Abstract
Purpose
To test the results for patients treated with combined photodynamic therapy (PDT) with vertiporfin (Visudyne, Novartis AG) and intravitreal bevacizumab for choroidal neovascularization (CNV) secondary to age-related macular degeneration (AMD).
Patients and methods
This is a prospective study including 18 eyes with subfoveal or juxtafoveal CNV secondary to AMD. Patients were treated with intravitreal bevacizumab 2.5 mg in the morning then PDT with vertiporfin in the evening of the same day. All patients were followed up for 6 months. The main outcome measures were stabilization (no change) or improvement of best corrected visual acuity (BCVA) with no leakage in fluorescein angiography (FLA) and reduction of central retinal thickness, and retreatment rate.
Results
At the end of 6 months follow up, all cases had either stabilization or improved BCVA. Fifteen eyes (80%) showed improved BCVA. The overall mean improvement in BCVA (n = 18) was 2.17 lines. Fifteen eyes (80%) required single combined treatment. Only 3 eyes (20%) required retreatment with the same protocol. No systemic or ocular complications were reported.
Conclusion
Combined intravitreal bevacizumab and PDT as a treatment of CNV secondary to AMD either for predominantly classic or occult subtypes has a positive therapeutic effect with stabilization or improvement of final BCVA and also might reduce the need for retreatment compared with literature retreatment rates of either modality alone.
Summary
Eighteen patients receiving combined therapy with PDT and intravitreal bevacizumab for CNV secondary to AMD, showed not only significant visual improvement but also reduction in the frequency of retreatment when compared to the results of monotherapy with each modality.
Keywords: intravitreal bevacizumab, photodynamic therapy, vertiporfin, choroidal neovascularization, age-related macular degeneration
Background
The beneficial effect of photodynamic therapy (PDT) treatment for choroidal neovascularization (CNV) secondary to age-related macular degeneration (AMD) has been addressed in several reports (TAP 1999). Other reports also addressed the beneficial effect of combined PDT and Triamcinolone acetonide in CNV secondary to AMD (Rechtman 2004; Spaide 2005). Recent reports addressed the beneficial effect of “off-label use of bevacizumab”, an antibody against vascular endothelial growth factor (VEGF), in treatment of CNV (Rosenfield 2005; Avery 2006).
Anti-VEGF agents have been used as a primary treatment for neovascular AMD (Gragoudas 2004; Rosenfield 2005; Avery 2006). This modality has gained wide spread use after the encouraging results of improvement of visual acuity in several published reports in spite the patient may receive injection every 4–6 weeks requiring frequent follow-up visits with increasing risks of endophthalmitis and also no guarantee of permanent cure of the CNV and its neovascular complex even with repeated injection (Gragoudas et al 2004; Gruewald et al 2006). Prevention of visual loss and improvement of the mean best corrected visual acuity (BCVA) were reported in 2 recent, double-blind, randomized, controlled studies carried out with ranibizumab (Rosenfeld et al 2006; Brown et al 2006). Even after the approval by the Food and Drug Administration of ranibizumab for the treatment of CNV due to AMD (in June 2006), bevacizumab is still used today in this off-label indication because its cost is considerably lower than that of ranibizumab (Rosenfeld 2006).
Combined treatment of bevacizumab (anti-VEGF monoclonal antibody) with PDT may have a synergistic effect that could reduce the need for retreatment. The VEGF inhibition in combined treatment not only will reduce the inflammation of the neovascularization but it will also limit the induced edema after the laser while allowing for closure of the neovascular complex. PDT itself will eradicate the accessory support cells that were not targeted by the VEGF inhibitor (Dhalla et al 2006; Spaide 2006).
