Abstract
Insulin resistance is prevalent in heart failure (HF) patients, and beta2 adrenergic receptors (β2-AR) are involved in glucose homeostasis. We hypothesized that β2-AR Gln27Glu and Arg16Gly polymorphisms affect insulin resistance in HF patients and explored if effects of β2-AR polymorphisms on glucose handling are modified by choice of beta blocker.
We studied 30 non-diabetic adults with HF and a history of systolic dysfunction, 15 on metoprolol succinate and 15 on carvedilol. We measured fasting glucose, insulin, and insulin resistance, and determined β2-AR genotypes at codons 27 and 16. The cohort was insulin resistant with a mean HOMA-IR score of 3.4 (95%CI 2.3-4.5, normal value=1.0). Patients with the Glu27Glu genotype exhibited higher insulin and HOMA-IR compared to individuals carrying a Gln allele (p=0.019). Patients taking carvedilol demonstrated lower insulin resistance if also carrying a wild type allele at codon 27 (fasting insulin 9.8±10.5 versus 20.5±2.1 for variant, p=0.072, HOMA-IR 2.4±2.7 versus 5.1±0.6, p=0.074, respectively); those on metoprolol succinate had high insulin resistance irrespective of genotype. The β2-AR Glu27Glu genotype may be associated with higher insulin concentrations and insulin resistance in patients with HF. Future studies are needed to confirm whether treatment with carvedilol may be associated with decreased insulin and insulin resistance in β2-AR codon 27 Gln carriers.
Keywords: Heart failure, insulin resistance, receptors, adrenergic, beta, genetic polymorphism
INTRODUCTION
Heart failure (HF) leads to alterations in the metabolic pathways involved in glucose metabolism and insulin resistance, and these have been associated with worse outcomes.[1-3] Diabetes mellitus is present in 20-25% of patients with chronic HF in large trials[4-6], and abnormal glucose metabolism and insulin resistance have been shown to be independent predictors of incident HF,[7-9] HF severity,[10] and HF mortality.[3] The exact mechanisms of insulin resistance in HF are unknown, although neurohormonal activation, particularly of the adrenergic system, is thought to play a role. Norepinephrine stimulates hepatic glucose production and affects glucose metabolism in part via the β2 adrenergic receptor (β2-AR).[11, 12] It also increases lipolysis in adipose tissue leading to elevated levels of free fatty acids.[13] In patients with HF, insulin mediated decreases in plasma free fatty acids are attenuated compared to individuals without HF.[14]
Another potential factor altering glucose handling is genetic variation within the β2-AR. Known single nucleotide polymorphisms (SNPs) in the β2-AR gene include amino acid substitutions at codons 16 (Arginine [Arg] or Glycine [Gly]), and 27 (Glutamine [Gln] or Glutamic acid [Glu]). These 2 SNPs are highly prevalent (Gln27Glu: approximately 43% in Caucasians, 27% in Blacks; Arg16Gly: 39% Caucasians, 50% in Blacks), and have known effects on receptor function.[15] Several studies in patients with insulin resistance, diabetes, and obesity have demonstrated elevated post-load plasma glucose and fasting insulin concentrations in patients homozygous for the variant allele at either the Arg16Gly or Gln27Glu codon.[16, 17] In healthy volunteers, the Gly16/Glu27 genotype has been associated with enhanced hepatic glucose production.[18]
Use of metoprolol succinate or carvedilol, both β-AR antagonists, is standard in the management of systolic dysfunction HF. While some studies indicate that beta blockers can worsen glycemic control,[19, 20] metoprolol succinate and carvedilol have different adrenergic receptor pharmacology. Metoprolol’s effect to worsen insulin resistance may be due to its β1 selectivity,[19] while carvedilol has the potential to improve insulin sensitivity due to its β receptor non-selectivity and α1 blockade.[19] A post-hoc analysis of the Carvedilol or Metoprolol European Trial (COMET) study demonstrated that new onset diabetes was more common in HF patients randomized to metoprolol tartrate than in those randomized to carvedilol.[21] It is therefore of interest to evaluate genetic factors influencing insulin resistance in HF patients, within the context of beta blockade with both metoprolol succinate and carvedilol at clinically relevant doses. We hypothesized that β2-AR polymorphisms influence insulin resistance in patients with HF on β blockers. As an exploratory aim, we also hypothesized that the influence of β2-AR polymorphisms on glucose handling can be modified by choice of beta blocker.
METHODS
Participants
We studied 30 non-randomized patients on target or maximally tolerated doses of β blocker therapy (15 metoprolol succinate and 15 carvedilol). Choice of β blocker was at the discretion of the treating clinician. Eligibility criteria included a history of systolic dysfunction (current or previous ejection fraction < 40%), with ACC/AHA Stage C, NYHA Functional Class II or III heart failure. All patients were on stable optimal medical therapy for heart failure for at least 30 days. Exclusion criteria included contra-indications to β-blockers; or concomitant use of scheduled inhaled β-agonists. In addition, patients with a prior diagnosis of diabetes mellitus, unstable angina, myocardial infarction or bypass surgery within the past 3 months, or underlying hypertrophic or restrictive cardiomyopathy were excluded. The protocol was approved by the University of Wisconsin institutional review board. All patients provided written informed consent in accordance with established guidelines for the protection of human subjects.
