons between age groups were performed for each brain region within the Age × Brain Region interaction, using Student’s T-tests. The alpha level for the ANOVA and planned comparisons was set at 0.05.
Data for each behavioral assessment made on the group of old mice are summarized in Table 1 in terms of mean, range, and standard deviation. Pearson correlation coefficients were calculated to determine the degree of linear relationship between performance of old mice on the different behavioral tests and CML and RAGE staining. A “strong” association between the measures was set at r = 0.5 or higher, which would account for at least 25% of the variance. Whole brain CML and RAGE were calculated by summation of the amounts of CML and RAGE in the different regions of the brain, adjusted for the percentage of the wet weight that each region accounted for in the whole brain.
An alternative data analytic approach was applied, based on the semiquantitative nature of the CML and RAGE immunostaining, to confirm that significant associations among the variables were not missed. In this approach, Degree of Impairment (high vs. low impairment based on a median split for each behavioral measure) was considered as a between-groups factor in one-way ANOVAs involving CML or RAGE relative intensity for each region. The alpha level was set at 0.01 for these analyses.
3. Results
3.1 Age-related accumulation of CML
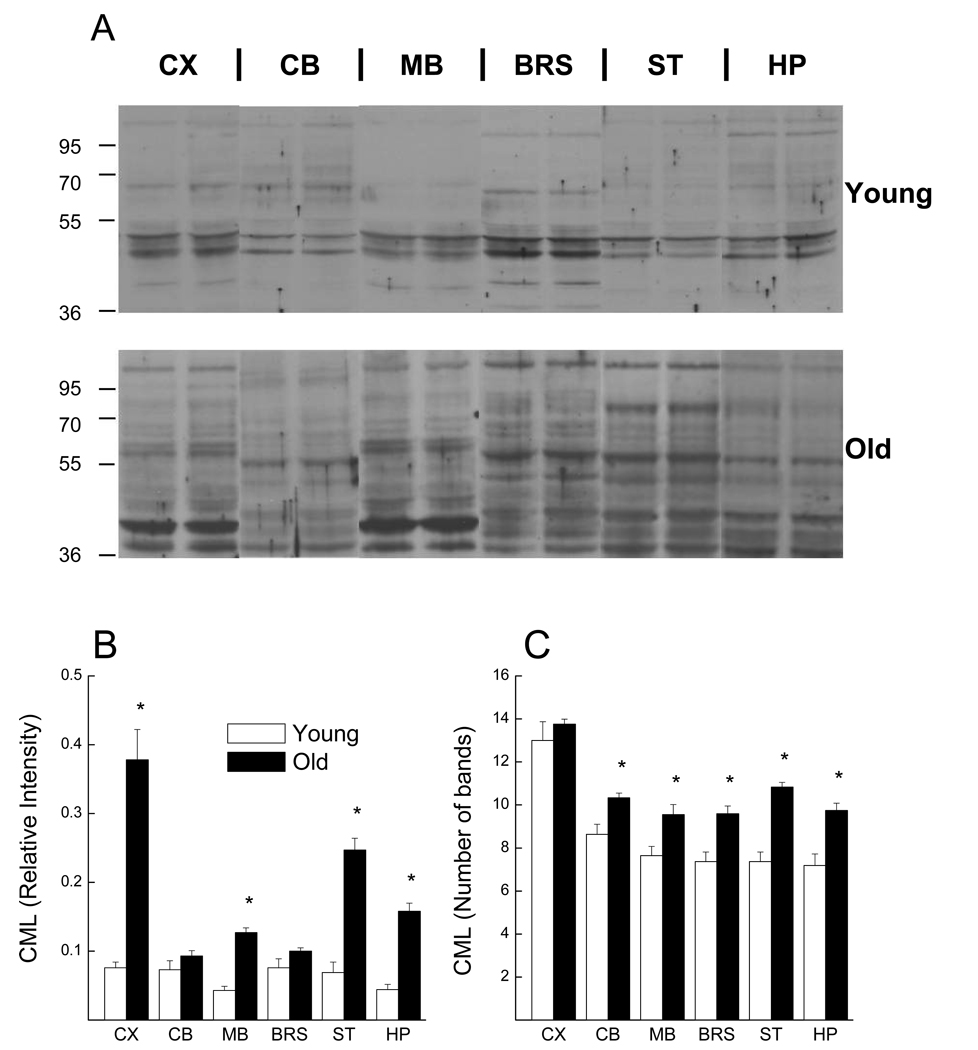
In general, there was an increase in the intensity of CML immunostaining in the old mice when compared to that of young mice (Fig. 1A). In the young mice, the whole lane relative intensity of CML was similar for cerebral cortex, cerebellum, brainstem, and striatum, whereas relatively lower CML intensity was observed in hippocampus and midbrain (Fig. 1B). In the old mice, however, the cerebral cortex contained the highest amount of CML, followed by striatum, hippocampus, and then midbrain. Cerebellum and brainstem contained the least amount of CML in the old mice. When CML intensity for each region was considered, levels in the cerebral cortex, midbrain, striatum and hippocampus of the old mice were significantly higher than in the same regions of the young mice. On the other hand, there were minimal age-related increases in CML of the cerebellum and brainstem. The region-dependent increases in CML relative intensity resulted in a significant Age × Brain Region interaction (F (5,170) = 4.397; p < 0.001)
Fig. 1.