Methods
We constructed a prospective study of 18 CNVs, of 18 patients secondary to AMD treated by combined intravitreal injection of bevacizumab and PDT with vertiporfin (Visudyne, Novartis AG). All patients completed follow up for 6 months after treatment. Inclusion criteria were as follows: only AMD patients with either subfoveal or juxtafoveal CNV and either predominantly classic or occult types were included. Lesion size was less than 5400 μm. Only patients with no previous treatment were included in the study. Exclusion criteria included patients with CNV under 55 years old, minimally classic membranes, cases that had been previously treated with any treatment modality, and base line intraocular pressure >21 mmHg. The study was approved by the ethics committee of the eye clinic. The off-label use of bevacizumab as well as its potential risks and benefits were discussed with each subject. All patients signed written informed consent. The treatment protocol is to inject 2.5 mg of bevacizumab in the vitreous of the treated eye under complete sterile condition in operating theater (in the morning) followed by PDT treatment in the evening of the same day. Pretreatment complete ophthalmic examination including BCVA, biomicroscopic fundus examination, and pretreatment fluorescein angiography (FLA) and optical coherence tomography (OCT) was done for all patients. All patients were examined at one day after treatment, at one week, at one month, and then monthly thereafter. FLA and OCT were performed twice; after 3 months and at the end of 6 months. The main outcome measures were stabilization (no change) or improvement of BCVA with no leakage in FLA and reduction of central retinal thickness (CRT), and retreatment rate as well as recording systemic and ocular complications. Retreatment was considered when there is persistent subretinal fluid according to OCT and persistent leakage on FLA. Retreatment was done according to the same combined protocol and no retreatment was done before 3 months after the initial therapy.
Bevacizumab injection
2.5 mg in 0.1 mL bevacizumab (Avastin, Genentech, San Francisco, CA) was injected intravitreal under complete sterile preparation in the operating theater in the usual sterile fashion (Aiello et al 2004) using topical anesthesia. Prophylactic topical antibiotic (gatifloxacine) was given for 2 days post-injection.
PDT
PDT (Visudyne, Novartis AG, Buelach, Switzerland) was performed as described previously by the TAP Study Group (1999).
Statistical analysis
Visual acuity was measured using Snellen’s chart and was converted to Log MAR for calculation of the means. Values were expressed as mean ± standard deviation. Lines of vision lost or gained were calculated as Snellen’s lines of vision (via Log MAR). Student’s t-test was used to compare visual acuity changes. p < 0.05 was considered statistically significant.
Results
Eighteen eyes in 18 consecutive patients were included in this study. All the patient characteristics are listed in Table 1. The study includes 10 male and 8 females. The age of patients ranged from 62 to 82 years with a mean of 72.8 ± 6.5 years.
Table 1.
Patient characteristics and results of combined photodynamic therapy and intravitreal bevacizumab