Study Protocol
This was a prospective cohort study in 30 individuals with a history of systolic dysfunction HF on guideline-based therapy, titrated to target (metoprolol succinate 200mg per day or carvedilol 25mg twice daily) or maximally tolerated beta blocker doses in the University of Wisconsin Hospital Heart Failure Management Program. The primary outcome variable was differences in fasting glucose and insulin concentrations, analyzed by genotype and by beta blocker therapy. Once patients gave informed consent, each had a fasting blood sample obtained by direct venipuncture for baseline glucose, insulin, and genotype analysis.
β2-AR genotyping
Genomic DNA was extracted from 10 mL of whole blood with a DNA isolation kit (DNA Wizard, Promega, Madison, WI) following the manufacturer’s suggested protocol. To determine genotype, polymerase chain reaction (PCR) was used to amplify the 250 base pair DNA region containing the β2-AR polymorphisms at positions 27 and 16 for pyrosequencing. Reaction Primers (Integrated DNA Technologies, Coralville, IA) were designed using Primer3 software; forward primer 5′-TACACCACAGCCGCTGAAT-3′ and reverse primer 5′-GGACGATGAGAGACATGACG-3′. PCR amplification was carried out in a volume of 37.5 μL containing 100 ng DNA, 5 pmol of each primer, and PCR master mix containing 1.0 U Taq DNA polymerase, 1.5 mM MgCl2, and 0.2 mM of each deoxynucleoside triphosphates (Promega, Madison, WI). The reactions were incubated at 94°C for 6 minutes, followed by 35 cycles of 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 60 seconds, with a final extension of 72°C for 5 minutes. Amplification was verified by electrophoresis on a 2% agarose gel and stained with ethidium bromide. PCR products were stored in a -70°C freezer until pyrosequencing reactions commenced after enrollment of the entire cohort. The pyrosequencing PSQ 96MA system (Biotage, Foxboro, MA) and the forward sequencing primer 5′-TTGCTGGCACCCAAT-3′ were used to determine genotypes.
Data Analyses
Baseline demographics, including characteristics by genotype, were compared to identify potential differences between groups using t-tests or Wilcoxon rank sum tests for continuous variables and Chi-Square or Fisher’s exact tests, as appropriate, for categorical variables. Hardy-Weinberg equilibrium was assessed by the Chi-Square test. Insulin resistance was calculated using the homeostatic model assessment of insulin resistance (HOMA-IR): fasting values of glucose (mmol/L) x insulin (μIU/mL) / 22.5.[22]
The primary study outcome variable for statistical analysis was mean fasting glucose and insulin levels at baseline. Assuming a 20% difference in fasting glucose and insulin concentrations (standard deviation = 15%[16]) by genotype, 30 subjects were needed to achieve 80% power at the 0.05 significance level based on published population genotype frequencies.[15] Power to detect differences in analyses stratified by β-blocker was 54%, assuming a 15% difference in fasting glucose and insulin by β blocker. It was determined a priori to compare participants with the homozygous variant genotype to heterozygous and wild type genotypes, as homozyogous individuals have been shown in previous studies to have worse cardiovascular and metabolic outcomes. Insulin, insulin resistance (HOMA-IR score), and glucose levels between homozygous variant and heterozygous participants for codons 27 and 16 were compared using the Wilcoxon rank sum test. Multivariable linear regression models were created to examine associations between genotype (homozygous variant vs. wild type carriers) and insulin and glucose measures while adjusting for specific beta-blocker treatment. Log-transformations of the outcome measures were performed where necessary. Separate models were created for each outcome variable (insulin, HOMA-IR score, and glucose levels) and for each codon (27 and 16). Each model was adjusted for body mass index (BMI as a continuous variable), and HF etiology (ischemic versus idiopathic). A genotype/beta-blocker interaction term was entered into the final multivariable model. P-values of <0.05 were considered significant. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
Patients
Thirty patients (9 females, 21 males) were enrolled, with a mean age of 57.8 ± 15.0 years (range 27-81 years). Mean ejection fraction was 34.6% ± 10.1% (range 15%-55%), and most were NYHA functional class II. Demographics, phenotypic and genotypic characteristics are shown in Tables 1 and 2. Arg16Gly and Gln27Glu frequencies in the sample were in Hardy-Weinberg equilibrium. No statistically significant demographic differences were observed at baseline between the beta blocker groups. For Arg16Gly, more males carried the homozygous genotype (Gly16Gly, p=0.018). Mean fasting glucose concentration in the sample was 101.5 mg/dL (95% CI 97.1-105.9), and mean insulin concentration was 13.1 μIU/mL (95% CI 9.2-17.0, normal range 3-19 μIU/mL). Mean HOMA-IR score was 3.4 (95% CI 2.3-4.5, normal value = 1.0), indicating a high prevalence of insulin resistance in the cohort.
