Abstract
Ranibizumab, a humanized antigen-binding fragment (Fab) that binds all isoforms of VEGF-A, significantly slows down loss of vision and causes significant visual improvement in many patients with choroidal neovascularization (CNV) due to exudative age-related macular degeneration (AMD). These benefits of intravitreal ranibizumab apply to all angiographic subtypes of neovascular AMD and across all lesion sizes when the drug is injected at monthly intervals as shown in two pivotal phase III trials (ANCHOR and MARINA). The results from the PrONTO study suggest that less frequent treatment with ranibizumab through a variable dosing regimen dependent on optical coherence tomography (OCT) findings is a treatment option that results in comparably favorable visual outcomes. Currently, it is unclear whether combination therapy of ranibizumab with photodynamic therapy (PDT) provides any significant advantage over ranibizumab monotherapy (FOCUS trial); however, the combination of PDT and ranibizumab may decrease the need for frequent retreatment. This question will be addressed in the SUMMIT trial. Therapy with ranibizumab is generally very well tolerated with a low rate of seriously adverse ocular events or systemic side-effects. The advent of vascular endothelial growth factor (VEGF) inhibitors has revolutionized the therapy of neovascular AMD. Ranibizumab at the moment appears to be the most effective approved treatment for neovascular AMD.
Keywords: Lucentis, ranibizumab, vascular endothelial growth factor (VEGF), age-related macular degeneration (AMD), neovascular, exudative AMD, treatment
Introduction
Age-related macular degeneration (AMD) is the most prevalent cause of moderate and severe vision loss in most developed countries. AMD is characterized by irreversible damage to the macula resulting in progressive loss of central vision. Two forms of AMD exist: non-exudative or non-neovascular (“dry”) AMD, the most common form, and exudative or neovascular (“wet”) AMD, which is characterized by the development of choroidal neovascularization (CNV). CNV can be subdivided into classic and occult forms according to its appearance on flourescein angiography. Both classic and occult components can occur within the same lesion. In predominantly classic lesions the disease progression is usually more rapid than in the predominantly occult forms. Although the neovascular forms of AMD account for only about 10% of the cases, they are responsible for 90% of blindness caused by the disease because the presence of CNV leads to vascular leakage and subretinal scar formation (Klein et al 1983; Green et al 1986).
The role of vascular endothelial growth factor (VEGF) in neovascular AMD
Vascular endothelial growth factor (VEGF) is a crucial regulator of vascular permeability and angiogenesis (Senger et al 1983; Leung et al 1989). Apart from its physiological functions it has a decisive role in tumour-angiogenesis and in the pathogenesis of neovascular eye diseases, including neovascular AMD, diabetic retinopathy, and retinopathy of prematurity (Adamis et al 1994; Pierce et al 1996; Rakic et al 2003). VEGF encompasses a group of proteins: VEGF-A, B, C, D, and placental growth factor, among which VEGF-A is the most relevant for angiogenesis and vascular permeability.
To date, 9 VEGF-A isoforms have been identified: VEGF121, VEGF145, VEGF148, VEGF165, VEGF165b, VEGF183, VEGF189, and VEGF206. VEGF165 is the isoform that is expressed most abundantly (Takahashi et al 2005; Pieramici et al 2006). However, VEGF121, VEGF183, and VEGF189 are also very frequently encountered in various tissues (Lei et al 1998; Ferrara et al 2003).
In animal models the inhibition of VEGF-A prevents the development of CNV, causes regression of existing CNV, reduces pathological vascular permeability and prevents the development of iris neovascularization due to retinal ischemia (Adamis et al 1996; Krzystolik et al 2002; Akiyama et al 2005). In humans VEGF-A levels are increased in the vitreous of patients with neovascular AMD as well as in excised CNV membranes (Wells et al 1996; Grossniklaus et al 2002).
Pharmacology of ranibizumab
Ranibizumab (Lucentis®, Genentech, Inc., San Francisco, CA, USA) is a humanized antigen-binding fragment (Fab) that neutralizes all VEGF-A isoforms. Ranibizumab was developed on the hypothesis that a full-size monoclonal antibody against VEGF-A, such as bevacizumab (Avastin®; Genentech, Inc., San Francisco, CA, USA), might not penetrate through the retina after intravitreal injection (Presta et al 1997). However, recently it has been demonstrated that the full-length antibody bevacizumab also penetrates the rabbit retina (Shahar et al 2006; Heiduschka et al 2007).
Ranibizumab (molecular weight 48 kDa) and bevacizumab (150 kDA) were derived from the murine monoclonal antibody A4.6.1. Ranibizumab was developed from a humanized Fab variant of A4.6.1, known as MB1.6. This Fab then underwent a series of modifications: Affinity selection using phage display technology increased the affinity of ranibizumab for VEGF-A by several times. Thus, ranibizumab cannot simply be described as a Fab of bevacizumab because their complementarity-determining regions (CDR) are markedly different (Muller et al 1998; Chen et al 1999). In contrast to a full-size antibody, ranibizumab cannot bind complement because it lacks the Fc (Fragment crystallizable) region. This may prevent the promotion of intraocular inflammation after intravitreal injection (Gaudreault et al 2005; Ferrara et al 2006).
The increased potency, the smaller molecular size compared to a full-length antibody for enhanced penetration into the retina and choroid, and the lack of the Fc region were considered to be advantageous for intravitreal efficacy. The systemic half-life of ranibizumab is a few hours compared to roughly 3 weeks for bevacizumab.
In the vitreous of monkeys the half-life of ranibizumab after a single intraocular injection is about 3 days and serum levels are very low, approximately 1000-fold lower than levels in the eye (Gaudreault et al 2005). The half-life after an intraocular injection of a full-length antibody, eg, trastuzumab, which is comparable to bevacizumab, is about 6 days (Mordenti et al 1999).
Established therapies for neovascular AMD prior to the introduction of ranibizumab
Laser photocoagulation
Laser photocoagulation was the first relevant treatment introduced to try to halt the progression of neovascular AMD. Photocoagulation remains an important treatment option for neovascular AMD patients with well-defined extrafoveal CNV.
The main benefit of the procedure has been described for the first 18 months after the treatment, with 24% of patients in the treatment group experiencing a visual loss of more than 6 lines compared with 41% in the observation group (Macular photocoagulation study group 1991). However, in the long term almost half of the patients treated will experience recurrence with development of subfoveal CNV and subsequent visual loss. Furthermore, only a small group of patients with neovascular AMD presents with extrafoveal CNV that is accessible by laser therapy (Zarbin et al 2007). With the advent of photodynamic therapy (PDT) and (later) modern pharmacological therapies, and concern for the impact of iatrogenic scotoma in subfoveal CNV, laser photocoagulation of peri- and subfoveal CNV is no longer recommended (Virgili et al 2000).
Photodynamic therapy
The concept of photodynamic therapy (PDT) is based on the high concentration of low-density lipoprotein (LDL) receptors in choroidal neovascular tissue. Verteporfin (Visudyne™, Novartis, Basel, Switzerland) when infused intravenously complexes with LDLs and thus accumulates in CNV membranes. Non-thermal laser activation (689 nm) of verteporfin induces endothelial damage with thrombus formation via reactive oxygen species formation without damage to the overlying retina (Fingar et al 1996; Zarbin et al 2007).
PDT is effective in reducing the rate of visual loss in patients with subfoveal predominantly classic CNV due to AMD. In these patients the mean loss of visual acuity after 2 years was 2.3 lines versus 4.5 lines in the neovascular AMD patients treated with placebo (Bressler et al 2002). The average patient requires 5–6 sessions during the first 2 years of treatment. PDT treatment encompasses a risk of up to 5% of visual loss of 4 or more lines within 7 days of treatment (Bressler et al 2005).