| Pre-treat. V/A
|
6-Mo. V/A
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Sex | Age (y) | CNV size (μm) | CNV type | CNV location | No. of retreatment | dicimal | Ft. 20/ | LogMAR | dicimal | Ft. 20/ | LogMAR | CRT basic (μm) | CRT final (μm) |
| 1 | F | 70 | 1,600 | PC | SF | 1 | 0.1 | 200 | 1 | 0.2 | 100 | 0.7 | 520 | 280 |
| 2 | F | 65 | 2,000 | PC | SF | 1 | 0.2 | 100 | 0.7 | 0.32 | 60 | 0.5 | 550 | 275 |
| 3 | M | 72 | 2,200 | O | SF | 1 | 0.12 | 160 | 0.9 | 0.25 | 80 | 0.6 | 650 | 240 |
| 4 | M | 80 | 2,300 | PC | JF | 2 | 0.05 | 400 | 1.3 | 0.05 | 400 | 1.3 | 850 | 400 |
| 5 | M | 65 | 1,800 | O | JF | 1 | 0.1 | 200 | 1 | 0.12 | 160 | 0.9 | 800 | 270 |
| 6 | M | 62 | 1,600 | PC | SF | 1 | 0.1 | 200 | 1 | 0.1 | 200 | 1 | 800 | 310 |
| 7 | F | 72 | 1,400 | O | SF | 1 | 0.2 | 100 | 0.7 | 0.32 | 60 | 0.5 | 620 | 230 |
| 8 | F | 73 | 2,000 | O | JF | 1 | 0.2 | 100 | 0.7 | 0.25 | 80 | 0.6 | 450 | 250 |
| 9 | M | 75 | 950 | PC | JF | 1 | 0.05 | 400 | 1.3 | 0.5 | 40 | 0.3 | 800 | 280 |
| 10 | M | 80 | 850 | PC | JF | 1 | 0.25 | 80 | 0.6 | 0.32 | 60 | 0.5 | 450 | 280 |
| 11 | M | 77 | 2,000 | PC | SF | 1 | 0.1 | 200 | 1 | 0.2 | 100 | 0.7 | 420 | 250 |
| 12 | F | 80 | 2,400 | O | SF | 1 | 0.1 | 200 | 1 | 0.25 | 80 | 0.6 | 500 | 230 |
| 13 | M | 70 | 2,400 | PC | SF | 1 | 0.1 | 200 | 1 | 0.2 | 100 | 0.7 | 520 | 220 |
| 14 | M | 69 | 3,000 | O | SF | 2 | 0.05 | 400 | 1.3 | 0.1 | 200 | 1 | 400 | 210 |
| 15 | M | 62 | 2,800 | O | SF | 2 | 0.05 | 400 | 1.3 | 0.05 | 400 | 1.3 | 715 | 320 |
| 16 | F | 80 | 2,700 | PC | JF | 1 | 0.12 | 160 | 0.9 | 0.2 | 100 | 0.7 | 400 | 270 |
| 17 | F | 82 | 2,600 | PC | SF | 1 | 0.05 | 400 | 1.3 | 0.1 | 200 | 1 | 580 | 280 |
| 18 | M | 77 | 2,000 | O | JF | 1 | 0.2 | 100 | 0.7 | 0.25 | 80 | 0.6 | 600 | 220 |
| Mean | 72.8 | 2,033 | 1.1667 | 0.11 | 20/190 | 0.98 | 0.18 | 20/112 | 0.75 | 590.278 | 267.5 | |||
| SD | 6.5 | 597.29 | 0.3835 | 2.4 lines | 0.24 | 2.8 lines | 0.277 | 149.5 | 45.25 | |||||
Abbreviations: CRT, central retinal thickness; SD, standard deviation; Ft., feet; CNV, subretinal neovascular membrane; V/A, best corrected visual acuity.
The CNV lesion size, among all lesion subtypes, ranged from 850 μm to 3000 μm with a mean of 2033 ± 597.3 μm. Ten membranes were predominantly classic type with a mean lesion size of 1900 μm wherein the classic CNV represents more than 50% of the lesion size. The other 8 had occult leakage pattern in FLA with a mean lesion size of 2200 μm. As regards the lesion site, subfoveal lesions (n = 11) had a mean lesion size of 2181 μm and while juxtafoveal lesions (n = 7) had a mean lesion size of 1800 μm.
All the patients (n = 18) included in this study got either stabilization (no change) or improvement of BCVA at the end of follow up period, both associated with no recurrent leakage in FLA and reduction of the pretreatment CRT by OCT due to absorption of pretreatment subretinal fluid (SRF) (Figures 1 and 2). The overall mean improvement in BCVA was 2.17 lines (p = 0.004, paired samples t test). Fifteen eyes (80%) showed improved BCVA by the end of follow up period while 3 eyes (20%) showed stabilization; all the 3 were of large lesion size as shown in Table 1.
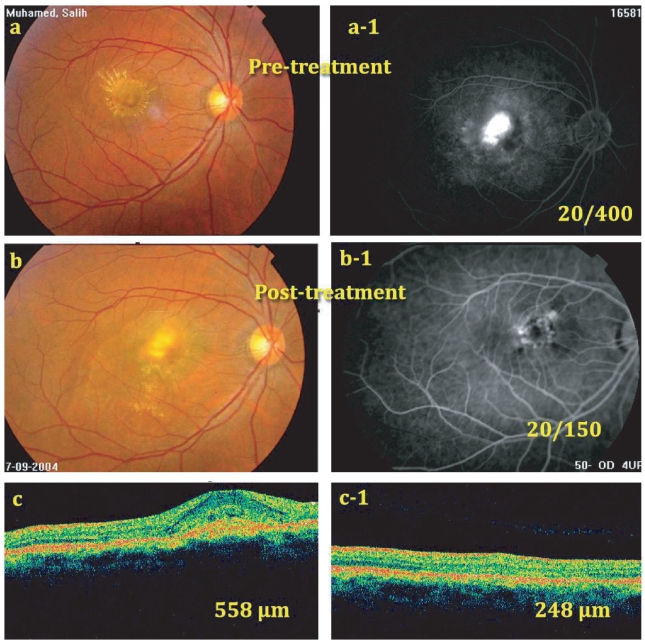
Figure 1.