CML immunostaining of protein from different regions of the brain in young and aged mice. (A) Representative immunoblots from homogenates of each brain region for two young (upper) and two old (lower) mice. Average whole lane intensity of CML immunoblots normalized with alpha-tubulin (B) and average number of visible CML-stained bands (C) are shown for the six brain regions. Each value represents the mean ± SE of 11 young or 24 old mice.
* indicates a significant difference between young and old groups (p<0.05).
Abbreviation: CX, cerebral cortex; CB, cerebellum; MB, midbrain; BRS, brainstem; ST, striatum; HP, hippocampus
As suggested by perusal of immunoblots from the different brain regions of young mice (Fig. 1A, upper), there was a relatively consistent banding pattern among the different brain regions. However, the CML immunostaining patterns of old mice were, at best, only partially reflective of simple quantitative increases in CML-staining in the same protein regions. Instead, there was an overall increase in the frequency of visible CML-staining bands within all regions of the brain, with the exception of the cerebral cortex (Fig. 1C). Depending upon the region of the brain considered, intensity of some bands staining for CML in young mice appear to be decreased with age, whereas other bands, not detected in young mice, exhibit intense CML-staining in older mice.
3.2 Regional differences in age-related expression of RAGE
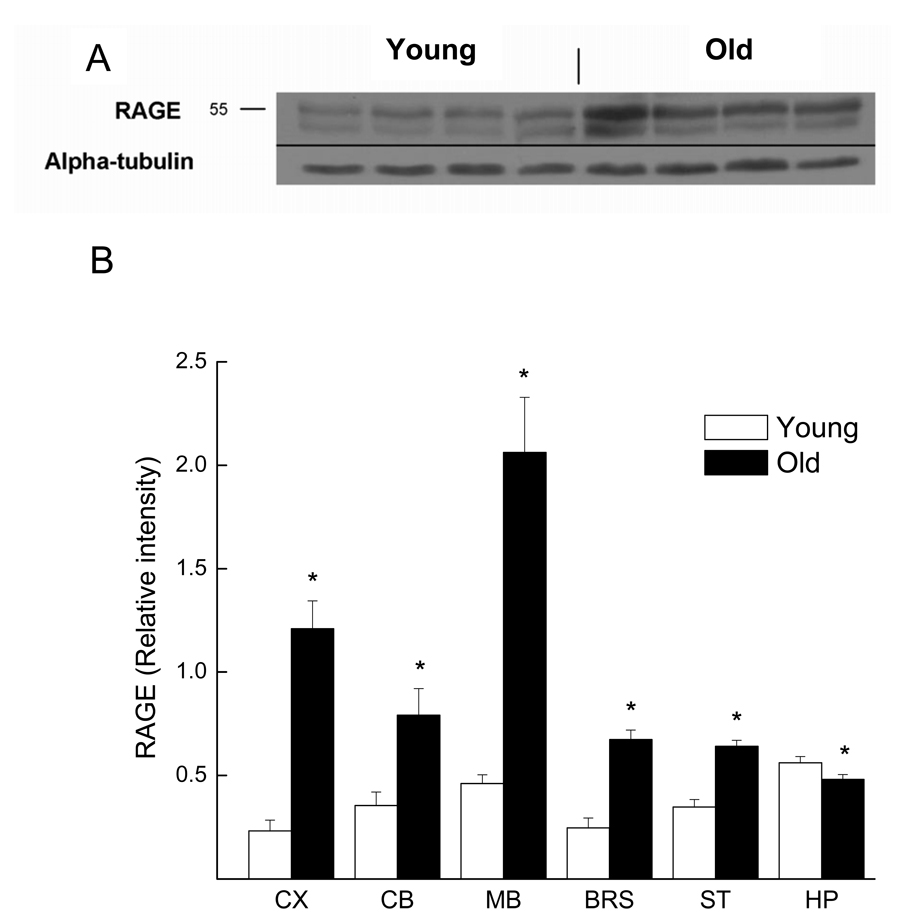
Similar to the results for total CML intensity, immunoblots for RAGE expression from the old mice were more intense than those from young mice in most regions of the brain (Fig. 2A). In young mice, expression of RAGE was highest in hippocampus, followed by midbrain, cerebellum, striatum, brainstem, and cerebral cortex (Fig. 2B). This pattern of RAGE expression was unlike that of the old mice, in which the midbrain was observed to have the highest RAGE protein expression; followed by cerebral cortex, cerebellum, brainstem, striatum, and hippocampus. These regional differences reflected different degrees of age-related increase in RAGE. It is noteworthy that a small age-related decrease in RAGE expression was apparent in the hippocampus (p = 0.038) (Fig. 2B). The regional differences in RAGE protein expression when comparing the young and old mice resulted in a significant interaction of Age with Brain Region (F (5,170) = 8.464; p < 0.001). Pearson correlation analysis revealed no significant degree of association between total CML and RAGE immunostaining within or among the different regions of the brain (data not shown).
Fig. 2.
RAGE protein expression in different brain regions of young and aged mice. (A) Representative RAGE immunoblots from cerebral cortex homogenates of four young and four old mice. Alpha-tubulin was used as loading control. (B) Densitometric analysis of RAGE immunoblots, normalized with alpha-tubulin. Each value represents the mean ± SE of 11 young or 24 old mice.
* indicates a significant difference between young and old mice (p<0.05).
Abbreviation: CX, cerebral cortex; CB, cerebellum; MB, midbrain; BRS, brainstem; ST, striatum; HP, hippocampus
3.3 Relationship between glycation status and behavioral test performance
To determine whether or not the increase in CML or RAGE in the old mice predicted cognitive or psychomotor dysfunction, the old mice were subjected to an established battery of behavioral tests that were sensitive to the effects of aging. The different functions for which the 25-month-old mice were tested are listed in Table 1, along with each group mean, the range, and the standard deviation of each measure of performance.