Table 1. Baseline demographics, Total and by beta blocker.
| Variable | All Subjects (N=30) | Carvedilol (N=15) | Metoprolol (N=15) | P carvedilol vs. metoprolol |
|---|---|---|---|---|
| Age (yrs) | 57.8 ± 15.0 | 54.8 ± 17.8 | 60.9 ± 11.3 | 0.38 |
| Men, n (%) | 21 (70) | 10 (67) | 11 (73) | 1.00 |
| White/black/Hispanic | 27 /1 / 2 | 14/0/1 | 13/1/1 | 0.89 |
| NYHA FC II/III | 27 (90%) / 3 (10%) | 15(100 %) / 0(0%) | 12(80%) / 3(20%) | 0.22 |
| Ischemic heart disease | 11 (37%) | 5 (33%) | 6 (40%) | 0.70 |
| Body mass index | 30.1 ± 5.1 | 29.6 ± 5.5 | 30.6 ± 4.9 | 0.46 |
| LVEF* (%), range | 34.6% ± 10.1%, 15-55% | 34.7 ± 10.1, 15-55% | 34.6 ± 10.6, 15-55% | 0.93 |
| Background therapy | ||||
| ACE inhibitor / ARB** | 29 (96.7%) | 15 (100%) | 14 (93%) | 1.0 |
| Furosemide | 21 (70%) | 11 (73%) | 10 (67%) | 0.99 |
| Digoxin | 15 (50%) | 9 (60%) | 6 (40%) | 0.27 |
| Spironolactone | 12 (40%) | 6 (40%) | 6 (40%) | 1.00 |
| Statin | 22 (73%) | 11 (73%) | 11 (73%) | 1.00 |
LVEF: left ventricular ejection fraction
ARB: angiotensin receptor blocker
Table 2. Baseline demographic characteristics, stratified by β2-AR genotype(homozygous variant versus wild type carriers).
| Variable | Glu27Glu (N=9) | Gln27 carriers (N=21) | Gly16Gly (N=17) | Arg16 carriers (N=13) |
|---|---|---|---|---|
| Age (yrs) | 57.3 ± 12.7 | 58.0 ± 16.1 | 55.7 ± 15.4 | 60.6 ± 14.5 |
| Men | 7 (78%) | 14 (67%) | 15 (88%)† | 6 (46%) |
| White/black/Hispanic | 8 / 1 / 0 | 19 / 0 / 2 | 16 / 1 / 0 | 11 / 0 / 2 |
| NYHA FC II/III | 9 / 0 | 18 / 3 | 15 / 2 | 12 / 1 |
| Ischemic heart disease | 4 (44%) | 7 (33%) | 8 (47%) | 3 (23%) |
| Body mass index | 32.2 ± 4.2 | 29.1 ± 5.3 | 29.6 ± 5.3 | 30.7 ± 4.9 |
| LVEF (%) | 40.0% ± 10.0% | 32.3% ± 9.5% | 34.1% ± 11.6% | 35.3% ± 8.2% |
| Background therapy | ||||
| Beta blocker (C or M#) | 4 / 5 | 11 / 10 | 7 / 10 | 8 / 5 |
| ACE inhibitor / ARB | 8 / 1 | 19 / 2 | 16 / 1 | 11 / 2 |
| Furosemide | 5 (56%) | 16 (76%) | 10 (59%) | 11 (85%) |
| Digoxin | 2 (22%) | 13 (62%) | 7 (41%) | 8 (62%) |
| Spironolactone | 4 (44%) | 8 (38%) | 6 (35%) | 6 (46%) |
| Statin | 5 (56%) | 17 (81%) | 12 (71%) | 10 (77%) |
Data are mean ± SD
C = carvedilol; M = metoprolol succinate
P=0.018 for sex comparing Gly16Gly and Arg16 carriers
Levels of fasting glucose, insulin, and insulin resistance by β2 polymorphisms
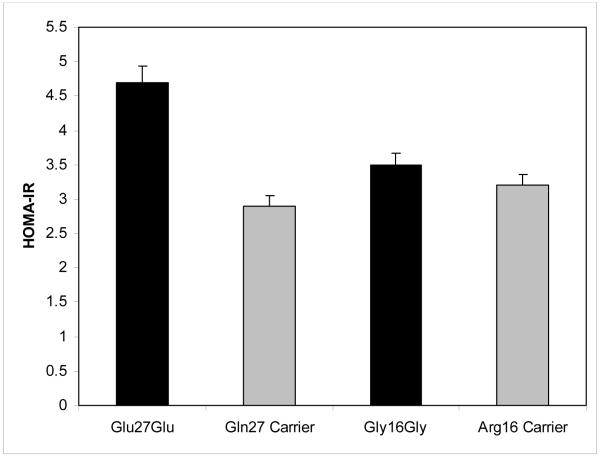
No significant differences in fasting glucose were detected between patients homozygous (n=9) for the variant Glu allele at codon 27 versus the 21 patients carrying at least one wild type Gln allele, (107.4 ± 13.4 mg/dL for Glu27Glu compared with 99.0 ± 10.4 mg/dL for Gln carriers, p=0.15). Nevertheless, homozygous variant patients had higher fasting insulin concentrations than those carrying at least one wild type allele (17.4 ± 6.8 μIU/mL versus 11.2 ± 11.4 μIU/mL, p=0.019). Similarly, HOMA-IR scores were higher for patients homozygous variant compared to wild type allele carriers at codon 27 (4.7 ± 2.0 versus 2.9 ± 3.2, p=0.019). (Figures 1 and 2) There were no differences in glucose, insulin or HOMA-IR levels between of genotype groups at codon 16.