The efficacy in patients with occult or minimal-classic type lesion is questionable. Recently, the VIO study failed to show any beneficial effect of PDT in patients with subfoveal occult CNV.
Thus, PDT only had some moderate effect in delaying visual loss in a subgroup of patients with neovascular AMD (Wormald et al 2005). The off-label use of intravitreal triamcinolone in combination with PDT yielded slightly better results (Arias et al 2006) or reduced the number of necessary repeat PDT treatments (Ergun et al 2006). However, this therapy is hampered by its side-effects – mostly steroid-induced glaucoma and cataract development.
Surgery for neovascular AMD
Because the formation of choroidal neovascular tissue is the hallmark of neovascular AMD, subretinal removal of CNV by pars plana vitrectomy has been proposed as a treatment modality. The Submacular Surgery Trial (SST), a prospective, randomized, multicenter clinical trial, evaluated submacular CNV excision versus laser photocoagulation for exudative and hemorrhagic subfoveal CNV due to AMD. However, CNV removal by submacular surgery was not superior to the less invasive laser photocoagulation of subfoveal CNV and thus this approach was abandoned with the advent of PDT (Bressler et al 2000).
The poor outcome of CNV excision was attributed to collateral damage to the retinal pigment epithelium (RPE), which is essential for the nutritional supply of the overlying macula. To avoid this problem it was suggested to combine CNV extraction with rotation of the macula to areas of undamaged RPE. This procedure was named macular translocation and is either performed as limited (LMT) or full macular translocation (FMT). In LMT the rotation is achieved by scleral folding whereas in FMT the retina is translocated after performing a 360° retinotomy. As demonstrated in several case series, LMT and FMT were able to prevent visual loss in many patients with neovascular AMD. Some patients even experienced a considerable increase of visual acuity (Aisenbrey et al 2002; Fujii et al 2002; Mruthyunjaya et al 2004). In one prospective, randomized mono-center trial FMT was compared with PDT in patients with subfoveal classic CNV due to AMD. In this study both FMT and PDT prevented visual loss of more than 3 Early Treatment Diabetic Retinopathy Study (ETDRS) lines in the same percentage of patients. However, the chance of visual improvement was significantly higher in the FMT group (Gelisken et al 2007).
Unfortunately, many patients who undergo FMT or LMT will complain of diplopia due to the rotated retina sometimes even after successful strabismus surgery. In addition, both FMT and LMT are complex retinal procedures that are prone to surgical complications. Thus, in the era of anti-VEGF therapy, macular translocation should not be performed as a standard primary procedure for neovascular AMD.
We currently offer FMT only to patients with acute loss of vision due to a tear of the RPE that involves the fovea or severe subfoveal hemorrhage, and to those who have not responded to anti-VEGF therapy, especially if the other eye already has end-stage neovascular AMD with subretinal scarring.
Other new surgical treatment modalities such as autologous transplantation of the retinal pigment epithelium and choroid are still experimental (MacLaren et al 2007; Heussen et al 2007).
Pegaptanib (Macugen®)
Pegaptanib is an oligonucleotide (aptamer) that binds within the heparin-binding domain of VEGF-A. It inactivates the VEGF165, VEGF189 and VEGF206 isoforms. However, it does not bind to other biologically active VEGF-A isoforms such as VEGF110, VEGF113, and VEGF121. The VISION trial, a randomized multicenter study, showed that 0.3 mg pegaptanib when injected intravitreally every 6 weeks, can reduce the rate of visual loss in patients with subfoveal neovascular AMD (after 1 year, 70% of the patients treated with pegaptanib lost less than three lines versus 55% in the control group) (Gragoudas et al 2004).
However, only 5% of the patients treated with pegaptanib experienced a moderate improvement of vision. This difference was not statistically significant. Thus, pegaptanib did not meet the high expectations that were associated with the introduction of this first anti-VEGF agent for neovascular AMD. Moreover, about 1% of patients who received the medication developed endophthalmitis, and a comparable number of patients had other serious ocular side-effects including retinal detachments and traumatic cataract (Gragoudas et al 2004).
Bevacizumab (Avastin)
Intravenous administration of bevacizumab, a humanized full-size antibody that inactivates all isoforms of VEGF-A, has been approved for the treatment of metastatic colorectal cancer as it has been shown to increase survival time when it is added to chemotherapy with 5-fluorouracil (Adams et al 2005; Hurwitz et al 2005).
Off-label systemically administered bevacizumab was studied in an uncontrolled open label trial in 18 patients with CNV due to AMD. It caused improvement in median visual acuity of 14 letters and substantial reduction in foveal thickness over a period of 24 weeks. However, in several patients there was a significant elevation in blood pressure that required adjustment or initiation of antihypertensive medicines (Moshfeghi et al 2006). To avoid these systemic complications, intravitreal injection of bevacizumab (the most commonly used dose being 1.25 mg) has subsequently been proposed and shown to yield similar positive results in a number of uncontrolled, mostly retrospective studies – with a very low rate of ocular and systemic side-effects (Rosenfeld et al 2005a; Avery et al 2006; Bashshur et al 2006; Costa et al 2006; Rich et al 2006; Spaide et al 2006; Aisenbrey et al 2007; Chen et al 2007; Emerson et al 2007; Goff et al 2007; Lazic et al 2007).
Unfortunately, no randomized clinical trials that address the efficacy of intravitreal bevacizumab in neovascular AMD have been performed so far.
Ranibizumab – a breakthrough in the treatment of neovascular AMD
Phase III trials – MARINA and ANCHOR
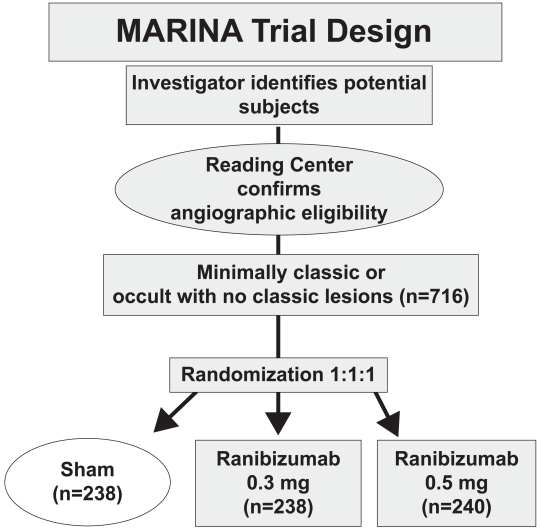
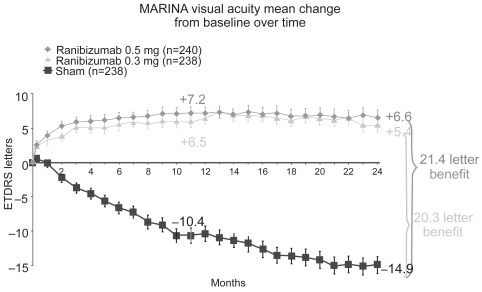
The MARINA study, a randomized, double-blind, controlled, multicenter phase III clinical trial, investigated the response of neovascular AMD patients with minimally classic or occult CNVs to ranibizumab (Rosenfeld et al 2006a). 716 patients were randomly assigned to receive 0.3 mg ranibizumab (n = 238), 0.5 mg ranibizumab or sham injections at monthly intervals for two years (Figure 1). After 24 months 90% of the ranibizumab-treated patients had lost fewer than 15 letters compared with 53% in the control group. Moreover, 33.3% of patients receiving 0.5 mg and 26.1% of patients receiving 0.3 mg ranibizumab gained at least 15 letters of visual acuity versus only 3.8% in the sham-injected patients. After 2 years, mean visual acuity had increased by 6.6 lines in the 0.5 mg ranibizumab group versus a decrease of 14.9 lines in the sham cohort (Figure 2). This favorable treatment outcome was independent of membrane type (minimally classic or purely occult CNV), the initial visual acuity or the lesion size. Another useful endpoint regarding function is the percentage of patients who achieve 20/40 vision or better, because that level of vision is sufficient for reading reasonably sized print. There were no differences among the groups at baseline (about 15%), but at 1 year, approximately 40% of patients in the ranibizumab groups achieved 20/40 compared to 11% in the sham group. Another interesting aspect regarding function was the percentage of patients that fell to vision 20/200 or worse which is equivalent to legal blindness in the United States. About 12% of patients in the ranibizumab groups had visual acuity of 20/200 or worse compared to 43% in the sham injection group. Thus, irrespective of different assessments, patients treated with 0.3 or 0.5 mg of ranibizumab did substantially better than those who received sham injections. The area of CNV lesion showed no change over the course of a year in patients treated with ranibizumab in contrast to an increase of about 2 disc areas in the control group. This indicates that although ranibizumab seems not to cause regression of neovascularization, it does seem to stop the growth of CNV.