A Pearson correlation matrix was generated, using the measures of performance on each test, and the relative intensity of CML or RAGE immunostaining in each of the six brain regions. The one-tailed power for this matrix was 82%, where n = 24, r = 0.5 and α = 0.05. The Pearson analysis of the interrelationships among the performances of old mice on the different behavioral tests confirmed that while several of the measures of learning/memory were correlated (r = 0.519 to 0.852), there were no consistently strong relationships between learning/memory measures and psychomotor/reflex performance (data not shown). Therefore, these behavioral dimensions were considered separately in relation to total CML or RAGE intensity. When psychomotor and reflexive behaviors were considered, the Pearson correlation analyses yielded no strong relationship between CML staining for any brain region (or in the whole brain) with any behavioral measure (Table 2). When measures of learning and memory were considered, correlation analyses again failed to indicate any strong relationships (Table 3). Expression of RAGE protein also failed to correlate with measures of psychomotor/reflex capability (Table 4) and learning/memory (Table 5). The strongest meaningful association in any given region was for CML in the hippocampus and the maximum spatial learning (r = 0.411), which accounted for approximately 20% of the variance.
Table 2.
Pearson correlation coefficients denoting association between RAGE immunostaining in different brain regions of old mice and psychomotor/reflex measurements
| Brain Region |
|||||||
|---|---|---|---|---|---|---|---|
| Psychomotor/Reflex | CX | CB | MB | BRS | HP | ST | WhBr |
| Wire tread (s) | −0.113 | 0.284 | 0.335 | −0.068 | 0.340 | 0.172 | −0.085 |
| Wire fall (s) | 0.122 | −0.204 | −0.234 | 0.111 | −0.197 | 0.302 | 0.124 |
| Bridge fall (s) | 0.044 | −0.115 | 0.068 | 0.014 | −0.077 | 0.302 | 0.032 |
| Rotorod fall (s) | 0.165 | −0.162 | 0.043 | 0.146 | −0.444 | 0.093 | 0.166 |
| Swim speed (s) | 0.105 | 0.053 | 0.317 | 0.403 | −0.063 | −0.183 | 0.311 |
| Auditory startle1 (force units) | −0.369 | 0.121 | 0.026 | −0.204 | 0.063 | −0.211 | −0.304 |
| Shock startle2 (force units) | 0.321 | 0.103 | −0.089 | 0.157 | −0.273 | 0.060 | 0.248 |
| Reaction time (ms) | −0.246 | −0.180 | 0.036 | −0.056 | 0.007 | 0.173 | −0.151 |
Response amplitude after 140-dB auditory stimulus
Response amplitude after 0.64-mA shock stimulus
Abbreviations: CX, cerebral cortex; CB, cerebellum; MB, midbrain; BRS, brainstem; HP, hippocampus; ST, striatum; WhBr, whole brain
Table 3.
Pearson correlation coefficients denoting association between CML immunostaining in different brain regions of old mice and measures of learning/memory
| Brain Region |
|||||||
|---|---|---|---|---|---|---|---|
| Learning/Memory | CX | CB | MB | BRS | HP | ST | WhBr |
| Swim Maze | |||||||
| Total learning index (cm) | −0.267 | −0.177 | 0.059 | 0.093 | 0.123 | −0.064 | −0.069 |
| Acquisition learning index (cm) | −0.092 | −0.121 | −0.147 | −0.026 | 0.206 | 0.060 | −0.061 |
| Reversal learning index (cm) | −0.321 | −0.163 | 0.207 | 0.159 | 0.014 | −0.145 | −0.052 |
| Maximum spatial learning (cm) | −0.220 | 0.094 | −0.184 | −0.233 | 0.411 | −0.054 | −0.251 |
| Target Quadrant (% time spent) | 0.167 | −0.111 | −0.227 | 0.391 | −0.270 | 0.210 | 0.321 |
| Annulus 40 (% time spent) | −0.156 | 0.056 | −0.268 | 0.152 | −0.065 | −0.063 | 0.015 |
| Annulus 20 (% time spent) | −0.053 | −0.026 | −0.402 | 0.070 | −0.010 | −0.103 | 0.009 |
| Platform entries (counts) | −0.176 | 0.070 | −0.178 | 0.102 | −0.077 | −0.125 | −0.025 |
| Avoidance learning | |||||||
| Session 1 (number of trials) | −0.077 | 0.053 | −0.013 | −0.033 | 0.120 | 0.259 | −0.050 |
| Session 2 (number of trials) | 0.009 | −0.130 | 0.303 | 0.246 | −0.324 | −0.341 | 0.154 |
Abbreviations: CX, cerebral cortex; CB, cerebellum; MB, midbrain; BRS, brainstem; HP, hippocampus; ST, striatum; WhBr, whole brain
Table 4.