(a) and (a-1) Pretreatment fundus photo and pretreatment FLA, late phase, showing filling of predominantly classic CNV occupying more than 50% of the lesion. Pretreatment BCVA = 20/400. (b) and (b-1) Post-treatment fundus photo and FLA, late phase, at 6 months showing closure of the CNV with only late leakage at its upper temporal edge (BCVA = 20/150). (c) Pretreatment OCT showing CNV with CRT = 558 μm. (c-1) OCT, 6 months post-treatment showing absorption of most of subretinal fluid and reduction of CRT to 248 μm. However, fovea did not return completely to normal configuration.
Abbreviations: BCVA, best corrected visual acuity; CNV, choroidal neovascularization; CRT, central retinal thickness; FLA, fluorescein angiography; OCT, optical coherence tomography.
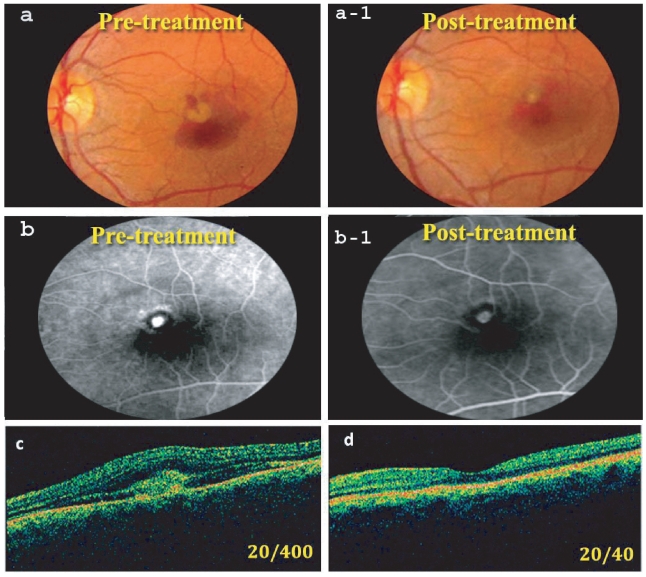
Figure 2.
(a) Pretreatment fundus photo showing juxtafoveal CNV with subretinal hemorrhage occupying about 50% of the lesion (BCVA = 20/400). (a-1) Post-treatment fundus photo at 6 months. Note regression of the membrane and absorption of most subretinal blood (BCVA = 20/40). (b) Pretreatment FLA, showing active CNV. (b-1) Post-treatment FLA showing CNV staining with no leakage. (c) Pretreatment OCT showing the CNV with fluid in the subretinal space (CRT = 800 μm). (d) OCT 6 months post-treatment with almost normal foveal configuration (CRT = 280 μm).
Abbreviations: BCVA, best corrected visual acuity; CNV, choroidal neovascularization; CRT, central retinal thickness; FLA, fluorescein angiography; OCT, optical coherence tomography.
Only 3 eyes (20%) required retreatment with the same protocol after 3 months from the initial treatment. No more cases required treatment at 6 months. Average retreatment rate was 1.17 ± 0.38.
For the predominantly classic membranes (n = 10), Mean improvement in BCVA was 2.5 lines (p = 0.014, paired samples t test), 2 eyes (20%) showed stabilization and 8 eyes (80%) showed improved BCVA by the end of follow up period and only 2 eyes (20%) required retreatment with the same protocol after 3 months from the initial treatment.
