Abstract
Purpose
This study was conducted to investigate whether retinoic acids (RAs) had any effect on apoptosis during the development of diabetic retinopathy.
Methods
To investigate whether RAs had any effect on apoptosis during the development of diabetic retinopathy, we housed 32 C57BL/6 male mice and induced diabetes in 24 by intra peritoneal injections of streptozotocin (STZ; Sigma, St Louis, MO) and treated 16 of the diabetic mice with the RAs, all-trans-retinoic acid (ATRA) (seven mice) and 4-[(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)carboxamido] benzoic acid (Am580) (nine mice). The other eight mice were used as diabetic controls. We then measured apoptosis in the retina by TdT-dUTP terminal nick-end labeling assay.
Results
RAs inhibited the apoptosis of retinal cells in diabetic retinopathy. Many apoptotic cells were observed in retinas of the eight diabetic control mice (mean value and SD: 37.8 ± 6.9), whereas when diabetic mice were treated with RAs, the number of apoptotic cells significantly decreased (mean value and SD: 9.9 ± 6.4 for the seven ATRA-treated diabetic mice and 9.8 ± 5.9 for the nine Am580-treated diabetic mice) (p < 0.05).
Conclusion
Treatment with RAs decreases apoptosis during the development of diabetic retinopathy.
Keywords: retinoic acids, apoptosis, diabetic retinopathy, glial cell line-derived neurotrophic factor
Introduction
In Japan, more than 12 million people are believed to suffer from diabetes (Kawamori 2002). The most common complication of diabetes is diabetic retinopathy, which is the leading cause of legal blindness among working-age adults in Japan. The functional components of diabetic retinopathy are vascular permeability and neural cell death. Recent studies have shown that most of the neural cell death in retinas associated with diabetic retinopathy is due to apoptosis (Barber et al 1998; Tezel et al 1999).
Some glial cells are well known to maintain survival of neural cells by production of various cytokines. Recently we reported that retinoic acid receptor stimulants such as all-trans-retinoic acid (ATRA) and 4-[(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)carboxamido] benzoic acid (Am580) improve diabetic retinopathy via changes of cytokine production in glial cells (Thang et al 2000; Nishikiori et al 2007a). In this study, we investigated whether RAs had any effect on apoptosis during the development of diabetic retinopathy.
Material and methods
To determine whether RAs had any effect on apoptosis during the development of diabetic retinopathy, 32 C57BL/6 male mice (5 weeks old, CLEA JAPAN, Tokyo, Japan) were housed and diabetes was induced in twenty-four mice by intra peritoneal (i.p.) injection of 40 mg/kg streptozotocin (STZ; Sigma, St Louis, MO) dissolved in 0.01M trisodium citrate buffer (pH 4.5) for 5 consecutive days. The cut-off levels in mice used to diagnose diabetes were more than 200 mg/dl in blood sugar measured using Gluco-card (ARKRAY, Kyoto, Japan) and positive urinal sugar measured using Rub-stick (Bayer, Leverkusen, Germany). Six weeks after the verification of diabetes, when the blood–retinal barrier breakdown was progressing as previously described (Nishikiori et al 2007a), mice were treated with 1.0 mg/kg ATRA every day (seven mice, 14 eyes) or 3.75 mg/kg Am580 every other day (nine mice, 18 eyes) for 1 week (Nishikiori et al 2007a, 2007b). Control mice (eight mice, 16 eyes) were i.p. injected with a solvent alone (250 μl of DMSO; Sigma). After the mice were sacrificed by dislocating the cervical vertebrae, enucleated mouse eyeballs were fixed in 1% paraformaldehyde/phosphate-buffered saline overnight. After the eyeballs were sliced in horizontal sections and flat-mounted onto a slide, TdT-dUTP terminal nick-end labeling (TUNEL) was performed using the In Situ Cell Death Detection Kit, Fluorescein (Roche, Basel, Switzerland). Using a laser-scanning confocal microscope (MRC 1024; Bio-Rad, Hercules, CA), the numbers of apoptotic cells in the retinas of all 64 eyes of the 32 mice were counted in 3~4 views of the retinas approximately 100 μm from the center of the optic nerve disk in each eye at the same magnification (×20) and averaged. All animal experiments followed the specifications of the Guidelines for Animal Experiments of Sapporo Medical University School of Medicine. All values are expressed as mean and SD. Data were statistically analyzed with the Fisher test for comparison of four groups. The levels of statistical significance was set at p < 0.05.