Pearson correlation coefficients denoting association between RAGE immunostaining in different brain regions of old mice and psychomotor/reflex measurements
| Brain Region |
|||||||
|---|---|---|---|---|---|---|---|
| Psychomotor/Reflex | CX | CB | MB | BRS | HP | ST | WhBr |
| Wire tread (s) | 0.359 | 0.018 | −0.139 | 0.051 | −0.001 | −0.278 | 0.119 |
| Wire fall (s) | 0.069 | −0.282 | −0.018 | −0.147 | 0.286 | 0.003 | −0.144 |
| Bridge fall (s) | 0.127 | −0.256 | 0.074 | −0.152 | 0.088 | 0.163 | −0.119 |
| Rotorod fall (s) | −0.309 | 0.081 | 0.030 | −0.040 | −0.159 | 0.173 | −0.131 |
| Swim speed (s) | −0.101 | 0.350 | 0.183 | −0.063 | −0.019 | −0.116 | −0.045 |
| Auditory startle1 (force units) | −0.067 | −0.126 | 0.125 | 0.166 | −0.285 | −0.039 | 0.165 |
| Shock startle2 (force units) | 0.295 | 0.379 | −0.086 | −0.202 | −0.122 | −0.151 | −0.130 |
| Reaction time (ms) | −0.025 | −0.456 | −0.177 | −0.014 | 0.306 | 0.016 | −0.038 |
denotes startle response to a 140-dB auditory stimulus
denotes response to a 0.64-mA shock stimulus
Abbreviations: CX, cerebral cortex; CB, cerebellum; MB, midbrain; BRS, brainstem; HP, hippocampus; ST, striatum; WhBr, whole brain
Table 5.
Pearson correlation coefficients denoting association between RAGE immunostaining in different brain regions of old mice and measures of learning/memory
| Brain Region | |||||||
|---|---|---|---|---|---|---|---|
| Learning/Memory | CX | CB | MB | BRS | HP | ST | WhBr |
| Swim Maze | |||||||
| Total learning index (cm) | −0.069 | −0.347 | 0.023 | 0.039 | −0.025 | 0.208 | 0.012 |
| Acquisition learning index (cm) | −0.154 | −0.352 | −0.023 | 0.034 | −0.058 | 0.168 | −0.022 |
| Reversal learning index (cm) | 0.024 | −0.226 | 0.053 | 0.029 | 0.011 | 0.170 | 0.036 |
| Maximum spatial learning (cm) | −0.200 | −0.123 | −0.038 | 0.367 | −0.392 | −0.085 | 0.308 |
| Target Quadrant (% time spent) | 0.175 | 0.224 | −0.056 | −0.242 | −0.025 | 0.233 | −0.200 |
| Annulus 40 (% time spent) | 0.103 | 0.063 | −0.127 | 0.099 | −0.066 | 0.124 | 0.108 |
| Annulus 20 (% time spent) | 0.021 | 0.178 | −0.087 | 0.149 | −0.315 | −0.017 | 0.148 |
| Platform entries (counts) | 0.011 | 0.107 | −0.047 | 0.197 | −0.289 | −0.120 | 0.198 |
| Avoidance learning | |||||||
| Session 1 (trials) | −0.222 | −0.093 | −0.178 | −0.080 | −0.148 | 0.000 | −0.171 |
| Session 2 (trials) | 0.262 | 0.140 | 0.091 | −0.322 | −0.047 | −0.051 | −0.240 |
Abbreviations: CX, cerebral cortex; CB, cerebellum; MB, midbrain; BRS, brainstem; HP, hippocampus; ST, striatum; WhBr, whole brain
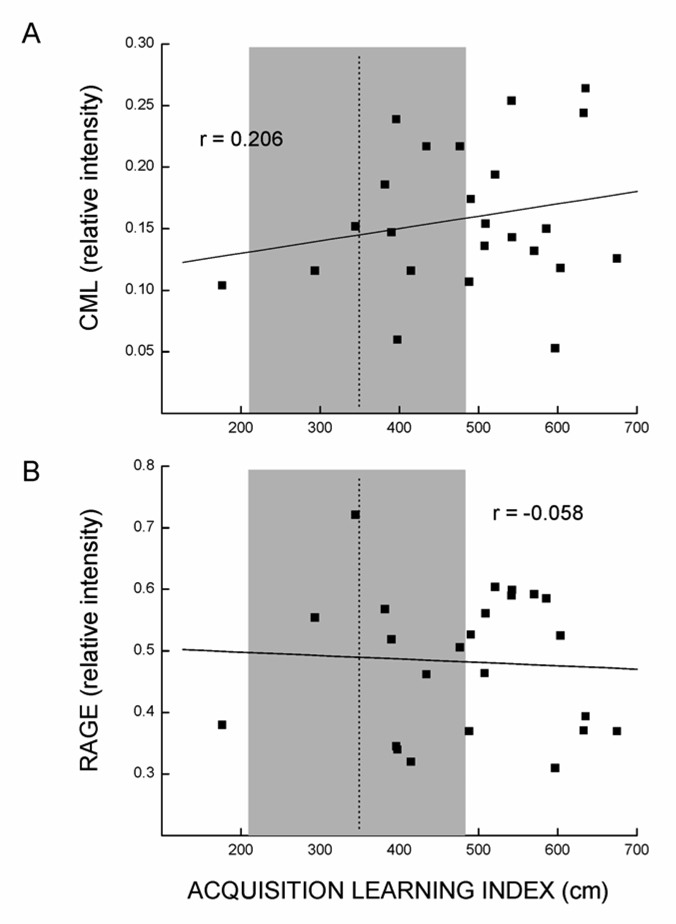
Of particular interest was the lack of relationship between CML or RAGE staining in the hippocampus, and spatial swim maze performance as illustrated in Fig. 3. The dotted line and shading in this figure represent the mean (± SD) acquisition learning index of a large young reference group (n=101) tested in this laboratory (de Fiebre et al., 2006), illustrating the varying degrees of impairment in spatial learning present in the old mice tested in the current study. As indicated in Table 3 and Table 5, hippocampal CML and RAGE did not predict any of the four parameters of impaired spatial swim maze performance.