Figure 1.
MARINA trial design.
Figure 2.
MARINA trial: after 2 years mean visual acuity had increased by 6.6 lines in the ranibizumab 0.5 mg group versus a decrease of 14.9 lines in the sham group. This favorable outcome was independent of membrane type (minimally classic or purely occult CNV), the initial visual acuity, or the lesion size. Adapted with permission from Rosenfeld PJ, Brown DM, Heier JS, et al. 2006a. Ranibizumab for neovascular age-related macular degeneration. N Eng J Med, 355:1419–31. Copyright © 2006. Massachusetts Medical Society. All rights reserved.
There was a risk of about 1% of developing presumed endophthalmitis in the treatment group. (0.00043 per individual injection). No statistically significant increase of hypertension or myocardial infarction could be observed. The risk of stroke was 0.8% in the sham-treated patients versus 1.3% (0.3 mg) and 2.5% (0.5 mg) in the ranibizumab-treated patients; these differences were not statistically significant. However, since the study was not powered to detect differences in adverse events, the absence of statistical significance should not be overrated.
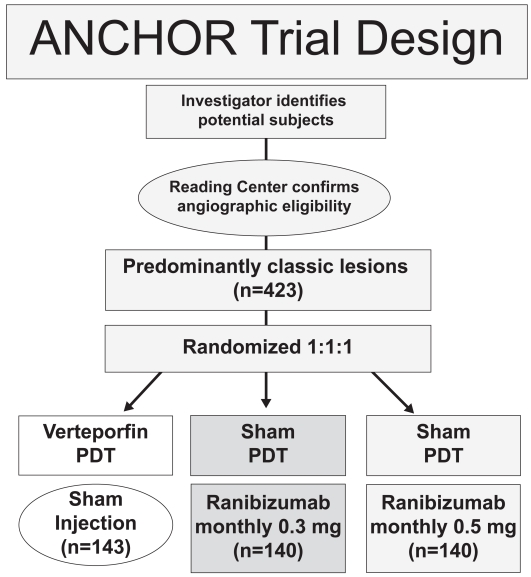
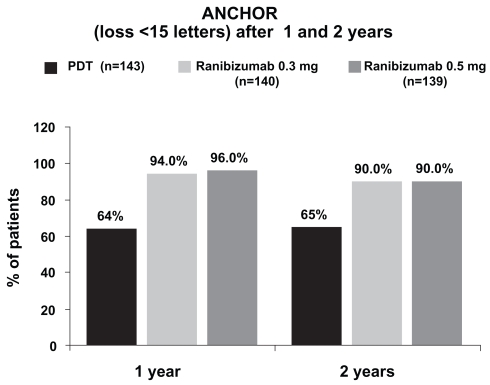
Another major randomized, double-blind, controlled, multicenter phase III clinical trial, the ANCHOR study, assessed the effect of ranibizumab in patients with predominantly classic CNVs (Brown et al 2006; Schmidt-Erfurth et al 2007). 432 patients were randomly assigned to receive either PDT with verteporfin every three months as needed plus a monthly sham injection (n = 143) or sham PDT as needed every 3 months plus a monthly injection of either 0.3 mg (n = 140) or 0.5 mg (n = 140) ranibizumab (Figure 3). After 24-months of follow-up, 90% of both ranibizumab treated groups lost less than 15 letters of visual acuity versus 65% in the PDT treated group (Figure 4). In the 0.5 mg ranibizumab cohort, 41%, and in the 0.3 mg group, 34% gained at least 15 letters of vision versus 6% in the PDT-treated patients (Figure 5). Mean visual acuity increased by 11.3 letters in the 0.5 mg ranibizumab plus sham PDT group whereas in the verteporfin PDT plus sham injection cohort it decreased by 10.4 letters. The risk of presumed endophthalmitis was about 1% among the ranibizumab-treated subjects. These results were similar to those seen in the MARINA trial and together they show that ranibizumab is the first agent that is able to cause significant visual improvement in a substantial number of patients with CNV due to AMD.
Figure 3.
ANCHOR trial design.
Figure 4.
In patients with classic subfoveal CNV due to AMD, ranibizumab prevented visual loss in a significantly higher number of patients than PDT (12-month results modified according to Brown et al 2006; 24-month results according to Schmidt-Erfurth et al 2007).
Figure 5.
In patients with classic subfoveal CNV due to AMD, ranibizumab improved vision significantly in up to 41% of patients (12-month results modified according to Brown et al 2006; 24-month results according to Schmidt-Erfurth et al 2007).
Although the studies were not designed to detect differences between the 0.3 mg and 0.5 mg doses, it was suggested that the 0.5 mg dose was superior. Moreover, there was no evidence of significant toxicity with either dose. Thus, 0.5 mg was the dose that was finally approved for intravitreal use in patients with neovascular AMD.
Patients who completed the ANCHOR or MARINA trial were offered the opportunity to participate in the HORIZON trial, an open-label extension trial in which re-injections of ranibizumab are given as needed. First results show that about half of the patients require re-injections within the first 6 months. Because the inhibition of VEGF is a non-curative approach, it is not foreseeable at any time in the future that patients will be able to definitely terminate the treatment.
No clinical head-to-head comparison between ranibizumab and pegaptanib, currently the only other anti-VEGF agent approved for the treatment of neovascular AMD, has been performed so far. However, the gains in mean visual acuity with ranibizumab (11.3 letters, ANCHOR 12 months; 7.2 letters, MARINA 12 months) versus a mean loss with pegaptanib (7.5 letters, VISION 12 month) are highly suggestive for the superiority of ranibizumab for the treatment of neovascular AMD (Stone et al 2006; Takeda et al 2007). However, there are other anti-VEGF medications on the horizon that may be even more effective than ranibizumab, eg, VEGF-Trap. Recently, a phase III clinical trial has started which will compare the effectiveness of VEGF-Trap and ranibizumab (VIEW1).
When adapting the data to real world conditions, the strict exclusion criteria of the ANCHOR and MARINA studies should be considered in order to avoid overestimation as well as an apples and oranges comparison (Rosenfeld et al 2006c): No lesions greater than 12 disc areas were included. For the lesion composition, the CNV portion within the lesion had to be 50% or more of the total lesion size. No subfoveal fibrosis or atrophy was allowed. No pre-treated eyes were considered and no concurrent intraocular condition was permitted. Only patients with signs of recent disease progression (≥10% increase in lesion size within 1 month, visual acuity loss >1 Snellen line, or subretinal hemorrhage ≤1 disc area) were included.