Figure 1.
Fasting insulin stratified by β2-AR genotype at codons 27 and 16 (homozygous variant versus wild type carrier). Data are means ± SE. * p=0.019 for comparison between Glu27Glu and Gln carriers. P=NS for comparison of Arg16Gly genotypes.
Figure 2.
HOMA-IR stratified by β2-AR genotype at codons 27 and 16 (homozygous variant versus wild type carrier). Data are means ± SE. * p=0.019 for comparison between Glu27Glu and Gln carriers. P=NS for comparison of Arg16Gly genotypes.
Levels of fasting glucose, insulin, and insulin resistance by beta-blocker
There were no statistically significant differences detected between the carvedilol and metoprolol succinate groups in fasting glucose (100.1 ± 8.7 mg/dL versus 102.9 ± 14.4 mg/dL,p=0.63) insulin (11.2 ± 10.4 μIU/mL versus 15.0 ± 10.7 μIU/mL, p=0.20), and HOMA-IR scores (2.8 ± 2.7 versus 4.0 ± 3.2, p=0.19).
Levels of fasting insulin and insulin resistance by β2 polymorphisms within beta blocker treatment subgroups
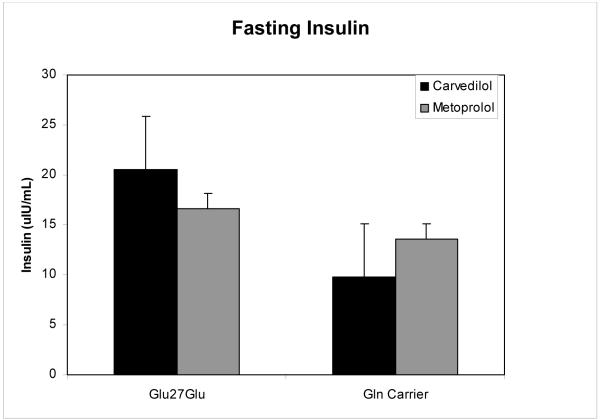
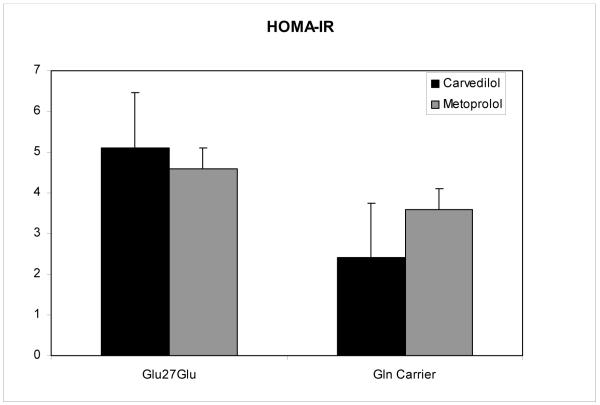
Results for fasting insulin and HOMA-IR were examined by genotype at codon 27 within beta blocker therapy subgroups. Patients on carvedilol, but not those on metoprolol succinate, had lower mean insulin and HOMA-IR values when carrying a wild type allele at codon 27 (Figures 3 and 4), a result short of statistical significance (fasting insulin 20.5 ± 2.1 versus 9.8 ± 10.5, p=0.072, and HOMA-IR 5.1 ± 0.6 versus 2.4 ± 2.7, p=0.074, respectively).
Figure 3.
Fasting insulin stratified by β2-AR Gln27Glu genotype and by beta blocker (carvedilol versus metoprolol). Data are means ± SE. * p=0.072 for comparison of Glu27Glu genotype to Gln carrier in the carvedilol cohort.
Figure 4.
HOMA-IR stratified by β2-AR Gln27Glu genotype and by beta blocker (carvedilol versus metoprolol). Data are means ± SE. * p=0.074 for comparison of Glu27Glu genotype to Gln carrier in the carvedilol cohort.
Multivariable models
Multivariable linear models were created for each outcome variable (insulin, HOMA-IR, and glucose) to determine if a genotype by beta blocker interaction was present after adjustment for BMI, NYHA FC, and etiology. Separate models were created for each of the 3 outcomes, and each SNP (Arg16Gly and Gln27Glu). No significant interactions were detected in any of the beta blocker models. As expected, BMI was a significant predictor of insulin levels and HOMA-IR in both codon 16 and codon 27 models (p=0.003). Ischemic etiology was a significant predictor of higher insulin levels in both SNP models (p=0.037).
DISCUSSION
Our results show that polymorphisms in codon 27 of the β2-AR may be associated with serum insulin levels and insulin resistance; moreover, the data suggest that choice of β-blocker may modify the relationship between β2-AR genotype and insulin resistance. In light of the high incidence of diabetes and insulin resistance in patients with HF, and that treatment with beta blockers may increase the risk of diabetes, the relationship between genetic predisposition to insulin resistance and beta blocker choice may have important clinical implications in HF patients.