Fig. 3.
Scatter plots illustrating lack of association between spatial swim maze learning and CML imunostaining (A) or RAGE protein expression (B) in the hippocampus (n = 24). The r values represent Pearson correlation coefficients; dotted lines and shaded areas indicate the performance level (mean ± SD) of a reference group of 101 young mice tested for spatial performance in this laboratory (de Fiebre et al., 2006).
As an alternative to the correlational approach applied above, the current data were recoded to generate groups with high or low impairment for each measure, based on a median split. This approach was applied based on the possibility that it might detect the presence of nonlinear associations not revealed by the Pearson correlation analysis (Baxter and Gallagher, 1996). One-way ANOVAs, performed to determine if CML or RAGE immunostaining in various brain regions was affected by high vs low impairment scores, failed to reveal any significant effects (data not shown).
4. Discussion
Previous investigations have indicated that AGEs accumulate in the aging brain and have the potential to promote neuronal dysfunction. To the best of our knowledge, the current studies are the first to address the potential for AGEs to account for individual differences in behavioral performance that are linked to declines in brain function. The main findings of this study were that (i) aging of the mouse brain involves a robust but region-specific increase in CML, a predominant AGE, as well as an increase in one receptor for AGEs (RAGE) and (ii) the relative amounts of CML and RAGE are not closely linked to the degree of age-related impairment of mice given behavioral tests of brain function.
CML was found to be increased significantly with age in four regions of the brain, the cerebral cortex, midbrain, striatum, and hippocampus. The finding that CML was elevated in the cerebral cortex and the hippocampus is consistent with previous reports (Dei et al., 2002; Kimura et al., 1996; Li et al., 1995; Pamplona et al., 2005). The current studies failed to indicate an increase of CML in the brainstem or cerebellum of the aged mice, although Weber and colleagues (1998) reported increased AGE immunoreactivity in these regions of the aged canine brain using a polyclonal antibody raised against a variety of AGE species. The latter findings could reflect a difference in the mammalian species studied or in the accrual of different species of AGE in different regions of the brain.
Accumulation of CML in tissues is a consequence of both advanced glycation and oxidation reactions (i.e. lipoxidation; glycoxidation) (Dunn et al., 1990), and thus it was expected that the presence of CML would correlate with oxidative stress and steady-state amounts of oxidative damage in different brain regions. Previous studies of aged C57BL/6 mice have clearly indicated the striatum, cortex, and hippocampus, those regions in which total CML accumulation was greatest, as areas where there is a robust age-related increase in oxidative stress (Rebrin et al., 2007) and accrual of protein oxidative damage (Dubey et al., 1996). Also in accordance with the present findings for total CML, the previous studies suggested that oxidative stress/damage was not altered as a function of age in the brainstem of mice. However, the lack of CML accumulation in the cerebellum would not be predicted, based upon the relatively low ratio of reduced to oxidized glutathione in this region for aged mice, suggestive of oxidative stress (Rebrin et al., 2007). Therefore, the correlation between oxidative stress/damage and accrual of CML among different regions of the brain appears to be strong, though imperfect.
In contrast to the accumulation of CML, expression of RAGE increased in an age-dependent manner in all regions of the brain except the hippocampus, where expression of RAGE protein was decreased. The relative amounts of RAGE in the different brain regions of the old mice was not correlated with the regional amounts of CML, or with previously reported regional differences in protein oxidation (Dubey et al., 1996). It is particularly noteworthy that while robust increases in CML were evident in the hippocampus of the old mice, there was a relative loss of RAGE expression. The significance of this decrease is not clear, as the functions and ligands for RAGE have not been well characterized in the brain. The interaction of RAGE with various ligands has been reported, including amphoterin (Horie et al., 1997), S100B (Huttunen et al., 2000), and beta-amyloid (Yan et al., 1998). It is possible that the absence of age related increases of RAGE protein expression in the hippocampus is influenced by the absence or the presence of different ligands in this region.
Although the age-related increases in CML concentration appear to coincide with oxidative damage in different brain regions, there was no strong association between total CML and the differing levels of behavioral performance among individuals in the group of old mice. The latter result was not expected, based on previous studies in which measures of protein or lipid oxidation were reported to strongly correlate with age-dependent psychomotor and cognitive decline (Forster et al., 1996; Liu et al., 2003; Nicolle et al., 2001). The degree of association between CML accumulation and protein oxidation among individual mice was not addressed directly in the current studies, although the apparent discrepancy may indicate that functionally relevant pools of protein or lipid are modified during oxidative stress, whereas accumulation of CML is reflective of different protein targets, perhaps less functionally relevant, that are not associated with behavioral impairment.
No strong association was noted involving RAGE and the behavioral measures and, overall, protein expression of RAGE was less predictive of impaired performance than CML. This was the case for age-related impairments across a number of important behavioral domains. It is perhaps most noteworthy that neither CML accumulation nor increased RAGE expression was predictive of impaired visually-mediated spatial learning/memory performance, as detected using the swim maze task. Numerous reports suggest that the degree of impaired spatial performance by old mice in this test is reflective of impaired plasticity in neural circuits within the aging hippocampus (Burke and Barnes, 2006; Gallagher and Rapp, 1997). Oxidative stress, as reflected in protein oxidation and other markers, is indeed predictive of impaired spatial performance (Forster et al., 1996; Nicolle et al., 2001) and has been implicated in both the physiological regulation and age-related impairment of synaptic plasticity (reviewed by Serrano and Klann, 2004). While accrual of AGEs or RAGE may, in part, be a cause or reflection of oxidative stress, the current studies suggest that neither CML nor RAGE are clearly implicated as a direct cause of age-impaired hippocampal plasticity.