One membrane of the occult type (n = 8) (12.5%) showed stabilization and the rest 7 membranes (87.5%) showed visual improvement by the end of follow up period. The mean improvement in BCVA was 1.92 lines. (p = 0.005, paired samples t test) One eye (12.5%) required another treatment according to the original protocol, 3 months after the first treatment. There was no significant difference between predominantly classic and occult subtypes concerning the visual improvement. (p = 0.853)
As regards the subfoveal location (n = 11), mean improvement in BCVA was 2.75 lines (p = 0.006, paired samples t test). Two eyes (18.2%) showed stabilization and 9 eyes (81.8%) showed visual improvement. Two eyes (18.2%) required another treatment also after 3 months from the initial treatment. As regard the juxtafoveal location (n = 7), mean improvement in BCVA was 2.33 lines (p < 0.1, paired samples t test). One eye (14.3%) showed stabilization and 6 eyes (85.7%) showed visual improvement. One eye (14.3%) required another treatment in the course of follow up. There was no significant difference between subfoveal and juxtafoveal subtypes concerning the visual improvement ( p = 0.856). The characteristics of different CNV subtypes are shown in Table 2.
Table 2.
Choroidal neovascularization subtype characteristics
| Lesion subtype | Mean lesion size (μm) | Patients with stabilization | Patient with visual gaining | Retreatment rate | p value between subgroups† |
|---|---|---|---|---|---|
| PC (10) | 1,900 | 20% | 80% | 20% | p = 0.853 |
| O (8) | 2,200 | 12.5% | 87% | 12.5% | |
| SF (11) | 2,181 | 18.18% | 81.8% | 18.18% | p = 0.856 |
| JF (7) | 1,800 | 14.28% | 85.7% | 14.28% |
Abbreviations: PC, predominantly classic; O, occult; SF, subfoveal; JF, juxtafoveal.
Notes: The difference in visual improvement between different subgroups was statistically insignificant (Student’s t-test for unpaired data).
Pretreatment visual acuity ranged from 20/80 to 20/400 and the post treatment visual acuity ranged from 20/40 to 20/400, the maximum visual gain was observed in one eye that improved from 20/400 to 20/40 after treatment and this membrane was relatively small (950 μm), and of the predominantly classic sub type with juxtafoveal location.
As regards the pretreatment central retinal thickness (CRT) by OCT it ranged from 400 to 850 μm with a mean of 590.3 ± 149.5 μm that significantly improved at the end of follow up period to a mean of 267.5 ± 45.3 μm with range of 210–400 μm (p < 0.001, paired samples t test).
There were no complications of treatment in this study group including endophthalmitis, ocular hypertension, uveitis, or any other systemic complications.
Discussion
According to this study there is very obvious beneficial effect of combined bevacizumab injection and PDT for the treatment of CNV secondary to AMD. It helps not only to stabilize but also to improve vision usually lost by this disease entity. Mean improvement in the post treatment visual acuity in our study (2.17 lines) is almost similar to the previously described newly treated patient who received monthly bevacizumab as a monotherapy (Miller 2005; Avery et al 2006).
Despite the demonstrated efficacy, a substantial proportion of eyes still developed moderate to severe visual loss after PDT. Our results are superior to the results of PDT alone where patients had loss of vision with an average of 2.2 lines in the Treatment of Age Related Macular Degeneration with PDT study (TAP 1999). In the VIP study (2001), average visual loss was 3.1 lines. Moreover, 50% of patients with occult CNV in AMD treated with PDT developed visual loss of 3 or more lines after 1 year as demonstrated in the VIP study (2001).
This gradual decline of vision in the course of therapy may be limited with the use of triamcinolone in conjunction with PDT. Nonrandomized studies by Spaide and colleagues (2003, 2005) and Rechtman and colleagues (2004), showed that after 6 to 12 months follow up, combined PDT/triamcinolone acetonide treatment of CNV due to AMD resulted in better outcome than PDT alone. The frequency of PDT is reduced with a certain degree of visual stabilization. The effect of triamcinolone is due to its anti-inflammatory and anti-angiogenic effect (Danis et al 1996; Ciulla et al 2001), as well as the reduction of choriocapillary damage after PDT (Chan et al 2006). However, the improvement of visual acuity seems to be limited (Spaide et al 2005; Chan et al 2006) in addition to several complications like secondary glaucoma and cataract progression that were encountered with its use (Junas et al 2003; Gillies et al 2004).
