Abstract
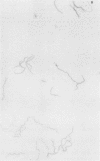
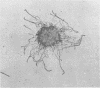
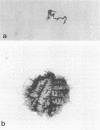
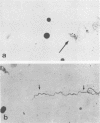
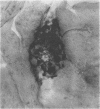
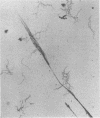
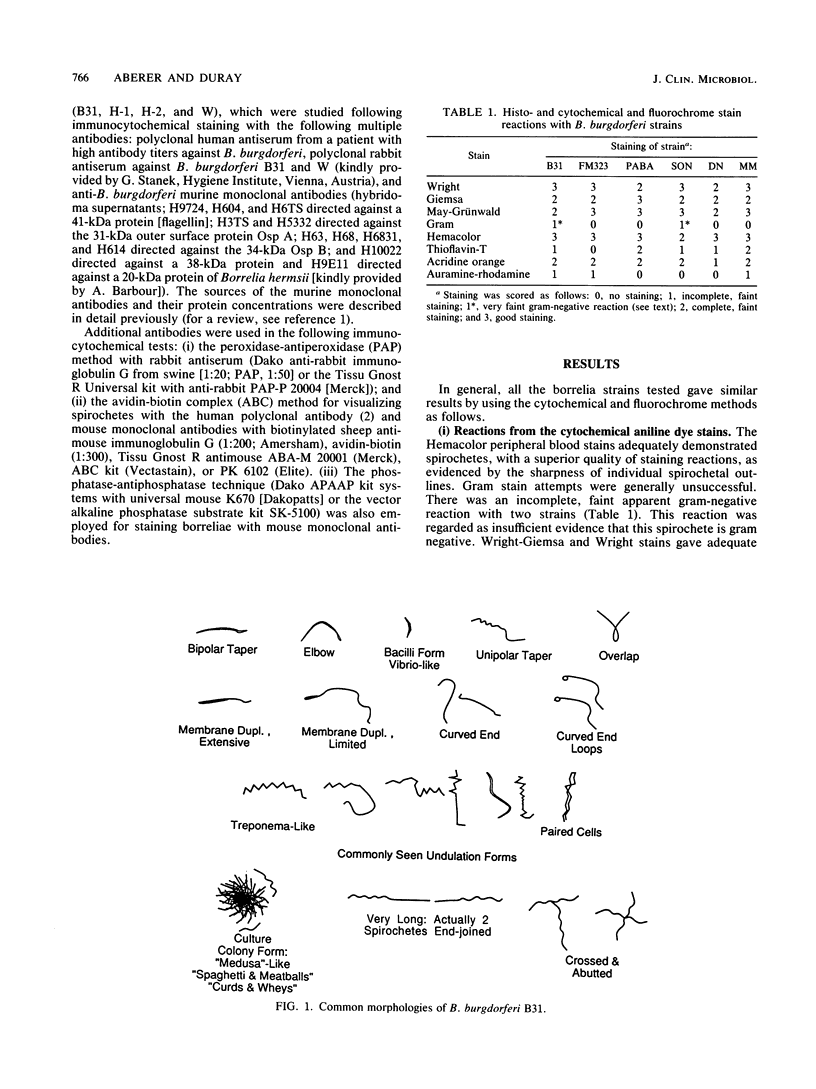
The microscopic recognition of Borrelia burgdorferi in biologic fluids and tissues is difficult and challenging because of low numbers of organisms occurring as single isolated spirochetes, the apparent lack of colony formation in tissues, and differing lengths and structural morphologies. To identify the most common morphologic forms, we studied numerous cultures by a variety of microscopic techniques. Culture suspensions of B. burgdorferi were stained by several different histochemical procedures (Gram, Wright, Wright-Giemsa, Giemsa, and polychromes), fluorochromes (thioflavin-T, acridine orange, and rhodamine), silver impregnation techniques (Warthin-Starry, modified Dieterle, modified microwave Dieterle, and Bosma-Steiner) and immunocytochemical methods with different polyclonal and monoclonal antibodies. Additionally, borrelia culture suspensions were embedded in agar and also injected into skin biopsies and processed for histologic study. The different staining properties were found to be influenced by fixation methods and the type of antibody in immunocytochemical stains. This study provides evidence for marked cytomorphic variations in individual spirochetes. Variations detected by these staining procedures provide a basis for future study of tissue sections and for how borreliae can be expected to appear in tissue sections.