Despite the improvement in mean visual acuity, a proportion of 59% (ANCHOR) to 76% of patients (MARINA) showed no improvement. In this group, the absence of a functional increase could be caused either by the pre-existing damage, eg, irreversible loss of photoreceptors, VEGF-independent disease progression, or insufficient VEGF-inhibition by ranibizumab.
Might this be a sign of treatment coming too late? Strangely, also the area occupied by the CNV in the fellow eye seems to influence the severity of AMD changes (Abugreen et al 2003). That may support the possibility of a patient-specific risk profile which corresponds to the lesion size. In the ANCHOR and the MARINA trials, in the group that lost 15 letters or more, a significant increase in total lesion area after monthly ranibizumab injection was reported. As this group also differed from the gainers in initial lesion size, but not in the decrease in leakage area, the authors postulated an overlaying process of geographic atrophy not responsive to ranibizumab (Rosenfeld et al 2006c).
Searching for an optimized strategy for re-injection – the PIER and the PRONTO studies
In the MARINA and ANCHOR trials, improvement in visual acuity appeared to reach a plateau after around 4 months. Moreover, monthly intraocular injections entail a certain risk and are expensive. These considerations lead to the presumption of two phases: a loading phase, during which 3 doses are needed to maximize the initial response, and a maintenance phase, in which a less frequent or a flexible treatment regimen can reduce injection related risks and costs.
A less frequent injection schedule was first investigated in the PIER study (Mieler et al 2006). Patients with subfoveal CNV were randomly assigned to receive either 0.5 mg ranibizumab (n = 61) or 0.3 mg ranibizumab (n = 60) or placebo (n = 63). Enrolled patients received three injections with ranibizumab or sham injections every 4 weeks followed by re-injections every 3 months. Patients treated with ranibizumab showed a mean improvement in visual acuity of 2.9 and 4.8 letters (0.3 and 0.5 mg, respectively) at 3 months, but after 12 months there was a mean reduction of 1.6 and 0.2 letters (0.3 and 0.5 mg, respectively). Although ranibizumab-treated patients still had a much better outcome than the sham-injected patients, who lost on average 16.3 letters, in comparison to the results with the monthly regimen that was used in the MARINA and ANCHOR trials the outcome was worse.
Comparable to the PIER study is the EXCITE trial in which 350 patients with classic or occult CNV were assigned to receive three injections every 4 weeks followed by re-injections every 3 months of either ranibizumab 0.3 mg or 0.5 mg . However, the results of the EXCITE trial have not been published yet.
The outcomes from the PIER study suggest that a fixed schedule of injections every 3 months is not an appropriate strategy.
However, if a variable re-injection schedule dependent on optical coherence tomography (OCT) and other clinical changes is used, the outcome seems to be better, as the results from a small open-label trial (PRONTO study) suggest (Fung et al 2007). In this study 40 patients with subfoveal CNV received three monthly injections of 0.5 mg ranibizumab and thereafter re-injections only when one of the following criteria was fulfilled: a) increase of central retinal thickness of 100 μm or more, b) loss of at least 5 letter of visual acuity, c) persistence of sub- or intraretinal fluid 1 month after the last injection, d) new hemorrhage in the macula, or e) new onset classic CNV. OCT was performed at baseline and at least monthly after injection. Fluorescein angiograms were obtained at baseline and every 3 months thereafter. The mean change in visual acuity was an improvement of 9.3 letters, 95% of the patients lost less than 15 letters, and 35% gained at least 15 letters. On average, central retinal thickness decreased by 178 μm compared with baseline.
These outcomes are similar to those observed in the MARINA and the ANCHOR trial. The mean number of injections in the PRONTO study over the first year was 5.6 (versus 13 in the MARINA study). The rationale for an OCT guided re-injection regimen is also supported by the OCT results of the MARINA study in which a close correlation of foveal thickness and response to ranibizumab therapy could be observed (Kaiser et al 2007).
While one cannot compare the results of a small open-label non-randomized trial with those of a large randomized trial, it seems likely that monthly injections may not be required to achieve optimal results. The safety and efficacy of ranibizumab administered on as-needed dosing regimen will be assessed in the SUSTAIN study. However, in this non-randomized, open-label and uncontrolled phase III clinical trial, the 0.3 mg dose of ranibizumab will be used, which is somehow problematic because 0.5 mg is the approved, and possibly more effective dose (www.clinicaltrials.gov/show/NCT00331864).
Ranibizumab plus PDT
Combining anti-VEGF therapy with other interventions could provide further improvement or may decrease the need for frequent re-injections. One approach to achieve this goal may be combination therapy of ranibizumab with verteporfin-PDT since after PDT the production of VEGF is increased (Schmidt-Erfurth et al 2003; Tatar et al 2006).
Most patients undergoing PDT have persistent CNV perfusion and gradual recanalization of the CNV leading to the need for re-treatment can be observed (Schmidt-Erfurth et al 2002).
Inactivating VEGF-A by ranibizumab or another anti-VEGF agent shortly after or before PDT with verteporfin may stop CNV growth and vascular leakage. Furthermore, decreasing CNV perfusion by PDT may decrease the number of required ranibizumab injections and thus reduce the likelihood of injection-related adverse ocular events. Monkey experiments demonstrated that the combination of ranibizumab and verteporfin-PDT reduces leakage from laser-induced CNVs more than PDT alone (Hussain et al 2005).
In the FOCUS study, a phase I/II, multicenter, randomized, single-masked, controlled study patients with predominantly classic CNV were randomly assigned to receive PDT plus monthly sham injection (n = 56) or PDT plus monthly 0.5 mg ranibizumab ( n = 105). PDT was performed 7 days before initial ranibizumab or sham treatment and then quarterly as needed according to established criteria (Heier et al 2006a).
Sham and true injections were administered every month unless PDT was given, in which case the intravitreal injection was skipped. At 12 months 90.5% of patients treated with ranibizumab and PDT lost less than 15 letters, 23.8% gained at least 3 lines (15 letters), and had a mean increase of visual acuity of 4.9 letters, compared to 67.9%, 5.4% and a mean visual loss of 8.2 letters, respectively, in the cohort treated with PDT alone. The percentage of patients receiving repeat PDT was significantly less in the group receiving ranibizumab. At 24 months, similar results were observed with 88% of patients in the combination treatment group losing less than 15 letters (versus 75% in the PDT-treated group), 25% gaining at least three lines (versus 7% in the PDT only group) and a 12.4 letter benefit in mean visual acuity change from baseline versus patients treated with PDT alone (Novartis unpublished data).
The visual acuity and the safety results were not as good as those seen in patients with predominantly classic CNV treated with ranibizumab alone in the ANCHOR trial. Moreover, 12% of patients treated with combination therapy experienced severe intraocular inflammation. However, one must be careful while comparing the more impressive results of the ANCHOR trial with those seen in the FOCUS study because the inclusion criteria were different: In the FOCUS study many patients had already undergone at least one session of PDT prior to enrolment in the study, whereas in the ANCHOR study previous PDT treatment was an exclusion criteria. In addition, the ranibizumab formulation that was used in the FOCUS study (lyophilized ranibizumab) was not the same as that in the ANCHOR trial (the liquid ranibizumab formulation that later received FDA approval). Thus, the increased rate of intraocular inflammation was likely a result of the formulation used. The formulation was switched to liquid ranibizumab during the course of the FOCUS which subsequently may decrease the rate of intraocular inflammation.