The use of anti VEGF is getting popularity as a primary treatment for wet AMD. This treatment has demonstrated improvement of vision. However, patients treated with anti-VEGF agent often receive intravitreal injections on regular 4–6 weeks interval with increasing potential risk of ocular complications (Rosenfield et al 2005; Avery et al 2006). Recent studies showed ranibizumab, an antibody fragment that inhibits VEGF, to be an effective treatment with visual improvement for exudative AMD but those patients still require multiple periodic injections (Heier et al 2006). The visual improvement in our study matches the results of Dhalla and colleagues (2006), who reported 80% stabilization and 67% improvement of vision with average 2.04 lines after 7 months following combined PDT and intravitreal bevacizumab. In our study, the visual positive effect involved all subtypes including subfoveal and juxtafoveal, classic and occult. This also matches the results of Dhalla and colleagues (2006). Eyes with subfoveal as well as classic CNV showed the greatest mean visual improvement (2.75, 2.5 lines). The TAP study (1999) also reported a better response of classic lesions to PDT. The visual improvement was statistically insignificant only in the juxtafoveal subgroup. This might be due to the fact that lesion does not involve the center of the fovea, which is affected only with subretinal fluid.
The visual improvement seen in our study as well as Dhalla and colleagues (2006) study is possibly due to the bevacizumab effect. This is supported by the findings of Lazic and Gabric (2007), in a randomized clinical trial, who reported significant visual improvement after 3 months in cases treated with bevacizumab alone as well as cases treated with combined bevacizumab and PDT and slight worsening in cases treated with PDT alone.
Another beneficial effect of combined therapy is the reduction of treatment frequency. For patients treated with PDT alone in the TAP study (1999), the need for retreatment at three months is 90.8% and slightly less at month 6 and required an average of 3.3 retreatments in the first year. Patients treated with anti-VEGF agents require intravitreal injections every 4 to 6 weeks (Gragoudas 2004; Miller 2005)
In our study we reported a retreatment rate of 20% of the whole case series after 6 months. Dhalla and colleagues (2006) reported a retreatment rate of 37% in 7 months using the same combined therapy. The frequency of PDT was found to be significantly reduced when combined with triamcinolone intravitreal injection with retreatment rate of 1.24 (Spaide 2005). Our results as well as those of Dhalla and colleagues (2006) showed that combined therapy reduces the frequency of both PDT and intravitreal injection thus reducing the economic cost of treatment and reduces the risk of complications of intravitreal injection as endophthalmitis and retinal detachment.
One of the reasons for less favorable outcome in treating CNV secondary to AMD with PDT might be because of collateral damage of adjacent structures as choriocapillary hypo perfusion and retinal pigment epithelium atrophy after PDT (Flower et al 2001; Moshfeghi et al 2003; Wachtlin et al 2003). The associated damage results in retinal edema and release of angiogenesis factors such as VEGF (Rogers et al 2002; Schmidt-Erfurth et al 2003).
Meanwhile, the neovascular beds that have mature smooth muscle and pericyte support don’t regress completely with VEGF inhibition and may lay dormant only as long as VEGF is suppressed (Bergers et al 2003; Erber et al 2004; Dhalla et al 2006), hence the need for frequent injections. So, nonthermal laser may be necessary to disrupt the anatomical component of CNV that do not respond to VEGF inhibition (Dhalla et al 2006), and anti-VEGF might be necessary to reduce the damage of the VEGF released by PDT. This might give justification and also an explanation for the efficacy of this combined treatment. Minimally classic and occult membranes are located more deeply than classical membranes and their exposure to light is reduced by surrounding factors. Because light activation of the drugs present in these membranes is less effective, this could explain the limited benefits of PDT in minimally classic and occult CNV (Lazic and Gabric 2007). A way to overcome this difficulty is to use intravitreal therapeutic agents that, by diffusion, a have better chance of reaching the deepest lesions; another reason for using combined therapy.
In our series, both treatments were given in the same day with the rationale of reducing the damage resulting from VEGF release after PDT. Intravitreal injection was given first to avoid exposure to strong light during the injection after having PDT. However, Dhalla and colleagues (2006), reported giving either treatment within 14 days interval with results are very close to ours. Lazic and Gabric (2007) reported that bevacizumab was given within one hour after PDT also with visual improvement. The optimal timing of bevacizumab injection in relation to the PDT procedure, therefore, warrants further investigation.