Heart failure and diabetes are related in a complex and incompletely understood fashion. The incidence of new diabetes in patients with HF is very high, ranging from 2-7% per year as compared with 0.1-0.4% in hypertensive populations.[21, 23-25] The HF patients in our cohort had markedly elevated insulin resistance, consistent with previous study findings.[2] We demonstrate that patients homozygous for the glutamate variant (Glu27Glu) at codon 27 of the β2-AR had higher fasting insulin concentrations and insulin resistance than patients carrying a wild type allele. The adrenergic system controls glucose metabolism in the liver, adipose tissues, and skeletal muscle, partially through β2-AR.[26] In addition to effects on glucose metabolism, the β2-AR has increased importance in HF. In normal hearts, the β1 receptor predominates, with a β1:β2 ratio of approximately 80:20. In HF, β1 receptors downregulate, decreasing the β1:β2 ratio to 60:40.[27, 28] It is logical then to theorize that β2-AR metabolic effects may have more importance in HF. Two non-synonymous single nucleotide polymorphisms (SNPs) at nucleotides 46 and 79 of the β2-AR gene result in changes in amino acid residues 16 (Arginine [Arg] or Glycine [Gly]), and 27 (Glutamine [Gln] or Glutamic acid [Glu]).[15, 29] The variant genotypes are resistant to agonist-induced receptor downregulation, whereas the wild type carriers have preserved downregulation.
Given their high prevalence, functional consequences, and the known physiologic links of adrenergic pathways to endocrine function, it is not surprising that the Gln27Glu polymorphism may be related to insulin resistance in HF patients. In a study of the effect of β2-AR haplotypes on terbutaline-mediated glucose production and insulin concentrations in healthy volunteers,[18] participants carrying the Glu27 homozygous genotype had more pronounced terbutaline-mediated glucose production and, similar to our findings, higher insulin concentrations compared with participants with a Gln27 allele. Similarly, in a study of 155 healthy individuals[30] the β2-AR Arg16Gly variant, specifically the Gly16 allele, was associated with increased insulin resistance. We did not find any association between the Arg16Gly SNP and insulin resistance. Haplotype analyses would be desirable for future studies as the β2-AR Arg16Gly and Gln27Glu variants are in tight linkage disequilibrium. As our study was exploratory, it was not designed to perform haplotype analyses. Interestingly, it is not uncommon to observe one SNP driving the biologic effect even in studies that include haplotype analyses. Our data would suggest that the Gln27Glu variant has primary importance in regulation of insulin resistance in HF. Future work should address the issue of β2-AR haplotypes more specifically.[15]
Additionally, we observed a trend for an association between β2-AR genotype at codon 27 and choice of β blocker on fasting insulin and insulin resistance, although our results were short of statistical significance. In patients prescribed carvedilol who had a wild type Gln allele at codon 27 insulin resistance was lower. Patients treated with metoprolol succinate irrespective of genotype at codon 27 and those homozygous for the variant allele treated with carvedilol all demonstrated higher insulin resistance. Although these results are intriguing, they should be considered exploratory, requiring confirmation in larger cohorts with power to test interactions of genotype with beta blocker treatment on metabolic outcomes. To our knowledge, this study is the first to examine a genotype by beta blocker interaction on metabolic outcomes in HF.
The mechanisms by which different beta blockers might affect insulin resistance in Glu27Glu patients remain unclear. Carvedilol is a non-specific adrenergic blocker at all doses, with blockade of β1, β2 and α1 receptors, while metoprolol succinate is β1 selective at lower doses and β1/ β2 nonselective at higher doses. The relative increase in importance of β2-AR specifically in HF and the role of the β2AR in glucose metabolism may provide a clue regarding the specificity of carvedilol to reduce insulin resistance by β2AR genotype. Glucose uptake is dependent on adequate blood flow, determined by both α1 and β2-AR tone. Blockade of β2-AR would potentially decrease peripheral blood flow, especially in the setting of unopposed α1 stimulation. Blockade of α1 receptors may lead to increased peripheral glucose utilization;[31] we did not directly examine α1 effects in this study. The relative importance of α and β-adrenergic receptors in mediating catecholamine-induced hepatic glucose handling is yet to be resolved. [26] It is possible that individuals carrying the variant Glu27Glu genotype treated with carvedilol have more pronounced β2-AR blockade, hence changing relative β1: β2: α1 effects, altering the balance of peripheral vasoconstriction and glucose uptake compared to the wild type Gln carriers.