In addition, the safety of liquid ranibizumab (0.5 mg) in combination with PDT is investigated in the PROTECT study. Patients were scheduled to receive liquid formulation ranibizumab one hour after PDT administration. Preliminary results showed a lower rate of intraocular inflammation than in the FOCUS study.
The role of PDT and ranibizumab combination therapy will be further assessed in the SUMMIT trial. This trial aims to evaluate the efficacy and safety of a combination therapy of PDT plus ranibizumab 0.5 mg (administered as needed based on pre-defined re-treatment criteria) versus ranibizumab 0.5 mg monotherapy. The SUMMIT trial encompasses three trials: The DENALI study, a 2-year phase IIIb trial which plans to enrol 300 patients with primary or active subfoveal CNV (all lesions) due to AMD in the United States and Canada. One arm of DENALI will also investigate the efficacy of combining ranibizumab with PDT with reduced fluence. The DENALI study is accompanied by the MONT BLANC study in Europe and the EVEREST trial in Asia (London New Drug Group, May 2007).
Finally, the notion of combining anti-VEGF medications with intravitreal triamcinolone (with or without PDT) and other treatments is already being explored by a number of investigators. However, if one includes variations in sequence, timing, and dose, the myriad possible combinations could easily become more confusing than helpful to ophthalmologists who are trying to select the best therapeutic option for their patients (Stone et al 2006).
Tolerability and safety of ranibizumab
The maximum tolerated single dose of lypophilized ranibizumab seems to be 0.5 mg. In a small series of patients (n = 27) with subfoveal CNV secondary to AMD, two experienced dose-limiting ocular inflammation with 1.0 mg (Rosenfeld et al 2005b). However, this inflammation usually is transient and self-limiting. An escalating injection scheme with increasing doses seems to be tolerated well, even if the dose is steadily increased up to 2.0 mg and injections are only 2 weeks apart (Rosenfeld et al 2006b). The approved 0.5 mg dose seems to be very well tolerated when given repeatedly (Heier et al 2006b). Results from the FOCUS and the PROTECT trials suggest that the liquid formulation (the currently approved formulation) is better tolerated than the reconstituted lyophilized formulation that caused uveitis in up to 4% of the patients (Heier et al 2006a).
However, 4 out of 5 presumed cases of endophthalmitis in the MARINA study were culture negative and there seems to be a concentration-dependent increase of intraocular inflammation.
Thus, a fair number of these cases of presumed endophthalmitis may be sterile and seem to have a good visual prognosis.
In addition, strict sterile injection conditions may decrease the rate of endophthalmitis well below 1%. We apply the same precautions for patients who receive intravitreal injection as for those who undergo cataract surgery (eg, using sterile gloves, sterile draping, irrigation of the conjunctiva with povidone-iodine, and performing the injection in the operating room). In the two pivotal phase III clinical trials, ranibizumab treatment was associated with a rate of less than 1.7% of serious adverse ocular events, including presumed endophthalmitis, retinal detachment, and uveitis. The endophthalmitis rate in the MARINA trial was 0.4% (0.3 mg) and 0.8% (0.5 mg) after 12 months, and 0.8% and 1.3% after 24 months, respectively. In the ANCHOR study endophthalmitis occurred at a comparable rate (Pieramici et al 2006).
The frequency of serious non-ocular adverse events was comparable for the ranibizumab-treated patients versus the sham or PDT-treated cohort in the MARINA, ANCHOR, and PIER trials.
The safety of ranibizumab (0.3 and 0.5 mg intravitreally) is further investigated in the SAILOR study, an ongoing phase IIIb clinical trial. In this study patients are randomly assigned to receive either 0.3 mg or 0.5 mg intravitreally every 4 weeks for 3 months and then as needed dependent on predefined retreatment criteria. Preliminary data from an interim safety analysis after 6 months showed a higher incidence of stroke in the 0.5 mg group (1.2% versus 0.3% in the 0.3 mg cohort). Patients with a history of stroke appeared to be at a higher risk of subsequent stroke (Genentech “Dear Health Care Provider” letter January 24, 2007).
However, the incidence of stroke in the 0.5 mg cohort was still well within the range that can be considered normal for the age group of the enrolled patients. But the rate of stroke for the 0.3 mg group was below the expected average incidence for the enrolled age group, therefore indicating less prevalent morbidity.
Further awareness seems necessary before surveillance data will allow a conclusive assessment.
Nevertheless, the findings of the 6-month interim analysis led Genentech to issue a letter to healthcare providers in the United States regarding the findings of this planned interim analysis. Currently the regulatory authorities in the European Union and other countries have not requested such a letter to be circulated.
There were no statistically significant differences between the two ranibizumab groups regarding other thromboembolic events such as myocardial infarction or vascular death. The fact that clinical trials are conducted on a carefully screened, relatively small group of patients under highly controlled circumstances limits the conclusions for moderately rare side-effects and the risks among understudied populations such as minorities and patients who have multiple health problems or chronic illnesses (Olson 2004).
Quality of life and anti-VEGF therapy
Quality-of-life data obtained during the MARINA trial by using the National Eye Institute Questionnaire (VFQ-25) (Maguire et al 2004) showed significant improvement in VFQ-25 scores in ranibizumab-treated patients (including vision specific dependency, near vision, distance vision, contrast sensitivity, and global vision – Chang et al 2007).
Using known disease-specific value-based medicine information (time trade-off utility) in patients with moderate and advanced neovascular AMD, therapy with ranibizumab appears to deliver an extraordinary degree of value compared to many other medical interventions (Brown et al 2007).
One should keep in mind that patients with moderate AMD have quality of life similar to those with moderate stroke or AIDS. Very severe AMD (visual acuity below 20/800 bilaterally) even approaches quality-of-life levels that are similar to those encountered with prostate cancer with uncontrolled pain or a stroke which leaves a patient bedridden and in need of constant care (Brown et al 2007).
Extrafoveal CNV due to AMD
No comparative trial of laser photocoagulation with ranibizumab (or other anti-VEGF agents) for extrafoveal CNV due to neovascular AMD has been performed so far. Laser photocoagulation remains a useful tool to halt progression of extrafoveal CNV.
There may be a rationale for combining laser with anti-VEGF therapy in the treatment of extrafoveal CNV due to neovascular AMD.
Ranibizumab (Lucentis) versus bevacizumab (Avastin)
Bevacizumab is substantially cheaper than ranibizumab (Steinbrook 2006; Rosenfeld 2006). A vast number of retrospective case series (Rosenfeld et al 2005a; Avery et al 2006; Bashshur et al 2006; Costa et al 2006; Rich et al 2006; Spaide et al 2006; Aisenbrey et al 2007; Chen et al 2007; Emerson et al 2007; Goff et al 2007; Lazic et al 2007) and one prospective non-randomized study (Costa et al 2006) found intravitreal bevacizumab to be very effective for subfoveal CNV due to neovascular AMD.
The intravitreal half-life of bevacizumab in humans is likely to be longer than that of ranibizumab (Mordenti et al 1999; Gaudreault et al 2005). That may be advantageous in respect to the number of required re-injections. On the other hand, the affinity of ranibizumab for VEGF-A is higher than that of bevacizumab (Ferrara et al 2006). It is unclear whether this has clinical implications. The presence of the Fc fragment in bevacizumab might render patients receiving bevacizumab more susceptible to the development of an immune response to the agent. This may pose the danger that recipients of repeat bevacizumab injections experience intraocular inflammation and that re-injections of the drug are less effective due to inactivation of the monoclonal antibody by the immune system.