Full text
PDF


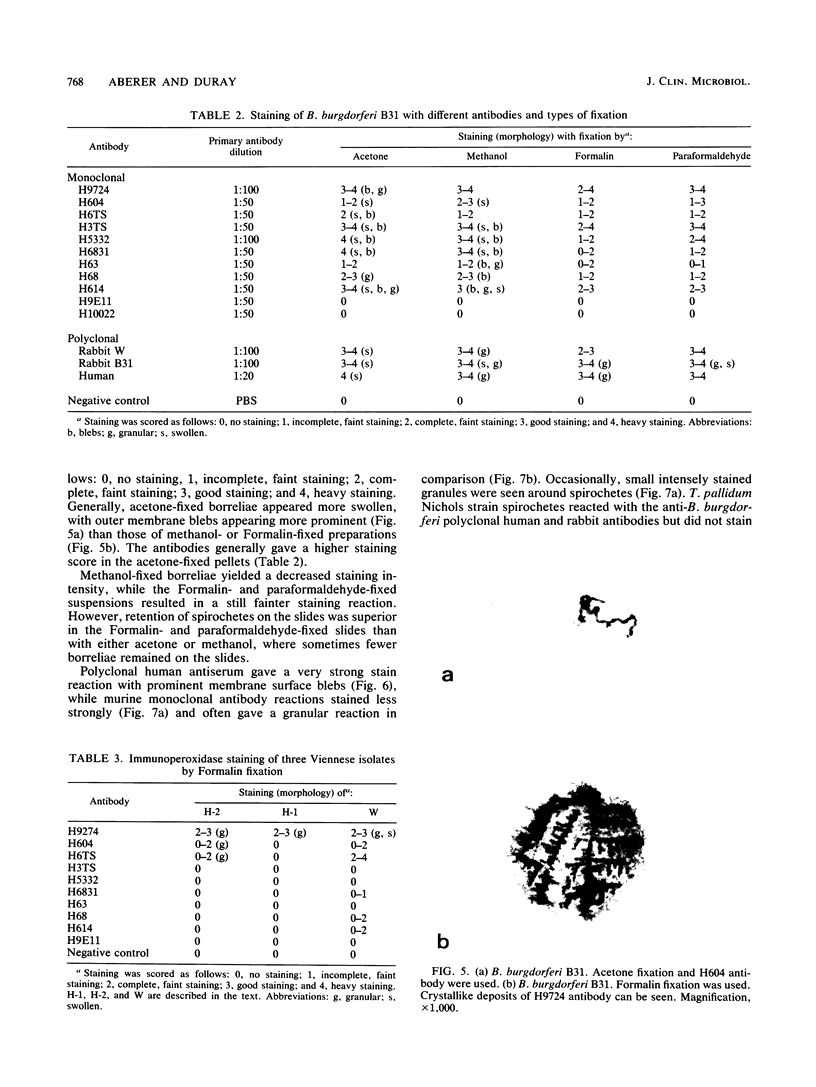

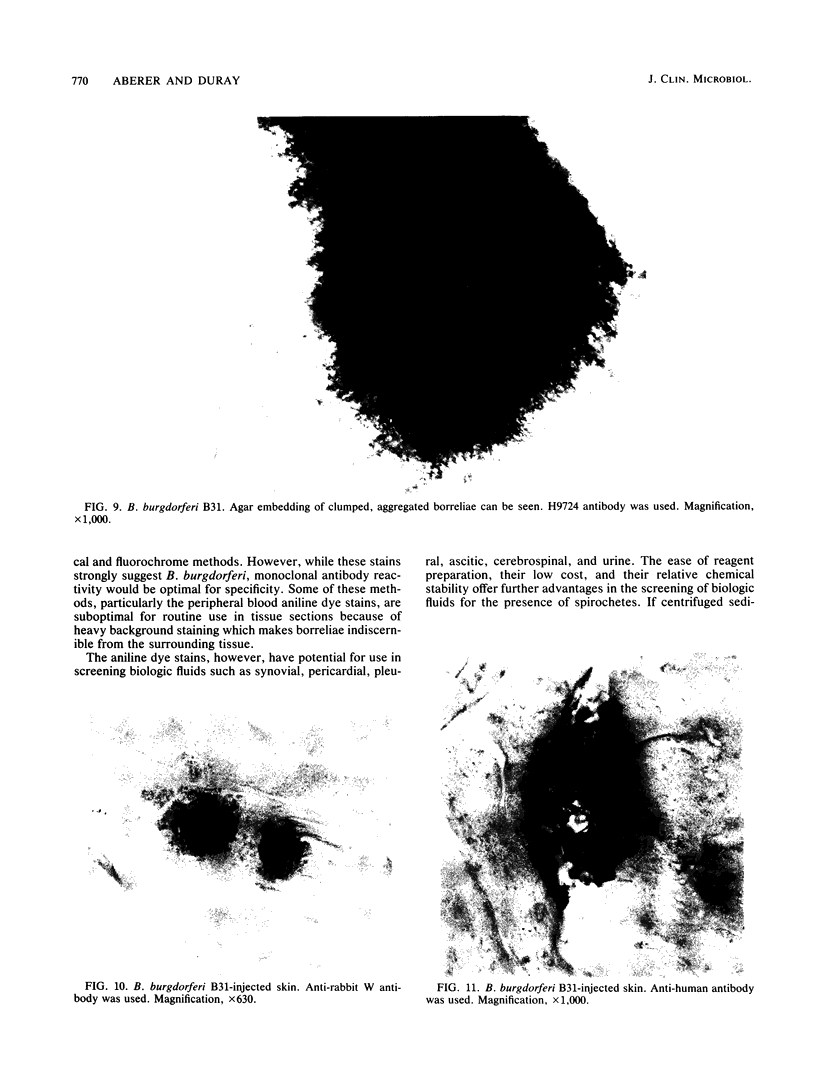


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aberer E., Brunner C., Suchanek G., Klade H., Barbour A., Stanek G., Lassmann H. Molecular mimicry and Lyme borreliosis: a shared antigenic determinant between Borrelia burgdorferi and human tissue. Ann Neurol. 1989 Dec;26(6):732–737. doi: 10.1002/ana.410260608. [DOI] [PubMed] [Google Scholar]
- Aberer E., Stanek G. Histological evidence for spirochetal origin of morphea and lichen sclerosus et atrophicans. Am J Dermatopathol. 1987 Oct;9(5):374–379. doi: 10.1097/00000372-198710000-00002. [DOI] [PubMed] [Google Scholar]
- Anderson J. F. Mammalian and avian reservoirs for Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:180–191. doi: 10.1111/j.1749-6632.1988.tb31852.x. [DOI] [PubMed] [Google Scholar]
- Asbrink E., Hovmark A. Successful cultivation of spirochetes from skin lesions of patients with erythema chronicum migrans Afzelius and acrodermatitis chronica atrophicans. Acta Pathol Microbiol Immunol Scand B. 1985 Apr;93(2):161–163. doi: 10.1111/j.1699-0463.1985.tb02870.x. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Hayes S. F. Biology of Borrelia species. Microbiol Rev. 1986 Dec;50(4):381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Bundoc V. G., Barbour A. G. Clonal polymorphisms of outer membrane protein OspB of Borrelia burgdorferi. Infect Immun. 1989 Sep;57(9):2733–2741. doi: 10.1128/iai.57.9.2733-2741.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Chaplin A. J., Jones S. E., Kirkpatrick P. Histological demonstration of spirochaetes: a method of preparing control sections. Med Lab Sci. 1985 Jul;42(3):283–284. [PubMed] [Google Scholar]