Results
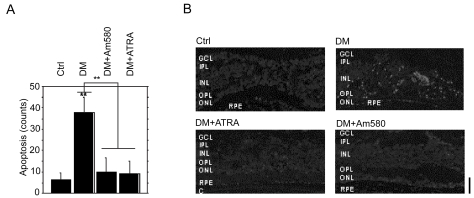
RAs inhibited the apoptosis during the development of diabetic retinopathy. To demonstrate this, we treated diabetic mice with ATRA or Am580 and measured apoptosis in the retina by TUNEL assay. Many apoptotic cells were observed in the retinas of the eight control diabetic mice (cell counts; max 60, minimum 30, mean value and SD: 37.8 ± 6.9), whereas when 16 diabetic mice were treated with ATRA or Am580, the number of apoptotic cells markedly decreased (cell counts; max 23, minimum 5, mean value and SD: 9.9 ± 6.4 in seven ATRA-treated diabetic mice and max 20, minimum 5, mean value and SD: 9.8 ± 5.9 in nine Am580-treated diabetic mice), and the level was suppressed nearly to the control level (cell counts; max 8, minimum 0, mean value and SD: 6.3 ± 2.6 in eight control mice) (Figures 1A and B).
Figure 1.
RAs inhibited the apoptosis during the development of diabetic retinopathy. A and B, Apoptosis of retinal cells in retina. (A, apoptotic cell counts, TdT-dUTP terminal nick-end labeling assay. B, laser-scanning confocal analysis.)
Abbreviations: Ctrl, control mice; ATRA, all-trans-retinoic acid (ATRA)-treated diabetic mice; Am580, Am580-treated diabetic mice; DM, diabetic mice.
Notes: **p < 0.01 Scale bars: 25 μm. The choroid (C), retinal pigment epithelium (RPE), outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL), and the ganglion cell layer (GCL) are labeled in the micrograph.
Discussion
In this study, we demonstrated a marked decrease of apoptosis during the development of diabetic retinopathy after treatment with RAs, though the mechanisms by which this occurred have not yet been clarified. RA, an essential signaling molecule throughout life (Mangelsdorf et al 1994; Kastner et al 1995), could play an important role in the induction of glial cell line-derived neurotrophic factor (GDNF) in glial cells (Thang et al 2000; Nishikiori et al 2007a). GDNF is a neurotrophic and differentiation factor in the retina (Koeberle et al 1998; Thang et al 2000; McGee Sanftner et al 2001; Politi et al 2001; Wu et al 2004) as well as the central nervous system (Lin et al 1993; Henderson et al 1994). Therefore one possible explanation is that RAs protect retinal cells in diabetic retinopathy by inducing GDNF production in glial cells in the retina (Nishikiori et al 2007a, 2007b). GDNF affects a wide variety of signaling pathways (Jing et al 1996; Treanor et al 1996; Airaksinen et al 2002) and there are many signaling pathways of apoptosis in retinal cells (Altucci et al 2001). Judging from the distribution of apoptosis in the retina, however, we cannot say anything about which cell type preferentially fell into apoptosis or which cell type was prevented from doing so by RAs, though several reports suggest that apoptosis in the retina preferentially occurs in retinal ganglion cells with photoreceptors (Koeberle et al 1998; Wu et al 2004).
Considering the observations of this study, we would like to make two proposals. One is that RAs are promising drugs for diabetic ophthalmic disease because they have anti-cataractogenic effects for diabetic cataracts (Nishikiori et al 2007b), blood–retinal barrier-protective effects for vascular integrity (Miyajima et al 2005; Nishikiori et al 2007a), and neuroprotective effects against apoptosis in diabetic retinopathy. The other is that glial cells in the retina can be a new target for treating the retinopathy. Although we i.p. injected RAs, another drug delivery assay such as intravitreal injections will be examined to make RAs more effective on glial cells. Future work along the lines of our proposals might provide a fundamental understanding of the treatment effects of RAs and the treatment targets of glial cells in the development of diabetic ophthalmic diseases. RAs might increase neurotrophic cytokines such as GDNF in glial cells, contributing to decreased apoptosis during the development of diabetic retinopathy. Hopefully, this study will help to improve the outcomes of patients with diabetes.