In addition to spatial learning/memory, cognitive function of the mice was also assessed by their ability to learn an active discriminative avoidance response and subsequently perform a reversal. A decline in ability to learn a discriminated avoidance response occurs in an age-dependent manner in C57BL/6 mice (Dubey et al., 1996; Forster and Lal, 1992; Forster et al., 1996; McDonald et al., 2005). The effect of age is especially pronounced when mice are required to reverse a response strategy that has been previously well trained (Dean et al., 1981; Forster and Lal, 1992; McDonald et al., 2005), a dimension of performance reflective of cognitive inflexibility (Voytko, 1999; Tsuchida et al., 2002) that has been linked to frontal cortical dysfunction (Schoenbaum et al., 2006). Neither CML accumulation nor increased RAGE expression predicted performance on any aspect of the discriminated avoidance task, suggesting that they are not likely a direct cause of age-related changes in frontal cortical functions.
Additional tests performed on the old mice in these studies were designed to detect age-related impairments in sensory and/or psychomotor performance that involve components of arousal, somatosensory function, auditory function, strength, balance and coordination. These tests clearly involve overlapping requirements for physical response and sensory capabilities, yet the performance deficits of individual old mice on the different psychomotor tests are not correlated (Sumien et al., 2006), suggesting they may be sensitive to aging of different neural substrates. For example, protein oxidation in the cerebellum, but not in other regions of the brain, is predictive of impaired balance as measured by the bridge-walking test employed in the current study (Forster et al., 1996). A similar association involving CML or RAGE was not detected in the current study and indeed, these markers were not predictive of impairment across several other relatively independent domains of psychomotor performance.
The current studies employed a statistical power of greater than 80% for detecting linear relationships of a magnitude comparable to those identified in other studies of brain aging using similar approaches (i.e., r > 0.5). An additional statistical approach involving analysis of variance was also unsuccessful in uncovering any significant relationship of CML or RAGE with performance on the behavioral measures. Notwithstanding, the ability of the current studies to fully address the role of AGEs in the senescent brain is subject to many limitations. Longitudinal studies in non-neural tissue have suggested that the rate of accumulation of AGEs may predict longevity, independently of their absolute values at a given age (Sell et al., 2000). While the cross-sectional data collected in the current studies suggest a significant accumulation of CML in older mice, the extent to which values for individual mice are reflective of accumulation rate cannot be determined. Further, AGEs describe a heterogeneous group of compounds with numerous protein targets and various receptors, which could elicit a variety of as yet uncharacterized effects on cellular function. Thus, AGE species other than CML, or any number of specific AGE-protein interactions, could contribute significantly to age-related brain dysfunction.
The observation in the current study of an age-related increase in frequency of CML-immunostaining bands is suggestive of an age-related difference in specific protein targets of CML. The significance of specific CML-protein interactions was not addressed in the current study and, if present, significant interactions would not necessarily be reflected in whole region assessment of CML quantified with regard to a variety of protein targets. However, at the same level of analysis, regional assessment of protein oxidation and other markers of oxidative stress are predictive of age-related impairment of cognitive and psychomotor function. Therefore, it seems clear that whole region accrual of CML and RAGE represent processes that are independent of oxidative stress and not similarly linked to brain dysfunction. Overall, our results are compatible with the view that accumulation of AGEs is a time-related process that is correlated with chronological age, but is not causally linked to brain aging.
Acknowledgements
This research was supported by National Institutes of Health-National Institute on Aging grant P01 AG022550. The authors would like to thank Dr. Mark Smith for generously providing the CML antibody. The authors also thank Dr. Margaret Rutledge for assistance during manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed MU, Brinkmann Frye E, Degenhardt TP, Thorpe SR, Baynes JW. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem. J. 1997;324(Pt 2):565–570. doi: 10.1042/bj3240565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Gallagher M. Neurobiological substrates of behavioral decline: Models and data analytic strategies for individual differences in aging. Neurobiol. Aging. 1996;17:491–495. doi: 10.1016/0197-4580(96)00011-5. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Castellani RJ, Harris PL, Sayre LM, Fujii J, Taniguchi N, Vitek MP, Founds H, Atwood CS, Perry G, Smith MA. Active glycation in neurofibrillary pathology of Alzheimer disease: N(epsilon)-(carboxymethyl) lysine and hexitol-lysine. Free Radic. Biol. Med. 2001;31:175–180. doi: 10.1016/s0891-5849(01)00570-6. [DOI] [PubMed] [Google Scholar]