Other investigators explored associations of β2-AR variants on the risk of sudden cardiac death in an older adult cohort, on mortality in individuals following an acute coronary syndrome, and on the risk of death and heart transplantation in patients with heart failure.[32-34] Elevated cardiovascular risk was associated with the Gln27Gln and Arg16Arg genotypes. In contrast, we detected higher insulin concentrations and insulin resistance in the Glu27Glu genotype, presumably correlating with negative cardiovascular outcomes. Differing outcome measures, study sample characteristics and cohort sizes, or additional genetic factors could all contribute to these discordant results. β1-AR variants have also been studied for associations with adverse cardiac risks in addition to interactions with beta blocker efficacy based on genotype.[35, 36]
Although we found differences in insulin concentrations and insulin resistance based on β2-AR genotype in this study, we did not see differences in fasting glucose concentrations. This is not necessarily unexpected, as early in the insulin resistance syndrome post-prandial glucose levels are affected while fasting glucose, measured in this study, is unchanged. Similarly, in the COMET study, there were no significant differences in blood glucose concentrations between carvedilol and metoprolol tartrate, despite a 20% higher incidence of new onset diabetes in the metoprolol treatment arm over the course of the trial.[21] The GEMINI study examining metabolic variables in patients with diabetes assigned to carvedilol or metoprolol, found similar mean blood glucose concentrations between the two agents over a follow up course of 5 months, yet higher HbA1c values in the metoprolol group.[20] We did not note significant differences between carvedilol and metoprolol succinate in fasting glucose, insulin, and HOMA-IR, but were not powered to detect them, and therefore recommend that future studies continue to examine potential differences.
A number of limitations should be noted. It is conceivable that selection bias influenced subject treatment with the different beta blockers, favoring including patients carrying a genotype that affords improved tolerability to target beta blocker doses studied. In prior studies, differences in beta blocker tolerability based on β2-AR polymorphisms have not been found.[37] We did not have baseline metabolic and body mass index data prior to beta blocker initiation. We therefore attempted to capture chronic metabolic changes from beta blocker therapy in patients with target or maximally tolerated beta blocker doses, which we thought is clinically relevant. This was a nonrandomized study, and as such, the clinician’s choice of beta blocker could have been influenced by his/her impression of their differing metabolic effects, which could have affected comparisons made between β blockers. It is also possible that higher number of patients who were NYHA functional class III in the metoprolol group compared to the carvedilol group could have affected the genotype by β blocker trends we observed on metabolic outcomes. However, mean ejection fractions were not significantly different between beta blocker groups. We did not account for the influence of ejection fraction on our outcomes, which could have influenced study results. The generalizability of our results to a wider group of patients, especially those with more advanced HF, is unknown. We cannot rule out that our findings among Gln27 carriers taking carvedilol are due to chance based on our small sample size, limited power to examine an interaction of genotype on beta-blocker effects, and negative beta blocker by genotype interaction term in our multivariable models. However, we felt beta blocker effect on insulin sensitivity was a secondary endpoint worthy of exploration. Due to issues of sample size, it was not feasible in this cohort to study β2-AR haplotypes at positions 16 and 27. This cohort study should not be used to infer causality but demonstrates an association between a common β2-AR polymorphism and insulin resistance which may be associated with specific choice of beta blocker therapy in HF patients. We do not currently support beta blocker choice determination based on β2-AR genotype based on this pilot study.
CONCLUSIONS
The β2-AR Glu27Glu genotype is associated with higher insulin concentrations and worse insulin resistance in patients with HF. Our data suggest that decreased insulin resistance in β2-AR codon 27 Gln carriers may be associated with treatment with carvedilol. Future studies should examine interactions of β2-AR genotype and beta blockers on glucose dynamics in heart failure.
ACKNOWLEDGMENTS
We gratefully acknowledge Drs. Walter Kao, David Murray, Peter Rahko, and Elaine Winkel for allowing their patients to participate in this study.
FUNDING SOURCES
Dr. Vardeny was supported by NIH (NCRR) 8K12RRO23268 and the American Association of Colleges of Pharmacy. Dr. Sweitzer was supported by NIH K23AG01022.
Footnotes
DISCLOSURES
The authors have no disclosures to declare.