However, these doubts may not be substantiated given the good clinical safety, tolerability and efficacy that have been documented in numerous patients including a database of thousands of bevacizumab-treated patients (Fung et al 2006; Maturi et al 2006; Michels 2006; Wu et al 2007; Ziemssen et al 2007).
In addition there is considerable experimental evidence that supports the safety of intravitreal bevacizumab (Luke et al 2006; Feiner et al 2006; Spitzer et al 2006; Spitzer et al 2007). However, this requires confirmation in controlled clinical trials, which are currently lacking.
Overall it seems likely that intravitreal bevacizumab and ranibizumab may have comparable visual benefits for patients suffering from neovascular AMD. Still one must keep in mind that intravitreal bevacizumab remains an off-label therapy that has not been approved by regulatory authorities for intraocular use. In addition, to date no standardized treatment protocols for re-evaluation and re-injection criteria for bevacizumab in neovascular AMD have been established.
It is unclear how bevacizumab and ranibizumab compare when given by intraocular injection, but a clinical trial sponsored by the NIH in the United States that will test this issue is underway (Comparison of AMD Treatments Trial – CATT).
However, even if a controlled clinical trial showed the two drugs to be equivalent, it remains unclear how bevacizumab might be authorized for intraocular use. Thus, even if the CATT trial should show the two drugs to be equivalent, the dilemma may go on for ophthalmologists who may feel pressured to continue using the more expensive but licensed alternative (Raftery et al 2007).
Conclusion
The impressive results with ranibizumab are far superior to those seen with PDT or pegaptanib. Therefore, monotherapy with ranibizumab has become the standard for care of patients with new onset of subfoveal CNV due to AMD regardless, of type and size of lesion. Ranibizumab can be administered either by monthly intravitreal injections or by three intraocular injections once a month followed by a re-injection schedule depending on changes of OCT findings and visual acuity. At this time, it cannot be predicted how long repeated intravitreal injections will have to be continued after 24 months. It is also unknown how ranibizumab and the many times cheaper bevacizumab compare when given by intraocular injection. Thus, head-to-head trials that assess comparative safety and efficacy of ranibizumab and bevacizumab are needed.
Currently, it is unclear whether combination therapy of ranibizumab with photodynamic therapy (PDT) provides any significant advantage over ranibizumab monotherapy.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose related to the manuscript.
References
- Abugreen S, Muldrew KA, Stevenson MR, et al. CNV subtype in first eyes predicts severity of ARM in fellow eyes. Br J Ophthalmol. 2003;87:307–11. doi: 10.1136/bjo.87.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–50. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- Adamis AP, Shima DT, Tolentino MJ, et al. Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularization in a nonhuman primate. Arch Ophthalmol. 1996;114:66–71. doi: 10.1001/archopht.1996.01100130062010. [DOI] [PubMed] [Google Scholar]
- Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotech. 2005;23:1147–57. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- Aisenbrey S, Lafaut BA, Szurman P, et al. Macular translocation with 360 degrees retinotomy for exudative age-related macular degeneration. Arch Ophthalmol. 2002;120:451–9. doi: 10.1001/archopht.120.4.451. [DOI] [PubMed] [Google Scholar]
- Aisenbrey S, Ziemssen F, Volker M, et al. Intravitreal bevacizumab (Avastin) for occult choroidal neovascularization in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2007;245:941–88. doi: 10.1007/s00417-006-0471-7. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Mohamedali KA, Silva RLE, et al. Vascular targeting of ocular neovascularization with a vascular endothelial growth factor121/gelonin chimeric protein. Mol Pharmacol. 2005;68:1543–50. doi: 10.1124/mol.105.015628. [DOI] [PubMed] [Google Scholar]
- Arias L, Garcia-Arumi J, Ramon JM, et al. Photodynamic therapy with intravitreal triamcinolone in predominantly classic choroidal neovascularization: one-year results of a randomized study. Ophthalmology. 2006;113:2243–50. doi: 10.1016/j.ophtha.2006.04.039. [DOI] [PubMed] [Google Scholar]
- Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–72. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Bashshur ZF, Bazarbachi A, Schakal A, et al. Intravitreal bevacizumab for the management of choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol. 2006;142:1–9. doi: 10.1016/j.ajo.2006.02.037. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Arnold J, Benchaboune M, et al. Verteporfin therapy of subfoveal choroidal neovascularization in patients with age-related macular degeneration: additional information regarding baseline lesion composition’s impact on vision outcomes-TAP report No. 3. Arch Ophthalmol. 2002;120:1443–54. doi: 10.1001/archopht.120.11.1443. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Bressler SB, Hawkins BS, et al. Submacular Surgery Trials Pilot Study Investigators. Submacular surgery trials randomized pilot trial of laser photocoagulation versus surgery for recurrent choroidal neovascularization secondary to age-related macular degeneration: I. Ophthalmic outcomes submacular surgery trials pilot study report number 1. Am J Ophthalmol. 130:387–407. doi: 10.1016/s0002-9394(00)00729-7. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Bressler SB, Haynes LA, et al. Verteporfin therapy for subfoveal choroidal neovascularization in age-related macular degeneration: four-year results of an open-label extension of 2 randomized clinical trials: TAP Report No. 7. Arch Ophthalmol. 2005;123:1283–5. doi: 10.1001/archopht.123.9.1283. [DOI] [PubMed] [Google Scholar]
- Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Eng J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- Brown MM, Brown GC, Brown H. Value-based medicine and interventions for macular degeneration. Curr Opin Ophthalmol. 2007;18:194–200. doi: 10.1097/ICU.0b013e3281377209. [DOI] [PubMed] [Google Scholar]
- Chang TS, Bressler NM, Fine JT, et al. Improved vision-related function after ranibizumab treatment of neovascular age-related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol. 2007;125:1460–9. doi: 10.1001/archopht.125.11.1460. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wiesmann C, Fuh G, et al. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol. 1999;293:865–81. doi: 10.1006/jmbi.1999.3192. [DOI] [PubMed] [Google Scholar]
- Chen CY, Wong TY, Heriot WJ, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration: a short-term study. Am J Ophthalmol. 2007;143:510–12. doi: 10.1016/j.ajo.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Costa RA, Jorge R, Calucci D, et al. Intravitreal bevacizumab for choroidal neovascularization caused by AMD (IBeNA Study): results of a phase 1 dose-escalation study. Invest Ophthalmol Vis Sci. 2006;47:4569–78. doi: 10.1167/iovs.06-0433. [DOI] [PubMed] [Google Scholar]
- Emerson MV, Lauer AK, Flaxel CJ, et al. Intravitreal bevacizumab (Avastin) treatment of neovascular age-related macular degeneration. Retina. 2007;27:439–44. doi: 10.1097/IAE.0b013e31804b3e15. [DOI] [PubMed] [Google Scholar]
- Ergun E, Maar N, Ansari-Shahrezaei S, et al. Photodynamic therapy with verteporfin and intravitreal triamcinolone acetonide in the treatment of neovascular age-related macular degeneration. Am J Ophthalmol. 2006;142:10–16. doi: 10.1016/j.ajo.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Feiner L, Barr EE, Shui YB, et al. Safety of intravitreal injection of bevacizumab in rabbit eyes. Retina. 2006;26:882–8. doi: 10.1097/01.iae.0000230717.85319.f5. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26:859–70. doi: 10.1097/01.iae.0000242842.14624.e7. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Fingar VH. Vascular effects of photodynamic therapy. J Clin Laser Med Surg. 1996;14:323–8. doi: 10.1089/clm.1996.14.323. [DOI] [PubMed] [Google Scholar]
- Fujii GY, de Juan E, Jr, Pieramici DJ, et al. Inferior limited macular translocation for subfoveal choroidal neovascularization secondary to age-related macular degeneration: 1-year visual outcome and recurrence report. Am J Ophthalmol. 2002;134:69–74. doi: 10.1016/s0002-9394(02)01511-8. [DOI] [PubMed] [Google Scholar]
- Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–83. doi: 10.1016/j.ajo.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Fung AE, Rosenfeld PJ, Reichel E. The International Intravitreal Bevacizumab Safety Survey: using the internet to assess drug safety worldwide. Br J Ophthalmol. 2006;90:1344–9. doi: 10.1136/bjo.2006.099598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudreault J, Fei D, Rusit J, et al. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46:726–33. doi: 10.1167/iovs.04-0601. [DOI] [PubMed] [Google Scholar]
- Gelisken F, Voelker M, Schwabe R, et al. Full macular translocation versus photodynamic therapy with verteporfin in the treatment of neovascular age-related macular degeneration: 1-year results of a prospective, controlled, randomised pilot trial (FMT-PDT) Graefes Arch Clin Exp Ophthalmol. 2007 Jan 12; doi: 10.1007/s00417-006-0524-y. (Epub) [DOI] [PubMed] [Google Scholar]
- Genentech “Dear Health Care Provider” letter January 24, 2007; available at http://www.gene.com./gene/products/information/tgr/lucentis/index.jsp.