References
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–94. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Altucci L, Rossin A, Raffelsberger W, et al. Retinoic acid-induced apoptosis in leukemia cells is mediated by paracrine action of tumor-selective death ligand TRAIL. Nat Med. 2001;7:680–6. doi: 10.1038/89050. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Lieth E, Khin SA, et al. The Penn State Retina Research Group: Neural apoptosis in the retina during experimental and human diabetes. J Clin Invest. 1998;102:783–91. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, et al. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–4. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Jing S, Wen D, Yu Y, et al. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 1996;85:1113–24. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- Kastner P, Mark M, Chambon P. Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell. 1995;83:859–69. doi: 10.1016/0092-8674(95)90202-3. [DOI] [PubMed] [Google Scholar]
- Kawamori R. Diabetes trends in Japan. Diabetes Metab Res Rev. 2002;18:9–13. doi: 10.1002/dmrr.296. [DOI] [PubMed] [Google Scholar]
- Koeberle PD, Ball AK. Effects of GDNF on retinal ganglion cell survival following axotomy. Vision Res. 1998;38:1505–15. doi: 10.1016/s0042-6989(97)00364-7. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, et al. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–2. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Umesono K, Evans RM. In: The Retinoids. Biology, Chemistry, and Medicine. Sporn MB, Roberts AB, Goodman DS, editors. Raven Press; New York: 1994. pp. 314–49. [Google Scholar]
- McGee Sanftner LH, Abel H, Hauswirth WW, et al. Glial cell line derived neurotrophic factor delays photoreceptor degeneration in a transgenic rat model of retinitis pigmentosa. Mol Ther. 2001;4:622–9. doi: 10.1006/mthe.2001.0498. [DOI] [PubMed] [Google Scholar]
- Miyajima H, Osanai M, Chiba H, et al. Glyceraldehyde-derived advanced glycation end-products preferentially induce VEGF expression and reduce GDNF expression in human astrocytes. Biochem Biophys Res Commun. 2005;330:361–6. doi: 10.1016/j.bbrc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Nishikiori N, Mitamura Y, Tashimo A, et al. Glial cell line-derived neurotrophic factor in the vitreous of patients with proliferative diabetic retinopathy. Diabetes Care. 2005;28:2588. doi: 10.2337/diacare.28.10.2588. [DOI] [PubMed] [Google Scholar]
- Nishikiori N, Osanai M, Chiba H, et al. Glial cell-derived cytokines attenuate the breakdown of vascular integrity in diabetic retinopathy. Diabetes. 2007a;56:1333–40. doi: 10.2337/db06-1431. [DOI] [PubMed] [Google Scholar]
- Nishikiori N, Osanai M, Chiba H, et al. Inhibitory effects of retinoic acid receptor alpha stimulants on murine cataractogenesis through suppression of deregulated calpains. Invest Ophthalmol Vis Sci. 2007b;48:2224–9. doi: 10.1167/iovs.06-1222. [DOI] [PubMed] [Google Scholar]
- Politi LE, Rotstein NP, Carri NG. Effect of GDNF on neuroblast proliferation and photoreceptor survival: additive protection with docosahexaenoic acid. Invest Ophthalmol Vis Sci. 2001;42:3008–15. [PubMed] [Google Scholar]
- Tezel G, Wax MB. Inhibition of caspase activity in retinal cell apoptosis induced by various stimuli in vitro. Invest Ophthalmol Vis Sci. 1999;40:2660–7. [PubMed] [Google Scholar]
- Thang SH, Kobayashi M, Matsuoka I. Regulation of glial cell line-derived neurotrophic factor responsiveness in developing rat sympathetic neurons by retinoic acid and bone morphogenetic protein-2. J Neurosci. 2000;20:2917–25. doi: 10.1523/JNEUROSCI.20-08-02917.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treanor JJ, Goodman L, de Sauvage F, et al. Characterization of a multicomponent receptor for GDNF. Nature. 1996;382:80–3. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- Wu WC, Lai CC, Chen SL, Sun MH, et al. GDNF gene therapy attenuates retinal ischemic injuries in rats. Mol Vis. 2004;10:93–102. [PubMed] [Google Scholar]