References
- [1].Swan JW, Anker SD, Walton C, Godsland IF, Clark AL, Leyva F, et al. Insulin resistance in chronic heart failure: relation to severity and etiology of heart failure. J Am Coll Cardiol. 1997 Aug;30(2):527–32. doi: 10.1016/s0735-1097(97)00185-x. [DOI] [PubMed] [Google Scholar]
- [2].Swan JW, Walton C, Godsland IF, Clark AL, Coats AJ, Oliver MF. Insulin resistance in chronic heart failure. Eur Heart J. 1994 Nov;15(11):1528–32. doi: 10.1093/oxfordjournals.eurheartj.a060425. [DOI] [PubMed] [Google Scholar]
- [3].Doehner W, Rauchhaus M, Ponikowski P, Godsland IF, von Haehling S, Okonko DO, et al. Impaired insulin sensitivity as an independent risk factor for mortality in patients with stable chronic heart failure. J Am Coll Cardiol. 2005 Sep 20;46(6):1019–26. doi: 10.1016/j.jacc.2005.02.093. [DOI] [PubMed] [Google Scholar]
- [4].The CONSENSUS Trial Study Group Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) The New England journal of medicine. 1987 Jun 4;316(23):1429–35. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- [5].The SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The New England journal of medicine. 1991 Aug 1;325(5):293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- [6].Packer M, Poole-Wilson PA, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, et al. ATLAS Study Group Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. Circulation. 1999 Dec 7;100(23):2312–8. doi: 10.1161/01.cir.100.23.2312. [DOI] [PubMed] [Google Scholar]
- [7].Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. Jama. 2005 Jul 20;294(3):334–41. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- [8].Amato L, Paolisso G, Cacciatore F, Ferrara N, Ferrara P, Canonico S, et al. The Osservatorio Geriatrico Regione Campania Group Congestive heart failure predicts the development of non-insulin-dependent diabetes mellitus in the elderly. Diabetes & metabolism. 1997 Jun;23(3):213–8. [PubMed] [Google Scholar]
- [9].Ingelsson E, Arnlov J, Sundstrom J, Zethelius B, Vessby B, Lind L. Novel metabolic risk factors for heart failure. J Am Coll Cardiol. 2005 Dec 6;46(11):2054–60. doi: 10.1016/j.jacc.2005.07.059. [DOI] [PubMed] [Google Scholar]
- [10].Suskin N, McKelvie RS, Burns RJ, Latini R, Pericak D, Probstfield J, et al. Glucose and insulin abnormalities relate to functional capacity in patients with congestive heart failure. Eur Heart J. 2000 Aug;21(16):1368–75. doi: 10.1053/euhj.1999.2043. [DOI] [PubMed] [Google Scholar]
- [11].Rizza RA, Cryer PE, Haymond MW, Gerich JE. Adrenergic mechanisms for the effects of epinephrine on glucose production and clearance in man. J Clin Invest. 1980 Mar;65(3):682–9. doi: 10.1172/JCI109714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sacca L, Morrone G, Cicala M, Corso G, Ungaro B. Influence of epinephrine, norepinephrine, and isoproterenol on glucose homeostasis in normal man. J Clin Endocrinol Metab. 1980 Apr;50(4):680–4. doi: 10.1210/jcem-50-4-680. [DOI] [PubMed] [Google Scholar]
- [13].Ng TB. Adrenergic control of lipolysis in adipocytes of several mammalian species. Comparative biochemistry and physiology. 1985;82(2):463–6. doi: 10.1016/0742-8413(85)90193-8. [DOI] [PubMed] [Google Scholar]
- [14].Marangou AG, Alford FP, Ward G, Liskaser F, Aitken PM, Weber KM, et al. Hormonal effects of norepinephrine on acute glucose disposal in humans: a minimal model analysis. Metabolism. 1988 Sep;37(9):885–91. doi: 10.1016/0026-0495(88)90124-2. [DOI] [PubMed] [Google Scholar]
- [15].Small KM, McGraw DW, Liggett SB. Pharmacology and physiology of human adrenergic receptor polymorphisms. Annu Rev Pharmacol Toxicol. 2003;43:381–411. doi: 10.1146/annurev.pharmtox.43.100901.135823. [DOI] [PubMed] [Google Scholar]
- [16].Gonzalez Sanchez JL, Proenza AM, Martinez Larrad MT, Ramis JM, Fernandez Perez C, Palou A, et al. The glutamine 27 glutamic acid polymorphism of the beta2-adrenoceptor gene is associated with abdominal obesity and greater risk of impaired glucose tolerance in men but not in women: a population-based study in Spain. Clin Endocrinol (Oxf) 2003 Oct;59(4):476–81. doi: 10.1046/j.1365-2265.2003.01871.x. [DOI] [PubMed] [Google Scholar]
- [17].Ikarashi T, Hanyu O, Maruyama S, Souda S, Kobayashi C, Abe E, et al. Genotype Gly/Gly of the Arg16Gly polymorphism of the beta2-adrenergic receptor is associated with elevated fasting serum insulin concentrations, but not with acute insulin response to glucose, in type 2 diabetic patients. Diabetes Res Clin Pract. 2004 Jan;63(1):11–8. doi: 10.1016/j.diabres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- [18].Lima JJ, Matsushima N, Kissoon N, Wang J, Sylvester JE, Jusko WJ. Modeling the metabolic effects of terbutaline in beta2-adrenergic receptor diplotypes. Clin Pharmacol Ther. 2004 Jul;76(1):27–37. doi: 10.1016/j.clpt.2004.03.006. [DOI] [PubMed] [Google Scholar]