With the use of bevacizumab neither cataract nor glaucoma was encountered in our study which makes it safer when compared with triamcinolone.
Our study with combined treatment of bevacizumab and PDT demonstrated that this treatment seems to be effective for treatment of CNV secondary to AMD whatever the type of this membrane (occult, classic and subfoveal, juxtafoveal). In spite of the small sample of the treated eyes but the findings seem to be encouraging. This observation matches the results of Dhalla and colleagues (2006). Lazic and Gabric (2007) reported a similar improvement in both occult and minimally classic CNV due to AMD at 3 months.
In summary, combined intravitreal bevacizumab and PDT as a treatment of CNV secondary to AMD either for predominantly classic or occult subtypes has a positive therapeutic effect with stabilization or improvement of final BCVA and also might reduce the need for retreatment compared to literature retreatment rates of either modality alone.
The main limitations in our study were the lack of randomization as well as small number of cases. In view of the encouraging results, further randomized controlled studies may be justified to determine the role of this combined therapy for neovascular AMD.
Footnotes
Disclosure
The authors have no proprietary interest or conflict of interest in this study. This work has been done in Magrabi Eye and Ear Hospitals, Oman and KSA and Saad Specialist Hospital KSA.
References
- Aiello LP, Brucker AJ, Chang S, et al. Evolving guidelines for intra-vitreal injections. Retina. 2004;24:S3–S19. doi: 10.1097/00006982-200410001-00002. [DOI] [PubMed] [Google Scholar]
- Avery RL, Pieramici DJ, Rabena MD, et al. Bevacizumab Intraviteal (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–72. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Bergers G, Song Sn, Meyer-Morse E, et al. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–95. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- Chan WM, Lai TY, Wong AL, et al. Combined photodynamic therapy and intravitreal triamcinolone injection for the treatment of subfoveal choroidal neovascularization in age-related macular degeneration: a comparative study. Br J Ophthalmol. 2006;90:337–41. doi: 10.1136/bjo.2005.081299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciulla TA, Criswell MH, Danis RP, et al. Intravitreal triamcinolone acetonide inhibits choroidal neovascularization in a laser-treated rat model. Arch Ophthalmol. 2001;119:399–404. doi: 10.1001/archopht.119.3.399. [DOI] [PubMed] [Google Scholar]
- Danis RP, Bingaman DP, Yang Y, et al. Inhibition of pre-retinal and optic nerve head neovascularization in pigs by itravitreal triamcinolone acetonide. Ophthalmology. 1996;103:2099–104. doi: 10.1016/s0161-6420(96)30383-7. [DOI] [PubMed] [Google Scholar]
- Dhalla MS, Gaurav K, Shah MD, et al. Combined photodynamic therapy with verteporfin and intravitreal bevacizumab for choroidal neovascularization in age-related macular degeneration. Retina. 2006;26:988–93. doi: 10.1097/01.iae.0000247164.70376.91. [DOI] [PubMed] [Google Scholar]
- Erber R, Thurnher A, Katsen AD, et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediate endothelial cell survival mechanisms. FASEB J. 2004;18:338–40. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- Flower RW, von Kerczek C, Zho L, et al. Theoretical investigation of the role of choriocapillaries blood flow in the treatment of subfoveal choroidal neovascularization associated with age-related macular degeneration. Am J Ophthalmol. 2001;132:85–93. doi: 10.1016/s0002-9394(01)00872-8. [DOI] [PubMed] [Google Scholar]
- Gillies MC, Simpson JM, Billson FA, et al. Safety of an intravitreal injection of triamcinolone: results from a randomized clinical trial. Arch Ophthalmol. 2004;122:336–40. doi: 10.1001/archopht.122.3.336. [DOI] [PubMed] [Google Scholar]