- De Koning J., Bosma R. B., Hoogkamp-Korstanje J. A. Demonstration of spirochaetes in patients with Lyme disease with a modified silver stain. J Med Microbiol. 1987 May;23(3):261–267. doi: 10.1099/00222615-23-3-261. [DOI] [PubMed] [Google Scholar]
- Kurtti T. J., Munderloh U. G., Johnson R. C., Ahlstrand G. G. Colony formation and morphology in Borrelia burgdorferi. J Clin Microbiol. 1987 Nov;25(11):2054–2058. doi: 10.1128/jcm.25.11.2054-2058.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Johnson R. C. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J Infect Dis. 1987 Jul;156(1):183–188. doi: 10.1093/infdis/156.1.183. [DOI] [PubMed] [Google Scholar]
- Preac Mursic V., Wilske B., Schierz G., Pfister H. W., Einhäupl K. Repeated isolation of spirochetes from the cerebrospinal fluid of a patient with meningoradiculitis Bannwarth. Eur J Clin Microbiol. 1984 Dec;3(6):564–565. doi: 10.1007/BF02013623. [DOI] [PubMed] [Google Scholar]
- Preac-Mursic V., Wilske B., Schierz G. European Borrelia burgdorferi isolated from humans and ticks culture conditions and antibiotic susceptibility. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):112–118. doi: 10.1016/s0176-6724(86)80110-9. [DOI] [PubMed] [Google Scholar]
- Schmidli J., Hunziker T., Moesli P., Schaad U. B. Cultivation of Borrelia burgdorferi from joint fluid three months after treatment of facial palsy due to Lyme borreliosis. J Infect Dis. 1988 Oct;158(4):905–906. doi: 10.1093/infdis/158.4.905. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Warkel R. L., Luna L. G., Helwig E. B. A modified Warthin-Starry procedure at low pH for melanin. Am J Clin Pathol. 1980 Jun;73(6):812–815. doi: 10.1093/ajcp/73.6.812. [DOI] [PubMed] [Google Scholar]