- [19].Jacob S, Rett K, Wicklmayr M, Agrawal B, Augustin HJ, Dietze GJ. Differential effect of chronic treatment with two beta-blocking agents on insulin sensitivity: the carvedilol-metoprolol study. Journal of hypertension. 1996 Apr;14(4):489–94. [PubMed] [Google Scholar]
- [20].Bakris GL, Fonseca V, Katholi RE, McGill JB, Messerli FH, Phillips RA, et al. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. Jama. 2004 Nov 10;292(18):2227–36. doi: 10.1001/jama.292.18.2227. [DOI] [PubMed] [Google Scholar]
- [21].Torp-Pedersen C, Metra M, Charlesworth A, Spark P, Lukas MA, Poole-Wilson PA, et al. Effects of metoprolol and carvedilol on preexisting and new on-set diabetes in patients with chronic heart failure {inverted exclamation}V data from the Carvedilol or metoprolol European Trial (COMET) Heart. 2007 Jan 19; doi: 10.1136/hrt.2006.092379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Duncan MH, Singh BM, Wise PH, Carter G, Alaghband-Zadeh J. A simple measure of insulin resistance. Lancet. 1995 Jul 8;346(8967):120–1. doi: 10.1016/s0140-6736(95)92143-5. [DOI] [PubMed] [Google Scholar]
- [23].Yusuf S, Ostergren JB, Gerstein HC, Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on the development of a new diagnosis of diabetes mellitus in patients with heart failure. Circulation. 2005 Jul 5;112(1):48–53. doi: 10.1161/CIRCULATIONAHA.104.528166. [DOI] [PubMed] [Google Scholar]
- [24].Vermes E, Ducharme A, Bourassa MG, Lessard M, White M, Tardif JC. Enalapril reduces the incidence of diabetes in patients with chronic heart failure: insight from the Studies Of Left Ventricular Dysfunction (SOLVD) Circulation. 2003 Mar 11;107(9):1291–6. doi: 10.1161/01.cir.0000054611.89228.92. [DOI] [PubMed] [Google Scholar]
- [25].Gress TW, Nieto FJ, Shahar E, Wofford MR, Brancati FL. Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus. Atherosclerosis Risk in Communities Study. The New England journal of medicine. 2000 Mar 30;342(13):905–12. doi: 10.1056/NEJM200003303421301. [DOI] [PubMed] [Google Scholar]
- [26].Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. 2000 May;43(5):533–49. doi: 10.1007/s001250051341. [DOI] [PubMed] [Google Scholar]
- [27].Bristow MR. beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000 Feb 8;101(5):558–69. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- [28].Yu XY, Lin SG, Wang XM, Liu Y, Zhang B, Lin QX, et al. Evidence for coexistence of three beta-adrenoceptor subtypes in human peripheral lymphocytes. Clin Pharmacol Ther. 2007 May;81(5):654–8. doi: 10.1038/sj.clpt.6100154. [DOI] [PubMed] [Google Scholar]
- [29].Reihsaus E, Innis M, MacIntyre N, Liggett SB. Mutations in the gene encoding for the beta 2-adrenergic receptor in normal and asthmatic subjects. Am J Respir Cell Mol Biol. 1993 Mar;8(3):334–9. doi: 10.1165/ajrcmb/8.3.334. [DOI] [PubMed] [Google Scholar]
- [30].Masuo K, Katsuya T, Fu Y, Rakugi H, Ogihara T, Tuck ML. Beta2-adrenoceptor polymorphisms relate to insulin resistance and sympathetic overactivity as early markers of metabolic disease in nonobese, normotensive individuals. Am J Hypertens. 2005 Jul;18(7):1009–14. doi: 10.1016/j.amjhyper.2005.01.006. [DOI] [PubMed] [Google Scholar]
- [31].Sarafidis PA, Bakris GL. Antihypertensive treatment with beta-blockers and the spectrum of glycaemic control. Qjm. 2006 Jul;99(7):431–6. doi: 10.1093/qjmed/hcl059. [DOI] [PubMed] [Google Scholar]
- [32].Sotoodehnia N, Siscovick DS, Vatta M, Psaty BM, Tracy RP, Towbin JA, et al. Beta2-adrenergic receptor genetic variants and risk of sudden cardiac death. Circulation. 2006 Apr 18;113(15):1842–8. doi: 10.1161/CIRCULATIONAHA.105.582833. [DOI] [PubMed] [Google Scholar]
- [33].Lanfear DE, Jones PG, Marsh S, Cresci S, McLeod HL, Spertus JA. Beta2-adrenergic receptor genotype and survival among patients receiving beta-blocker therapy after an acute coronary syndrome. Jama. 2005 Sep 28;294(12):1526–33. doi: 10.1001/jama.294.12.1526. [DOI] [PubMed] [Google Scholar]
- [34].Shin J, Lobmeyer MT, Gong Y, Zineh I, Langaee TY, Yarandi H, et al. Relation of beta(2)-adrenoceptor haplotype to risk of death and heart transplantation in patients with heart failure. The American journal of cardiology. 2007 Jan 15;99(2):250–5. doi: 10.1016/j.amjcard.2006.08.020. [DOI] [PubMed] [Google Scholar]
- [35].Shin J, Johnson JA. Pharmacogenetics of beta-Blockers. Pharmacotherapy. 2007 Jun;27(6):874–87. doi: 10.1592/phco.27.6.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liggett SB. Beta2-adrenergic receptor polymorphisms and sudden cardiac death: a signal to follow. Circulation. 2006 Apr 18;113(15):1818–20. doi: 10.1161/CIRCULATIONAHA.105.618967. [DOI] [PubMed] [Google Scholar]
- [37].Terra SG, Pauly DF, Lee CR, Patterson JH, Adams KF, Schofield RS, et al. beta-Adrenergic receptor polymorphisms and responses during titration of metoprolol controlled release/extended release in heart failure. Clin Pharmacol Ther. 2005 Mar;77(3):127–37. doi: 10.1016/j.clpt.2004.10.006. [DOI] [PubMed] [Google Scholar]