- deFiebre NC, Sumien N, Forster MJ, deFiebre CM. Spatial learning and psychomotor performance of C57BL/6 mice: Age sensitivity and reliability of individual differences. AGE. 2006;28:235–253. doi: 10.1007/s11357-006-9027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean RL, 3rd, Scozzafava J, Goas JA, Regan B, Beer B, Bartus RT. Age-related differences in behavior across the life span of the C57BL/6J mouse. Exp. Aging Res. 1981;7:427–451. doi: 10.1080/03610738108259823. [DOI] [PubMed] [Google Scholar]
- Dei R, Takeda A, Niwa H, Li M, Nakagomi Y, Watanabe M, Inagaki T, Washimi Y, Yasuda Y, Horie K, Miyata T, Sobue G. Lipid peroxidation and advanced glycation end products in the brain in normal aging and in Alzheimer's disease. Acta Neuropathol. 2002;104:113–122. doi: 10.1007/s00401-002-0523-y. [DOI] [PubMed] [Google Scholar]
- Dubey A, Forster MJ, Lal H, Sohal RS. Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral functions of the mouse. Mech. Ageing Devel. 1996;333:189–197. doi: 10.1006/abbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- Dunn JA, Ahmed MU, Murtiashaw MH, Richardson JM, Walla MD, Thorpe SR, Baynes JW. Reaction of ascorbate with lysine and protein under autoxidizing conditions: Formation of N epsilon-(carboxymethyl)lysine by reaction between lysine and products of autoxidation of ascorbate. Biochemistry. 1990;29:10964–10970. doi: 10.1021/bi00501a014. [DOI] [PubMed] [Google Scholar]
- Dunn JA, McCance DR, Thorpe SR, Lyons TJ, Baynes JW. Age-dependent accumulation of N epsilon-(carboxymethyl)lysine and N epsilon-(carboxymethyl)hydroxylysine in human skin collagen. Biochemistry. 1991;30:1205–1210. doi: 10.1021/bi00219a007. [DOI] [PubMed] [Google Scholar]
- Dunn JA, Patrick JS, Thorpe SR, Baynes JW. Oxidation of glycated proteins: Age-dependent accumulation of N epsilon-(carboxymethyl)lysine in lens proteins. Biochemistry. 1989;28:9464–9468. doi: 10.1021/bi00450a033. [DOI] [PubMed] [Google Scholar]
- Dyer DG, Dunn JA, Thorpe SR, Bailie KE, Lyons TJ, McCance DR, Baynes JW. Accumulation of maillard reaction products in skin collagen in diabetes and aging. J. Clin. Invest. 1993;91:2463–2469. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc. Natl. Acad. Sci. USA. 1996;93:4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster MJ, Lal H. Within-subject behavioral analysis of recent memory in aging mice. Behav. Pharmacol. 1992;3:337–349. [PubMed] [Google Scholar]
- Forster MJ, Lal H. Estimating age-related changes in psychomotor function: Influence of practice and of level of caloric intake in different genotypes. Neurobiol. Aging. 1999;20:167–176. doi: 10.1016/s0197-4580(99)00041-x. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu. Rev. Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Horie K, Miyata T, Yasuda T, Takeda A, Yasuda Y, Maeda K, Sobue G, Kurokawa K. Immunohistochemical localization of advanced glycation end products, pentosidine, and carboxymethyllysine in lipofuscin pigments of Alzheimer's disease and aged neurons. Biochem. Biophys. Res. Commun. 1997;236:327–332. doi: 10.1006/bbrc.1997.6944. [DOI] [PubMed] [Google Scholar]
- Huttunen HJ, Kuja-Panula J, Sorci G, Agneletti AL, Donato R, Rauvala H. Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J. Biol. Chem. 2000;275:40096–40105. doi: 10.1074/jbc.M006993200. [DOI] [PubMed] [Google Scholar]
- Kikuchi S, Shinpo K, Takeuchi M, Yamagishi S, Makita Z, Sasaki N, Tashiro K. Glycation--a sweet tempter for neuronal death. Brain Res. Brain Res. Rev. 2003;41:306–323. doi: 10.1016/s0165-0173(02)00273-4. [DOI] [PubMed] [Google Scholar]
- Kimura T, Takamatsu J, Ikeda K, Kondo A, Miyakawa T, Horiuchi S. Accumulation of advanced glycation end products of the maillard reaction with age in human hippocampal neurons. Neurosci.Lett. 1996;208:53–56. doi: 10.1016/0304-3940(96)12537-4. [DOI] [PubMed] [Google Scholar]
- Lee C, Yim MB, Chock PB, Yim HS, Kang SO. Oxidation-reduction properties of methylglyoxal-modified protein in relation to free radical generation. J.Biol.Chem. 1998;273:25272–25278. doi: 10.1074/jbc.273.39.25272. [DOI] [PubMed] [Google Scholar]
- Li JJ, Surini M, Catsicas S, Kawashima E, Bouras C. Age-dependent accumulation of advanced glycosylation end products in human neurons. Neurobiol.Aging. 1995;16:69–76. doi: 10.1016/0197-4580(95)80009-g. [DOI] [PubMed] [Google Scholar]
- Liu JH, Ho SC, Lai TH, Liu TH, Chi PY, Wu RY. Protective effects of Chinese herbs on D-galactose-induced oxidative damage. Methods Find. Exp. Clin. Pharmacol. 2003;25:447–452. doi: 10.1358/mf.2003.25.6.769650. [DOI] [PubMed] [Google Scholar]
- McDonald SR, Sohal RS, Forster MJ. Concurrent administration of coenzyme Q10 and alpha-tocopherol improves learning in aged mice. Free Radic.Biol.Med. 2005;38:729–736. doi: 10.1016/j.freeradbiomed.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Nicolle MM, Gonzalez J, Sugaya K, Baskerville KA, Bryan D, Lund K, Gallagher M, McKinney M. Signatures of hippocampal oxidative stress in aged spatial learning-impaired rodents. Neuroscience. 2001;107:415–431. doi: 10.1016/s0306-4522(01)00374-8. [DOI] [PubMed] [Google Scholar]