- Goff MJ, Johnson RN, McDonald HR, et al. Intravitreal bevacizumab for previously treated choroidal neovascularization from age-related macular degeneration. Retina. 2007;27:432–38. doi: 10.1097/IAE.0b013e318042b53f. [DOI] [PubMed] [Google Scholar]
- Gragoudas ES, Adamis AP, Cunningham ET, et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–16. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- Green WR, Wilson DJ. Choroidal neovascularization. Ophthalmology. 1986;93:1169–76. doi: 10.1016/s0161-6420(86)33609-1. [DOI] [PubMed] [Google Scholar]
- Grossniklaus HE, Ling JX, Wallace TM, et al. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol Vis. 2002;8:119–26. [PubMed] [Google Scholar]
- Heiduschka P, Fietz H, Hofmeister S, et al. 7. Penetration of bevacizumab through the retina after intravitreal injection in the monkey. Invest Ophthalmol Vis Sci. 2000;48:2814–23. doi: 10.1167/iovs.06-1171. [DOI] [PubMed] [Google Scholar]
- Heier JS, Antoszyk AN, Pavan PR, et al. Ranibizumab for treatment of neovascular age-related macular degeneration: a phase I/II multicenter, controlled, multidose study. Ophthalmology. 2006b;113:633–642.e1-4. doi: 10.1016/j.ophtha.2005.10.052. [DOI] [PubMed] [Google Scholar]
- Heier JS, Boyer DS, Ciulla TA, et al. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration: year 1 results of the FOCUS Study. 2006a;Arch Ophthalmol;124:153215–42. doi: 10.1001/archopht.124.11.1532. Erratum in: Arch Ophthalmol, 2007;125:138. [DOI] [PubMed] [Google Scholar]
- Heussen FM, Fawzy NF, Joeres S, et al. Autologous translocation of the choroid and RPE in age-related macular degeneration: 1-year follow-up in 30 patients and recommendations for patient selection. Eye. 2007 Jul 20; doi: 10.1038/sj.eye.6702823. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Eng J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- Husain D, Kim I, Gauthier D, et al. Safety and efficacy of intravit-real injection of ranibizumab in combination with verteporfin PDT on experimental choroidal neovascularization in the monkey. Arch Ophthalmol. 2005;123:509–16. doi: 10.1001/archopht.123.4.509. [DOI] [PubMed] [Google Scholar]
- Kaiser PK, Blodi BA, Shapiro H, et al. Angiographic and Optical Coherence Tomographic Results of the MARINA Study of Ranibizumab in Neovascular Age-Related Macular Degeneration. Ophthalmology. 2007 Jul 11; doi: 10.1016/j.ophtha.2007.04.030. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BEK, Linton KP. The Beaver Dam Eye Study: the relation of age-related maculopathy to smoking 1993. Am J Epidemiol. 1993;137:190–200. doi: 10.1093/oxfordjournals.aje.a116659. [DOI] [PubMed] [Google Scholar]
- Krzystolik MG, Afshari MA, Adamis AP, et al. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol. 2002;120:338–46. doi: 10.1001/archopht.120.3.338. [DOI] [PubMed] [Google Scholar]
- Lazic R, Gabric N. Intravitreally administered bevacizumab (Avastin) in minimally classic and occult choroidal neovascularization secondary to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2007;245:68–73. doi: 10.1007/s00417-006-0466-4. [DOI] [PubMed] [Google Scholar]
- Lei J, Jiang A, Pei D. Identification and characterization of a new splicing variant of vascular endothelial growth factor: VEGF183. Biochim Biophys Acta. 1998;443:400–6. doi: 10.1016/s0167-4781(98)00240-1. [DOI] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–9. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- London New Drugs Group. May 2007. Ranibizumab, pegaptanib and bevacizumab for the treatment of age-related macular degeneration. APC/DTC Briefing.
- Luke M, Warga M, Ziemssen F, et al. Effects of bevacizumab on retinal function in isolated vertebrate retina. Br J Ophthalmol. 2006;90:1178–82. doi: 10.1136/bjo.2006.094995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren RE, Uppal GS, Balaggan KS, et al. Autologous transplantation of the retinal pigment epithelium and choroid in the treatment of neovascular age-related macular degeneration. Ophthalmology. 2007;114:561–70. doi: 10.1016/j.ophtha.2006.06.049. [DOI] [PubMed] [Google Scholar]
- Macular photocoagulation study group. Argon laser photocoagulation for neovascular maculopathy. Five-year results from randomized clinical trials. Arch Ophthalmol. 1991;109:1109–14. [PubMed] [Google Scholar]
- Maguire M Complications of Age-Related Macular Degeneration Prevention Trial Research Group. Baseline characteristics, the 25-Item National Eye Institute Visual Functioning Questionnaire, and their associations in the Complications of Age-Related Macular Degeneration Prevention Trial (CAPT) Ophthalmology. 2004;111:1307–16. doi: 10.1016/j.ophtha.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Maturi RK, Bleau LA, Wilson DL. Electrophysiologic findings after intravitreal bevacizumab (Avastin) treatment. Retina. 2006;26:270–4. doi: 10.1097/00006982-200603000-00003. [DOI] [PubMed] [Google Scholar]
- Michels S. Is intravitreal bevacizumab (Avastin) safe? Br J Ophthalmol. 2006;90:1333–4. doi: 10.1136/bjo.2006.102293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieler WF. PIER: Year 1 FA/OCT Results in a Study of Ranibizumab (Lucentis™) for Choroidal Neovascularisation (CNV) due to Age related Macular Degeneration (AMD). Program and abstract of the 24th Annual American Society of Retina Specialists and 6th Annual European Vitreoretinal Society Meeting; September 9–13, 2006; Cannes, France. 2006. Abstract 507. [Google Scholar]
- Mordenti J, Thomsen K, Licko V, et al. Intraocular pharmacokinetics and safety of a humanized monoclonal antibody in rabbits after intravit-real administration of a solution or a PLGA microsphere formulation. Toxicol Sci. 1999;52:101–6. doi: 10.1093/toxsci/52.1.101. [DOI] [PubMed] [Google Scholar]
- Moshfeghi AA, Rosenfeld PJ, Puliafito CA, et al. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration. Twenty-four-week results of an uncontrolled open-label clinical study. Ophthalmology. 2006;113:2002–11. doi: 10.1016/j.ophtha.2006.05.070. [DOI] [PubMed] [Google Scholar]
- Mruthyunjaya P, Stinnett SS, Toth CA, et al. Change in visual function after macular translocation with 360 degrees retinectomy for neovascular age-related macular degeneration. Ophthalmology. 2004;111:1715–24. doi: 10.1016/j.ophtha.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Muller YA, Chen Y, Christinger HW, et al. VEGF and the Fab fragment of a humanized neutralizing antibody: crystal structure of the complex at 2.4 A resolution and mutational analysis of the interface. Structure. 1998;6:1153–67. doi: 10.1016/s0969-2126(98)00116-6. [DOI] [PubMed] [Google Scholar]