- Gragoudas ES, Adamis AP, Cunningham ET, et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–16. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- Grunewald M, Avraham I, Dor Y, et al. VEGF-induced adult neovascularization: recrutement, retension, and role of accessory cells. Cell. 2006;124:175–89. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Heier JS, Antoszyk AN, Pavan PR, et al. Ranibizumab for treatment of neovascular age-related macular degeneration: a phase I/II multicenter, controlled, multidose study. Ophthalmology. 2006;113:633–42. doi: 10.1016/j.ophtha.2005.10.052. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Kreissig I, Hugger P, et al. Intravitreal triamcinilone acetonide for exudative age related macular degeneration. Br J Ophthalmol. 2003;87:462–8. doi: 10.1136/bjo.87.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazic R, Gabric N. Verteporfin therapy and intravitreal bevacizumab combined and alone in choroidal neovascularization due to age-related macular degeneration. Ophthalmology. 2007;114:1179–85. doi: 10.1016/j.ophtha.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Miller JW. Randomized, controlled phase III of ranibizumab (Lucentis) for minimally classic or occult neovascular age-related macular degeneration. Presented at American Society of Retina Specialists Annual Meeting; July 2005; Montreal, Quebec, Canada. 2005. [Google Scholar]
- Moshfeghi DM, Kaiser PK, Grossniklaus HE, et al. Clinicopathologic study after submacular removal of choroidal neovascular membranes treated with vertiporfin ocular photodynamic therapy. Am J Ophthalmol. 2003;135:343–50. doi: 10.1016/s0002-9394(02)01936-0. [DOI] [PubMed] [Google Scholar]
- Rechtman E, Danis RP, Pratt LM, et al. Intravitreal Triamcinolone with photodynamic therapy for subfoveal choroidal neovascularization in age related macular degeneration. Br J Ophthalmol. 2004;88:344–7. doi: 10.1136/bjo.2003.027177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers AH, Martidis A, Greenberg BP, et al. Optical coherence tomography findings following photodynamic therapy of choroidal neovascularization. Am J Ophthalmol. 2002;134:566–67. doi: 10.1016/s0002-9394(02)01566-0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ. Intravitreal Avastin: the low cost alternative to Lucentis? Am J Ophthalmol. 2006;142:141–3. doi: 10.1016/j.ajo.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Rosenfield PJ, Moshfeghi AA, Puliqfito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–5. [PubMed] [Google Scholar]
- Schmidt-Erfurth U, Schlutzer-Schrehard U, Cursiefen C, et al. Influence of photodynamic therapy on expression of vascular endothelial growth factor (VEGF), VEGF receptor 3, and pigment epithelium driven factor. Invest Opthtalmol Vis Sci. 2003;44:4473–80. doi: 10.1167/iovs.02-1115. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Sorenson J, Maranan L. Combined photodynamic therapy with verteporfin and intravitreal triamcinolone acetonide for choroidal neovascularization. Ophthalmology. 110:1517–25. doi: 10.1016/S0161-6420(03)00544-X. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Sorenson J, Maranan L. Photodynamic therapy with verteporfin combined with intravitreal injection of triamcinolone acetonide for choroidal neovascularization. Ophthalmology. 2005;112:301–4. doi: 10.1016/j.ophtha.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Spaide RF. Rationale for combination therapies for choroidal neovascularization. Am J Ophthalmol. 2006;141:149–56. doi: 10.1016/j.ajo.2005.07.025. [DOI] [PubMed] [Google Scholar]
- [TAP] Treatment of Age-Related Macular Degeneration With Photodynamic Therapy study group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials – TAP report. Arch Ophthalmol. 1999;117:1329–45. [PubMed] [Google Scholar]
- [VIP] Verteporfin in Photodynamic Therapy Report 2. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: Two-year results of randomized clinical trial inkling lesions with occult and no classic choroidal neovascularization. Am J Ophthalmol. 2001;131:541–60. doi: 10.1016/s0002-9394(01)00967-9. [DOI] [PubMed] [Google Scholar]
- Wachtlin J, Behmi T, Heinmann H, et al. Concentric retinal pigment epithelium atrophy after single photodynamic therapy. Graefes Arch Clin Exp Opthtalmol. 2003;241:518–21. doi: 10.1007/s00417-003-0650-8. [DOI] [PubMed] [Google Scholar]