- Pamplona R, Dalfo E, Ayala V, Bellmunt MJ, Prat J, Ferrer I, Portero-Otin M. Proteins in human brain cortex are modified by oxidation, glycoxidation, and lipoxidation. effects of Alzheimer disease and identification of lipoxidation targets. J. Biol. Chem. 2005;280:21522–21530. doi: 10.1074/jbc.M502255200. [DOI] [PubMed] [Google Scholar]
- Qian M, Liu M, Eaton JW. Transition metals bind to glycated proteins forming redox active "glycochelates": Implications for the pathogenesis of certain diabetic complications. Biochem. Biophys. Res. Commun. 1998;250:385–389. doi: 10.1006/bbrc.1998.9326. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: A common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15:16R–28R. doi: 10.1093/glycob/cwi053. [DOI] [PubMed] [Google Scholar]
- Rebrin I, Forster MJ, Sohal RS. Effects of age and caloric intake on glutathione redox state in different brain regions of C57BL/6 and DBA/2 mice. Brain Res. 2007;1127:10–18. doi: 10.1016/j.brainres.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S, Bichler J, Wells-Knecht KJ, Thorpe SR, Baynes JW. N epsilon-(carboxymethyl)lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry. 1995;34:10872–10878. doi: 10.1021/bi00034a021. [DOI] [PubMed] [Google Scholar]
- Sasaki N, Fukatsu R, Tsuzuki K, Hayashi Y, Yoshida T, Fujii N, Koike T, Wakayama I, Yanagihara R, Garruto R, Amano N, Makita Z. Advanced glycation end products in Alzheimer's disease and other neurodegenerative diseases. Am.J.Pathol. 1998;153:1149–1155. doi: 10.1016/S0002-9440(10)65659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim. Biophys. Acta. 2000;1498:99–111. doi: 10.1016/s0167-4889(00)00087-2. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding changes in orbitofrontal cortex in reversal-impaired aged rats. J. Neurophysiol. 2006;95:1509–1517. doi: 10.1152/jn.01052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano F, Klann E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res. Rev. 2004;3:431–443. doi: 10.1016/j.arr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Sell DR, Kleinman NR, Monnier VM. Longitudinal determination of skin collagen glycation and glycoxidation rates predicts early death in C57BL/6NNIA mice. FASEB J. 2000;14:145–156. doi: 10.1096/fasebj.14.1.145. [DOI] [PubMed] [Google Scholar]
- Sobal G, Menzel J, Sinzinger H. Why is glycated LDL more sensitive to oxidation than native LDL? A comparative study. Prostaglandins Leukot. Essent. Fatty Acids. 2000;63:177–186. doi: 10.1054/plef.2000.0204. [DOI] [PubMed] [Google Scholar]
- Sumien N, Sims MN, Taylor HJ, Forster MJ. Profiling psychomotor and cognitive aging in four-way cross mice. AGE. 2006;28:265–282. doi: 10.1007/s11357-006-9015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumien N, Heinrich KR, Sohal RS, Forster MJ. Short-term vitamin E intake fails to improve cognitive or psychomotor performance of aged mice. Free Radic. Biol. Med. 2004;36:1424–1433. doi: 10.1016/j.freeradbiomed.2004.02.081. [DOI] [PubMed] [Google Scholar]
- Tohgi H, Utsugisawa K, Nagane Y, Yoshimura M, Ukitsu M, Genda Y. Decrease with age in methylcytosines in the promoter region of receptor for advanced glycated end products (RAGE) gene in autopsy human cortex. Brain Res. Mol. Brain Res. 1999;65:124–128. doi: 10.1016/s0169-328x(98)00351-9. [DOI] [PubMed] [Google Scholar]
- Tsuchida J, Kubo N, Kojima S. Position reversal learning in aged Japanese macaques. Behav. Brain Res. 2002;129:107–112. doi: 10.1016/s0166-4328(01)00336-9. [DOI] [PubMed] [Google Scholar]
- Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, Bijlsma JW, Lafeber FP, Baynes JW, TeKoppele JM. Effect of collagen turnover on the accumulation of advanced glycation end products. J. Biol. Chem. 2000;275:39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- Vlassara H. The AGE-receptor in the pathogenesis of diabetic complications. Diabetes Metab. Res. Rev. 2001;17:436–443. doi: 10.1002/dmrr.233. [DOI] [PubMed] [Google Scholar]
- Voytko ML. Impairments in acquisition and reversals of two-choice discriminations by aged rhesus monkeys. Neurobiol. Aging. 1999;20:617–627. doi: 10.1016/s0197-4580(99)00097-4. [DOI] [PubMed] [Google Scholar]
- Weber K, Schmahl W, Munch G. Distribution of advanced glycation end products in the cerebellar neurons of dogs. Brain Res. 1998;791:11–17. doi: 10.1016/s0006-8993(97)01421-2. [DOI] [PubMed] [Google Scholar]
- Yan H, Harding JJ. Glycation-induced inactivation and loss of antigenicity of catalase and superoxide dismutase. Biochem. J. 1997;328(Pt 2):599–605. doi: 10.1042/bj3280599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SD, Stern D, Kane MD, Kuo YM, Lampert HC, Roher AE. RAGE-Abeta interactions in the pathophysiology of Alzheimer's disease. Restor. Neurol. Neurosci. 1998;12:167–173. [PubMed] [Google Scholar]
- Yim MB, Yim HS, Lee C, Kang SO, Chock PB. Protein glycation: Creation of catalytic sites for free radical generation. Ann. N. Y. Acad. Sci. 2001;928:48–53. [PubMed] [Google Scholar]