- Olson MK. Are novel drugs more risky for patients than less novel drugs? J Health Econ. 2004;23:1135–58. doi: 10.1016/j.jhealeco.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Pieramici DJ, Avery RL. Ranibizumab: treatment in patients with neovascular age-related macular degeneration. Expert Opin Biol Ther. 2006;6:1237–45. doi: 10.1517/14712598.6.11.1237. [DOI] [PubMed] [Google Scholar]
- Pierce EA, Foley ED, Smith LE. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol. 1996;114:1219–28. doi: 10.1001/archopht.1996.01100140419009. [DOI] [PubMed] [Google Scholar]
- Presta LG, Chen H, O’Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–9. [PubMed] [Google Scholar]
- Rakic JM, Lambert V, Devy L, et al. Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3186–93. doi: 10.1167/iovs.02-1092. [DOI] [PubMed] [Google Scholar]
- Raftery J, Clegg A, Jones J, et al. Ranibizumab (Lucentis) versus bevacizumab (Avastin): modelling cost effectiveness. Br J Ophthalmol. 2007;91:1244–6. doi: 10.1136/bjo.2007.116616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich RM, Rosenfeld PJ, Puliafito CA, et al. Short-term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Retina. 2006;26:495–511. doi: 10.1097/01.iae.0000225766.75009.3a. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ. Intravitreal avastin: the low cost alternative to lucentis? Am J Ophthalmol. 2006;142:141–3. doi: 10.1016/j.ajo.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Eng J Med. 2006a;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heijer JS, et al. Supplementary Appendix to Ranibizumab for neovascular age-related macular degeneration. N Eng J Med. 2006c;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Heier JS, Hantsbarger G, et al. Tolerability and efficacy of multiple escalating doses of ranibizumab (Lucentis) for neovascular age-related macular degeneration. Ophthalmology. 2006b;113:623–32.e1. doi: 10.1016/j.ophtha.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Moshfeghi AA, Puliafito CA, et al. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005a;36:331–35. [PubMed] [Google Scholar]
- Rosenfeld PJ, Schwartz SD, Blumenkranz MS, et al. Maximum tolerated dose of a humanized anti-vascular endothelial growth factor antibody fragment for treating neovascular age-related macular degeneration. Ophthalmology. 2005b;112:1048–53. doi: 10.1016/j.ophtha.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Schmidt-Erfurth U, Michels S, Barbazetto I, et al. Photodynamic effects on choroidal neovascularization and physiological choroid. Invest Ophthalmol Vis Sci. 2002;43:830–41. [PubMed] [Google Scholar]
- Schmidt-Erfurth UM, Richard G, Augustin A, et al. Guidance for the treatment of neovascular age-related macular degeneration. Acta Ophthalmol Scand. 2007;85:486–94. doi: 10.1111/j.1600-0420.2007.00979.x. [DOI] [PubMed] [Google Scholar]
- Schmidt-Erfurth U, Schlotzer-Schrehard U, Cursiefen C, et al. Influence of photodynamic therapy on expression of vascular endothelial growth factor (VEGF), VEGF receptor 3, and pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2003;44:4473–80. doi: 10.1167/iovs.02-1115. [DOI] [PubMed] [Google Scholar]
- Senger DR, Galli SJ, Dvorak AM. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–5. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Shahar J, Avery RL, Heilweil G, et al. Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin) Retina. 2006;26:262–9. doi: 10.1097/00006982-200603000-00002. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Laud K, Fine HF, et al. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006;26:383–90. doi: 10.1097/01.iae.0000238561.99283.0e. [DOI] [PubMed] [Google Scholar]
- Spitzer MS, Wallenfels-Thilo B, Sierra A, et al. Antiproliferative and cytotoxic properties of bevacizumab on different ocular cells. Br J Ophthalmol. 2006;90:1316–21. doi: 10.1136/bjo.2006.095190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer MS, Yoeruek E, Sierra A, et al. Comparative antiproliferative and cytotoxic profile of bevacizumab (Avastin), pegaptanib (Macugen) and ranibizumab (Lucentis) on different ocular cells. Graefes Arch Clin Exp Ophthalmol. 2007 Mar 9; doi: 10.1007/s00417-007-0568-7. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Steinbrook R. The price of sight – ranibizumab, bevacizumab, and the treatment of macular degeneration. N Engl J Med. 2006;355:1409–12. doi: 10.1056/NEJMp068185. [DOI] [PubMed] [Google Scholar]
- Stone EM. A very effective treatment for neovascular macular degeneration. N Engl J Med. 2006;355:1493–5. doi: 10.1056/NEJMe068191. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 2005;109:227–41. doi: 10.1042/CS20040370. [DOI] [PubMed] [Google Scholar]
- Takeda AL, Colquitt JL, Clegg AJ, et al. Pegaptanib and ranibizumab for neovascular age-related macular degeneration: a systematic review. Br J Ophthalmol. 2007 doi: 10.1136/bjo.2007.118562. (Epub ahead of print; May 2 2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar O, Adam A, Shinoda K, et al. Expression of VEGF and PEDF in choroidal neovascular membranes following verteporfin photodynamic therapy. Am J Ophthalmol. 2006;142:95–104. doi: 10.1016/j.ajo.2006.01.085. [DOI] [PubMed] [Google Scholar]
- Virgili G, Bini A. Laser photocoagulation for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2000;8:CD004763. doi: 10.1002/14651858.CD004763.pub2. [DOI] [PubMed] [Google Scholar]
- Wells JA, Murthy R, Chibber R, et al. Levels of vascular endothelial growth factor are elevated in the vitreous of patients with subretinal neovascularisation. Br J Ophthalmol. 1996;80:363–6. doi: 10.1136/bjo.80.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormald R, Evans J, Smeeth L, Henshaw K. Photodynamic therapy for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2005;19:CD002030. doi: 10.1002/14651858.CD002030.pub2. [DOI] [PubMed] [Google Scholar]
- Wu L, Martinez-Castellanos MA, Quiroz-Mercado H, et al. Twelvemonth safety of intravitreal injections of bevacizumab (Avastin(R)): results of the Pan-American Collaborative Retina Study Group (PACORES) Graefes Arch Clin Exp Ophthalmol. 2007 doi: 10.1007/s00417-007-0660-z. Aug 3 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- www.clinicaltrials.gov/show/NCT00331864.
- Zarbin M, Szirth B. Current treatment of age-related macular degeneration. Optom Vis Sci. 2007;84:559–72. doi: 10.1097/OPX.0b013e3180de4dd7. [DOI] [PubMed] [Google Scholar]
- Ziemssen F, Luke M, Messias A, et al. Safety monitoring in bevacizumab (Avastin) treatment: retinal function assessed by psychophysical (visual fields, colour vision) and electrophysiological (ERG/EOG) tests in two subgroups of patients. Int Ophthalmol. 2007 July 20; doi: 10.1007/s10792-007-9122-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]